Small laboratory animal models of anterior cruciate ligament reconstruction
Abstract
Anterior cruciate ligament (ACL) injuries are common knee ligament injuries. While generally successful, ACL reconstruction that uses a tendon graft to stabilize the knee is still associated with a notable percentage of failures and long-term morbidities. Preclinical research that uses small laboratory species (i.e., mice, rats, and rabbits) to model ACL reconstruction are important to evaluate factors that can impact graft incorporation or posttraumatic osteoarthritis after ACL reconstruction. Small animal ACL reconstruction models are also used for proof-of-concept studies for the development of emerging biological strategies aimed at improving ACL reconstruction healing. The objective of this review is to provide an overview on the use of common small animal laboratory species to model ACL reconstruction. The review includes a discussion on comparative knee anatomy, technical considerations including types of tendon grafts employed amongst the small laboratory species (i.e., mice, rats, and rabbits), and common laboratory evaluative methods used to study healing and outcomes after ACL reconstruction in small laboratory animals. The review will also highlight common research questions addressed with small animal models of ACL reconstruction.
1 INTRODUCTION
Anterior cruciate ligament (ACL) injuries are common sport-related knee ligament injuries. The general population risk of ACL injury has been estimated to be 68.6 per 100,000 people,1 whereas the estimated the sports risk of ACL injury ranges from 0.25 to 2.62/10,000 athletic exposures.2 A separate analysis estimated the overall athlete ACL injury sport risk to be 3.5% among female athletes (1 in 29 female athletes) and 2.0% amongst male athletes (1 in 50 male athletes).3 The current standard of care for ACL injuries is ligament reconstruction, which includes the implantation of a tendon graft into bone tunnels. A recent US commercial claims and encounters database suggest that the prevalence of ACL injury and reconstruction is increasing over time. The study reported an increase of 22% for ACL reconstructions between 2002 and 2014, particularly in the under-18 population.4
While generally successful, the failure rates after ACL reconstruction are not negligible. A systematic review identified the long-term ACL graft rupture risk to be 7.9% (range 3.2%–11.1% among individual studies).5 A separate study reported the long-term rates of clinical failure after ACL reconstruction (i.e., >2+ pivot, IKDC grade C or D Lachman, and/or >5 mm on KT arthrometer) to be 10.3%.6 The causes of failures after ACL reconstruction can be related to various factors including abnormal mechanical loads, errors in graft tunnel placement, failure of graft fixation or incorporation, unaddressed associated injuries, or multifactorial.7-9 In addition to failure of ACL reconstruction, the long-term morbidity due to posttraumatic osteoarthritis (OA) following ACL reconstruction is also notable. A recent systematic review reported a rate of symptomatic tibiofemoral and patellofemoral OA to be 35% and 15%, respectively.10
Due to a notable rate of failure and morbidity with ACL reconstruction, in vivo research that attempts to identify underlying reasons for failure or elucidating biological factors (i.e., cells or cellular mediators) and cellular mechanisms that may improve healing continue to be areas of research interest. Small animal laboratory species are not only used to model both noninvasive and invasive models of ACL injury,11-15 but they are also commonly used to model ACL reconstruction to study healing and posttraumatic OA after ACL reconstruction.15-18 Common small animal species used for laboratory research include mice, rats, guinea pigs, and rabbits.19-21 ACL injury models have been described in all four aforementioned small animal species; however, mice, rats, and rabbits are the most applicable small animal species for ACL reconstruction research.
In addition to evaluating factors that impact healing and long-term morbidity after ACL reconstruction, small animal species are also used to develop novel treatments. As new treatment strategies emerge around ACL reconstruction, preclinical models to assess for feasibility, safety, and efficacy of new interventions around ACL reconstruction are needed. Development and advances in biomaterials and application of orthobiologics that improve graft incorporation or mitigate OA risk are areas of active investigation using small animal models of ACL reconstruction.22-24 Typically, longitudinal in vivo studies in laboratory animals provide investigators with the most effective models for ethical translation of new treatments before application in humans.25 In general, small laboratory animal ACL models can be effective for biochemical, biomechanical, and histologic analyses while large animal models (i.e., dogs, pigs, and goats/sheep) combine these outcome measures with additional ease to better evaluate knee stability and function, kinetic and kinematic analyses, biomarkers, arthroscopic assessments, and clinically relevant diagnostic imaging.26-28
While the foundational principles of experimental design for animal models of ACL reconstruction are consistent regardless of the animal species, ACL reconstruction models can vary widely with respect to specie graft source, surgical technique, postoperative management, validated outcome measures, and healing periods. Differences in anatomy, biomechanics, associated costs, and clinical applicability amongst different species used to model ACL surgery should also be considered.29, 30 Consequently, selecting the appropriate animal model that has the appropriate end-point analyses to allow for effective hypothesis testing and valid clinical application for ACL research is key to a successful translational research strategy. In addition, compliance with animal research guidelines such as PREPARE and ARRIVE are key factors to guarantee quality, reproducibility, and translatability as well as implementing the 3Rs (Replacement, Reduction, and Refinement) for performing humane and ethical animal research.31-33 The objectives of this review are to (1) highlight the comparative knee anatomy among common small animal species used in ACL reconstruction (i.e., mice, rats, and rabbits), (2) discuss the technical considerations including types of tendon grafts employed amongst the different small animal species, (3) review the common laboratory evaluative methods used to study different research questions surrounding ACL reconstruction in small laboratory animals, and (4) provide an overview of common research questions addressed with small animal models of ACL reconstruction.
2 TRANSLATIONAL CONSIDERATIONS IN MODELING ACL RECONSTRUCTION IN SMALL LABORATORY ANIMALS
The common small animal species used for laboratory research include mice, rats, guinea pigs, and rabbits.19-21 There are several factors that should be considered as it relates to using small laboratory animals to model ACL reconstruction. These include how to best represent the complexity of ACL injury, how to employ grafts and surgical techniques that most closely mimic the human clinical scenario, and incorporating outcome measures that consider relevant timing and clinical relevance to answer the research question. Researchers will have to balance these factors with regard to the conclusions they hope to achieve following the study.
2.1 Methods of inducing ACL injury in small laboratory animals
Some key considerations in modeling ACL reconstruction amongst various small animal species for preclinical research include the nature of the ACL injury, type and timing of treatment, and clinical relevance of the outcome measures. Many preclinical models of ACL injury that are combined with reconstruction employ surgical transection of the ligament followed by immediate treatment via reconstruction or repair. This approach has been described for mice, rats, and rabbits, but not guinea pigs.34-39 In addition to studying ACL reconstruction, ACL complete or partial surgical transection is also used to induce posttraumatic OA to study the effects of joint degeneration, which has been described in rodents, guinea pigs, and rabbits.13, 40-44 While surgical-induced injury is an ethical, cost-effective, and biomechanically relevant approach, an important limitation of an ACL surgical transection injury model when compared to an induced ACL rupture model is the lack of whole-joint injury that occurs after an acute rupture (i.e., injury to the articular cartilage, meniscus, subchondral bone, and joint capsule) and the associated intraarticular responses (i.e., hemarthrosis, inflammation, and edema). The immediate reconstruction at the time of transection also does not recapitulate what occurs in human scenarios.
Alternatively, mechanically induced ACL rupture models in small animal species have been described with mice and rats, but not in rabbits or guinea pigs.11, 12 Noninvasive means of inducing ACL injury produce some of the associated intraarticular injuries and responses associated with ACL injury. Mechanical rupture models have been used to evaluate a range of topics including the effects of ACL injury on muscle atrophy and progression of posttraumatic OA.45, 46 While noninvasive means of inducing ACL injury permit a timing to reconstruction that may better replicate the human condition (i.e., surgical reconstruction that occurs separately from the ACL injury), to the best of our knowledge, there has not been a preclinical study that combined a noninvasive method of inducing ACL rupture followed by ACL reconstruction in small laboratory animals.
2.2 ACL graft ligamentization in small laboratory animals
Successful ACL graft remodeling, incorporation, and function depend on a complex interplay among factors including inflammation, joint comorbidities, graft biologic and material properties, tunnel placement and fixation method, and postoperative management protocol.47 Animal studies can engender widely different durations and magnitudes for functional graft healing based on each of these primary factors. In general, early healing and proliferation phases for tendon grafts used for ACL reconstruction in animals exhibit a greater extent of initial degeneration compared to what is reported for human patients, such that mechanical properties of the ACL reconstruction may be more compromised initially.48 In contrast, graft integration and remodeling are typically completed within 6–12 months in animal models, encompassing a much shorter period compared to the 3-year or longer period reported for humans.49
There should therefore be a general recognition that much of what is known on the timing of various phases of graft ligamentization after ACL reconstruction is established in larger animal species (i.e., rabbits, sheep, and dogs), and not in smallest laboratory species such as mice and rats. Hence, there is a need for additional information on the timing of various phases of graft ligamentization amongst the smallest laboratory animal species. This has resulted in the lack of standardization on defined postoperative endpoint time points amongst mice and rat ACL reconstruction studies. For example, common study endpoints for rodents are 2 and 4 weeks whereas others have used 3 and 6 weeks.36, 37, 50 It is unclear what phases of graft ligamentization this time point corresponds to amongst mice and rats. The lack of long-term ligamentization studies among mice and rats suggests that current endpoint analyses may be due to established laboratory practice and study constraints rather than defined ligamentization healing phases.
In contrast to mice and rats, there are longer-term ligamentization studies in rabbits that may guide investigators on the relative timing of graft maturation phases in this species. Giordano et al.38 histologically evaluated the ligamentization of semitendinosus (ST) autograft amongst New Zealand white rabbits.38 One month after ACL reconstruction, numerous disorganized fibroblasts and collagenous fibers were identified during the early phases of ligamentization. A marked cellular necrosis stage was noted by 3–4 weeks after graft placement. This was then followed by a reduction of cellular necrosis that was observed in the early phase of the neo-ligament healing process. By 8 weeks, the collagen fibers had become aligned in parallel with newly formed capillaries and highly differentiated fibroblasts. At 40 days after surgery, the tissue necrosis stage was no longer present, and vascularization of the graft was readily seen. At 24 and 48 weeks the transplanted tendon still differed histologically from both semitendinosus tendon (ST) and the native ACL. Given there were no further significant histologic changes seen after 24 weeks when compared to 48 weeks, the authors concluded that the ligamentization process in rabbits may be complete by 24 weeks with semitendinosus autografts in New Zealand White rabbits.
Similar to semitendinosus autograft, a long-term rabbit study on patellar tendon (PT) autograft has also been performed. Ballock et al.39 evaluated ACL reconstruction using PT autograft over 52 weeks in New Zealand white rabbits.39 At 6 weeks, the graft was hypercellular with rounded or ovoid cells with some central area of hypocellularity. The graft collagen orientation was haphazard and there were no vascular channels noted at 6 weeks. At 30 weeks, the graft had areas of longitudinal fiber orientation with variable crimp patterns and occasional vascular channels along the periphery of the graft. At 52 weeks, the graft had an overall parallel alignment with vascular segments in the periphery and mid-substance of the graft. A notable finding in this study was that the anteroposterior (AP) laxity of the reconstructed knees at 52 weeks among the rabbits was greater than ACL deficient knees (5.2 ± 0.7 mm vs. 3.4 ± 0.3 mm, respectively). These findings may suggest the grafts evaluated in Ballock et al. may be clinically failed grafts that were under significant strain and stretched over the course of the study rather than successful stable grafts. In summary, investigators may need to consider what is known in terms of the timing of tendon graft healing amongst different small laboratory animal species when choosing the appropriate species for their study.
2.3 Tendon graft considerations in small animal ACL reconstruction models
The intrinsic characteristics of the tendon graft used to reconstruct the injured ligament along with the variation among the small animal species should be another primary consideration for preclinical study design.18 The superficial digital flexor (SDF)—also known as long digital flexor (LDF) in bipedal animals—and the semitendinosus (ST) tendon autografts are frequently the tendon graft used for ACL reconstruction in mice, rats, and rabbits. Comparatively, tendon grafts are more commonly used for human patients including patellar (PT), hamstring (HT), and quadriceps (QT) tendons. Traditional tendon graft choices for human ACL reconstructions are commonly more effectively employed and clinically relevant for large animal models of ACL reconstruction.51, 52 While time-zero tissue biomechanical properties of human tendon graft used for ACL reconstruction are widely available and typically exceeds native ACL properties,53 such time-zero data regarding the material properties of tendon grafts used for small animal ACL reconstruction relative to the species' native ACL are largely lacking. It should also be recognized that the biological properties of tissue chosen for animal ACL reconstruction may vary, and therefore, need to be considered during the analysis.54
It has been suggested that all tissue grafts used for ACL reconstruction experience a decrease in strength of more than half during the first stages of healing and only recover between 50% and 60% of the native ACL's material properties after remodeling is complete.48 Importantly, differences between autografts and allografts in terms of differences in material properties, incorporation, and healing noted in human patients hold true in animal models as long as harvest, processing, and storage methods are similar.51, 55, 56 In addition, species-specific differences in knee morphology, ACL graft geometry, and composition affect biomechanical properties, increasing the importance of graft choice in each species for comparisons with human grafts. One anatomic variation amongst species worth noting that may be relevant to studying the ACL is the functional bundles concept. It has been a long-held belief that the human ACL has two main functional bundles (anteromedial and posterolateral bundles).57 This however has been recently revisited where some have described additional bundles or contend that the ACL is actually a single ribbon-like structure.58-61 Regardless of the number of bundles, it is likely that tendon graft reconstruction of the ACL likely does not recapture all of the exquisite detail of the native ACL. The main comparative morphological variations amongst mice, rats, and rabbits relative to humans are summarized in Table 1.
| Morphological feature | Rodent (mice)62 | Rabbit61, 63 | Human61, 63 |
|---|---|---|---|
| Number of ACL bundles | Two | One | Two57/Three59 |
| Tibial plateau angle | 24.32 ± 1.50a | 24 ± 5 | 7 ± 4 |
| 32.46 ± 2.64b | |||
| ACL width (mm) | N/A | 4.84 ± 1.24 | 12.73 ± 1.35 |
| ACL length (mm) | N/A | 10.6 ± 1.38 | 37.78 ± 3.17 |
| PCL width (mm) | N/A | 4.34 ± 1.27 | 14.23 ± 5.17 |
| PCL length (mm) | N/A | 10.0 ± 1.52 | 40.30 ± 7.45 |
| Medial meniscus width (mm) | N/A | 2.4 ± 0.35 | 9.5 ± 0.71 |
| Medial meniscus length (mm) | N/A | 9.2 ± 0.4 | 39.8 ± 3.71 |
| Lateral meniscus width (mm) | N/A | 4.06 ± 0.47 | 9.83 ± 0.69 |
| Lateral meniscus length (mm) | N/A | 10.0 ± 1.17 | 33.28 ± 3.53 |
| Notch width (mm) | 0.5 ± 0.1 | 3.88 ± 0.51 | 21.9 ± 5.0 |
- Abbreviations: ACL, anterior cruciate ligament; N/A, not available; PCL, posterior cruciate ligament.
- a Range of postero-lateral tibial slope.
- b Posteromedial tibial slope.
2.4 Desired outcome analyses in small animal ACL reconstruction
The final consideration in designing a preclinical study is the available study outcome analyses that may be used to answer the study question. Serial clinical exams to assess ACL graft laxity (i.e., Lachman exam or anterior drawer), which is possible in larger animal species,25, 51, 52, 64 are not as easily performed in the smaller laboratory animal species, such as mice and rats. Similarly, functional assessment, such as gait analysis, is achievable but may be comparably less established after ACL reconstruction in small animal laboratory species when compared to certain larger animal models, particularly dogs where it is commonly used in canine clinical care.51, 64 Biomechanical endpoint analyses of small animal femoral-ACL-tibia-complexes however are commonly performed including time-zero and/or initial endpoint measures of load-to-failure and stiffness.18, 34-37, 65 Less commonly, Young's modulus, absorbed energy, and displacement are reported. High-resolution imaging including microcomputed tomography (μCT), magnetic resonance imaging (MRI; ≥7 T), and micro-polyethylene terephthalate (PET) have all been used to evaluate graft healing after small animal models of ACL reconstruction.66-68 A variety of histological endpoint assessments including the intraarticular ACL graft, graft tunnels, and chondral surfaces are also readily performed in small animal models. The main characteristics of animal studies involving ACL reconstruction in small animal species, including type of procedure, graft used, postoperative management, and outcome measures are summarized in Supporting Information: Table S1.
3 SMALL ANIMAL MODELS OF ACL RECONSTRUCTION
The common small laboratory animal species used for ACL reconstruction are mice, rats, and rabbits. We are not aware of ACL reconstruction studies using the guinea pig species. The below sections will summarize the characteristics of ACL reconstruction models in mice, rats, and rabbits.
3.1 Rodent ACL reconstruction models
Mice and rats can serve as important models for biomedical research, particularly mechanistic studies targeting ACL injury and reconstruction.69, 70 The anatomical, physiological, and genetic similarities to humans in combination with their small size, ease of handling, capabilities for genetic manipulation, and abundant availability foster the use of rodents in ACL research.69 Further, rodents present economic advantages based on costs for animals, housing, and maintenance as well as shorter timeframes to reach skeletal maturity. Mice have been recently used for ACL reconstruction studies,71, 72 and its inherent advantages relative to other small animal species include several transgenic species of mice that are available, which permits evaluation of impact of specific genes in healing after ACL reconstruction via knockout models. Based on its larger relatively larger size and associated technical advantages, rats have traditionally served as a more attractive rodent model with the Sprague–Dawley strain being the most popular.17, 37, 73, 74
3.2 Tendon graft choices in rodent ACL reconstruction
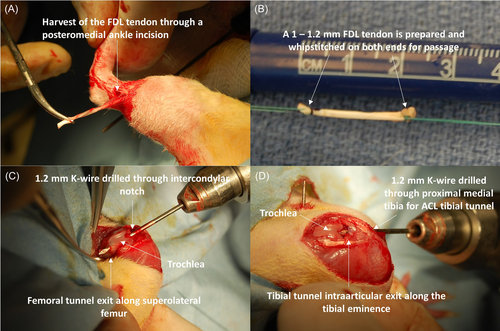
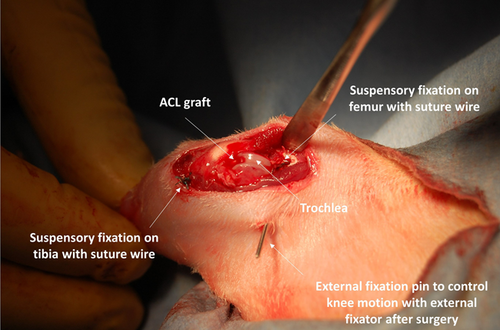
The SDF tendon autograft is the primary choice for ACL reconstruction in both mice and rats based on access, harvest technique, and size (Figures 1 and 2).17, 35-37, 50, 72, 75-77 None of the tendons typically used for human ACL autografts nor any type of allografts have been commonly used in both rodent models, which hinders direct comparison of graft properties across species.
3.3 Outcome measures in rodent ACL reconstruction models
The “grimace scale” provides a standardized, semiautomated method to evaluate pain severity in rodents.78, 79 To assess function, gait analysis methods for rodent models measure stride width, stride length, and velocity by applying stamp-pad ink to the hind paws before the animal walking through a tunnel lined with white paper.80 Semiautomated methodologies for kinematic gait analysis that incorporate video analysis, custom arthrometers, and fluoroscopic analysis to evaluate flexion-extension and anterior-posterior translation have been specifically designed for small laboratory animals.81-87 Additionally, controlled loading after surgery while the animal is chemically restrained has been performed to make comparisons among physical therapy protocols and activity regimes.35, 72 Other functional outcome measures used in rodents, such as lameness scores, have also being adapted from other larger species and applied in several studies.81-90
Imaging outcomes in rodents usually include the evaluation of tendon-to-bone integration assessed using μCT. Bone volume fraction, bone density, and area metrics for bone tunnels provide valuable information in regards to the tendon graft integration with the bone.35, 37, 72, 75, 91 The recent advancement and availability of high-field MRI scanners along with improved designs in radiofrequency and more efficient pulse sequences have made high-quality images feasible for acquisition in small rodent knees.67 High-strength (≥7 T) MRI can detect cartilage thickness changes and arthritic features that are challenging at lower strengths.67, 68 High magnetic fields with specifically adapted measurement strategies permit direct or indirect measures for rodent cartilage pathobiology.92 Goebel et al.93 used 7 T MRI after ACL transection (ACLT) to evaluate cartilage thickness in weight-bearing areas of the knee to compare control and OA-induced rats. In addition, techniques such as ultra-short-echo time along with contrast agents (gadolinium) facilitate the assessment of hydration status and estimation of glycosaminoglycan content that could indicate pathological processes within the joint.94 However, due to specific anatomic and histologic variations in rodents with respect to humans, such as persistent epiphyseal cartilage, increased posterior curvature of the femoral condyles, and a distally fused tibiofibular joint, direct extrapolation of findings may be limited.71, 95, 96
In addition to imaging analysis, other data related to cartilage and joint health can be obtained from rodent ACL models. While serum, urine, and synovial fluid (SF) biomarkers may be useful to assess joint inflammation and cartilage degradation in humans, rodents are often limited by the volume of body fluids that can be collected. As an example, only 0.2–0.4 and 1–2 ml of blood can be collected from mice and rats, respectively, using jugular vein access under anesthesia, and only 0.5–1 ml of blood can be collected from mice, or 5–10 ml from rats, when using cardiac puncture at study termination.97, 98 SF collection is much more complicated and only ~1 and ~100 µl can be recovered after joint lavage in mice and rats respectively, after adjusting for dilution.83, 84, 89, 90, 97, 98 Alternative methods of SF recovery have been described including Whatman Paper Recovery, Polyacrylate Bead recovery, and calcium sodium alginate compound recovery, which may facilitate extracting small amounts of SF for biomarker analysis.89
Biomechanical testing is a common procedure that evaluates the material properties of grafts before and after surgery. AP displacement of the tibia with respect to the femur, load to failure, and stiffness are the main outcome measures assessed. However, lack of consistency in testing methods has resulted in some degree of variability in reported measures. Fu et al.36 used a 70° flexion and an anterior pulling force (2 N) applied to the tibia at a displacement rate of 20 mm/min to test AP displacement, and tested load to failure at 50 N with a displacement rate of 40 mm/min. Kawakami et al.50 tested load to failure at a 0.25 mm/s (15 mm/min) displacement rate, while the Rodeo et al. uses a 0.167 mm/s (10 mm/min) rate.37, 75 Hence, such discrepancies may affect interpretation of biomechanical results, especially when added to other confounding variables such as animal sex, age, or timepoint differences among studies. The biomechanical results of mice and rat ACL reconstructions are listed in Table 2.
| Species | Ligament/graft | Load to failure (N) | Stiffness (N/mm) | References |
|---|---|---|---|---|
| Mice | Native ACL | 5.60 ± 0.75 | 3.44 ± 1.47 | 34 |
| LDF | 1.79 ± 0.40a | 2.59 ± 0.87a | ||
| Rat | Native ACL | ~35.5 | ~25.6 | 36, 37 |
| LDF | 12.51–24.16 | 9.44–18.86 |
- Abbreviations: ACL, anterior cruciate ligament; LDF, long digital flexor.
- a Four-week postoperative.
Graft healing within the bone tunnel is commonly evaluated using histological scores that have been adapted from other species and are now well established for rodent tissues.36, 99 A graft degeneration scale from 0 to 3 was used in the study by Fu et al. under bright field and polarized illumination.36 In addition, this score evaluates healing in the bone-tendon interface and adverse peri-graft bone changes (defined as the presence of bone intrusion into the tendon graft, peri-graft bone erosion, or the presence of cysts in peri-graft interface).36 Another histologic score developed for rabbits has also been used to assess ACL graft incorporation in rats.99, 100 Histologic scores for tendon-bone healing assessment in rodents are summarized in Supporting Information: Table S2.
3.4 Clinical questions evaluated using rodent ACL models
The rat model has been particularly valuable for improving ACL reconstruction techniques by assessing the effects of tunnel location, ACL tendon graft pretension magnitude, and postoperative activity protocols on graft strain, tendon-to-bone integration, graft remodeling, and graft survival.17, 18, 37, 75, 101-103 Similarly, murine models of ACL reconstruction have been used to study the tendon-to-bone formation and the impact of postoperative mechanical load on graft tunnel incorporation after ACL reconstruction.34, 35 Although these studies have significantly enhanced our understanding regarding pretension magnitude, graft positioning, and the importance of controlling the postoperative mechanical load on reconstructed rodent knees, consensus in experimental design still needs to be reached. Moreover, the use of high graft pretension magnitudes relative to body weight poses an additional challenge in how to determine a correct comparison method that allows for valid translation to clinical application. Augmentation techniques to enhance graft-bone integration and to mitigate bone tunnel diameter expansion around the graft along with the improved histological appearance and higher tensile load to failure are focuses of current work using rodent models.50
3.5 Rabbit ACL reconstruction models
Rabbit models have been effectively used with reported advantages over rodent models, including animal size, graft selection, and sampling capabilities.18, 65, 104-107 While numerous variations of lapin ACL reconstruction have been described, a New Zealand White rabbit model using long digital extensor (LDE) tendon autograft is the most used model.108
3.6 Graft choices for rabbit ACL reconstruction
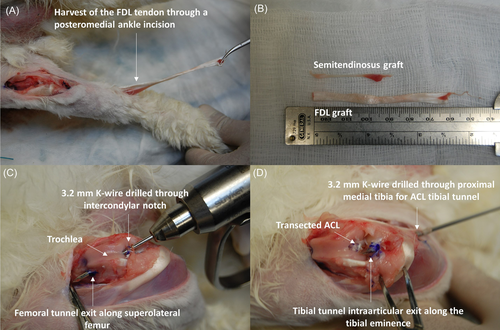
Common grafts used to reconstruct the ACL in rabbits include PT, ST, and LDE autografts.18, 65, 104-106, 108, 109 However, due to the variation in size, especially cross-sectional area, the mechanical properties of rabbit tendon grafts vary significantly (Table 3). While the PT graft can be harvested similar to human patients, it is not as commonly used as ST and LDE grafts. While the ST can be harvested without complications, the graft is significantly smaller and shorter when compared to the LDE graft, making the surgical procedure more challenging (Figure 3B). On the other hand, LDE grafts are more easily harvested due to their increased length and more superficial distribution along the distal hindlimb (Figure 3A). Although the tendon autografts used in rabbits may be similar to those used in humans, the need for open arthrotomy and lack of specific instrumentation for implantation and fixation used to reconstruct the ACL, could limit the translational potential of this model (Figure 4).
| Graft | Load to failure (N) | Stiffness (N/mm) | References |
|---|---|---|---|
| Native ACL | 351.8 (±41.6) | 66.6 (±11.2) | 18 |
| PT | 87.2–178.9 | 20.1–49.9 | 18 |
| HT (ST) | 28.2–69.33 | 5.24–10.68 | 104 |
| LDE | ~8–34 | N/A | 105 |
- Abbreviations: HT (ST), hamstring tendon (semitendinosus); LDE, long digital extensor; N, Newtons; N/A, not available; PT, patellar tendon.
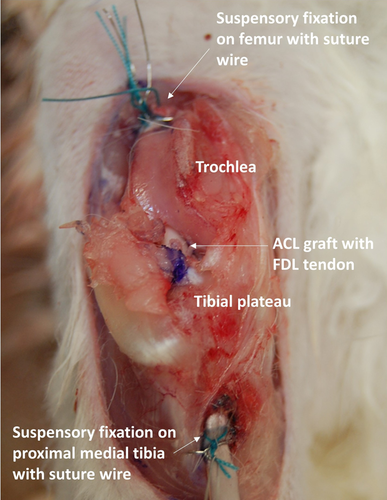
3.7 Outcome measures in rabbit ACL reconstruction models
Like rodents, the grimace scale is often used as an assessment of pain severity in rabbits.110, 111 Due to the natural position and structural biology of rabbit hindlimbs, which ambulate through hopping instead of walking, forces applied to the ACL graft might not be comparable to other species. Kinematic gait analysis is therefore only accurate when using complex methods such as infrared emitting diodes implanted through intracortical pins in the tibia and the femur in rabbit ACL models.112 Functional evaluation in the rabbit is also assessed by adapting the Lachman test, range of motion, and lameness score from humans and larger species.112-114
High-resolution MRI along with adapted grading has been used in rabbit models of ACLT to evaluate cartilage integrity.115 Kajabi et al.116 used multiparametric 9.4 T MRI to demonstrate early degenerative changes in the superficial cartilage of an ACLT rabbit model. Furthermore, Chai et al.117 used 7.1 T MRI to evaluate ligament-bone integration, tunnel diameter, and signal-to-noise ratio in a rabbit model of ACL reconstruction using a synthetic PET ligament.117 Additional imaging techniques, such as µCT scans, have also been implemented in the rabbit model to assess tendon-bone integration. For example, Bi et al.65 utilized µCT to demonstrate that reconstructing the rabbit ACL with silk-collagen scaffold decreased the average femoral bone tunnel area compared to the semitendinosus autograft at 4 weeks postoperatively.65 Quantitative computed tomography was used to determine that a 3-week COX-2 inhibitor (celecoxib) treatment decreased area of trabecular bone, cortical density, and new bone formation.104 Moreover, µCT was also used to evaluate new bone formation between an additive manufactured (AM) porous-titanium interference screw implant and a conventional interference screw.105
In contrast to rodents, evaluation of ACL healing and OA biomarkers is more feasible in the lapin model due to larger volumes of blood and SF.118, 119 Moreover, Luo et al.120 demonstrated significant differences in SF proteome between ACL transected and control rabbits, including adiponectin, pyruvate kinase, fibrinogen, paraoxonase-1, and carboxilesterase-2. Further, the concentrations of inflammatory mediators (IL-1β and IL-17) and gene expression of the degradative enzyme, MMP-13, were also significantly higher in antero-medial bundle and posterolateral bundle resection groups compared to controls.13
With regard to biomechanical testing, Woo et al.121 described a detailed protocol to evaluate the structural properties of the rabbit femur-anterior cruciate ligament-tibia complex (FATC). However, there is not a consensus on the most appropriate biomechanical testing protocol for the rabbit FATC, with published reports using different knee flexion angles ranging from 45° to 90° flexion during testing and different displacement rates ranging from 0.5, 5, 10, 20, 25, to 60 mm/min (1 mm/s).18, 65, 104-106, 122 Because of the variation in testing technique, it is difficult to compare biomechanical outcomes among studies.
In terms of quantitative histological assessment, a four-point histological grading score has been developed for ACL reconstruction in the rabbit.123 Demirag et al.123 used a semiquantitative histological score to assess the healing process at the bone-tendon interface based on the presence of fibrovascular tissue consisting of fibroblasts and inflammatory cells and newly formed Sharpey-like collagen fibers (Supporting Information: Table S2).123
3.8 Clinical questions evaluated with rabbit ACL models
Like rodents, lapin models have been used to evaluate the effects of surgical technique on ACL healing. Graft tension magnitudes in rabbits range from 1 to 17 N, the latter being associated with initial (2-week postoperative) occurrence of necrosis and lower cellularity.18 However, careful interpretation of results is recommended, especially when applying high pretension to the graft, which could potentially lead to cartilage damage as seen in humans when magnitudes above 40 N are implemented.124 Therefore, adequate scaling of tension magnitudes to each animal model is needed.
In addition, rabbit model studies have been able to examine the effects of inflammatory inhibition, growth factors, and bio-enhancement on tendon graft-bone integration.104-106 Based on their relative size and knee anatomy, clinically relevant fixation devices have been evaluated and SF biomarkers have been assessed in rabbits.104-106, 122 Consequently, the rabbit model holds promising potential for cost- and time-effective preclinical ACL reconstruction studies aimed at screening methods for improving graft tendon healing and incorporation.
4 LIMITATIONS AND FUTURE DIRECTIONS
There are notable limitations when evaluating the existing literature on use of mice, rats, rabbit models of ACL reconstruction. Many of the ACL reconstruction studies in small laboratory animals use surgical transection as the mode of injury. This does not recreate what occurs with human ACL injury including the subsequent inflammatory cascade, which occurs via nonsurgical means. While noninvasive rupture models have been described in mice and rats, they have not been combined with ACL reconstruction. Furthermore, unlike human surgery, surgical reconstructions in small animals are done via an open arthrotomy and the relative standardization of how ACL reconstructions are performed amongst small laboratory animals and postoperative regiment is not done. Small animal studies have shown that outcomes from graft positioning, tensioning, and limb immobilization can all impact biomechanical and histological outcomes. Furthermore, while biomechanical and histological scoring systems have been described in both rodents and rabbits, a universal protocol is not used in small animal ACL reconstructions. Finally, the defined ligamentization phases of ACL graft are not established in small animal ACL reconstruction studies. This has resulted in studies using different endpoint analyses based likely on established laboratory practice rather than defined healing phases of ACL reconstruction in small animals. The lack of standardization regarding how ACL reconstruction studies are conducted limits the ability the perform interstudy comparisons in small animal models of ACL reconstruction.
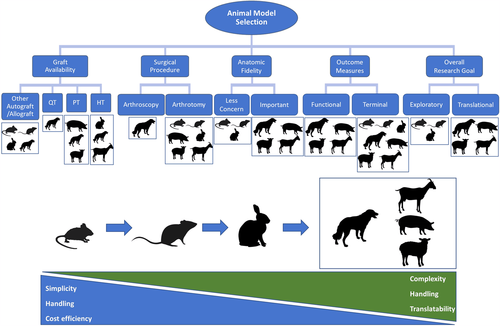
Figure 5 provides a few generalizations regarding the selection of the appropriate animal species to model ACL reconstruction. As an example, if a particular research study was to evaluate differences in a particular objective outcome-based on grafts that are used in humans (i.e., quadriceps, PT, or hamstring tendon), dogs are the only species that have been used in the literature to model each graft type with ACL reconstruction. If the ACL research goal is to obtain data that are to be interpreted to be translational, a larger animal species such as dogs, pigs, sheep, or goats are more likely to achieve that goal. While larger animal species have similar translational potential, the handling of sheep, pigs, and goats may be more complex than dogs. Researchers should also recognize that while larger animal species may solve some of the aforementioned small animal limitations (i.e., better defined ligamentization phases, use of human instrumentation and tendon graft choices, possible arthroscopic surgery), large animal species also share similar limitations (i.e., graft positioning, how ACL injury is induced). Therefore, it is imperative for researchers to design studies with these limitations acknowledged, use standardized methods for outcome measures when possible,125 employ comprehensive assessments with correlations, and move to larger animal models if translational applications are the ultimate goal particularly if defined phases of ACL ligamentization and fidelity to human surgery are important.
5 CONCLUSIONS
Small animal models for reconstruction offer ethical methods for mechanistic and screening studies on clinical questions surrounding ACL reconstructions. Studies on ACL graft positioning, graft tensioning, and changes in the postoperative mechanical environment of the reconstructed knee are readily accomplished in small animal laboratory species. Evaluation of new biologics or biomaterials that may improve healing after ACL reconstruction can also be assessed in small laboratory species. There are established evaluative methods to evaluate healing including biomechanical testing, imaging with CT and high-resolution MRI, and histological scoring systems in both rodents and rabbits. The advantages of small laboratory animals relative to larger animal species include its relatively significant decrease in costs, which typically affords the ability to achieve larger sample sizes. While there are notable limitations amongst small animal ACL reconstruction models, it can effectively inform and optimize experimental designs for pivotal large animal model studies such that the 3 Rs for ethical use of animals in research are addressed and the safety and efficacy of preclinical translational studies are optimized.
AUTHOR CONTRIBUTIONS
Sebastian Cardona-Ramirez, Aaron M. Stoker, James L. Cook, and Richard Ma: Manuscript conception and design; Sebastian Cardona-Ramirez, Aaron M. Stoker, James L. Cook, and Richard Ma: Draft manuscript preparation. All authors reviewed the manuscript and approved the final version of the manuscript.
CONFLICT OF INTEREST
James L. Cook is a paid consultant, paid presenter or speaker and receives research support from Arthrex, Inc; is a paid consultant for Bioventus; receives research support from the Coulter Foundation; receives research support from DePuy, A. Johnson & Johnson company; receives research support from GE Healthcare; is on the editorial or governing board of the Journal of Knee Surgery; receives research support from Merial; is a board or committee member for Midwest Transplant Network; is a board or committee member, receives IP royalties and research support from Musculoskeletal Transplant Foundation; receives research support from National Institutes of Health (NIAMS and NICHD); receives research support from Purina; receives publishing royalties, financial or material support from Thieme; is a paid consultant for Trupanion; and receives research support from the U.S. Department of Defense. Aaron M. Stoker receives IP royalties from Musculoskeletal Transplant Foundation. Richard Ma is a board or committee member for the American Orthopaedic Association; is a board or committee member for the American Orthopaedic Society for Sports Medicine; receives research support from Cartiheal; is on the editorial or governing board of the Journal of Bone and Joint Surgery—American; receives research support from Moximed; receives research support from Novocart; is a board or committee member for the Orthopaedic Research Society; is a board or committee member for Rugby Research Injury Prevention Group. Sebastian Cardona-Ramirez has no conflict of interest to disclose.