Methodology, selection, and integration of fracture healing assessments in mice
All authors have read and approved the final submitted manuscript.
Abstract
Long bone fractures are one of the most common and costly medical conditions encountered after trauma. Characterization of the biology of fracture healing and development of potential medical interventions generally involves animal models of fracture healing using varying genetic or treatment groups, then analyzing relative repair success via the synthesis of diverse assessment methodologies. Murine models are some of the most widely used given their low cost, wide variety of genetic variants, and rapid breeding and maturation. This review addresses key concerns regarding fracture repair investigations in mice and may serve as a guide in conducting and interpreting such studies. Specifically, this review details the procedures, highlights relevant parameters, and discusses special considerations for the selection and integration of the major modalities used for quantifying fracture repair in such studies, including X-ray, microcomputed tomography, histomorphometric, biomechanical, gene expression and biomarker analyses.
1 BACKGROUND
In the United States, there were an estimated 2 million osteoporosis-related fractures with an associated total cost of $17 billion in 2005.1 By 2025, these numbers are expected to surpass 3 million osteoporotic fractures with anticipated total costs over $25 billion annually.2, 3 Contributing to this total economic burden are costs associated with lost productivity due to long recovery periods. For instance, an expected recovery from a closed tibial fracture with intramedullary stabilization is 3–6 months. This duration is often even longer in the 5%–33% of fractures in the United States that result in delayed or nonunion.4, 5 Studies investigating the mechanisms underlying repair and evaluating potential interventions are pivotal to expediting and improving fracture healing outcomes.
Endochondral ossification is essential to long bone fracture healing. The first stage of this process is hematoma formation, at which point ruptured vessels release immune cells and nutrients, and granulation and fibrous tissue begins to form. In this stage, hypoxic and biomechanical strain attenuates osteoblast differentiation in the fracture gap and promotes differentiation of chondrocytes leading to the second stage of healing, fibrocartilaginous callus formation. These cells proliferate and secrete a cartilaginous matrix serving as an early scaffold for further repair. As the callus matures, chondrocytes undergo apoptosis or trans-differentiate into osteoblasts.6-8 Concurrently, neovascularization proceeds at the cartilage–bone transition providing oxygen so that osteoblasts may generate mineralized bone, leading to the third stage of healing, bony callus formation. Once mineralized bone bridges the fracture gap, the final and longest stage of repair begins, bone remodeling. At this point, osteoclasts degrade recently generated bone at the fracture site to allow for revisions of physiological contour and structure.6, 9, 10 As endochondral ossification occurs immediately adjacent to the fracture line, intramembranous ossification may proceed subperiosteally, producing a hard callus which supports rigidity.6, 11
There are several factors believed to influence the structure and composition of the final product of fracture repair. For instance, there is a perceived correlation between the amount of cartilage that forms and the interfragmentary motion during repair, with abundant motion resulting in higher cartilage content and vice versa. Due to this phenomena, the choice of fixators in mice studies may have a profound impact on fracture callus composition.12 This entire process generally occurs within 3–6 months in humans and 5 weeks in mice and occurs via similar mechanisms between species.13 It is important to keep this paradigm of fracture healing in mind when considering the various modalities used to study fracture healing to understand what each modality may provide depending on time postinjury.
Animal models are frequently used to adequately monitor and characterize this healing process, as they are one of the most effective simulations of in vivo bone healing available. These studies allow precise fractures to be created without the numerous confounding variables present in human fracture studies. Bones may also be obtained from humanely euthanized animal subjects at various stages of the healing process to measure the outcomes of healing bone. Of animals, mice are particularly useful as they mature quickly, are inexpensive to breed and maintain high populations, and have many genetic variants and molecular probes readily available.14 A variety of fracture models have been proposed in mice, including fractures created by closed three-point bending, impact loading, or open surgical approaches.15, 16
To characterize underlying mechanisms, identify deterministic factors, and develop interventions to expedite and improve fracture healing outcomes, diverse sets of fracture repair assessments must be tactfully selected and synthesized. This review details the procedures, highlights relevant parameters, and discusses special considerations for the selection, integration, and planning of the major modalities of quantifying fracture repair, including X-ray, microcomputed tomography (µCT), histomorphometric, biomechanical, gene expression, and biomarker analyses.
2 RADIOGRAPHS
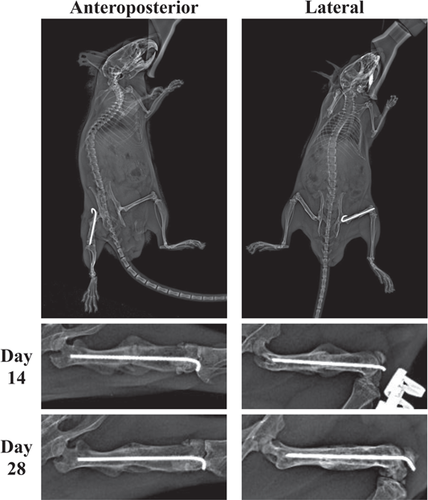
Radiographic evaluation of fractures involves taking X-rays throughout the course of fracture repair and may offer a noninvasive approximation of fracture repair progress and success (Figure 1). Traditionally, they have not often been used to generate numerical data; however, this may change following validation of new scoring systems such as the standard and modified radiographic union scale in tibia, radiographic union scale in tibia (RUST) and modified radiographic union scale in tibia (mRUST) respectively.
2.1 Procedure
These evaluations are the least invasive and time intensive, and therefore are generally performed immediately following fracture induction, periodically throughout the repair process, and before euthanasia. For RUST/mRUST scoring, two orthogonal or perpendicular views should be obtained for each time point. Mice need to be anesthetized for the duration of X-ray collection to minimize motion artifact, which may require specialized radiography systems.
For X-ray scoring methods such as RUST/mRUST, at least two or three blinded reviewers is preferred to generate scores.17-19 Training of the scorers in advance may also be necessary, as orthopedic surgeons who use RUST/mRUST scores in human clinical practice may need time to acclimate to murine models.
2.2 Outcomes
2.2.1 Traditional
Traditionally, X-rays in fracture studies serve to display the progression of fracture healing, with minimal attempts to generate numerical data. Corrales et al.20 found that the most commonly evaluated X-ray criteria in animal studies were bridging of fracture by callus, bone or trabeculae; bridging of the fracture at three or more cortices; and obliteration of the fracture line and/or cortical continuity. However, these were not deemed reliable measures due to high intra and interclass variability.20 Other studies have found that callus size, measured from radiographs, correlates with µCT measures such as cross-sectional area and bone mineral density (BMD),21, 22 while radiographically determined cortical continuity seems to be an accurate indicator of mechanical strength.23 Promising new approaches to quantifying X-ray are RUST and mRUST (Table 1).
| Radiographic criteria | |||
|---|---|---|---|
| Callus | Fracture line | Scorea | |
| RUST | Absent | Visible | 1 |
| Present | Visible | 2 | |
| Present | Invisible | 3 | |
| mRUST | Absent | Visible | 1 |
| Present | Visible | 2 | |
| Bridging | Visible | 3 | |
| Remodeled | Invisible | 4 | |
- a A score is given to each of 4 of the orthogonal cortices as viewed from anteroposterior and lateral radiographs. These values are summed together to give a final standard and modified radiographic union scale in tibia (RUST and mRUST, respectively) score.
2.2.2 RUST and mRUST
The RUST scoring system, first developed by Whelan et al.,24 was designed to reproducibly grade tibia fracture healing in humans. RUST scoring is judged from orthogonal radiographic images of the tibial fracture site, with each cortex being graded from 1 to 3 then summing all cortices for a total between 4 (no healing) and 12 (completely healed) (Table 1).24 Previous radiographic scoring methods, such as those proposed by Hammer et al., predicted healed fractures at a relatively low rate of 50%, with an interclass correlation coefficient (ICC) of 60%. RUST has been found to have significantly higher ICCs of 70% reported by Litrenta et al.25 and 86% by Kooistra et al.18 RUST scoring also has the benefit of being relatively simple, asking the assessor to only comment on the presence or absence of a fracture line or callus, as opposed to the Hammer scale, which asked assessors to rate the degree of fracture line and the size of callus.
Modified RUST scoring, or mRUST, is an altered RUST score model that asks assessors to judge if a callus is present, bridging, or remodeled rather than only if it is present or absent. As such, the mRUST score ranges from 1 to 4 for each cortex rather than 1–3, with total values between 4 and 16 (Table 1). mRUST scoring has demonstrated higher ICC than RUST scoring.17 Both of these scoring methods have been validated in human tibial fractures, but evidence supporting use in animal femur fracture models is present.17, 25
In murine fracture studies, evidence supporting the implementation of RUST and mRUST is limited but promising. Cooke et al. found both RUST and mRUST to have an excellent relationship to structural and biomechanical metrics.19 Both positively correlate with BMD, bone volume/tissue volume (BV/TV), callus strength, and callus rigidity, with mRUST generally having slightly higher correlation coefficients, with an ICC of 0.71 in mRUST and an ICC of 0.63 in RUST. Additionally, 90% of scorers agreed that scans with mRUST scores of 13 were “healed,” and 100% agreed that scans with mRUST scores of 8 were “not healed.”19
3 BIOMARKERS
Biomarkers offer noninvasive, inexpensive means of assessing fracture healing stage and underlying processes and have shown potential as independent predictors of fracture healing success. Selected biomarkers are generally serum peptides that represent either systemic or local processes facilitating fracture repair, or extracellular matrix components that are produced or degraded during repair.26
3.1 Procedure
Serum biomarkers are quantified using a variety of immunoassay techniques. Investigations begin with serum collection targeted to specific stages of repair or periodically throughout a study. Serum is then assessed, often with a form of enzyme-linked immunoassay (ELISA) such as sandwich assays, competitive assays, and antigen down assays. Sandwich assays involve using two antibodies that bind different sites on an antigen or ligand of the molecule of interest. The first antibody, the capture antibody, binds the molecule of interest with high specificity and agglutinates it to the assay tray. The second antibody binds at a separate site on the molecule of interest, and is conjugated to a reporter molecule, often an enzyme that catalyzes a visualizable reaction or possesses innate fluorescence. Depending on what is conjugated to the detection antibody, spectrophotometric, fluorescence, or chemiluminescence readers are employed to detect a signal proportionate to the amount of target antigen present in the sample. From these measures, serum concentration of the molecule of interest may be determined. Sandwich assays tend to be sensitive and robust and are of the most commonly used, however assay format will likely depend on what reagents are available and the dynamic range required for the assay.27
3.2 Outcomes
Hussein et al. reports 50 serum proteins of interest that exhibit predictable trends throughout fracture repair.28 Examples of these markers, often reported in fracture repair studies, include tartrate-resistant acid phosphatase (TRAP), receptor activator of nuclear factor kappa beta ligand (RANKL), and c-terminal telopeptide (CTX) reflecting osteoclast activity, alkaline phosphatase, procollagen type 1 N-terminal polypeptide, and osteocalcin reflecting osteoblast activity, CXM and collagen X reflecting chondrocyte activity and transforming growth factor beta, tumor necrosis factor-a (TNF-a), and vascular endothelial growth factor reflecting immune activity and angiogenesis.29-32
Assessment of these biomarkers may elucidate how underlying fracture repair processes are altered in various circumstances, manifesting in distinct repair outcomes.29-32 Furthermore, recent reviews have investigated the potential of these biomarkers to serve as independent predictors of fracture repair success.33-35 This is of particular interest as reliable correlations could enable earlier clinical identification of nonunion, improving clinical outcomes.
4 MICROCOMPUTED TOMOGRAPHY
µCT involves taking a series of X-ray images of the sample at different rotations, and then using computer algorithms to reconstruct a 3D image stack for analysis. This reconstruction enables high-resolution visualization of fracture callus architecture. These assessments are generally performed ex vivo for the quantification of mineralized bone, however, may be adapted for in vivo and/or soft tissue analysis (e.g., cartilage and vasculature).
4.1 Procedure
4.1.1 Sample preparation
Ex vivo sample preparation for µCT often depends on subsequent testing planned for each sample, although each sample within a set for µCT should be fixed in the same manner. For instance, sets to undergo later mechanical testing should not be fixed, as this can alter structural properties of bone.36 Freezing in sterile phosphate-buffered saline (PBS) is a less deleterious form of storage for samples destined for mechanical testing.37 Sets to undergo other assessments (e.g., histological staining) may be fixed, usually using formalin or organic solvents such as alcohols or acetone. Before scanning, bones are cleaned of surrounding tissue and are generally placed in a holder filled with a medium such as saline or ethanol and wrapped in low-density foam or gauze to limit desiccation and motion artifact.
µCT may also be conducted in vivo with additional considerations such as animal placement, anesthesia, and image registration.38 In vivo µCT is advantageous for its ability to generate longitudinal data without requiring mouse sacrifice. However, its resolution is limited by anesthetic time and the negative effects of radiation exposure may disrupt bone homeostasis and fracture repair.39, 40
4.1.2 Acquiring projection images
Image quality and scan times are dependent on a number of adjustable parameters including X-ray tube voltage, intensity, rotation step, integration time, and voxel size.41 There is not great consensus among murine fracture studies on ideal values for these settings. General ranges for these values in such studies are voltage (50–80 kV), intensity (80–200 µA), rotation step (0.2–0.7°), integration time (250–1500 ms), and voxel size (5-20 µm).15, 21, 42-45 Large deviations from these ranges may distort derived measures. For instance, when the voxel size is too large there may be overestimation of material size/density, but when it is too small there may be excessive noise.46
During scans, samples are generally aligned with the long axis of the scanner, aluminum, or copper filters may be placed in the beam path to reduce beam hardening, and pixel binning may be used to improve contrast at the cost of reduced resolution.41
4.1.3 Computerized reconstruction and processing
After scanning, 3D image stacks are reconstructed from the produced rotation image projections, using software such as NRecon or image processing language (IPL) and processed. Processing primarily involves selecting the volume of interest (VOI), voxel thresholding, and applying filters.
Generally, in fracture studies, the VOI is the fracture callus. This region may be selected as the volume within an absolute length or fraction of total bone length above and below the fracture line center, or by manually tracing the proximal and distal boundaries using semi-automated segmentation tools. The cortex and intramedullary canal are usually excluded from the final VOI, although may be included in studies using µCT to quantify fracture bridging (Figure 2).15, 21, 42-45
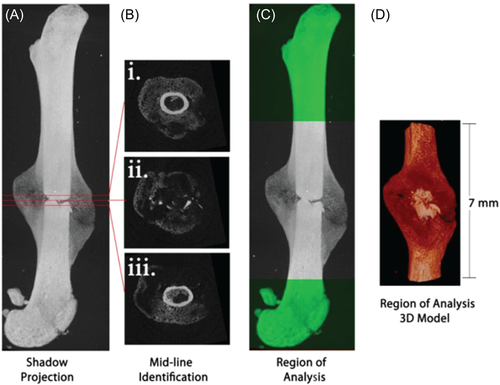
Once the VOI has been identified, threshold values are selected to differentiate tissue types within based on voxel intensity. A single threshold may be selected if the desire is only to delineate between bone and nonbone, however additional thresholds may be necessary if, for instance, bone must also be distinguished from a scaffold or implanted device, or differentiation between various degrees of mineralization is desired. Thresholds are generally either carefully selected absolute values or percentages of maximum gray value (Figure 3).15, 21, 41-45
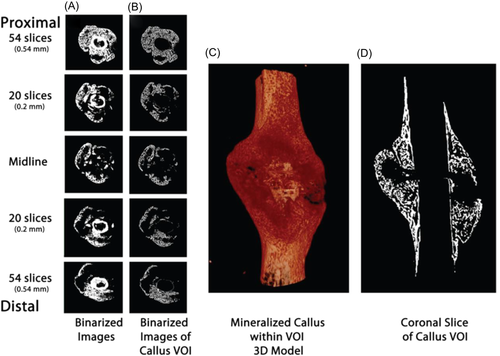
Lastly, filters may be carefully implemented to minimize noise without blurring images, and to correct potential ring artifacts and beam hardening.41
4.2 Outcomes
µCT parameters pertinent to fracture healing include TV, tissue mineral density (TMD), bone volume (BV), bone volume fraction (BV/TV), BMD, and the polar moment of inertia (pMOI) (Table 2). TV, TMD, BV/TV, and BMD measure the volume or fractional volume of mineralized and nonmineralized tissues as well as their respective hydroxyapatite densities. pMOI, is the resistance of an object to change in rotational velocity based solely on its geometry. Briefly stated, the more area an object possesses in a certain cross-section, and the further displaced that area is from the center of the rotational axis, the more resistant it is to changes in rotational velocity (including bending and torsion) at that cross-section.47
| Units | |
|---|---|
| Radiograph | |
| Callus size | µm2 |
| Cortical continuity | − |
| Microcomputed tomography | |
| Tissue volume (TV) | mm3 |
| Tissue mineral density (TMD) | mg HA/cm3 |
| Bone volume (BV) | mm3 |
| Bone volume fraction (BV/TV) | % |
| Bone mineral density (BMD) | mg HA/cm3 |
| Polar moment of inertia (pMOI) | m4 |
| Histomorphometry | |
| Osseous area (OAr) | mm2 |
| Cartilage area (CgAr) | mm2 |
| Fibrous area (FbAr) | mm2 |
| Total callus area (CAr) | mm2 |
| Void area (VAr) | mm2 |
| Biomechanics (torsion) | |
| Ultimate torque | N |
| Torsional stiffness | N/deg |
| Twist to failure | deg |
| Toughness | N*deg |
| Biomechanics (bending) | |
| Ultimate force | N |
| Stiffness | N/mm |
| Strain | − |
| Toughness | N*mm |
Each of these measures has been found to show aberrancy following fracture and resolution throughout repair, as well as correlation to biomechanical values. pMOI, BV, and BMD tend to correlate most with biomechanical properties, while TV and TMD tend to show the most consistent convergence to nonfractured values throughout repair.21, 44, 45
Murine fracture studies less consistently include µCT measures of trabecular microstructure such as trabecular separation (Tb. Sp,), trabecular thickness (Tb. Th), and trabecular number (Tb.N). Data analyzing trabecular microstructure throughout fracture repair in mice is limited but appears to follow predictable trends throughout the progression of endochondral ossification in mice. Recent studies have found trabecular number and thickness to increase most drastically in the first 1–3 weeks postfracture, as cartilage is converted to woven, trabecular and cortical bone, with apparent tapering to still greater than prefracture values by Week 5, as bone is resorbed and remodeled.48-51 Trends in trabecular separation exhibit roughly inversely proportional changes.48-50 These values have also been found to correlate with mechanical functionality throughout repair.50 Imaging the wide range of trabecular mineralization during repair may require smaller voxel sizes and more imaging time.52 Additionally, with the use of contrast, µCT may also be utilized to assess soft tissues such as cartilage and vascularization.53, 54
5 HISTOMORPHOMETRY
Histological staining followed by histomorphometry offers another avenue for assessment of the fracture callus that is best for differentiating between tissue types (cartilage, fibrous, bone, and marrow). It may only be performed ex vivo and is destructive of samples. These assessments involve staining thin sections with a variety of dyes, many of which selectively color certain structures and tissue types, allowing for straightforward identification and computer analysis. Data gathered from these analyses may include the area or relative percentage of area consisting of cartilage, bone, fibrous tissue, or marrow.
5.1 Procedure
5.1.1 Slide preparation
Histologic analysis begins with removing the bone to be analyzed from the humanely euthanized mouse. The sample is then fixed in formalin or a high percentage ethanol solution to halt proteolytic enzymes and bacterial proliferation, then it is dehydrated by further increasing the ethanol percentage to drive out excess water. It should be noted that while formalin (typically 10% neutral buffered formalin and 4% paraformaldehyde) is simple, fast, and easily available, it does decrease the biomechanical strength of bone over time, degrades nucleic acids, and is both toxic and carcinogenic.55, 56 One alternative is glyoxal, which provides similar aldehyde-based fixation with less toxicity.56, 57 Some patented glyoxal-based formulas, such as HistoChoice, have been shown to improve staining of bone tissue samples using stains such as Toluidine blue, Von Kossa, and alkaline phosphatase staining.58 For typical histologic sectioning, bones are decalcified in ethylenediaminetetraacetic acid (EDTA) or another decalcifying agent, and then embedded in paraffin, or are embedded into poly(methyl methacrylate) if preservation of calcified/mineralized bone is desired. EDTA is typically the agent of choice as it preserves the antigenicity of the sample for immunohistochemical staining. However, EDTA requires a long incubation period (around 21 days), so more rapid acting agents such as nitric acid or formic acid may be used if time is limited, as these agents work more rapidly (around 8 days). This rapid action, however, decreases the preservation and staining of immunohistochemistry (IHC) markers.59 A study utilizing fluorescence marking may wish to avoid paraffin embedding as it may disrupt the desired fluorescent emission.60 In this case, samples may be prepared using cryoembedding and cyrosectioning, in which samples are frozen in a solution such as Optimal Cutting Temperature (O.C.T.) compound, a mixture of glycols and resins, to maintain fluorescence after sectioning.60 Blocks containing embedded tissues are then sliced into micrometer wide sections and stained before analysis.
5.1.2 Staining
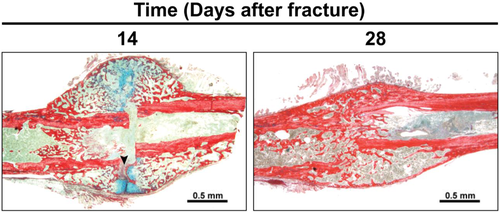
Structural histomorphometric staining protocols are selected to consistently distinguish cartilage, bone, and fibrous tissue, for quantification with semi-automated measurement techniques. The most frequently used staining method is a hematoxylin and eosin stain, which stains based on the acidophilic or basophilic properties of the tissue. Also available are alcian blue and picrosirius red, which stains proteoglycan and collagen, respectively (Figure 4). There are many other stains for bone tissues such as Von Kossa, Gömöri trichrome, and so on, safranin O for cartilage, and endomucin for angiogenesis.61 A list of common stains and their uses is shown in Table 3. After staining, slides are assessed using semi-automated techniques such as open-source GNU Image Manipulation Program (www.gimp.org). This free select tool can be used to demarcate each stained area to generate associated pixel counts, which are then converted to micrometer square using a standard scale. Other available tools include Adobe Photoshop and ImageJ.62
| Use | |
|---|---|
| Stain | |
| Hematoxylin and eosin | Acidophilic/basophilic |
| Collagen stains | |
| Picrosirius Red | Collagen |
| Herovici | Young versus mature collagen |
| Collagen I, II, X | Collagen types |
| Cartilage stains | |
| Alcian Blue | Proteoglycans in cartilage |
| Safranin O | Cartilage |
| Mineralized bone stains | |
| Von Kossa | Calcium/potassium mineralization |
| Alizarin Red | Calcium mineralization |
| Osteocalcin | Bone matrix synthesis |
| Differential stains | |
| Gömöri Trichrome | Muscle fibers, collagen, nuclei |
| Hall Brundts Quadruple | Cartilage and mineralized bone |
| Osteoclast stains | |
| Tartrate-resistant acid phosphatase (TRAP) | Osteoclast activity |
| Endothelium | |
| Endomucin | Angiogenesis |
| Other | |
| Osteopontin | Multiple |
Certain studies may also conduct IHC, which involves using antibodies linked to fluorescent or otherwise identifiable proteins that bind specific molecular markers of repair processes, enabling quantification of their temporal and spatial presence, absence or relative abundance.9, 10, 60 These techniques generally utilize antibodies conjugated to enzymes that catalyze detectable reactions, for the visualization and localization of targeted molecules.63 Common IHC stains for bone are included in Table 3.
5.2 Outcomes
Histomorphometric parameters typically used for fracture repair outcomes include the total or fractional areas of osseous, cartilage, and fibrous tissue, as well as the total callus area and void area (Table 2). These values have been shown to correlate with other methods of assessment throughout fracture repair and indicate key stages of fracture healing. Histologic measures of callus size, diameter, and area—independent of tissue composition—can be used to predict or corroborate biomechanical properties, including strength, stiffness, and moments of inertia.64-66 Histologic measures of mineralized tissue in callus are highly correlated with µCT outcomes of BMD, BV/TV, and mechanical stiffness,64, 67 and increased presence of cartilaginous or fibrous tissue within the fracture defect has been linked to increased incidence of union and nonunion respectively.68
IHC measures may be included to further elucidate the underlying molecular processes which manifest in repair outcomes, as well as observe early stages of bone healing, such as hematoma and chondrogenesis, as µCT and X-ray would fail to detect this nonmineralized tissue.16, 69, 70
6 BIOMECHANICS
Biomechanical testing offers the most direct quantification of bone's ability to support efficient locomotion and is thus pivotal for determining the functionality of the end product of fracture repair. These tests involve applying force, chiefly through bending or torsion, while tracking displacement until material failure occurs. Simultaneous recording of force and displacement produces a curve from which numerous mechanical properties can be derived.
6.1 Procedures
6.1.1 Bending
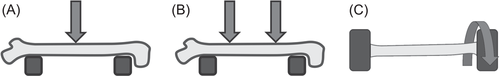
Mechanical testing is most commonly conducted by bending, of which there are two main varieties: three point and four point. In both three- and four-point bending, the bone is set on two supports separated by just less than the sample length. In three-point bending, a steadily increasing downward force is then applied at the center of the two supports until fracture occurs (Figure 5A). With four-point bending, the procedure is similarly conducted, except two downward forces are applied each equidistant from the center of the bone (Figure 5B). The theoretical advantage to four-point bending is that it more uniformly spreads the moment of bending between the two points of force application to account for callus heterogeneity. However, in practice, it is difficult to position most bones so both prongs make equal contact, so it has not been used as often as three-point bending.47, 71
6.1.2 Torsion
Torsion tests are performed less frequently than bending tests; however, they are preferred, especially for fracture studies, as they best account for bone heterogeneity by distributing the force most equally throughout the entire sample and are less prone to experimental variation.72 For these tests, samples are first fixed on both ends into circular or rectangular grips of the testing device. Then one end is twisted while the other is held firmly, or both ends are twisted in opposite directions producing torsional stress until material failure (Figure 5C). The amount of torque required to complete each incremental turn (usually of about half a degree) is recorded, and these data are used to generate a torque–angular displacement curve similar to that of the bending tests.47, 71
6.2 Outcomes
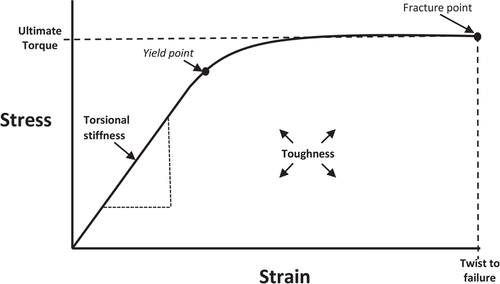
Primary measures of torsional tests include ultimate torque, torsional stiffness, twist to fail, and toughness (Table 2). Ultimate torque is the torque at which material failure occurs. Torsional stiffness is the torque required to make each individual rotation (the slope of the force–displacement relationship) before the yield point (the point of delinearization of the force–displacement relationship where small increments of force provoke large displacements of bone due to compounding micro cracks decreasing stiffness). Twist to fail is the degrees of rotation completed to produce fracture, and toughness is the total energy to fracture (area under the curve of force–displacement relationship) (Figure 6).
Primary measures of bending tests are derived from a force–displacement relationship rather than a torque–angular displacement relationship but are otherwise conceptually similar and include maximum force, stiffness, and toughness (Table 2).
Differences in fracture repair stage and/or success may manifest in key failure patterns on biomechanical testing. For instance, fracture calluses with a higher portion of cartilage or granulation tissue tend to withstand higher interfragmentary strain (change in fracture gap width divided by original fracture gap width) before failure, and rupture with a more ductile pattern (ultimate stress greater than fracture stress) compared with calluses with a higher portion of mature bone. Specifically, fully bridged calluses tend to rupture under strains of <2%, cartilaginous calluses under strains of 2%–10%, and granulation tissue or nonunions under strains of up to 100%.12, 73 Furthermore, specific modes of bone failure, such as at the bone–graft interface in critical size defect repairs, often have characteristic patterns on biomechanical testing.74
7 GENE EXPRESSION
Genetic approaches aim to elucidate the intricate cellular and molecular processes underlying fracture repair and are often included to explain other structural, compositional, and mechanical outcomes or to test specific hypotheses. Among fracture repair investigations, genetic approaches encompass a particularly wide breadth of possibilities. Two common techniques are using reporter mice and performing gene expression analyses. A summary of common reporter mice has recently been published to which we refer the interested reader.75
7.1 Procedure
Reporter mice are genetically modified, linking easily measurable “reporter genes” to genes of interest. They are often generated using cre/loxP systems in which certain DNA sequences are flanked by two loxP sites. DNA between these sites will be excised in the presence of activated cre recombinase (which may be linked to other genes to localize its expression to certain cells, tissues, events, etc). The net effect of this deletion in reporter mice is often activation of the expression of reporter genes which may encode fluorescent (green fluorescent protein and red fluorescent protein) or nonfluorescent (chloramphenicol acetyltransferase and lacZ) proteins, linked to the expression of genes of interest. Imaging technologies may then be used for a variety of assessments including spatiotemporal gene expression, live cell imaging, lineage tracing, and analysis of cell morphology.76
Another commonly implemented genetic technique in fracture repair studies is quantitative real-time polymerase chain reaction (qRT-PCR). qRT-PCR is a variant of standard PCR, and measures the relative abundance of mRNA of specific genes within a sample. It involves reverse transcribing RNA into DNA followed by several rounds of DNA amplification in the presence of DNA binding fluorescent molecules. This fluorescence is then measured to determine the quantity of amplified DNA which will be directly proportionate to the initial quantity of mRNA.77
Newer methods of gene expression analyses are also becoming more widely available, including ribonucleic acid sequencing (RNA-seq), single cell ribonucleic acid sequencing (scRNA-seq), and ATAC-seq. RNA-seq and scRNA-seq enable efficient quantification of genome wide expression within a sample or individual cells, respectively. In general, RNA sequencing involves isolation and purification of RNA followed by fragmentation and reverse transcription to generate complementary DNA (cDNA). Sequencing adapters are then ligated to the ends of these fragments, and they are read by a sequencing platform.78 scRNA involves additional considerations for capturing single cells and unbiased cDNA amplification.79 ATAC-seq works similarly except adapters are ligated to accessible segments of DNA allowing investigators to assess chromatin accessibility.80 There are many sequencing platforms available such as Illumina HiSeq for traditional RNA seq, 10x Genomics Chromium or BD Rhapsody for scRNA seq and Illumina KAPA library quant kit for ATAC-seq.
7.2 Outcomes
Potential targets of these investigations in fracture repair studies are vast, including genes related to the extracellular matrix, growth and differentiation factors, matrix metalloproteinases, and angiogenic factors.78 Hadjiargyrou et al. have identified 704 genes of particular significance to the fracture repair process.79 Fracture healing investigations often utilize qRT-PCR, RNA sequencing, and reporter mice to study the role of these genes in the molecular and cellular processes of fracture repair.80-85
8 SELECTION, INTEGRATION, AND PLANNING OF FRACTURE REPAIR ASSESSMENTS
Generally, multiple or all mentioned methodologies are included in fracture repair studies, as they tend to complement one another so that their combined application offers more than the sum of their individual assessments. Examples of this principle are evident in studies examining the impact of bisphosphonate and alcohol administration on fracture repair. Bisphosphonate administration has been shown to concurrently increase mechanical strength and decrease material quality throughout repair, and it was concluded this was due to increased callus volume compensating for decreased quality.86 In these studies, if only mechanical testing had been performed, they may have concluded that bisphosphonates improved fracture repair outcomes, however this is not necessarily the case. Furthermore, a study investigating alcohol administration on fracture repair observed decreased mechanical stiffness despite unchanged TVs, likely as a manifestation of compositional rather than structural aberrancy.42 In this study, if just structural and mechanical assessments had been performed, there would not have been a satisfactory explanation for observed mechanical deficits.
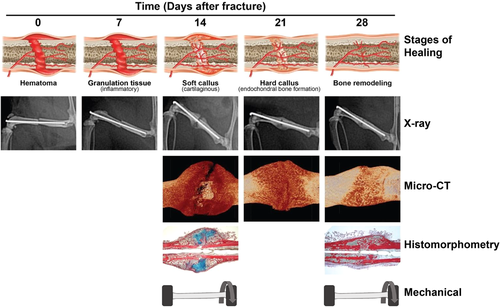
For reasons such as these, in fracture repair studies it is often valuable to include a combination of mechanical, structural, and compositional assessments, although method selection will largely vary depending on specific hypotheses being tested. All forms of assessment discussed in this review fit their own niche for investigating fracture repair and together act synergistically, each expanding the significance that may be derived from the rest. Table 2 provides an overview of relevant parameters of each methodology and Figure 7 offers an approximate timeline for conducting assessments throughout repair process.
Though beyond the scope of this review, statistical analysis is essential in designing experiments and interpreting their results and must be tailored to the hypothesis being tested. This requires careful consideration of the experimental design, procedures for randomization, blinding of investigators, statistical methods, and power of analysis before initiating any experiment, often necessitating the expertise of an experienced statistician.87, 88
8.1 Radiograph
X-rays are the least time intensive method and can be performed in vivo without risk of disrupting healing processes (unlike µCT). For these reasons, they are often performed once or twice a week throughout the course of the study, as well as immediately following fracture creation and before euthanasia (Figure 7). This method offers a clinically relevant approximation of fracture healing progression, although tends to be limited due to subjectivity and high intra and interobserver variability.20 RUST and mRUST offer potentially more reliable, consistent approaches to scoring X-rays, with both human and murine studies giving ICC values between 0.63 and 0.86,17-19 which indicates substantial agreement in assessment89; however, X-ray still cannot wholly replace µCT and histomorphometry as it is a much lower resolution view of the architecture and cannot adequately assess soft tissue (cartilage and fibrous).89
8.2 Biomarkers
Biomarkers offer noninvasive and inexpensive means of assessing fracture healing stage and local and systemic processes related to fracture healing. Due to the relative convenience of this methodology, biomarkers are often measured once or twice a week throughout the repair process.26, 28 Beyond their role in tracking fracture repair progress in these studies, many biomarkers may possess direct clinical utility in early identification of nonunions.33-35
8.3 Microcomputed tomography
µCT offers the highest resolution visualization of fracture callus architecture and is the only structural assessment to offer precise volumetric measures. It may be conducted in vivo, yet this may result in lower resolution and altered repair.39, 40
Typical µCT is limited in that it cannot distinguish cartilage from fibrous tissue and, before callus bridging, has trouble distinguishing any form of soft tissue from the surrounding apparatus.45 For these reasons, it is best suited for analysis of mineralized bone, and therefore is most effectively implemented at and after the expected onset of the hard callus stage of repair, 2–3 weeks postfracture (Figure 7). However, it is nondestructive so it can be completed on bones before either histology or mechanical testing (which are both destructive and cannot be conducted on the same bones), regardless of time point.
8.4 Histomorphometry
Structural histologic assessments offer the best distinction of tissue types (bone, cartilage, fibrous, and marrow) and observation of tissues unseen on radiograph or µCT, particularly during early healing (hematoma, chondrogenic, calcified cartilage phase, and early bone formation). The distinction between cartilage and fibrous tissue is of particular importance because fibrous tissue filling the fracture defect may indicate poor ossification, while cartilage indicates the opposite, and excessive fibrous tissue formation is a key sign of nonunion.68
Due to this ability to distinguish soft tissues and nonmineralized tissues, histomorphometric assessments complement typical µCT well (as typical µCT cannot differentiate these) and are generally most usefully employed at and after the anticipated onset of the cartilaginous stage of repair, 1–2 weeks postfracture (Figure 7). One promising alternative to traditional histomorphometric assessment that can measure soft tissue in vivo is magnetic resonance imaging (MRI).90
Additionally, IHC offers the ability to localize the expression of proteins of interest at the fracture site. While quantitation is possible, data generated primarily point to the mechanisms underlying bone healing progression.
8.5 Biomechanics
Biomechanical tests offer the most direct measures of bone functionality, however, they are limited due to their high degree of variability, especially in bending tests.45 Due to this high variability and the fact that both mechanical testing and histology are destructive (and therefore cannot both be conducted on the same samples), it is usually best to designate larger portions of each experimental group for biomechanical testing. These tests are usually reserved toward the anticipated end of the repair process, 3–4 weeks postfracture (Figure 7).
8.6 Gene expression
Gene expression investigations utilizing qRT-PCR, RNA sequencing, and reporter mice elucidate the cellular and molecular processes underlying fracture repair. These approaches may be tailored to any point throughout repair depending on specific hypotheses being tested and are often conducted ex vivo requiring fracture callus extraction followed by imaging (reporter mice) or callus tissue homogenization and RNA isolation and purification (qRT-PCR).76, 79 Both these assessments are destructive so investigators must consider what portion of samples to allocate to these tests rather than histologic or biomechanical tests. This decision will depend on the emphasis of the study and the anticipated intraclass variance in gene expression under investigation (greater variance may necessitate larger sample sizes).
9 CONCLUSION
This article presents an overview of quantifying fracture repair outcomes in mice including descriptions of the major methodologies, the interpretation of relevant outcome parameters, and key considerations for tactfully selecting, integrating, and planning these assessments. Thoughtful coordination and synthesis of these approaches is critical for evaluating the efficacy of new fracture healing interventions. Moving forward, more data must be gathered and assessed to ascertain the validity of new techniques (e.g., mRUST, in vivo µCT, MRI, and biomarkers) and their potential for improving the efficiency, clinical relevance, and capacity for precise evaluation of outcomes in fracture repair investigations. Novel assessment modalities may at some point become surrogates for their more labor intensive and expensive traditional counterparts. Continual reassessment and reconsolidation of investigative approaches will be necessary.
ACKNOWLEDGMENTS
This project was funded, in part by the Indiana University School of Medicine (RMN, MAK, CDC), and by T35HL110854 (AMK), T32AR065971 (ACM), and R01AG060621 (MAK)from the National Institutes of Health. This material is also the result of work supported with resources and the use of facilities at the Richard L. Roudebush VA Medical Center, Indianapolis, IN: VA Merit #BX003751.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Writing original draft, approval of submitted version: Adam M. Knox. Writing original draft, approval of submitted version: Anthony C. McGuire. Conceptualization, critical revisal, approval of submitted version: Roman M. Natoli. Conceptualization, critical revisal, approval of submitted version: Melissa A. Kacena. Conceptualization, critical revisal, approval of submitted version: Christopher D. Collier.