Early Deterioration of the Right Ventricular Pacing Threshold Predicts the Increase in His-Bundle Pacing Threshold During the Chronic Phase: A Single-Center Retrospective Study
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
His-bundle pacing (HBP) facilitates physiological ventricular activation. However, concerns about long-term threshold deterioration persist. The predictors of chronic threshold elevation are not yet well established.
Methods
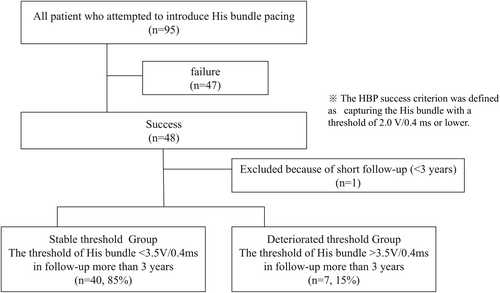
Between February 2018 and December 2021, HBP was attempted in 95 patients undergoing pacemaker implantation. Strict success criteria (threshold ≤ 2.0 V/0.4 ms) were applied, and 47 patients with successful HBP were monitored for over 3 years. We assessed pacing thresholds for both the His-bundle and right ventricle (RV) at implantation, 1 week, 1 month, and annually thereafter. Lead shape was evaluated using chest radiography. Patients were categorized into two groups based on their His-bundle pacing threshold: stable (≤ 3.5 V/0.4 ms) and deteriorated (> 3.5 V/0.4 ms).
Results
Deterioration in His-bundle pacing thresholds was associated with increased RV pacing thresholds. Deterioration in RV pacing occurred earlier, with significant differences observed at 1 week post-implantation (median RV: 1.87 vs. 3.25 V/0.4 ms, p = 0.032; His-bundle: 1.0 vs. 1.25 V/0.4 ms, p = 0.212). Multivariate analysis identified an RV threshold ≥ 3.0 V/0.4 ms at 1 week (OR 10.7, p = 0.036) and lead bending on chest radiography (OR 12.8, p = 0.018) as independent predictors of chronic His-bundle pacing threshold deterioration.
Conclusion
An elevated RV pacing threshold at 1 week post-implantation and lead flexion at implantation may serve as early indicators of long-term deterioration in His-bundle pacing thresholds. When the RV pacing threshold increase is detected, it is important to closely monitor the patient and frequently adjust the output settings to prevent pacing failure.
Abbreviations
-
- AUC
-
- area under the curve
-
- CRT
-
- cardiac resynchronization therapy
-
- HBP
-
- His-bundle pacing
-
- LBBAP
-
- left bundle branch area pacing
-
- LVEF
-
- left ventricular ejection fraction
-
- OR
-
- odds ratio
-
- ROC
-
- receiver operating characteristic
-
- RV
-
- right ventricle
-
- RVP
-
- right ventricular pacing
1 Introduction
Conduction system pacing, known for its physiological conduction pattern and cardiac contraction, is increasingly preferred over traditional pacing methods. Among these techniques, His-bundle pacing (HBP) directly stimulates the His-bundle and is recognized as the pioneering method, first proposed in the literature [1]. Previous studies have demonstrated that HBP significantly lowers mortality rates and reduces heart failure hospitalizations compared with conventional pacing methods [2]. Advances in delivery sheath technology have led to improvements in the success rate of HBP. Despite these advancements, challenges such as early battery depletion and the need for lead repositioning (7%–11%) due to threshold deterioration in the chronic phase persist [2-4]. Prior research has identified several factors that contribute to increases in HBP threshold, including the shape of the lead at implantation and changes in His-bundle potential [5, 6]. On the other hand, we focused on the association between right ventricular myocardial pacing threshold and His-bundle pacing threshold in this study, because the assessment can be conducted relatively easily with a 12-lead electrocardiogram and a programmer. No studies have yet explored the relationship between alterations in right ventricular myocardial threshold and His-bundle pacing threshold changes. This study aims to determine whether changes in right ventricular myocardial threshold are associated with, or can predict, chronic phase deterioration in His-bundle pacing threshold.
2 Methods
2.1 Study Design
This study was a single-center, retrospective observational study. Patients included were those who underwent His-bundle pacing (HBP) at Bell Land General Hospital in Sakai City, Osaka, Japan, from February 2018 to December 2021. The standard success criterion for HBP is defined as capture at 2.5 V/1.0 ms [7]. However, to account for magnetic resonance imaging compatibility and to minimize early battery depletion, our hospital adopted a stricter criterion, setting the success threshold at a His-bundle pacing threshold of 2.0 V/0.4 ms or below. Patients with < 3 years of follow-up post-implantation were excluded. Informed consent was obtained from all participants. This study adhered to the principles of the Declaration of Helsinki.
2.2 Implant Procedure
The implantation technique for HBP followed protocols outlined in previous studies [8, 9]. The procedure involved the use of a specific lead (Select Secure TM 3830; Medtronic Inc.) and a delivery sheath (C315HIS, Medtronic Inc., Minneapolis, USA). The sheath was inserted through the subclavian vein and advanced to the right ventricular anterior septum. To locate the atrioventricular node, contrast injection was administered. The lead tip was then directed toward the right ventricular septum from the sheath, and pacing was performed to confirm His-bundle capture before the lead was secured. Due to the lack of necessary equipment, His-bundle potentials could not be evaluated in the electrophysiology laboratory; therefore, pacing assessment was performed following lead implantation. Successful His-bundle capture was determined by observing the waveform on the surface 12-lead electrocardiogram to assess whether it represented selective HBP, non-selective HBP, or only right ventricular pacing (RVP). The pacing output was initially set at 10.0 V with a pulse width of 0.4 ms and was gradually reduced to the minimum required to achieve His-bundle or right ventricular myocardium capture. If His-bundle capture was not possible at or below the 2.0 V/0.4 ms threshold, up to five re-implantation attempts were made. Failing this, the strategy was switched to right ventricular septal pacing. All procedures were conducted by a single experienced physician.
2.3 Follow-Up and Outcomes
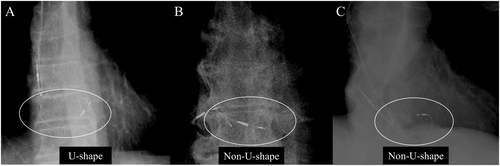
Patient medical histories, comorbidities, electrocardiograms, and echocardiogram findings were extracted from medical records. Post-implantation chest radiographs were used to assess lead slack. According to Beer et al. [5], lead shapes were classified as either U-shape or non-U-shape. As depicted in Figure 1A, a U-shape configuration shows the RV lead descending toward the right atrium, tricuspid valve annulus, and base of the right heart, before curving upward toward the His-bundle insertion site. Non-U-shape configurations, illustrated in Figure 1B,C, are characterized by insufficient lead sag or an inverted lead within the right ventricle, anchored at the His-bundle. Inter- and intra-observer variabilities were assessed according to the Bland and Altman method. Patients were followed up at 1 week, 1 month, and annually post-implantation. During each outpatient visit, His-bundle and RV pacing thresholds, wave amplitude, and lead impedance were measured. The ventricular lead's output was progressively reduced to assess the His-bundle and RV pacing thresholds using intracardiac electrograms and surface 12-lead ECGs before and after any changes in the QRS waveform. The pulse width was consistently set at 0.4 ms for these evaluations. The pacing output was generally set high enough to ensure reliable His-bundle capture, typically at least twice the His-bundle pacing threshold. If His-bundle pacing threshold deterioration occurred, the threshold was re-assessed at a pulse width of 0.4 ms, followed by an adjustment in pulse width and output to minimize battery consumption. Should early battery depletion be anticipated, strategies were adjusted to either maintain RV capture only or to undertake additional lead placement or replacement.
3 Statistical Analysis
Continuous variables are presented as mean ± standard deviation or median (first and third quartiles), while categorical variables are shown as counts and percentages. Differences between two groups were analyzed using the Student's t-test for normally distributed data and the Mann–Whitney U-test for non-normally distributed data. Categorical variables were assessed using either Fisher's exact test or the Chi-square test, depending on the data distribution. To identify predictors of outcomes, multivariate logistic regression analyses were conducted. Factors that showed significance in the univariate analysis were included in the multivariate model using a forward stepwise selection method. In logistic regression analyses, variables with a p-value of < 0.05 in the univariate analysis were incorporated into the multivariate model. The RV pacing threshold at 1 week post-implantation and the lead configuration at the time of implantation were evaluated using Kaplan–Meier curves, incorporating information on the timing of His-bundle threshold deterioration. These two variables were further analyzed using both univariate and multivariate analyses with the Cox proportional hazards model. Statistical significance was set at p < 0.05. All statistical analyses were performed using EZR [10], a software that enhances the functionalities of R and R Commander.
4 Results
4.1 Baseline Characteristics and Procedural Results
Among the 95 patients who underwent HBP at our institution, 48 were able to achieve His-bundle capture at 2.0 V/0.4 ms or lower. One patient was excluded from the study due to early death, leaving a total of 47 patients for analysis with a follow-up period of at least 3 years. Considering the pacemaker's nominal output setting of 3.5 V/0.4 ms, patients were classified into two groups: the stable threshold group, where the His-bundle pacing threshold was maintained at or below 3.5 V/0.4 ms throughout the follow-up, and the deteriorated threshold group, where the threshold exceeded 3.5 V/0.4 ms at any point. Forty patients (85.0%) were in the stable threshold group, and seven patients (15.0%) were in the deteriorated threshold group (Figure 2). The baseline characteristics of both groups are summarized in Table 1. The average age of the cohort was 76.0 ± 6.4 years, with 28 patients (59.6%) being male. The primary indications for pacemaker implantation included complete atrioventricular block in 40 patients (85.1%), sinus node dysfunction in three patients (6.3%), and bradycardic atrial fibrillation in two patients (4.2%). Additionally, two patients (4.2%) exhibited complete left bundle branch block with impaired cardiac function and underwent cardiac resynchronization therapy (CRT) using a left ventricular lead placed within the coronary sinus. There were no significant differences in the underlying conditions between the two groups. The QRS duration of junctional beats also did not show significant differences between groups. Comorbid conditions included atrial fibrillation in six patients (12.8%), hypertension in 31 patients (66%), diabetes mellitus in 11 patients (23.4%), chronic renal failure in 11 patients (23.4%), hyperlipidemia in 15 patients (31.2%), and coronary artery disease in eight patients (17%); no significant differences in these comorbidities were observed between the groups. The median left ventricular ejection fraction (LVEF) at the time of implantation was 55.4% ± 9.3%, with no significant difference in left ventricular function between the groups. Table 2 presents a comparison of procedural and pacing characteristics between the stable and deteriorated threshold groups. No significant differences were found in terms of selective HBP at the minimum output, procedure duration, ventricular pacing rate, or paced QRS duration. However, a U-shaped lead configuration was associated with stable His-bundle pacing thresholds, unlike other configurations. Lead revision occurred in two cases within the deteriorated threshold group during battery replacement, more than 3 years post-implantation, where left bundle branch area pacing (LBBAP) was introduced. Rates of heart failure hospitalization post-implantation did not differ significantly between the groups.
| Total (n = 47) | Stable threshold (n = 40) | Deteriorated threshold (n = 7) | p | |
|---|---|---|---|---|
| Age | 76.0 ± 6.43 | 76.7 ± 5.34 | 71.7 ± 10.3 | 0.252 |
| Male (%) | 28 (59.6) | 23 (57.6) | 5 (71.4) | 0.685 |
| Indication (%) | ||||
| SSS | 3 (6.3) | 3 (7.5) | 0 (0.0) | 1 |
| CAVB | 40 (85.1) | 34 (85.0) | 6 (85.7) | 1 |
| Brady AF | 2 (4.2) | 2 (5.0) | 0 (0.0) | 1 |
| CLBBB | 2 (4.2) | 1 (2.5) | 1 (14.3) | 0.278 |
| Junction QRS (ms) | 114.2 ± 27.0 | 113.0 ± 25.6 | 120.9 ± 35.6 | 0.486 |
| Infra nodal block (%) | 24 (51.0) | 18 (45.0) | 6 (85.7) | 0.097 |
| Medical history (%) | ||||
| AF | 6 (12.8) | 5 (12.5) | 1 (14.3) | 1 |
| HT | 31 (66.0) | 26 (65.0) | 5 (71.4) | 1 |
| DM | 11 (23.4) | 10 (25.0) | 1 (14.3) | 1 |
| CKD | 11 (23.4) | 11 (27.5) | 0 (0.0) | 0.175 |
| DLP | 15 (31.2) | 14 (35.0) | 1 (14.3) | 0.404 |
| IHD | 8 (17.0) | 8 (20.0) | 0 (0.0) | 0.328 |
| BNP (pg/nL) | 148.8 (72.1–221.5) | 120.5 (74.2–243.0) | 136.0 (71.0–213.5) | 0.988 |
| LVEF (%) | 55.4 (52.7–60) | 60 (58.7–60) | 55 (42.5–60) | 0.072 |
| pEF (> 50%) | 39 (83.0) | 35 (87.5) | 4 (57.1) | 0.08 |
- Note: Values are mean ± SD, n (%) or median (quartile 1–3). p-values < 0.05 were considered statistically significant.
- Abbreviations: AF, atrial fibrillation; CAVB, complete atrioventricular block; CKD, chronic kidney disease; DLP, dyslipidemia; DM, diabetes mellitus; HT, hypertension; IHD, ischemic heart disease; LVEF, left ventricular ejection fraction; pEF, preserved ejection fraction; SSS, sick sinus syndrome.
| Total (n = 47) | Stable threshold (n = 40) | Deteriorated threshold (n = 7) | p | |
|---|---|---|---|---|
| Pacing morphology (at the minimum output) | ||||
| Selective HBP | 37 (78.7) | 30 (75.0) | 7 (100) | 0.318 |
| Non selective HBP | 10 (21.3) | 10 (25.0) | 0 (0.0) | |
| RV lead shape | ||||
| U-shape | 42 (89.4) | 38 (95.0) | 4 (57.1) | 0.018 |
| Non U-shape | 5 (10.6) | 2 (5.0) | 3 (42.9) | |
| Operation time (min) | 159.4 ± 37.9 | 159.8 ± 37.8 | 138.6 ± 31.7 | 0.203 |
| Ventricular pacing burden (%) | 100 (89–100) | 100 (89–100) | 99.5 (74.5–100) | 0.999 |
| Paced QRS (ms) | 118.7 ± 16.6 | 118.8 ± 17.0 | 118.3 ± 15.5 | 0.935 |
| Lead revision | 2 (4.3) | 0 (0.0) | 2 (28.6) | 0.01 |
| Hospitalization because of HF after HBP induction | 5 (10.6) | 3 (7.5) | 2 (28.6) | 0.154 |
- Note: Values are mean ± SD, n (%) or median (quartile 1–3). p-values < 0.05 were considered statistically significant.
- Abbreviations: HB, His-bundle; HBP, His-bundle pacing; HF, heart failure; RV, right ventricular.
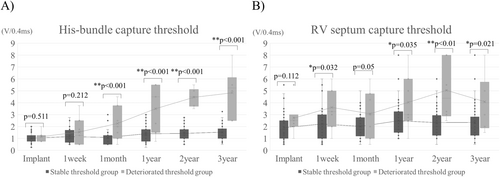
4.2 Lead Threshold Trend
The changes in His and RV pacing thresholds over time for both the stable threshold group and the deteriorated threshold group are presented in Table 3 and Figure 3. Initially, at the time of implantation, there was no significant difference in the His-bundle pacing threshold between the groups. However, a statistically significant difference emerged 1 month post-implantation, and this gap in thresholds continued to widen progressively each year thereafter. In contrast, the RV pacing threshold was already higher in the deteriorated threshold group at implantation, although this difference was not statistically significant (p = 0.112). By 1 week post-implantation, the gap had further widened, with a significant increase in the RV pacing threshold noted in the deteriorated threshold group. This pattern persisted in subsequent follow-ups, and an increase in the RV pacing threshold was found to correlate with an increase in the His-bundle pacing threshold. No significant differences were observed between the two groups in terms of amplitude or impedance values (Figure S1).
| Total (n = 47) | Stable threshold (n = 40) | Deteriorated threshold (n = 7) | p | |
|---|---|---|---|---|
| His-bundle pacing capture threshold (V/0.4 ms) | ||||
| Implant | 1.0 ± 0.44 | 1.02 ± 0.44 | 1.14 ± 0.45 | 0.511 |
| 1 week | 1.21 ± 0.74 | 1.15 ± 0.62 | 1.53 ± 1.23 | 0.212 |
| 1 month | 1.28 ± 0.96 | 1.08 ± 0.70 | 2.25 ± 1.45 | < 0.001 |
| 1 year | 1.70 ± 1.22 | 1.38 ± 0.68 | 3.50 ± 1.96 | < 0.001 |
| 2 year | 1.82 ± 1.20 | 1.42 ± 0.6 | 4.45 ± 0.74 | < 0.001 |
| 3 year | 1.98 ± 1.51 | 1.52 ± 0.74 | 4.83 ± 2.08 | < 0.001 |
| RV pacing capture threshold (V/0.4 ms) | ||||
| Implant | 2.03 ± 1.23 | 1.91 ± 1.18 | 2.71 ± 1.38 | 0.112 |
| 1 week | 2.41 ± 1.61 | 2.20 ± 1.50 | 3.60 ± 1.80 | 0.032 |
| 1 month | 2.18 ± 1.31 | 2.0 ± 1.07 | 3.07 ± 2.03 | 0.05 |
| 1 year | 2.72 ± 1.80 | 2.48 ± 1.60 | 4.03 ± 2.40 | 0.035 |
| 2 year | 2.67 ± 1.75 | 2.32 ± 1.33 | 5.04 ± 2.51 | < 0.001 |
| 3 year | 2.58 ± 1.74 | 2.34 ± 1.52 | 4.08 ± 2.41 | 0.021 |
- Note: Values are mean ± SD. p-values < 0.05 were considered statistically significant.
- Abbreviation: RV, right ventricular.
4.3 Predictor of High His-Bundle Pacing Capture Threshold
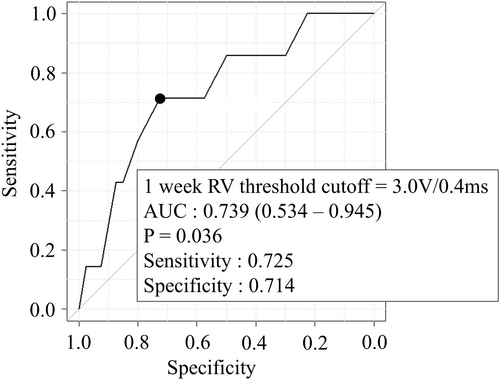
In this study, we investigated factors associated with a long-term increase in His-bundle pacing threshold during follow-up. In the deteriorated threshold group, the RV pacing threshold significantly increased at 1 week post-implantation, preceding the rise in His-bundle pacing threshold. Based on this observation, we conducted a receiver operating characteristic (ROC) analysis for the RV pacing threshold at 1 week post-implantation. The results are displayed in Figure 4. The area under the curve (AUC) was 0.739 (95% confidence interval: 0.534–0.945), and the cutoff value was established at 3.0 V/0.4 ms (sensitivity: 72.5%, specificity: 71.4%, p = 0.036). Univariate analysis indicated that lead shape (U-shape vs. non-U-shape) and RV pacing threshold at 1 week post-implantation (> 3.0 V/0.4 ms) were associated with an increase in His-bundle pacing threshold. Multivariate analysis confirmed these two factors as independent predictors (Table 4). No significant correlations were found between His-bundle pacing threshold deterioration and factors such as age, diabetes, hypertension, hyperlipidemia, atrial fibrillation, history of heart failure, chronic renal failure, infra-nodal block, ischemic heart disease, or reduced ejection fraction. Additional analyses incorporating the timing of His-bundle threshold deterioration are presented in Figures S2 and S3. Both an RV pacing threshold ≥ 3.0 V/0.4 ms at 1 week post-implantation (p = 0.042) and the lead shape at implantation (p = 0.009) were statistically significantly associated with chronic-phase His-bundle pacing threshold deterioration. These two variables were further analyzed using a multivariate Cox proportional hazards model, which demonstrated that both were independent predictors (C-index = 0.81).
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds rates (95% CI) | p | Odds rates (95% CI) | p | |
| Male sex | 1.82 (0.25–21.3) | 0.685 | ||
| Age | 2.72 (0.2–23.7) | 0.276 | ||
| Diabetes mellitus | 0.50 (0.0–5.03) | 0.99 | ||
| Hypertension | 1.33 (0.18–15.7) | 0.99 | ||
| Hyperlipidemia | 0.31 (0.0–3.02) | 0.404 | ||
| Atrial fibrilliation | 1.16 (0.02–13.5) | 0.99 | ||
| Lead shape (U or non U) | 12.8 (1.13–198) | 0.018 | 24.9 (1.81–343.0) | 0.016 |
| History of heart failure | 0.21 (0.01–3.12) | 0.154 | ||
| Chronic kidney disease | 0 (0.0–2.20) | 0.175 | ||
| Infra-nodal block | 7.06 (0.75–351.6) | 0.097 | ||
| Ischemic heart disease | 0 (0–3.48) | 0.329 | ||
| Reduced EF | 3.06 (0.04–68.0) | 0.391 | ||
| RV threshold > 3.0 V/0.4 ms after 1 week HBP induction | 6.29 (0.87–75.6) | 0.036 | 10.7 (1.09–105.0) | 0.042 |
- Note: p-values < 0.05 were considered statistically significant.
- Abbreviations: EF, ejection fraction; HBP, His-bundle pacing; RV, right ventricular.
5 Discussion
The main findings of our study are as follows: (1) Despite applying a stricter criterion for successful His-bundle pacing, the proportion of cases experiencing an increase in His-bundle pacing threshold aligns with previous reports. (2) There is a tendency for the RV pacing threshold to increase alongside the His-bundle pacing threshold, with the rise in RV pacing threshold typically preceding the increase in His-bundle pacing threshold. (3) A right ventricular pacing threshold of 3.0 V/0.4 ms or higher, observed 1 week post-implantation, predicts an increase in the His-bundle pacing threshold during the chronic phase. (4) Non-U-shaped lead flexion post-implantation is associated with an increase in the His-bundle pacing threshold in the chronic phase.
The escalation of the His-bundle pacing threshold in the later stages of HBP remains a significant challenge. Previous studies report that the incidence of threshold increases after HBP implantation ranges from 5.7% to 30.0% [2, 4, 5, 9, 11-15]. It is crucial to select the optimal implantation site to achieve the best possible His-bundle pacing threshold. Watanabe et al. suggested that a His-bundle pacing threshold of ≥ 1.25 V/1.0 ms at implantation is linked to an increased occurrence of clinical events, such as lead replacement or removal [16]. In our study, we implemented a stringent success criterion for His-bundle capture at ≤ 2.0 V/0.4 ms during HBP implantation. As a result, the majority of patients maintained an adequate threshold; however, approximately 15% experienced worsening of the threshold. This rate is consistent with the previously reported incidence of threshold increases, demonstrating that such increases occur with a certain probability, even under a more stringent success criterion. In cases where the His-bundle pacing threshold deteriorated during the chronic phase, a higher RV pacing threshold was also observed. This trend persisted throughout the 3-year follow-up period, with the increase in RV pacing threshold occurring earlier than the increase in His-bundle pacing threshold and showing significant differences between the stable and deteriorated threshold groups as early as 1 week post-implantation.
Various factors may contribute to the deterioration of RV pacing thresholds, including age-related changes and micro-dislodgement of the lead; however, we primarily consider inflammatory changes at the lead tip as the most likely cause. When only non-steroid-eluting leads were used, it was demonstrated that pacing thresholds tended to rise within the first month after implantation and then remain elevated, a phenomenon attributed to localized inflammation and fibrosis [17]. Additionally, patients who develop keloids at the pacemaker pocket site have been reported to exhibit elevated inflammatory biomarkers, which are associated with increased pacing thresholds during the chronic phase. It has been suggested that similar inflammatory responses may occur in both the skin and myocardial tissue [18]. In the context of HBP as well, the degree of inflammatory changes at the lead tip may similarly influence the likelihood of chronic deterioration of the His-bundle pacing threshold [8, 19]. The observed increase in RV pacing threshold over time following implantation suggests localized inflammatory changes in the myocardial tissue surrounding the lead tip. We hypothesize that this inflammation leads to local fibrosis around the lead during the chronic phase, subsequently resulting in a deterioration of the His-bundle pacing threshold. Consistent with this hypothesis, our study found that an RV pacing threshold of ≥ 3.0 V/0.4 ms 1 week post-implantation is predictive of a subsequent increase in the His-bundle pacing threshold during long-term follow-up.
Another predictive factor identified for an increase in the His-bundle pacing threshold was the shape of the lead at the time of insertion. Beer et al. [5] reported that among cases with an increased His-bundle pacing threshold, two-thirds exhibited this increase early in the follow-up, and the shape of the inserted lead was associated with this deterioration. The target sites for HBP are exceptionally narrow, and even minor misalignments can affect the His-bundle pacing threshold. The primary issue appears to be that lead insertion methods other than the U-shape create unstable contact between the lead and the His-bundle, leading to increased thresholds. In our study, cases where the lead was inserted in configurations other than the U-shape tended to show worsening His-bundle pacing thresholds. These findings highlight the critical importance of lead orientation during insertion, given the narrow confines of the His-bundle region.
Recently, LBBAP has gained popularity due to its simplicity and the ability to maintain stable pacing thresholds over the long term. Moreover, clinical outcomes with LBBAP have been reported to be comparable to those achieved with HBP [20-22]. At present, LBBAP has largely supplanted HBP as the predominant method for conduction system pacing. Nonetheless, HBP is still regarded as the pacing method that most closely replicates physiological conduction. In the future, the development of leads that can capture the distal His-bundle, coupled with systems designed to minimize inflammation at the lead insertion site, could maintain stable thresholds and potentially prompt a reassessment of HBP's value.
6 Study Limitation
This study has several limitations, including a small sample size and its nature as a retrospective observational study conducted at a single institution. Additionally, the procedure for HBP was performed by a single operator. Given that HBP is technically demanding and the operator's experience can significantly influence long-term outcomes [14], it would be beneficial to consider data from multiple operators. The success criterion for HBP in this study was defined as His-bundle capture with a threshold of 2.0 V/0.4 ms or lower. However, the standard success criteria for HBP in general clinical practice are often set at ≤ 2.5 V/1.0 ms. Utilizing the standard success criteria might yield different results if the same follow-up were conducted. Nevertheless, by reducing the pulse width, the differences between the RV and His-bundle pacing thresholds became more pronounced, and the separate evaluation of these thresholds proved to be informative.
In our study, the deteriorated threshold group included patients whose His-bundle pacing threshold exceeded 3.5 V/0.4 ms at least once during follow-up, using the standard pacemaker output setting of 3.5 V/0.4 ms as a benchmark. This definition, unique to our study, means that patients whose threshold increased by 1–2 V during follow-up but remained below 3.5 V were classified into the ‘stable threshold’ group, depending on their initial implantation threshold. Previous studies have classified deterioration using an increase of more than 1 V [5]; in contrast, our study used absolute values rather than relative changes, potentially leading to an underestimation of threshold deterioration compared to previous studies.
We hypothesize that the chronic deterioration of the His-bundle pacing threshold is attributable to inflammatory changes at the lead tip. However, we did not obtain objective imaging data—such as MRI, gallium scintigraphy, or PET—to support this hypothesis. In the future, incorporating such objective assessments into the analysis may enhance the credibility of our findings.
Lastly, the electrophysiology laboratory was unavailable during implantation due to environmental constraints. Sato et al. reported that a negative component of the His potential during lead insertion of ≥ 0.06 mV, coupled with current injury to the His-bundle, indicates a stable His-bundle pacing threshold 1 year later [6]. Unfortunately, we did not assess the local His potential at implantation, which could be a significant oversight.
7 Conclusion
We found that the worsening of the RV pacing threshold at 1 week post-implantation and the orientation of lead contact with the His-bundle are associated with, and may predict, chronic deterioration of the His-bundle pacing threshold. The increase in the RV pacing threshold observed 1 week post-implantation likely reflects local inflammatory responses. When conducting HBP, it is crucial not only to monitor changes in the His-bundle pacing threshold but also to carefully observe changes in the RV pacing threshold over time. When the RV pacing threshold increase is detected, it is important to closely monitor the patient and frequently adjust the output settings—such as increasing the output—to prevent pacing failure.
Acknowledgments
The authors have nothing to report.
Ethics Statement
The study was fully anonymous, and no sensitive data were collected or managed. The project was conducted in accordance with local laws and regulations.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data used in this report are available.




