The application of in vitro-derived human neurons in neurodegenerative disease modeling
Abstract
The development of safe and effective treatments for age-associated neurodegenerative disorders is an on-going challenge faced by the scientific field. Key to the development of such therapies is the appropriate selection of modeling systems in which to investigate disease mechanisms and to test candidate interventions. There are unique challenges in the development of representative laboratory models of neurodegenerative diseases, including the complexity of the human brain, the cumulative and variable contributions of genetic and environmental factors over the course of a lifetime, inability to culture human primary neurons, and critical central nervous system differences between small animal models and humans. While traditional rodent models have advanced our understanding of neurodegenerative disease mechanisms, key divergences such as the species-specific genetic background can limit the application of animal models in many cases. Here we review in vitro human neuronal systems that employ stem cell and reprogramming technology and their application to a range of neurodegenerative diseases. Specifically, we compare human-induced pluripotent stem cell-derived neurons to directly converted, or transdifferentiated, induced neurons, as both model systems can take advantage of patient-derived human tissue to produce neurons in culture. We present recent technical developments using these two modeling systems, as well as current limitations to these systems, with the aim of advancing investigation of neuropathogenic mechanisms using these models.
Significance
Reprogramming technology has pioneered the use of human neurons for in vitro studies of neurologic disease. These models allow mechanistic approaches to understanding uniquely human drivers of disease in relevant cell types. We highlight advantages and present challenges of both human induced pluripotent stem cell (hiPSC)-derived neurons and directly converted, or induced, neurons for in vitro disease modeling. We summarize results from recent experiments, review methodological developments, and discuss experimental design. These models provide valuable opportunities to study neurologic disease in the appropriate human genetic background and will significantly advance neurologic disease research in the coming years.
1 INTRODUCTION
As the population ages, neurodegenerative diseases, such as Alzheimer's disease (AD) and related dementias (ADRD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS), pose an increasingly greater threat to human health and burden on the health-care system (Heemels, 2016). As an example, nearly 6 million Americans suffer from AD, with estimated costs nearing $290 billion annually (“Alzheimer's Association Report: 2019 Alzheimer's disease facts and figures,” 2019). A study looking at AD clinical trials involving 244 candidate compounds between 2002 and 2012 reported that only one of the 244 compounds was approved, resulting in a failure rate of 99.6% (for comparison, cancer had a failure rate of 81%; Cummings, Morstorf, & Zhong, 2014). Thus, there is a substantial need for safe and effective therapeutic interventions to improve patient outcomes.
Lack of effective therapeutics is due, in part, to the complex underlying etiology of many neurodegenerative disorders. For ADRD and other age-related neurodegenerative disorders, the complete cellular and molecular mechanisms underlying neuronal dysfunction and degeneration have not been fully elucidated, despite the ongoing identification of disease-associated genes and variants.
Animal models, particularly rodents, have significantly advanced our understanding of the underlying mechanisms of neurodegenerative diseases. However, inherent differences between laboratory rodents and human subjects, such as genetic background, brain anatomy, and pathological processes associated with aging, can make it difficult to predict drug efficacy in humans based on success in rodent models. In the case of AD, many transgenic mouse models only show neuropathologic and behavioral phenotypes when multiple combinations of genetic mutations that lead to autosomal-dominant disease are expressed at extremely high levels (Drummond & Wisniewski, 2017). Even then, AD mouse models do not consistently replicate many pathological hallmarks, such as the extensive neuronal loss observed in human patients (LaFerla & Green, 2012).
The selection of appropriate models in which to study the mechanisms underlying early disease pathogenesis is key to successful identification of therapeutic targets. Thus, models that have the ability to incorporate (a) the complexity of human genetics, (b) include cellular phenotypes that resemble the pathology of the disease, and (c) can be deployed in therapeutic screening paradigms, and that include cellular phenotypes that resemble the pathology of the disease represent powerful tools in understanding and treating age-related neurodegenerative disorders.
Recent technological advances have made cell culture platforms, such as human-induced pluripotent stem cell-derived neurons (hiPSC-Ns), induced neural stem cells (iNSCs), and induced neurons (iNs), a promising complement to rodent models of neurodegenerative diseases. These human neuronal models have rapidly become critical tools for the investigation of specific genes, proteins, and molecular pathways involved in disease pathology. In particular, over the last 10 years there has been increasing interest in the use of postmortem tissue to generate hiPSCs and iNs (Bliss et al., 2012; Iovino et al., 2014; Rose et al., 2018; Sproul et al., 2014). Use of postmortem tissue allows researchers to perform mechanistic experiments in living cells with a genetic background unique to each individual, while also allowing for correlative insights stemming from each research subject's clinical and neuropathologic data.
Here, we will review and compare methods used to generate human neurons in vitro, specifically neurons derived from pluripotent stem cells versus those generated through direct conversion of somatic cells. We will highlight some of the recent findings emerging from this field, as they apply to a range of neurodegenerative diseases, and discuss current limitations and future applications of these technologies.
2 INDUCED PLURIPOTENT STEM CELLS
Human pluripotent stem cells (hPSCs), including embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs) allow for the generation of human neurons in vitro in a scalable manner. The basic principle behind the generation of hiPSCs involves the introduction of exogenous transcription factors into fully differentiated cells, thus reverting the cells back to an embryonic-like state (Mahmoudi & Brunet, 2012). This technology was pioneered by Takahashi and Yamanaka who demonstrated that expression of four transcription factors (OCT4, SOX2, KLF4, and c-MYC, termed the “Yamanaka factors”) could successfully reprogram mouse and human fibroblasts into pluripotent cells with a gene expression profile, morphology and growth profile similar to that of ESCs (Takahashi et al., 2007; Takahashi & Yamanaka, 2006). Ensuing protocols included the addition of NANOG and LIN28 (Yu et al., 2007). Current protocols for generating hiPSCs largely use non-integrating methods to ensure that the reprogramming factors are not permanently established in the host genome. hiPSC technology has rapidly progressed with hiPSCs being derived from somatic cell sources as diverse as keratinocytes from hair (Linta et al., 2012), CD34+ cells from peripheral blood (Loh et al., 2009) and renal proximal epithelial cells from urine (Zhou et al., 2011). hiPSCs lines have also been established from postmortem tissue including dura mater (Bliss et al., 2012; Sproul et al., 2014) and leptomeninges (Rose et al., 2018). As the field has expanded, multiple differentiation protocols have been developed to generate central nervous system cell types, including various neuron subtypes, astrocytes, oligodendrocytes, and microglia, from hiPSCs using small molecule-based methods and transcription factor-based methods, as shown in Table 1 (Abud et al., 2017; Ehrlich et al., 2017; Ladewig et al., 2012; Perriot et al., 2018), and this remains a very active area of ongoing research.
| Cell type | Differentiation method | Key protocol steps | References |
|---|---|---|---|
| Neurons | Small molecules in stepwise defined media (NPCs: N2, B27, Noggin, SB431542, FGF; Neurons: N2, B27, BDNF, GDNF, cAMP) | iPSCs → neural stem cells → neuroprogenitor cells (NPCs) → neurons | Chambers et al. (2009); Shi, Kirwan, Smith, Robinson, and Livesey (2012) |
| Forced transcription factor expression of NGN2 using lentivirus + defined media (BDNF, NT3, B27, NT3, Ara-C, laminin) | iPSCs → iNs | Zhang et al. (2013) | |
| Astrocytes | Small molecules in stepwise defined media (GPCs: AM, Noggin, PDGF-AA; Astrocytes: N2, B27, FBS, LIF) | iPSCs → embryoid bodies → glial progenitor cells (GPCs) → astrocytes | Santos et al. (2017); methods reviewed in Suga, Kondo, and Inoue (2019) |
| Oligodendrocytes | Small molecules in stepwise defined media (NSCs: SB431542, LDN193189, RA; OPCs: RA, smoothened agonist, PDGF, IGF-1, HGF, NT3, Insulin, T3, Biotin, cAMP; Oligodendrocytes: Insulin, T3, Biotin, cAMP, ascorbic acid) | iPSCs → neural stem cells (NSCs) → oligodendrocyte progenitor cells (OPCs) → oligodendrocytes | Douvaras et al. (2014); Douvaras and Fossati (2015) |
| Microglia | Small molecules in stepwise defined media (iHPCs: FGF2, BMP4, Activin A, LiCl, VEGF, TPO, SCF, IL-3, IL-6; Microglia differentiation: CSF-1, IL-34, TGFβ1, insulin; Microglia maturation: CX3CL1, CD200) | iPSCs → hematopoietic progenitor cells (iHPCs) → microglia, differentiation step → microglia, maturation step | Abud et al. (2017); McQuade et al. (2018); Shi et al. (2012) |
hiPSCs are an attractive in vitro model for neurodegenerative diseases because of their human patient-specific origin, expandability, ability to differentiate into diverse human cell types, and potential to provide personalized medicine therapeutic solutions all while avoiding ethical issues associated with ESCs (Shi, Inoue, Wu, & Yamanaka, 2017). This approach is invaluable for studies exploring the mechanistic aspects of human neurons, as it is challenging to isolate and expand human primary neurons from the brain. hiPSC-derived neurons have been used to study disease processes involved in multiple neurodegenerative diseases, as reviewed in several recent publications (Berry, Smith, Young, & Mack, 2018; Penney, Ralvenius, & Tsai, 2020; Zhang, Hu, Shang, & Qi, 2019). Such hiPSC models have enabled significant scientific progress, especially in elucidating the pathogenesis of sporadic forms of neurodegenerative diseases and in drug discovery, with compounds identified using iPSC-derived neuronal models of AD (Bright et al., 2015) and ALS (Wainger et al., 2014) progressing to phase II clinical trials.
An ongoing challenge in neurodegenerative disease modeling is the ability to understand selective vulnerability, where for a particular disorder a specific neuronal subtype is more affected than others. Refinements in neuronal differentiation protocols have made significant advances in producing distinct neuronal subtypes such as CA3 hippocampal neurons (Sarkar et al., 2018), cortical interneurons (Nestor et al., 2015), medium spiny neurons (MSNs; Stanslowsky et al., 2016), dopaminergic neurons (Mahajani, Raina, Fokken, Kugler, & Bahr, 2019; Theka et al., 2013; Zhang, Xia, & Reijo Pera, 2014), motor neurons (Hester et al., 2011; Shimojo et al., 2015), and Purkinje neurons (Watson, Wong, Vowles, Cowley, & Becker, 2018). Furthermore, studies that transplant hiPSC-derived neurons into slice cultures show that the transplanted cells take on the specific properties of the region where they were transplanted, such as CA1 or the dentate gyrus (Hiragi et al., 2017). Large-scale single cell transcriptome studies of the human brain, such as those done by Hodge and colleagues (Hodge et al., 2019), provide data about specific neuronal subtypes and will certainly influence hiPSC differentiation protocols to further refine in vitro cell types.
hiPSCs have great utility as a tool to generate neurons to model neurodegenerative diseases; however, as with any model there are important limitations to consider. The generation of hiPSCs can be expensive and labor-intensive (Nicholas et al., 2013; Schlachetzki, Saliba, & Oliveira, 2013); hiPSCs also have potential for tumorigenicity (Miura et al., 2009) and aberrant genetic and epigenetic modifications (Gore et al., 2011; Lister et al., 2011). While many cell types of the CNS can be differentiated from hiPSCs, such as neurons, astrocytes, oligodendrocytes, and microglia (Table 1), protocols vary widely in their ease and efficiency; many differentiation protocols can take weeks to months to generate the cell type of interest (Espuny-Camacho et al., 2013; Krencik, Weick, Liu, Zhang, & Zhang, 2011; Nicholas et al., 2013; Wang et al., 2013). For neurons, this can be circumvented to some extent by expressing the neural transcription factor NGN2 in hiPSCs, generating excitatory neurons in under 1 month (Zhang et al., 2013).
The strongest risk factor for most neurodegenerative diseases is age (Niccoli & Partridge, 2012). Modeling age-related neurodegenerative disorders using hiPSCs presents a unique set of challenges, since hiPSC-derived neurons are fetal in nature and gene expression profiles are characteristic of an early embryonic stage (Israel et al., 2012). Despite this, however, phenotypes classically associated with age-related neurodegenerative disease have been consistently reported in experiments using hiPSC-derived neurons. For example, recent work demonstrates that hiPSC-derived neural cells show APOE genotype-dependent phenotypes consistent with AD pathophysiological changes, including tau phosphorylation, endosome enlargement, and impaired Aβ clearance in hiPSC-derived neurons, astrocytes, and microglia (Lin et al., 2018; Wang et al., 2018). This work highlights the utility of using hiPSC-derived cells to understand cell type-specific mechanisms, especially those that may have a genetic driver. Aging is a multifactorial process driven by genetics and environment and affects diverse cellular pathways (Lopez-Otin, Blasco, Partridge, Serrano, & Kroemer, 2013; Rodriguez-Rodero et al., 2011). The progressive dysfunction and degeneration of neurons in age-associated neurodegenerative diseases is also multifactorial and takes decades to develop; therefore using hiPSCs to model neurodegenerative diseases may not adequately address these processes, because the reprogramming protocols for these cells appear to reverse many of the cellular and molecular hallmarks of aging (Lo Sardo et al., 2017; Mertens et al., 2015; Studer, Vera, & Cornacchia, 2015). If and how cells age in vitro is not well understood and has prompted an intriguing line of research to introduce the effects of aging into hiPSC-derived cellular models of neurodegenerative disease.
Progerin overexpression has been studied as a potential way to create an aged phenotype in hiPSC-derived neurons. Progerin is a pathogenic protein that results in the human premature aging disorder Hutchinson-Gilford progeria syndrome (HGPS; Ashapkin, Kutueva, Kurchashova, & Kireev, 2019). When progerin was overexpressed in hiPSC-derived midbrain dopaminergic neurons, these cells displayed a phenotype that resembled late-onset PD (Miller et al., 2013), including progressive loss of tyrosine hydroxylase-positive cells, Lewy body-precursor inclusions, dendrite degeneration, neuromelanin accumulation, and mitochondrial function defects. However, because progerin represents a specific type of premature aging disorder, the molecular mechanism of how age-related phenotypes are induced may not recapitulate all aspects of normal aging. The need exists to identify molecular drivers of non-syndromic aging as well. Furthermore, the brain is relatively spared in HGPS patients due to low expression of endogenous progerin protein levels (Frost, 2016; Zhang et al., 2011). It remains unclear if progerin overexpression can be used to accurately model other age-related neurodegenerative diseases, such as AD. Recently, overexpression of the SCNA gene that encodes α-synuclein was also reported to induce nuclear aging phenotypes in iPSC-derived neurons, including age-related changes in nuclear architecture and global DNA methylation (Tagliafierro, Zamora, & Chiba-Falek, 2019). Interestingly, aging phenotypes in neurons derived from higher passage neural progenitor cells were also reported to display aging phenotypes, indicating that non-genetic methods of inducing aging in vitro may be possible (Tagliafierro et al., 2019).
3 INDUCED NEURONS
Human neurons can also be generated in vitro through a process called direct conversion, or transdifferentiation, effectively bypassing the reprogrammed, pluripotent stage and directly converting one somatic cell type into another (Figure 1). These directly converted neurons are known as iNs. iNs are distinct from neurons differentiated from iPSCs, neurons differentiated from other pluripotent stem cells, such as ES cells, or somatic cells programmed into a neural progenitors, which then undergo further differentiation into a more mature neuron (Drouin-Ouellet et al., 2017; Mollinari et al., 2018). This is a rapidly evolving field and various methods for generating iNs have been recently reviewed (Traxler, Edenhofer, & Mertens, 2019). Diverse cell types have been used to generate iNs, including fibroblasts, blood cells, glial cells, and pericytes (summarized in Traxler et al., 2019). We recently demonstrated that cells cultured from postmortem-derived leptomeningeal tissue will also successfully convert to iNs (Rose et al., 2018).
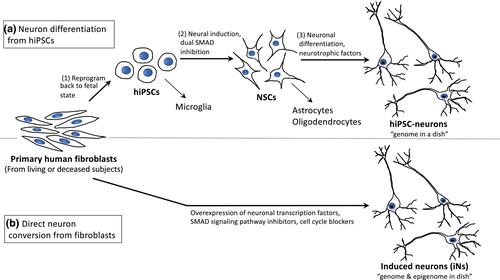
The first directly converted neurons were created by Vierbuchen and colleagues in 2010 from mouse fibroblasts. Starting with 19 neural-lineage-specific transcription factors, they found that a combination of three factors, ASCL1 (the master gene for inducing neuronal fate) as well as BRN2 and MYT1L (both of which enhance neuronal conversion), were able to rapidly and efficiently transform mouse embryonic fibroblasts and perinatal tail tip fibroblasts into iNs (Vierbuchen et al., 2010). Now commonly referred to as “BAM reprogramming factors” it has been shown by single-cell and genome-wide expression analyses that these factors not only induced transcription of neuronal genes, but also silenced the starting cell type transcriptome over time. Thus, reprogramming with the BAM factors led to a binary lineage switch rather than an induction of a hybrid phenotype (Marro et al., 2011). The BAM factors were also used to directly convert human fibroblasts into iNs (Pang et al., 2011; Pfisterer et al., 2011). Since this seminal work by Vierbuchen and colleagues in 2010, iNs have been generated through a number of strategies involving the use of various combinations of transcription factors, miRNAs and small molecule cocktails (SMCs; Table 2), also reviewed in (Traxler et al., 2019). Subsequent additions of other transcription factors, including NEUROD1 and SOX2 have improved the conversion efficiency resulted in complex neuronal morphologies and expression of neuron-specific proteins, for example β-III tubulin and MAP2 (Karow et al., 2012; Pang et al., 2011; Wang et al., 2014). Nanopattern topography has also been shown to improve direct conversion efficiency in mouse and human fibroblast cells, in which the cells are plated on defined nanoscale grooved patterns during the direct conversion process (Kim et al., 2017), and combinations of miRNAs can also directly convert fibroblasts (Yoo et al., 2011). Finally, knockdown of human polypyrimidine tract binding protein (PTBP), an RNA-binding protein that can block neuronal differentiation, has also been shown to directly convert fibroblasts to iNs (Liu et al., 2014).
| Disease | Donor cell type (human) | Specific conversion components | iN characteristics | Disease phenotype(s) observed | References |
|---|---|---|---|---|---|
| Alzheimer's disease (AD) | Dermal fibroblasts, fAD subjects with APP or PSEN1 mutations |
|
|
APP mutation line:
PSEN1 mutation lines:
|
Hu et al. (2015) |
| Fibroblasts, AD, and control subjects (with in vitro mutant APP overexpression) |
|
|
|
Kim et al. (2017) | |
| Parkinson's disease (PD) | Embryo-derived fibroblasts |
|
|
|
Pfisterer et al. (2011) |
| Fibroblasts, genetic forms PD, and control subjects |
|
|
|
Caiazzo et al. (2011) | |
| Fetal lung-derived fibroblasts |
|
|
|
Liu et al. (2012) | |
| Dermal fibroblasts, early onset PD subject with genetic mutation in PINK1 gene, and healthy controls |
|
|
|
Fiesel et al. (2015) | |
| Dermal fibroblasts, PD subjects with homozygous and heterozygous PINK1 mutations, compared to healthy controls with WT PINK1 |
|
|
|
Puschmann et al. (2017) | |
| Huntington's disease (HD) | Fibroblasts, two HD subjects, compared to healthy control |
|
|
|
Liu et al. (2014) |
| Dermal fibroblasts |
|
|
|
Victor et al. (2014) | |
| Amyotrophic lateral sclerosis (ALS) | Fibroblasts, ALS subjects with three different FUS mutations |
|
|
|
Lim, Choi, Oh, Xue, et al. (2016) |
| Dermal fibroblasts, c9FTD/ALS subjects with C9ORF72 mutation and controls |
|
|
|
Su et al. (2014) | |
| Dermal fibroblasts, three ALS subjects with FUS mutations, and controls |
|
|
|
Liu et al. (2016) | |
| Spinal muscular atrophy (SMA) | Embryonic fibroblasts |
|
|
|
Son et al. (2011) |
| Fibroblasts, SMA, and control subjects |
|
|
|
Zhang et al. (2017) | |
| Frontotemporal dementia with parkinsonism-17 (FTDP-17T) | Dermal fibroblasts, FTDP-17T subject (PMI 2 hr), and control subjects |
|
|
|
Iovino et al. (2014) |
| Pantothenate kinase-associated neurodegeneration (PKAN) | Dermal fibroblasts, 3 PKAN and 3 control subjects |
|
|
|
Santambrogio et al. (2015) |
| Krabbe disease (KD) | Dermal fibroblasts, KD subjects with mutations in GALC gene, and control subjects |
|
|
|
Lim, Choi, Oh, Choi, et al. (2016) |
Since iN reprogramming methods bypass the pluripotent state, iNs may not undergo the rejuvenation reported in hiPSC-Ns. Indeed, several studies have shown that iNs appear to retain transcriptomic and epigenetic characteristics similar to the aged donor somatic cells (Huh et al., 2016; Mertens et al., 2015). This makes iNs an intriguing model of aged, patient/disease-specific neurons in culture, which may provide unique opportunities to study the neuropathological mechanisms underlying neurodegenerative diseases in the context of aging. However, recent work shows extensive de novo DNA methylation occurs in mouse fibroblasts directly converted to neurons using BAM factors (Luo et al., 2019). While this study suggests that this epigenetic remodeling promotes a neuronal epigenetic landscape, more detailed analysis of epigenome remodeling that may occur in directly converted human neurons in aged or disease states is warranted.
In the following sections we focus on current applications of iN technology in neurological disease modeling research. iNs have been used to research mechanisms involved in many neurodegenerative diseases, outlined in greater detail in Table 2. iNs have also been used to model other nervous system disorders, including autism spectrum disorder (Yoshimizu et al., 2015), schizophrenia (Passeri et al., 2015; Siegert et al., 2015), Dravet syndrome and mild febrile seizures (Jiao et al., 2013), and glaucoma (Meng, Wang, Gu, Wang, & Guo, 2013). The wide range of research fields utilizing iNs to study neurologic disease suggests a promising and lasting role for this technology in the study of human neuronal function and dysfunction, particularly in age-associated diseases. Here we highlight some of the interesting findings from the studies of neurodegenerative disease mechanism using iN culture models.
3.1 Alzheimer's disease
AD is a neurodegenerative disorder that affects memory as well as other cognitive abilities. AD is characterized neuropathologically by the presence of neurofibrillary tangles containing hyperphosphorylated tau (pTau) and amyloid beta plaques (Montine et al., 2012). Hu and colleagues developed a protocol to robustly convert human fibroblasts derived from patients with familial AD (fAD) into functional iNs using a SMC and a neural maturation media (Table 2; Hu et al., 2015). In this study, iNs derived from fAD patients displayed increased extracellular Aβ42 and an elevated Aβ42/Aβ40 ratio, compared to iNs derived from control subjects. In addition, levels of phosphorylated tau (pTau) and total tau were higher in iNs from fAD patients with an APP mutation relative to controls, but this difference was not observed in iNs from fAD patients with the presenilin 1 mutation (Hu et al., 2015), suggesting this important pathologic characteristic may be influenced differently in neurons harboring different fAD mutations. In a separate study, iNs generated from sporadic and fAD patients were used to study the effects of the APOE 3/4 versus 3/3 genotype on neuron health and pathophysiology when mutant APP (670/M671 Swedish mutation) was overexpressed in these lines. APOE 3/4 iNs faithfully replicated the disease features seen in APOE 3/4 AD patients such as altered APP processing, increased levels of Aβ42 and tau hyperphosphorylation. Furthermore, gene expression profiling and network analysis of these iNs indicated dysregulation of desmoglein 2 (DSG2), a putative AD risk gene (Karch & Goate, 2015). Functional studies of DSG2 knockdown demonstrated that inhibition of DSG2 reduced Aβ aggregation induced by overexpression of APP in APOE 3/4 iNs (Kim et al., 2017). There are fewer studies using iNs from sporadic AD subjects than familial genetic AD subjects, in part due to immense genetic and phenotypic variability between sporadic AD subjects. Recent genetic studies highlight major cell biological pathways that are implicated in AD such as lipids, endocytosis, and inflammation (Karch & Goate, 2015). Therefore, one strategy to mitigate some variability would be to stratify patient-derived cell lines based on polygenic AD risk in specific cellular pathways, and to focus on relevant in vitro assays to assess phenotypic presentation of the identified risk variants in neurons.
3.2 Parkinson's disease
Several researchers have also developed iN-based models of PD, a disorder involving both motor and non-motor symptoms. PD is characterized neuropathologically by the loss of dopaminergic neurons in the substantia nigra as well as the aggregation of pathological forms of α-synuclein in Lewy bodies and Lewy neuritis (Kalia & Lang, 2015). Pfisterer and colleagues used BAM transcription factors together with two genes involved in dopaminergic neuron generation, LMX1A and FOXA2, to convert human fibroblasts into iNs comparable to dopaminergic neurons (Pfisterer et al., 2011). Caiazzo and colleagues also used transcription factors to generate functional dopaminergic iNs from the fibroblasts of patients with genetic forms of PD and healthy donors. Fibroblasts from PD patients and normal controls both showed a similar ability to transform into dopaminergic iNs (Caiazzo et al., 2011). In another study, dopaminergic iNs, generated from IMR90 human fibroblasts, were injected into the striatum of a PD rat model. These iNs demonstrated functional properties of dopaminergic neurons such as dopamine release and re-uptake and proper electrophysiologic profiles. When these iNs were injected into a rat model of PD, the authors documented improved motor symptoms in the animals along with evidence of long-term engraftment and the retention of dopaminergic properties of the iNs (Liu et al., 2012). iN models have also been used to investigate ubiquitin phosphorylation in PD (Fiesel et al., 2015) and to assess the PTEN-induced putative kinase 1 (PINK1) p.G411S mutation as a risk factor for PD (Puschmann et al., 2017). Currently, much of the literature relevant to the use of iNs in modeling PD has been focused on methodological development and validation of dopaminergic iNs. Investigation of disease-specific mechanisms in dopaminergic iNs from PD patients is required, including idiopathic, or sporadic, PD and more broadly in Lewy body disease, to see if dopaminergic and cortical iNs recapitulate disease phenotype(s) seen in human disease, such as pathological post-translational alterations to α-synuclein.
3.3 Huntington's disease
iNs have also been used to model Huntington's disease (HD), a neurodegenerative disease caused by a genetic mutation, a CAG repeat, in the Htt gene that results in expanded polyglutamine repeats in the N terminus of the huntingtin protein (Htt; Liu et al., 2014). Pathological hallmarks of HD include Htt protein inclusions, as well as neuron loss in defined regions of the cortex and in the neostriatum affecting MSNs (Liu et al., 2014). Fibroblasts derived from HD patients showed a similar capacity for iN conversion compared to healthy controls, using knockdown of PTBP. HD patient-derived iNs display abnormal neuritic branching and degeneration, shrunken soma and increased cell death compared to iNs derived from normal individuals, as well as mutant Htt protein inclusions in their soma and neuropils (Liu et al., 2014). Victor and colleagues used a combination of transcription factors and miRNAs to create a population of iNs analogous to MSNs. Upon transplantation into the brains of mice, the MSN-like iNs survived for over 6 months, displayed similar membrane properties as MSNs and formed axonal projections to the anatomical targets of MSNs (Victor et al., 2014). Based on the studies reviewed above, iNs may be a promising in vitro model of HD, given the presence of Htt pathological protein inclusions similar to those found in HD patients, among other similarities in disease phenotype. In contrast, the hallmark pathological protein aggregates in AD and PD (amyloid plaques/neurofibrillary tangles and Lewy bodies, respectively) have not been observed in iN models of these diseases. As a future research direction, it may be interesting to generate both iNs and iPSC-Ns from the same HD individuals to identify possible epigenetic contributors involved in disease progression or initiation, as HD is an age-associated disease in which symptom onset occurs during adulthood, even though mutant Htt protein is expressed ubiquitously throughout life (Li & Li, 2015).
3.4 Motor neuron diseases
Several iN models of motor neurons have been applied to the studies of ALS and spinal muscular atrophy (SMA). Lim and colleagues created iNs from the fibroblasts of ALS patients with different FUS mutations using a combination of shRNAs against PTB and neurotrophic factors. In ALS patient-derived iNs, FUS was located predominantly in cytoplasmic regions, with oxidative stress leading to the accumulation of cytoplasmic FUS into cytoplasmic granules, consistent with observations in ALS patients (Lim, Choi, Oh, Xue, et al., 2016). One ALS patient line generated in this study was contained a novel FUS variant, demonstrating the value of these iN models in furthering our understanding of the mechanisms involved in newly identified gene mutations. In a different study using ALS patient-derived iNs, researchers found that ALS patient-derived iNs displayed significantly higher FUS protein aberrantly localized in the cytoplasm, decreased soma size, deficits in action potential firing, and an increased susceptibility to death compared to controls (Liu, Zang, & Zhang, 2016). In this study a chemical screen of small molecules showed that a 1 μM concentration of kenpaullone was the most effective at promoting the survival of ALS iNs (Liu et al., 2016). It is worth noting that FUS mutations only comprise 4% of familial and 1% of sporadic ALS cases (Lim, Choi, Oh, Xue, et al., 2016), thus it will be important to generate iN models of ALS that represent a broader proportion of ALS cases. One such study has used shRNA inhibition of PTBP to develop iNs from a frontotemporal dementia and amyotrophic lateral sclerosis (c9FTD/ALS) subjects, an important addition to the field, as mutations in C9ORF72 are the most common genetic cause of ALS and FTD (Su et al., 2014).
SMA is an autosomal recessive disorder leading to full or partial loss of the SMN1 gene. This disorder results in loss of anterior horn or α motor neurons. SMA iNs were created by BAM and other transcription factors, including NEUROD1, ISL1, and HB9, to reprogram fibroblasts from SMA patients and controls into motor iNs. The SMA iNs had a similar conversion efficiency as control iNs, but displayed reduced neurite outgrowth, neurite fracturing, and overt degeneration after 60 days in vitro. While treatment with a histone deacetylase inhibitor SAHA unfortunately did not rescue the neurodegeneration, the recapitulation of neurodegeneration makes it a promising model for other drug screening (Zhang et al., 2017).
3.5 Frontotemporal Lobar degeneration
Mutations in the microtubule-associated protein tau (MAPT) gene can cause frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17T; Boeve & Hutton, 2008). In a pioneering study, Iovino and colleagues used autopsy-derived dermal fibroblasts from a FTDP-17T subject with a 2-hr postmortem interval (PMI), and cultured the tissue explant for 2 weeks until fibroblasts started to appear. The resultant fibroblasts were then exposed to the BAM, LMX1A, and FOXA2 transcription factors. The FTPD-17T iNs expressed 3R and 4R isoforms of tau with expression levels comparable to human adult fibroblast-derived iNs, while human embryonic fibroblast-derived iNs only expressed 3R tau (Iovino et al., 2014).
FTLDs are a diverse and varied group of neurodegenerative diseases, including tau, TDP-43, and FUS proteinopathies. As a relatively large proportion of FTLDs are hereditary (20%–30%; Iovino et al., 2014) but are also aging-related diseases, using iNs to model these diseases in vitro could provide the combination of genetic and epigenetic factors representative of the conditions present in the human disease.
3.6 Other neurodegenerative diseases
Pantothenate kinase-associated neurodegeneration (PKAN) is a neurodegenerative disease characterized by high iron accumulation in the brain. Fibroblasts taken from PKAN patients displayed altered iron homeostasis, as evidenced by significantly elevated toxic-free iron in the mitochondrial compartment, increased levels of carbonylated protein and significant reductions in glutathione and heme content (Santambrogio et al., 2015). These PKAN patient fibroblasts were exposed to MASH1, NURR1, and LMX1A transcription factors in order to generate PKAN iNs. These iNs contained higher levels of reactive oxygen species, lower levels of glutathione, and significantly lower membrane potentials, compared to control iNs (Santambrogio et al., 2015). Although mitochondrial iron was not measured in the iNs, the authors concluded that the altered oxidative status and mitochondrial dysfunction seen in PKAN iNs were likely due to iron mishandling.
Krabbe disease (KD) is a neurodegenerative disorder caused by defective β-galactosylceramidase, a lysosomal enzyme responsible for cleavage of several substrates including psychosine. Fibroblasts from KD patients and healthy controls were exposed to shRNAs against human PTBP and then treated with neurotrophic factors to generate iNs. Relative to controls, KD patient-derived iNs displayed significantly reduced β-galactosylceramidase enzymatic activity, increased psychosine levels, enlarged and irregularly shaped lysosomal vesicles, neurite fragmentation, abnormal neuritic branching, disorganized actin filaments, and defective axonal outgrowth (Lim, Choi, Oh, Choi, et al., 2016).
Taken together, the studies summarized above use iNs in the context of specific neurodegenerative diseases. Recent work is beginning to incorporate a greater number of specific patient-derived lines, although to date much of the research has focused on the development of direct conversion protocols, for example to create neuronal subtypes and to improve conversion efficiency (Caiazzo et al., 2011; Pfisterer et al., 2011; Son et al., 2011; Victor et al., 2014). Currently, studies of neurodegenerative diseases have included use of iNs in a limited capacity, primarily as proof-of-concept, to show that patient-derived somatic cells can be successfully converted to iNs, and that the resultant iNs display important disease-specific phenotypes. Several studies have begun to use iN disease models to test novel compounds that may be able to rescue such disease phenotypes (Liu et al., 2016; Su et al., 2014; Zhang et al., 2017). Currently, at most only two to three diseased lines per study have been included, providing important and promising examples of how iNs can be applied to research on human neurodegenerative disease mechanisms and drug development. It will be critical in future studies to expand the number of different patient-derived lines when testing such compounds, to demonstrate that these potential therapeutic targets are effective across a broader disease population. There is vast potential in this field for expansion, to use iNs as a physiologically relevant in vitro neuronal model of genetic, epigenetic and molecular contributors to neurodegenerative disease mechanisms, especially as larger patient iN cohorts become economically and technologically feasible, especially in order to overcome inherent variability present in human sporadic aging-related neurodegenerative diseases.
4 DISCUSSION
Developing effective therapies or preventive strategies for ADRD and other neurodegenerative diseases is the principle goal of research in these areas, yet most interventions fail to reach the market due to safety or efficacy issues. A major hurdle in effective therapeutic development is limitations in the models used to identify potential drug targets and test candidate therapies, which in some cases have not accurately predicted efficacy in patients (Young, D'Souza, Lemischka, & Schaniel, 2012). Genetically modified animal models (which are often based on Mendelian forms of the disease) and immortalized human cell lines have long been utilized in neurodegenerative disease research; both hiPSC-N and iN models have significant advantages when compared to animal models or immortalized cells. Each method provides access to human neurons that may not otherwise be feasible to grow in primary culture. Both hiPSC-Ns and iNs can be directed into distinct neuronal subtypes, although hiPSCs also have added benefit of being differentiated into non-neuronal central nervous system cell types, including astrocytes and microglia (Table 1). Direct conversion of human somatic cells to CNS cell types other than neurons has not been as successful (Traxler et al., 2019). Both hiPSC-Ns and iNs capture human genetic background in a dish, which is essential in understanding unique human aspects of disease. However, in the case of hiPSCs much of the epigenome, which contains important environmental and aging information, is erased. Thus, iNs, which do not undergo full reprogramming, may be a useful tool to study mechanisms involved in cellular aging and which are integrally associated with the cellular epigenome. Indeed, iNs may retain more epigenetic characteristics of the donor cell, which can affect several aspects of cellular function including mitochondrial function, cell senescence, DNA damage, nuclear pore permeability, and protein localization (Huh et al., 2016; Mertens et al., 2015; Mertens, Reid, Lau, Kim, & Gage, 2018). As such, iNs not only capture the genome in a dish but some level of the epigenome in the dish as well, and may therefore have tremendous potential for investigating how age affects neuronal function and toxicity in age-associated neurodegenerative diseases.
In terms of disease modeling, human iNs have been shown to display many of the neuropathologic phenotypes present in the patients from which the cells were derived. For example, abnormalities in iron homeostasis and energetic dysfunction seen in PKAN patient-derived iNs and fibroblasts were not observed in animal models (Santambrogio et al., 2015). Similarly, in ALS patient-derived iNs, the mutant FUS protein was located in the cytoplasm, rather than the nucleus—a disease phenotype that was not observed in primary rat neurons, HEK-293 cells, or fibroblasts with the same mutation (Lim, Choi, Oh, Xue, et al., 2016). iNs derived from fAD patients had higher levels of Aβ42 and pTau, a disease phenotype not observed the corresponding patient fibroblasts (Hu et al., 2015). Lastly, while both fibroblasts and iNs from patients with KD displayed lysosomal abnormalities, additional phenotypic changes including neuronal branching were also observed in Krabbe iNs, a phenotype that cannot be assessed in fibroblasts (Lim, Choi, Oh, Choi, et al., 2016). For future studies, availability of in vitro models for mechanistic studies that can be related back to matched neuropathologic tissue will yield great insights into both early and late stages of the disease process. We have published a method to freeze and cryoprotect the leptomeningeal samples taken at autopsy from subjects that will undergo a comprehensive neuropathologic work up. Using these cells, we can simultaneously reprogram the leptomeningeal cells to hiPSC and differentiate neurons as well as directly convert the cells to iNs. This model gives us two forms of in vitro-derived neurons from which we can compare phenotypes in living cells to the pathology of the subject's brain (Rose et al., 2018; Figure 1). However, this protocol requires access to high-quality postmortem tissue, may be sensitive to PMI, and could require specific informed consent. Patient-specific iNs with comprehensive clinical and pathologic characterization of the donors and their tissue will likely play an important role in the identification of candidate drug targets and drug screening, contributing to a precision medicine approach for novel neurodegenerative disease therapeutics.
In this review we have highlighted several key advantages of iNs, focusing on epigenome integrity and the retention of cellular age. However, there are cautionary aspects to this technology as well. Since iNs are newer and less commonly used compared to hiPSCs, some aspects of the technology are not as well developed as with hiPSCs. For example, a greater number of neuronal and neural cell types have been established using hiPSCs (Table 1) compared to iNs. Indeed, many protocols for differentiating hiPSCs are well standardized and often the reagents are commercially available. For iNs, however, the efficiency of conversion is reduced and the cocktails of necessary transcription factors are less standardized. An additional consideration is the epigenetic state of the starting cell type, which may have a large effect on the efficiency of conversion (Wapinski et al., 2013) thus presenting an additional challenge depending on available patient material. Furthermore, somatic cells have not been directly converted to other neural cell types, such as microglia. While iNs may be a less tumorigenic cell source for development of therapeutic transplantation technology compared to hiPSC-derived cells, there is still some risk of genomic integration and potential disruption of endogenous genes, including tumor suppressor genes, by the lentivirus used in many iN protocols (Liu et al., 2012). Significant work has been done to minimize retro- and lentiviral reprogramming-associated integrations of DNA sequences into the genome in hiPSC reprogramming, and the use of commercially available, non-integrating Sendai viruses is becoming common practice in the field (Mertens et al., 2018). However, integration-free iN generation has received far less attention, with Sendai viruses for iN factors such as the BAM factors or NEUROD1 not yet commercially available (Mertens et al., 2018). While some studies suggest that the epigenome of iNs may be similar to that of the parental somatic cell, recent work shows that the BAM factors can induce global de novo DNA methylation (Luo et al., 2019). There are also limitations to the application of gene editing technologies using iNs, such as CRISPR/Cas9, which has not to our knowledge been successfully carried out in an iN model system even though it has been used extensively in hiPSC experiments. Mature iNs are not an expandable cell population and require a new induction process for every experiment, which places limitations on the starting material since primary fibroblasts cannot be expanded indefinitely. In contrast, hiPSCs and progenitor populations are renewable and can be banked for future applications, which can decrease labor and material costs overtime. Several groups have reported low fibroblast-to-neuron efficiency rates and limitations due to neuronal purity, with only 1%–6% of the starting somatic cell population converting successfully to iNs expressing mature neuronal markers (Liu et al., 2012; Santambrogio et al., 2015; Zhang et al., 2017). The variability in conversion efficient appears to be both cell line-dependent and protocol-dependent, thus there is a great amount of method optimization that is warranted in the use of iNs to study neurodegenerative diseases.
A clear advantage of both iNs and hiPSC-derived neural cells is the ability to generate patient-specific cell lines, effectively capturing the unique genetic background in vitro. However, this strategy also lends itself to the inherent phenotypic variability present in human samples. Indeed, we and others have reported phenotypes from hiPSCs that are present in some, but not all, cell lines studied (Israel et al., 2012; Kondo et al., 2013; Young et al., 2015). Therefore, the number of unique patient cell lines needed for in vitro experiments in order to ascertain signal from noise is an important factor in experimental design. In studies where the expected phenotypic result is not yet known, estimates of the expected effect size from similar studies in the literature can be used in order to determine power and ideal sample size. However, the effect of a particular gene mutation or variant may also be subtle at the level of the cell but quite detrimental for development of disease. For example, only a single copy duplication of the amyloid precursor protein, which results in only 1.5X the expression of APP in the cell, is enough to cause aggressive, early onset AD. A survey of recent papers modeling AD (Flamier et al., 2018; Foveau et al., 2019; Meyer et al., 2019; Ochalek et al., 2017) shows that most studies are using cell lines derived from three to five patients and three to five controls. Experimental cost and the labor-intensive methods for this type of work currently limit the number of cell lines from different individuals that can be studied. The use of isogenic cell lines where variant or mutations of interest are introduced or corrected using gene editing techniques can mitigate intrinsic variability, and in these studies typically two to four clones of isogenic cell lines are used (Lin et al., 2018; Wang et al., 2018; Woodruff et al., 2013). In general, these studies use sample sizes that correspond to recent work analyzing the technical and biological variability in hiPSCs (Germain & Testa, 2017). Most of the work in neurodegenerative disease modeling has been performed using hiPSC-derived cells; in the iN studies summarized in this manuscript, typically only two to three patient cell lines are used, therefore a more careful analysis of the variability in iN studies is warranted.
Finally, while simple monogenetic diseases can often be modeled using a single cell with a defined phenotype, modeling more complex, multifactorial neurodegenerative diseases is more challenging since multiple factors contribute to development of the phenotype and pathology over time and space that are impossible to model in a single cell type. Thus, to more closely replicate the complex environment in the brain, there may be value in coculturing patient-derived iNs with other cell types in the brain. With the development of protocols to generate patient-specific microglia (Abud et al., 2017) and patient-specific astrocytes (Caiazzo et al., 2015), these cells could be cocultured together with patient-specific iNs, as well as culturing these cells in a three-dimensional culture to capture the spatiotemporal context of the brain environment. This 3D culture system could either be an engineered system, whereby cells are cultured on a supporting material (such as a biodegradable scaffold or gel; Kim et al., 2015; Soman et al., 2012; Zhang, Pekkanen-Mattila, et al., 2014) or a structure-free system, whereby the different cell types form self-organizing, discrete structures for example, spheroids or organoids (Lancaster et al., 2013; Qian et al., 2016; Raja et al., 2016).
5 CONCLUSION
Both hiPSC-derived neurons and iNs are valuable and relevant human in vitro cell culture modeling systems that can be used to help elucidate disease mechanisms, improving our understanding of genetic risk factors in neurodegenerative diseases and screen for therapeutic targets. iNs in particular hold promise in the study of diseases in which advanced age is a primary risk factor, such as the neurodegenerative diseases discussed in this study, as they retain more of the epigenetic “aging” signature than neurons differentiated from hiPSCs. iN protocols continue to be developed and refined and the different protocols used in neural reprogramming including the vast array of different factors, each with their own function, serve to confirm previous research showing that neuronal identity is highly specific and closely controlled by a unique combination of intrinsic and extrinsic signals (Liu et al., 2013). Further studies investigating mechanisms of direct reprogramming are needed to elucidate the relationship between reprogramming factors and the final cell product in order to optimize the methodology (e.g., reprogramming efficiency, purity, cell yield, cell type specificity), which will help drive the study of neuron function in the context of brain aging and neurodegeneration.
ACKNOWLEDGMENTS
This work was supported by the BrightFocus Foundation (A2018656S to JEY), the NIH (K01AG054841 and R01AG062148 to JEY; P50AG005136 to CDK), and the Nancy and Buster Alvord endowment (to CDK).
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization, G.D.; Writing – Original Draft, G.D., S.E.R., A.K., and J.E.Y.; Writing – Review & Editing, G.D., S.E.R., A.K., D.A.N., C.D.K., and J.E.Y.; Visualization, G.D., S.E.R., and J.E.Y.; Supervision, J.E.Y.; Funding Acquisition, J.E.Y. and C.D.K.




