Glial source of nitric oxide in epileptogenesis: A target for disease modification in epilepsy
Funding information: This research was supported by startup funds, the Presidential Initiative on Interdisciplinary Research (Big Data Brain Initiative) fund to T. Thippeswamy, Iowa State University, Iowa (grant 721-18-17-IR-172E), and the NIH grant NS099007 to T. Thippeswamy.
Significance: Neuroinflammatory mediators are emerging as therapeutic targets for epilepsy. Status epilepticus–induced neuroinflammation in epileptogenesis is characterized by reactive gliosis and production of reactive oxygen and nitrogen species. Glial-mediated nitric oxide (NO) production in the central nervous system, in contrast to the peripheral nervous system, is cytotoxic. Therefore, the agents that target the glial source of NO could be neuroprotective and may restore brain function. This approach can be beneficial to modify/prevent epileptogenesis and the disease. In this review, we discuss the potential disease-modifying role of the inducible NO synthase inhibitor, in contrast to neuronal NO synthase inhibitors, in epilepsy.
Abstract
Epileptogenesis is the process of developing an epileptic condition and/or its progression once it is established. The molecules that initiate, promote, and propagate remarkable changes in the brain during epileptogenesis are emerging as targets for prevention/treatment of epilepsy. Epileptogenesis is a continuous process that follows immediately after status epilepticus (SE) in animal models of acquired temporal lobe epilepsy (TLE). Both SE and epileptogenesis are potential therapeutic targets for the discovery of anticonvulsants and antiepileptogenic or disease-modifying agents. For translational studies, SE targets are appropriate for screening anticonvulsive drugs prior to their advancement as therapeutic agents, while targets of epileptogenesis are relevant for identification and development of therapeutic agents that can either prevent or modify the disease or its onset. The acute seizure models do not reveal antiepileptogenic properties of anticonvulsive drugs. This review highlights the important components of epileptogenesis and the long-term impact of intervening one of these components, nitric oxide (NO), in rat and mouse kainate models of TLE. NO is a putative pleotropic gaseous neurotransmitter and an important contributor of nitro-oxidative stress that coexists with neuroinflammation and epileptogenesis. The long-term impact of inhibiting the glial source of NO during early epileptogenesis in the rat model of TLE is reviewed. The importance of sex as a biological variable in disease modification strategies in epilepsy is also briefly discussed.
1 INTRODUCTION
Epilepsy is the fourth most common neurological disorder in humans and animals worldwide (Beghi, 2016; Bernstein, Bilheimer, & Makuc, 2010; De Risio et al., 2015; Kirkley, Madl, Duncan, Gulland, & Tjalkens, 2014; Pakozdy, Halasz, & Klang, 2014; Vos et al., 2015; WHO Executive Board, 2015). It is a chronic and debilitating disorder that affects both men and women of all ages (Vos et al., 2015; WHO Executive Board, 2015). The lifetime prevalence of active epilepsy worldwide is ∼2% ( > 65 million people) (WHO Executive Board, 2015). According to a report from the Centers for Disease Control and Prevention, it is estimated that ∼10% of Americans experience a seizure during their lifetime and that ∼3% of these develop epilepsy by the time they are 80 years of age. In the United States alone, about 150,000 new cases of epilepsy are reported annually (Austin, Hesdorffer, Liverman, & Schultz, 2012; Hesdorffer et al., 2011; Hesdorffer & Begley, 2013; Koh et al., 2014). Epilepsy affects nearly 3.2 million Americans, and its management, including caregiver expenses, costs an estimated $15.5 billion annually (Bernstein et al., 2010). Apart from the direct impact of epilepsy on the economy, the epilepsy disorder spectrum has a huge secondary impact on physical, psychological, and social issues (Austin et al., 2012; Krumholz et al., 2015). Although more than 30 antiseizure or antiepileptic drugs (ASDs/AEDs) are available to treat epilepsy, > 50% of people with epilepsy (PWE) experiencing their first seizure do not become seizure-free with the first ASD/AED therapy. Moreover, about 17% of PWE require combination therapy (Krumholz et al., 2015; Kwan & Brodie, 2000). Whether the first seizure is appropriately treated or not, or the second seizure is treated, AEDs do not reduce/prevent the long-term probability of seizure freedom and comorbidity. Furthermore, about one-third of PWE are refractory to the current AEDs (Kwan, Schachter, & Brodie, 2011; Musicco, Beghi, Solari, & Viani, 1997). Although AEDs control seizures in the other two-thirds of PWE, they do not cure or modify the disease process in temporal lobe epilepsy (TLE) (Kwan et al., 2011). TLE is the most common type among all epilepsies with cognitive dysfunction as a prevailing comorbidity (Pearson et al., 2015; Rzezak et al., 2016; Thompson et al., 2016). The majority of AEDs (including 47 failed drugs in human trials) that are not very effective, or do not cure the disease, are ion channel–targeted drugs (Temkin et al., 2001; Varvel, Jiang, & Dingledine, 2015). This suggests the need for the development of drugs that target alternative pathways to cure/modify the disease. To achieve this, a better understanding of the mechanisms of epileptogenesis in animal models is required.
2 SEVERITY OF STATUS EPILEPTICUS DETERMINES EPILEPTOGENESIS
Convulsive status epilepticus (SE) that is experimentally induced in rodents by neurotoxins such as kainate or pilocarpine or diisopropylfluorophosphate, an organophosphate, causes irreversible brain damage if not adequately treated immediately (Brandt, Bankstahl, Töllner, Klee, & Löscher, 2016; Furtado, Rossetti, Chanda, & Yourick, 2012; Iyer, Iken, & Leon, 2015; Lemercier, Carpentier, Sentenac-Roumanou, & Morelis, 1983; Puttachary, Sharma, Thippeswamy, & Thippeswamy, 2016; Puttachary, Sharma, Verma, et al., 2016). The duration and severity of SE determine the outcome of epileptogenesis. The definition of SE depends on the context and has been constantly changing (Manno, 2011; Seinfeld, Goodkin , & Shinnar, 2016). According to the recommendation of the Commission on Classification and Terminology and the Commission on Epidemiology of the International League Against Epilepsy (ILAE), the SE is defined as “a condition resulting either from the failure of the mechanisms responsible for seizure termination or from the initiation of mechanisms, which lead to abnormally, prolonged seizures (after time point t1). It is a condition, which can have long-term consequences (after time point t2), including neuronal death, neuronal injury, and alteration of neuronal networks, depending on the type and duration of seizures” (Trinka et al., 2015). Traditionally, it has been accepted that the duration of convulsive seizures (CSs) during SE is sufficient to cause long-term brain injury/damage to enable the brain to generate spontaneous seizures—that is, “an enduring epilepticus” (Gastaut, 1983). The initial duration of SE for humans was 60 min, which was then reduced to 30 min. This duration is now widely accepted for studies that investigate the long-term consequences of SE—that is, epileptogenesis and epilepsy (Dodson et al., 1993; Guidelines for epidemiologic studies on epilepsy, 1993). Seizures normally self-terminate by activating the inhibitory mechanism; however, if this mechanism fails it can lead to prolonged seizures (i.e., SE), which may then require administration of intervention drugs to terminate SE (Manno, 2011). Interestingly, the clinical trial guidelines recommend ∼5 min to intervene in the case of continuous CSs (Dodson et al., 1993; Lowenstein, Bleck, & Macdonald, 1999; Seinfeld et al., 2016; Shinnar, Berg, Moshe, & Shinnar, 2001). In chemoconvulsant animal models, it has been known that a minimum of 10 min of convulsive SE is sufficient to cause brain injury and to induce TLE (Nairismagi et al., 2004; Puttachary, Sharma, Tse, et al., 2015).
It is also important to note that in some mouse models, even though the initial SE is severe and prolonged, the development of epilepsy may be compromised and may not manifest the classical features of epileptogenesis, such as progressive increase in frequency of spontaneous CSs. For example, the SE induced by repeated low doses of kainate (i.p.) in a C57BL/6J mouse model produced severe SE, and continuous video electroencephalography (EEG) confirmed that they developed epilepsy in less than 5 days (Puttachary, Sharma, Tse, et al., 2015). A similar rapid epileptogenesis has been reported in the mouse pilocarpine model (Mazzuferi, Kumar, & Kaminski, 2012). The spontaneous CSs persist for about 4 to 6 weeks, but they become infrequent thereafter. However, electrographic nonconvulsive seizures (NCSs) persist for a longer period, up to 4 months post-SE (Puttachary, Sharma, Tse, et al., 2015). Intrahippocampal kainate administration in the mouse model also produced similar results with respect to infrequent CS, but frequent electrographic NCS (Klee, Brandt, Töllner, & Löscher, 2017) and widespread granule cell dispersion have been reported (Hester & Danzer, 2014; Murphy & Danzer, 2011; Murphy, Hofacer, Faulkner, Loepke, & Danzer, 2012; Suzuki et al., 2005). This could be due to the direct impact of intrahippocampal injection of kainate. It is also important to note that the kainate-induced SE via the intraperitoneal route in transgenic mice, bred on C57 genetic background (e.g., eGFP-expressing mice), caused inconsistent epileptogenesis resulting in ∼5 spontaneous CSs in the first month, and the CSs were completely absent in subsequent months (Figure 1). However, as in the C57BL/6J mice, large numbers of electrographic NCSs persisted in transgenic mice in a 3-month continuous video EEG study (data not shown). Therefore, mouse kainate (i.p.) models are not suitable for chronic studies if the experimental objective is to determine the effects of a disease modifier on the frequency of CSs. However, they can be useful to assess the impact of drugs on epileptiform spikes and/or electrographic NCSs in the absence of a CS paradigm. Therefore, in such scenarios, one could consider NCS and spike trains as variables, instead of CS, to compare between control and drug-treated groups. To mimic human TLE for translational purposes, a rat kainate model is more suitable in terms of the progressive nature of the disease because the frequency of spontaneous CSs increases consistently over time in rats in contrast to mouse kainate models (Figure 1).
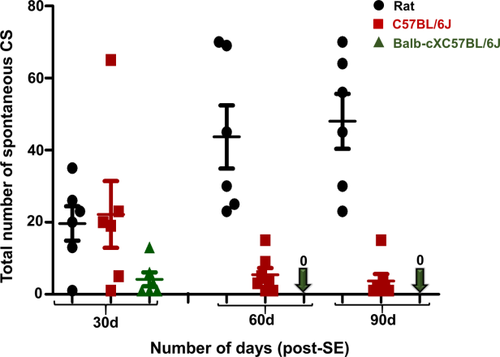
Comparison of spontaneous CS frequency between the kainate models of rat, C57BL/6J, and cross-bred wild-type mice. The seizures were quantified from 3 months of continuous video EEG recordings. The behavioral spontaneous CSs were verified against EEG pattern and the power spectrum as described previously for the rat and mouse kainate model of TLE (Puttachary, Sharma, Tse, et al., 2015; Puttachary, Sharma, Verma, et al., 2016). CSs were progressive in the rat, while they decreased over time in the mouse models. Mann–Whitney test, *p < 0.05, n = 6–8. CS = convulsive seizures
In a recent review article by Löscher et al., the impact of inter- and intrastrain differences in rats and mice for epilepsy research has been thoroughly discussed (Löscher, Ferland, & Ferraro, 2017). According to this review, the following variables should be controlled to achieve reproducibility and rigor, and to minimize experimental bias in epilepsy research. These are environmental (housing, enrichment, food, water, and litter size); experimental (seizure frequency and duration, and seizure threshold); biological (age and sex); and genetic (genetic background and gene manipulation). Because the focus of our review is to address the targets of epileptogenesis for disease modification, we considered the initial SE severity as an important factor to initiate epileptogenesis. We largely overcame variability in severity of SE in inbred rats (Sprague–Dawley) and mice (C57BL/6J) by choosing the same vendor; selecting similarly aged animals (6–8 weeks); appropriate training of experimenters to minimize stress during handling of animals; and implementing methodological rigor—for example, 2–3 tiered blinded behavioral analyses by both direct observation and a secondary validation by analyses of recorded videos to distinguish between NCS and CS and to accurately determine the exact duration of CS during the SE. This type of methodological rigor is essential for selecting post-SE animals for unbiased grouping for vehicle control and test drug treatments in experiments. This will avoid confounding results in the long-term studies aimed at determining the disease-modifying effect of the test drug (Puttachary, Sharma, Thippeswamy, et al., 2016).
In rats, the route of kainate administration, use of anesthesia while administering the kainate, and the strain used in the experiments also impact SE and epileptogenesis. With respect to sensitivity and variability of response to a single dose of kainate by subcutaneous route, the Fischer-344 (F-344) rats are reported as a reliable strain (Golden, Smith, Ferraro, & Reyes, 1995; Sharma, Jordan, Reams, Hall, & Snyder, 2008). In the adult male F-344 rats study, a single dose of kainate at 9 mg/kg (s.c.) induced SE in 93%, of which 95% survived and 80% developed epilepsy (Sharma et al., 2008). In another long-term study in F-344 rats, the kainate (3 mg/kg) was administered repeatedly at 1-hr intervals for 4 hr to induce SE (Rao, Hattiangady, Reddy, & Shetty, 2006). In this F-344 rat model, the duration and severity of seizures increased from the third to fourth month, and the average number of seizures was 2.57 to 2.63 per hour. Therefore, F-344 rats may be appropriate to model the extreme severe spectrum of the disease. In our pilot studies in F-344 rats, the mortality was too high ( > 30%) in both repeated low-dose (RLD) and single high-dose intraperitoneal method of kainate administration at 12 to 17.5 mg/kg (unpublished). Moreover, for expensive long-term telemetry experiments (continuous 6-month study), high mortality is neither economical nor justifiable from ethical perspective. Therefore, we chose Sprague–Dawley rats for our long-term studies. However, these rats are less sensitive and showed more variable responses to a single dose of kainate by subcutaneous or intraperitoneal routes (Cosgrave et al., 2008; Golden et al., 1995). To overcome this advantage, we used the RLD method of kainate administration (i.p.) as described in a previous publication (Puttachary, Sharma, Thippeswamy, et al., 2016). It is also worth noting that the administration of kainate via intrahippocampal route under isoflurane anesthesia impacts epileptogenesis in rats. Interestingly, isoflurane did not affect the SE severity, but it dampened the epileptogenesis in rats (Bar-Klein et al., 2016).
The diazepam is commonly used to terminate behavioral seizures. It is well documented that diazepam indeed controls behavioral seizures, but it has little or no impact on electrographic events if the SE is severe (Apland et al., 2014; Goodkin, Joshi, Mtchedlishvili, Brar, & Kapur, 2008; Kadriu, Guidotti, Costa, Davis, & Auta, 2010; Pibiri et al., 2008; Qashu, Figueiredo, Aroniadou-Anderjaska, Apland, & Braga, 2009; Todorovic, Cowan, Balint, Sun, & Kapur, 2012). However, diazepam does suppress epileptiform activity in animals with mild SE (Figure 2). Diazepam has been known to reduce neurodegeneration after prolonged SE, but not in the hippocampus during epileptogenesis (Apland et al., 2014; Qashu et al., 2009). However, diazepam administration minimizes mortality to some extent and controls variability in the duration of behavioral SE between animals. Therefore, it is a common practice in our laboratory to administer diazepam at 2 hr after the onset of first CS (stage ≥ 3), and the exact duration of CS during this 2-hr period is calculated to determine the severity of SE as described in our previous publications (Puttachary, Sharma, Tse, et al., 2015; Puttachary, Sharma, Thippeswamy, et al., 2016; Tse, Puttachary, Beamer, Sills, & Thippeswamy, 2014).
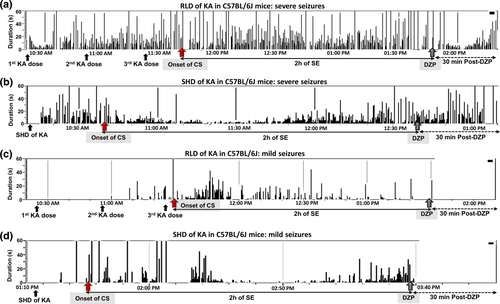
(a–d) SHD and RLD methods of kainate administration in C57BL/6J mice and their impact on SE, and diazepam treatment on spiking activity. (a,b) In the severe group, diazepam treatment was effective on behavioral seizures, but not on the electrographic seizures. (c,d) In the mild group, it was effective on both behavioral and electrographic seizures. Each vertical bar within the box represents a spike train that could contain epileptiform spikes, spike clusters ( < 12 s), and/or a NCS or CS seizure ( > 12 s). The spike trains can be continuous for several minutes or intermittent. The red arrow represents the onset of first convulsive seizure (i.e., stage ≥ 3), and the gray arrow represents the time when diazepam was administered 2 hr after onset of the first CS. RLD = repeated low-dose; SE = status epilepticus; SHD = single high-dose; NCS = non-convulsive seizure
3 THE HALLMARKS OF EPILEPTOGENESIS
Traditionally, epileptogenesis was limited to the “latent period,” the duration between the brain insult and the onset of spontaneous recurrent seizures. As per the new guidelines of ILAE, epileptogenesis extends from the time of first brain insult, such as SE, with a consequence of structural (cellular and molecular) and functional changes in the brain, leading to a decreased seizure threshold for onset of spontaneous recurrent seizures, which continue to progress thereafter (Hellier, Patrylo, Buckmaster, & Dudek, 1998; Kadam, White, Staley, & Dudek, 2010; Pitkanen & Engel, 2014; Pitkanen, Lukasiuk, Dudek, & Staley, 2015; Williams et al., 2009). The process of epileptogenesis follows immediately after SE, and the brain changes continue to progress beyond the first couple of spontaneous seizures. Several studies have shown that epileptogenesis could start as soon as the SE begins (Bumanglag & Sloviter, 2008; Loscher & Brandt, 2010; Sloviter, 2008). The hyperexcitability of neurons, manifested by epileptiform spiking, occurring during SE triggers a series of overlapping molecular and cellular changes in the brain (Aronica et al., 2017; Barker-Haliski, Löscher, White, & Galanopoulou, 2017; Klee et al., 2017). In the rat and mouse kainate models, though the behavioral seizures stopped after diazepam treatment, the electrographic seizures persisted for several hours (Figure 3) (Puttachary, Sharma, Thippeswamy, et al., 2016; Puttachary, Sharma, Verma, et al., 2016). This could be due to the residual effects of kainate in the brain. In the C57BL/6J mouse kainate model, we detected high concentrations of kainate in the hippocampal tissues at 4 hr post injection (i.p. route). Interestingly, the kainate residues were also detected at 24 hr post injection (Figure 4). Therefore, the initial epileptiform activity during the post-SE period could be due to the direct effects of kainate receptors’ activation. The hyperexcited neurons modify the internal and external milieu as a result of altered membrane potential for exchange of ions, which ultimately sensitizes the glial cells (Vezzani, French, Bartfai, & Baram, 2011; Vezzani, Pascente, & Ravizza, 2017). The intrinsic changes that occur in neurons during SE, and thereafter, determine the fate of the neurons and the brain as a whole (Varvel et al., 2015). The altered neurons' intrinsic properties and their subsequent communication with glial cells can trigger either a compensatory survival mechanism or neurodegeneration. In a recent in vivo 2-photon live imaging study, it was shown that the microglia, the resident macrophages of the brain, become activated as early as 30 min following an insult to the brain. The microglia migrate to the synaptic terminals and engage in dendritic pruning to limit the damage (Parkhurst et al., 2013; Szalay et al., 2016). In addition, microglia initially tend to support neuronal survival by producing trophic agents (Vezzani et al., 2011). The astrocytes, being close to the synaptic terminals and the blood vessels, tend to support neurons by uptaking the extra synaptic glutamate and potassium, and by transporting glucose from blood vessels to the neurons (David et al., 2009; Murphy, Binder, & Fiacco, 2017; Puttachary, Sharma, Stark, & Thippeswamy, 2015; Vezzani et al., 2011). It is also suggested that astrocytes, in concurrence with endothelial cells and pericytes, regulate blood-brain barrier (BBB) function, which is compromised during SE (Puttachary, Sharma, Thippeswamy, et al., 2016). If these compensatory mechanisms fail, miscommunication between neurons and glia can turn the normal brain into an epileptic brain. Therefore, identification of molecules that play a critical role in such miscommunication could be a potential therapeutic target for epileptogenesis. One such molecule is a gaseous signaling agent, nitric oxide (NO) (Thippeswamy, McKay, Quinn, & Morris, 2006).
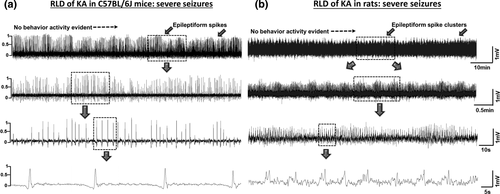
Examples of 30-min EEG traces, after the DZP treatment, from mouse (a) and rat (b) that had severe SE after kainate administration. The DZP administration did not control epileptiform spiking in the animals that had severe SE but it did control behavior seizures after administration. EEG traces showing epileptiform spikes (a) and epileptiform spike clusters (b). The expanded EEG traces are also shown (second, third and fourth EEG trace)
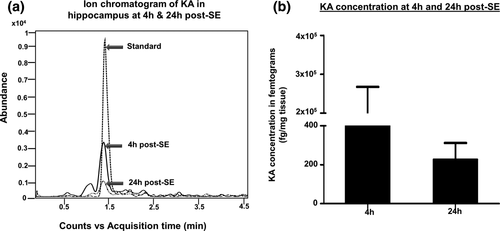
Ion chromatogram showing the relative abundance of kainate in the hippocampus (a). Liquid chromatography-mass spectrometry (LC-MS) analysis confirmed that the kainate was present in the hippocampus at higher levels at 4 hr, but persisted even at 24 hr post administration (b). n = 4
The consequences of SE are reactive gliosis, excessive production of reactive oxygen/nitrogen species and proinflammatory cytokines and chemokines, increased epileptiform spiking, neurodegeneration, excessive neurogenesis (with aberrant migration of neuroblasts and inappropriate integration), and spontaneous recurrent CS with or without mossy fiber sprouting (Bertram, 2013; Buckmaster, 2010; Goldberg & Coulter, 2013; Ryan, Liang, Rivard, & Patel, 2014; Vezzani et al., 2011). These are some of the well-known hallmarks of epileptogenesis (Figure 5). It is imperative to assume that intervening in some or all of these will either prevent or modify epilepsy. The majority of AEDs do impact some of these components of epileptogenesis, especially by targeting the neuronal ion channels to control seizures, but they do not cure the disease completely. Therefore, there is a need to develop new drugs with a different mechanism of action that can act on multiple targets to prevent or modify the course of development of epilepsy.
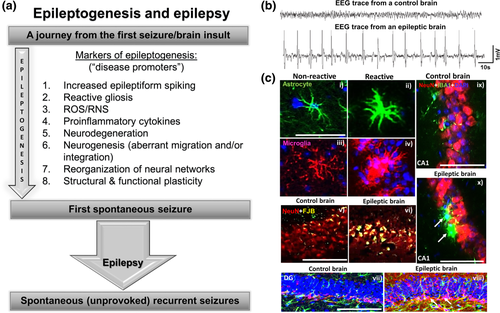
(a) Common features of epileptogenesis that occur soon after SE. (b) An example of epileptiform spikes on EEG from an epileptic brain is compared with a normal brain. (c) IHC images from the hippocampus (i–vi and ix–x) and the dentate gyrus (vii, viii). The astrocytes [green in (i) and (ii)] and microglia [red in (iii) and (iv), and green in (ix) and (x)] become reactive (ii, iv, x) in an epileptic brain. Reactive gliosis causes production of proinflammatory cytokines, chemokines, and reactive oxygen/nitrogen species to induce neurodegeneration [FJB+ NeuN (vi)]. Red-labeled cells are NeuN+ in panels v, vi, ix, and x. Yellow in (v) and (vi) represents FJB+ cells. Epileptic brain showed increased production of neuroblasts (pink-labeled cells) in the subgranular zone of the dentate gyrus (white arrows in viii) in contrast to the control brain (vii). Further details on these parameters and the quantified data can be found in Puttachary, Sharma, Thippeswamy, et al., 2016, and Puttachary, Sharma, Verma, et al., 2016. Scale bar, all 100 µm
4 AEDs, ANTIEPILEPTOGENIC AGENTS, AND ANIMAL MODELS
The vast majority of currently available anticonvulsant drugs have been, presumably, thoroughly screened using a battery of preclinical high-throughput tests. The most common methods used are acute seizure mouse/rat models and electrophysiology of brain slices, and in recent years, the zebra fish model has been proposed (Baraban, 2013; Rowley & White, 2010; White, 2002). The test drug of interest is administered either before inducing seizures or coadministered with chemoconvulsants to understand the therapeutic effect of the drug in controlling seizures. Indeed, this approach is useful for screening test compounds intended for the discovery of anticonvulsive drugs, but the outcome does not reveal whether the chosen compound would be useful as a potential antiepileptogenic and/or antiepileptic agent. Therefore it is important to screen any potential antiepileptic or antiepileptogenic test drug in relevant and highly reproducible preclinical/animal models of epilepsy to further advance it as a therapeutic agent. In our studies, we found that the rat kainate chronic model of TLE is the most appropriate model for testing disease-modifying agents post SE. Unlike certain mouse models, the rat kainate model has several advantages for studying epileptogenesis. The early epileptogenic hallmarks (the “disease promoters”), such as reactive gliosis, proinflammatory cytokine production, and neurodegeneration, are consistent, and spontaneous CSs are progressive in nature in the rat model, in contrast to the mouse model, especially with respect to frequency of CSs (Bertram, Lotham, & Lenn, 1990; Jorgensen et al., 1993; Puttachary, Sharma, Thippeswamy, et al., 2016; Puttachary, Sharma, Verma, et al., 2016; Rao et al., 2006; Vezzani et al., 2011; Williams, Hellier, White, Staley, & Dudek, 2007; Williams et al., 2009).
5 NO AND EPILEPSY
NO is a diffusible gaseous molecule that cannot be stored in the cells as such; therefore, its role as a therapeutic target remains debatable. The effects of NO are largely mediated either through the activation of soluble guanylyl cyclase and/or nitrosylation of cytosolic and membrane proteins. For example, S-nitrosylation modulates NMDA receptor activity (Lei et al., 1992; Manzoni et al., 1992) and limits excessive Ca2+ influx to protect neurons. However, the role of NO as a protective or toxic molecule depends on the time and the source of NO production following an insult and, most important, the isoform of NO synthase (NOS) involved. The substrate for NO production is one of the essential amino acids, arginine, which is also a limiting factor for NO production/regulation. Three major isoforms of NOS catalyze the formation of NO. Based on the abundance of NOSs in various cell types, NOSs are classified as neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). Pharmacological inhibitors have been used to target these enzymes to regulate the levels of NO in various in vitro and in vivo models with conflicting outcomes (Thippeswamy et al., 2006). Several studies have also highlighted the controversial pathophysiological roles of NO in the peripheral and central nervous systems (Chung, Dawson, & Dawson, 2005; Cosgrave et al., 2008; Dawson & Dawson, 1998; Dawson & Snyder, 1994; Hobbs, Higgs, & Moncada, 1999; Moncada & Erusalimsky, 2002; Puttachary, Sharma, Stark, et al., 2015; Thippeswamy et al., 2006). In epilepsy models, the controversies could be due to inappropriate use of NOS inhibitors with respect to selectivity, dose and time of treatment (pre or post insult), solvents used as vehicle and method of reconstitution, and route/method of administration (Beamer, Otahal, Sills, & Thippeswamy, 2012; Cosgrave et al., 2008; Hagioka, Takeda, Zhang, Sato, & Morita, 2005; Kato, Sato, Yokoyama, Kayama, & Yoshimura, 2005; Kovacs et al., 2009; Takei et al., 2001). NO has been shown to have both anticonvulsive (Kendrick et al., 1996; Penix, Davis, & Subramaniam, 1994; Royes et al., 2007; Sardo & Ferraro, 2007) and proconvulsive actions (De Sarro & De Sarro, 1993; Tutka, Klonowski, Dzieciuch, Kleinrok, & Czuczwar, 1996), which seems to depend on the species and the types of chemoconvulsants employed in the study (Cosgrave et al., 2008). All three NOS isoforms are expressed during epilepsy, but at different stages of seizures or epileptogenesis. For example, eNOS is upregulated in a rodent model of SE within 3 to 24 hr of intracranial injection of kainate (Chuang et al., 2007), while nNOS and iNOS are upregulated in the mouse model of electrically induced SE (Catania et al., 2003). In the rat kainate model, nNOS and iNOS are upregulated in less than 3 days post SE and persist for a longer duration (Cosgrave et al., 2008; Vezzani et al., 2011). In this review, anticonvulsive and/or disease-modifying effects of two NOS inhibitors (nNOS-specific inhibitor, Nw-propyl-l-arginine [L-NPA]; iNOS-specific inhibitor, 1400W) in the mouse and rat model of TLE are discussed.
6 nNOS IN SE AND EPILEPTOGENESIS
It is challenging to develop a single drug to act on multiple targets without having any adverse effects. All drugs have off-targets to a variable proportion, which causes side effects accordingly. It is customary to weigh the benefits of a drug against side effects in a given situation. A drug that targets an event or molecules during SE may not be effective during the post-SE period if the target molecule of interest is not expressed in a significant amount during epileptogenesis. For example, the nNOS-mediated NO production was thought to play an important role during SE. The brain slice experiments have demonstrated the neuronal source of endogenous NO as a key promoter for initiating seizure-like events in the hippocampal formation and entorhinal cortex (Kovacs et al., 2009). We tested the hypothesis in the mouse kainate model with the nNOS-specific inhibitor L-NPA, which has 149- and 3158-fold greater selectivity over eNOS and iNOS, respectively (Beamer et al., 2012; Zhang, Fast, Marletta, Martasek, & Silverman, 1997). Initially we tested the anticonvulsive effect of nNOS inhibition in mice by treating with L-NPA (20 mg/kg, i.p.) 30 min prior to the induction of SE with kainate. Kainate is a glutamate analogue that causes seizures with a hippocampal focus and is widely used to develop models of TLE in rodents (Ben-Ari & Cossart, 2000). L-NPA pretreatment significantly reduced the severity and duration of CSs, gamma EEG power, and epileptiform spike rate during SE and also during the first 7 days post SE (Beamer et al., 2012). Moreover, the histology of brain sections revealed a significant reduction of c-Fos, a cellular marker of neuronal hyperexcitability, in dentate granule cells at 2 hr post SE (Beamer et al., 2012). These results suggest that nNOS also facilitates seizure generation during SE.
Reactive gliosis, neurodegeneration, and neurogenesis are the most commonly recognized features of epileptogenesis (Parent & Lowenstein, 2002; Puttachary, Sharma, Thippeswamy, et al., 2016; Puttachary, Sharma, Verma, et al., 2016; Vezzani et al., 2011). To get a better understanding of these, we did immunohistochemistry (IHC) on brain sections collected at 3 days post SE from the L-NPA experiments. As expected, we observed a significant reduction in hippocampal microgliosis and astrogliosis in the L-NPA–pretreated group compared with the vehicle control group, since the initial SE severity was compromised in the L-NPA–pretreated animals (Beamer et al., 2012). However, neurodegeneration and neurogenesis were not significantly changed at 3 days post SE. It was suggested that C57BL/6J mice are resistant to kainate-induced neurodegeneration (Schauwecker, 2012; Schauwecker & Steward, 1997). However, we have demonstrated neurodegeneration in C57BL/6J mice using Fluoro-Jade B and NeuN costaining in the hippocampal formation, entorhinal cortex, and amygdala at 7 days post SE (Puttachary, Sharma, Thippeswamy, et al., 2016). Likewise, gliosis (both astrogliosis and microgliosis) reached its maximum at 7 days post SE, but decreased at later time points, in contrast to the rat kainate model of TLE (Puttachary, Sharma, Thippeswamy, et al., 2016; Puttachary, Sharma, Verma, et al., 2016). These are important differences between the C57BL/6J mouse kainate model and the rat kainate model. In cross-bred mice and rats, we observed reactive gliosis and neurodegeneration as early as 24 hr post SE (personal observation, unpublished work), and in the rats they persisted throughout the period of epileptogenesis and also during the chronic phase (Puttachary, Sharma, Verma, et al., 2016). Interestingly, neurogenesis persistently increased in C57BL/6J mice during epileptogenesis (at 7 days post SE), perhaps to compensate for the ongoing neurodegeneration (Puttachary, Sharma, Thippeswamy, et al., 2016). It is also interesting to note that SE increases activity-dependent synaptogenesis in the outer and middle molecular layers of the dentate gyrus in the early stage of epileptogenesis, observed at 72 hr post SE in C57BL/6J mice, which was suppressed in the L-NPA–treated group (Beamer et al., 2012). The suppressed gliosis and neo-synaptogenesis at 3 days post SE in the L-NPA–treated group were not due to the direct effects of nNOS inhibition but, instead, were due to compromised initial severity of SE owing to the pretreatment approach. The results from pretreatment experiments of test drugs provide proof-of-concept for their antiseizure efficacy and confirm that the decreased severity of seizures during SE dampens the events of epileptogenesis. A similar conclusion can be drawn from gene knockout or transgenic mice experiments. Therefore, to confirm the antiepileptogenic or antiepileptic effects of a pharmacological agent or gene of interest from the translational aspect, intervention strategies during the post-SE period are appropriate for investigating disease-modifying agents in epilepsy. An example for intervention of epileptogenesis soon after SE, targeting the NO signaling, and its long-term impact on epilepsy, is further discussed.
7 iNOS INHIBITOR AND EPILEPTOGENESIS
The complex role of NO in epilepsy owes to cell-specific expression of three different isoforms of NOS expressed at various stages of epileptogenesis. As mentioned earlier, the neuronal source of NO produced by an nNOS-mediated mechanism promotes seizures. Both in vitro and in vivo studies have confirmed a proconvulsive role of NO in acute models (Beamer et al., 2012; Kovacs et al., 2009). IHC of the brain sections at 3 days post SE in the rat kainate model of TLE showed a significant increase in nNOS levels in neurons and iNOS (iNOS/NOS2) in microglia (Cosgrave et al., 2008). Because we knew that L-NPA pretreatment reduced SE severity and epileptiform spikes, we further tested whether posttreatment would have a similar beneficial effect. Surprisingly, we observed a marginal increase, rather than the anticipated significant decrease, in epileptiform spike rate and gliosis at 24 hr and 72 hr post SE between the vehicle and L-NPA–treated groups. This may suggest a beneficial role of nNOS during the early stages of epileptogenesis in contrast to its proconvulsive role during acute seizure onset (i.e., SE). It has been shown that a transient increase in nNOS, following insult to the brain, protects neurons by S-nitrosylation of the NR2B subunit of NMDAR to control excessive calcium influx (Campelo et al., 2012; Gidday et al., 1999; Gonzalez-Zulueta et al., 2000). Because we did not observe expected modifications in early epileptogenesis by inhibiting nNOS with L-NPA, we focused our investigation on the role of iNOS in epileptogenesis. We first tested a highly potent iNOS inhibitor, 1400W [N-{3-(aminomethyl) benzyl} acetamidine], on brain slices to understand whether it suppresses epileptiform spiking activity before it was tested in animal models. The 1400W indeed significantly suppressed kainate-induced epileptiform spikes in the brain slices (Puttachary, Sharma, Verma, et al., 2016). 1400W is a slow, tight-binding, and highly selective pharmacological inhibitor of iNOS with a Kd value of 7 nM. It is > 5000-fold and > 200-fold selective for iNOS than eNOS and nNOS, respectively (Alderton, Cooper, & Knowles, 2001; Garvey et al., 1997; Jafarian-Tehrani et al., 2005; Parmentier et al., 1999; Perez-Asensio et al., 2005). It is biologically active in vivo and has no pulmonary or cardiovascular side effects, and the physiological activities mediated by eNOS and nNOS are not compromised at the optimum dose of 20 mg/kg (Eissa and Huston, 2003; Garvey et al., 1997). 1400W is BBB permeable and has shown to suppress abnormal levels of NO metabolites in rodents (Crowell, Steele, Sigman, & Fay, 2003; Garvey et al., 1997; Parmentier et al., 1999; Perez-Asensio et al., 2005). It is 100 times more potent than other iNOS inhibitors (ED50 ≈ 0.3 mg) in reducing delayed vascular injury in the rat lipopolysaccharride model (Garvey et al., 1997). Importantly, studies have shown that rats tolerated a dose of 120 mg/day for a 7-day period when 1400W was administered as intravenous infusion; however, it was lethal at 50 mg/kg when given as a single intravenous bolus (Garvey et al., 1997). As discussed earlier, this is an important finding considering the reported controversial roles of NO in brain pathology (Chung et al., 2005; Dawson & Dawson, 1998; Dawson & Snyder, 1994; Hobbs et al., 1999; Moncada & Erusalimsky, 2002; Thippeswamy et al., 2006).
Treatment with 1400W, at 20 mg/kg, effectively ameliorated brain pathology and suppressed seizures by > 90% in the rat kainate model of chronic epilepsy in a 6-month continuous video EEG study (Puttachary, Sharma S, Verma, et al., 2016). In traumatic brain injury (TBI) and stroke models, it reduced brain injury by decreasing glutamate release (Jafarian-Tehrani et al., 2005; Parmentier et al., 1999; Perez-Asensio et al., 2005). 1400W also protected BBB integrity, which is compromised soon after SE (Boje, 1996; Puttachary, Sharma, Verma, et al., 2016). Serum albumin (SA) is considered a biomarker for BBB leakage (Frigerio et al., 2012). Increased SA and glutamate levels induce reactive astrogliosis that causes hyperexcitability of neurons and, hence, seizures (Boje, 1996; Frigerio et al., 2012; Liu, Li, Lein, & Ford, 2012; Salar et al., 2014; Weissberg et al., 2015). Our previous studies from the rat and mouse epilepsy models have shown increased serum albumin and glial fibrillary acidic protein (GFAP) levels in the hippocampus at 7 days post SE, and 1400W treatment reduced their levels (Puttachary, Sharma, Thippeswamy, et al., 2016; Puttachary, Sharma, Verma, et al., 2016). 1400W also reversed SE-induced suppression of Kir 4.1 and GLT1 levels, perhaps by reducing reactive gliosis, which could also be due to decreased levels of glutamate and SA (Puttachary, Sharma, Verma, et al., 2016). Overall, these changes attenuated neuronal excitability, which was evident from a significant reduction in the epileptiform spike rate and seizures in the rat kainate model (Puttachary, Sharma, Verma, et al., 2016). 1400W reduced neurodegeneration, as indicated by a reduction in the FJB+ neurons, both at 7 days and at 6 months post SE (Puttachary, Sharma, Verma, et al., 2016). These findings provide evidence for the long-term neuroprotective role of 1400W and its disease-modifying properties in epilepsy.
1400W is commercially available as a pure ( > 99%) water-soluble compound. It has been tested in healthy human volunteers, patients with heart failure (Dover et al., 2006), and liver cirrhosis (Ferguson et al., 2006). There were no adverse effects of 1400W in humans, and no toxicity has been reported. It effectively suppressed inflammatory mediators in human cartilage derived from osteoarthritic patients and in a rat neuropathic pain model (Jarvinen et al., 2008; Makuch, Mika, Rojewska, Zychowska, & Przewlocka, 2013). Other iNOS inhibitors such as L-NIL-TA (referred to as SC-51 in the literature), VAS203 (Vasopharm, 2013), and KD7040 are in clinical trials for asthmatic humans (and healthy volunteers), TBI patients, and neuropathic pain, respectively (Hansel et al., 2003; Vasopharm, 2013). Furthermore, in our ongoing studies on organophosphate neurotoxicity rat model, we observed similar effects of neuroprotection by 1400W and its long-term seizure suppression as observed in the rat kainate model of TLE. Collectively, these studies strongly support the therapeutic potential of iNOS inhibitors, and 1400W in particular, as a promising drug for disease modification not only in epilepsy but also in various other neurodegenerative disorders.
8 SEX AS A BIOLOGICAL VARIABLE IN EPILEPSY RESEARCH
Overdependence on male animals in preclinical research obscures the key sex differences that could create a huge gap in the knowledge base, and it undermines the quality of data (Clayton & Collins, 2014). For translational studies, this is unjustified because women experience higher rates of adverse drug reactions than men do (Clayton & Collins, 2014; Franconi, Brunelleschi, Steardo, & Cuomo, 2007; Zucker & Beery, 2010). A population-based study on epilepsy in women in the United States revealed that out of a million women of childbearing age treated with AEDs every year, about 33% did not respond to the drugs, and some even had severe side effects (Katz, Levy, Wiznitzer, & Sheiner, 2006). The males responded 75% better than the females to the same class of drugs with the least side effects (Arroyo et al., 2004; Beydoun et al., 2005; Elger, Brodie, Anhut, Lee, & Barrett, 2006; French, Kugler, Robbins, Knapp, & Garofalo, 2003; Pulman, Hemming, & Marson, 2014; Tomson & Battino, 2011; Whitley & Lindsey, 2009). The bias toward the choice of male animals versus females in preclinical studies is due to the undisputed influence of sex hormones, during the female reproductive cycle, on experimental results (Backstrom et al., 2003; Herzog & Frye, 2003; Herzog et al., 2004, 2011). Indeed, this is an important variable that has been left out of experimental designs in most preclinical studies so far. This negligence seems to have had a huge impact on the success/failure of certain drugs in females during clinical trials and on the withdrawal of FDA-approved drugs from the market (Parekh, Fadiran, Uhl, & Throckmorton, 2011). In 1993, the National Institutes of Health (NIH) introduced the Revitalization Act requiring the inclusion of women in all NIH-funded clinical research (Clayton & Collins, 2014). The National Institute of Mental Health (NIMH) recently conducted a workshop to highlight the impact of sex differences in the brain on behavior and neurological disorders. The NIMH has emphasized the need for more neuroscientists to incorporate sex as a variable in experimental designs (NIMH, 2011). The new NIH policy requires applicants to report their plans for the balance of male and female animals in preclinical studies in all future applications (Clayton & Collins, 2014). The challenge for epilepsy researchers in using female animals as models is the influence of sex hormones on seizure threshold during various stages of the reproductive cycle (Backstrom et al., 2003; Herzog & Frye, 2003; Herzog et al., 2004, 2011).
The differences in male and female responses to a drug or an insult to the brain are mainly due to the effects of neurosteroids and sex hormones on neurons and glial cells. The neurosteroids alter neuron–glial communication in normal brain development and function and also during neuropathological conditions (Backstrom et al., 2003; Herzog & Frye, 2003; Herzog et al., 2004, 2011). It is plausible that this communication is compromised if the levels of hormones in females are altered during different phases of the reproductive cycle (Bellefontaine et al., 2011; Carver, Wu, Gangisetty, & Reddy, 2014; Wu, Gangisetty, Carver, & Reddy, 2013). It has been known for some time that the neurons primed by estrogen are protected against neurodegeneration and that females live longer and experience less pain than males do (Arevalo, Azcoitia, & Garcia-Segura, 2014; Tenenbaum, Azab, & Kaplanski, 2007). On the flip side, in some women higher estrogen and lower levels of progesterone excite neurons and trigger seizures, and their response to AEDs is compromised (Backstrom et al., 2003; Herzog & Frye, 2003; Herzog et al., 2004, 2011), which has been demonstrated in rat models (Carver et al., 2014; Wu et al., 2013).
During the follicular phase (the first half of the estrus cycle), the brain is under the influence of estradiol, while during the luteal phase (the second half of the cycle) progesterone influences brain function (Herzog et al., 2011; Wu et al., 2013). Estradiol peaks at midcycle, and progesterone declines before menstruation begins (Backstrom et al., 2003; Herzog & Frye, 2003; Herzog et al., 2004, 2011). These alterations influence receptor plasticity in the brain and cause premenstrual syndrome, migraine, and “catamenial” epilepsy in humans (Amour et al., 2015; Backstrom et al., 2003; Herzog et al., 2011). Low progesterone levels during the perimenstrual period or before ovulation trigger frequent seizures, and these could become refractory to conventional AEDs (Herzog & Frye, 2003; Herzog et al., 2004). Until recently, these factors were not adequately considered in the vast majority of preclinical drug discovery experiments in epilepsy research. Female rats attain puberty by 4 weeks of age and maintain regular estrous cycles thereafter. Each cycle is 4 to 5 days long and occurs throughout the year (Maguire, Stell, Rafizadeh, & Mody, 2005; Ojeda, Urbanski, Costa, Hill, & Moholt-Siebert, 1990; Westwood, 2008). There are four stages of the estrous cycle in rats as in humans: diestrus, proestrus, estrus, and metestrus. Progesterone levels are high during diestrus and pregnancy but low during estrus, which is comparable to the cycle in humans (Westwood, 2008; Wu et al., 2013). Progesterone has anticonvulsant, anxiolytic, and neuroprotective properties (Reddy, 2003, 2004, 2009, 2010; Scharfman & MacLusky, 2006), and therefore it plays a significant role in epilepsy, anxiety, and depression (Reddy, 2003; Reddy & Jian, 2010; Smith et al., 1998; Van Broekhoven and Verkes, 2003). In the brain, progesterone is converted into allopregnanolone, a neurosteroid that rapidly alters the excitability of neurons through direct interaction with the inhibitory pathway via the gamma amino butyric acid (GABA)-A receptors (Hosie, Wilkins, & Smart, 2007; Reddy & Mohan, 2011; Scharfman & MacLusky, 2006; Scharfman et al., 2005). Several reports suggest the critical role of NO in modulating neurosteroid-mediated signaling between neurons and glial cells in the brain (Chakraborti, Gulati, & Ray, 2014; Chamniansawat & Chongthammakun, 2014; Cheepsunthorn, Mairaue, & Nasee, 2006; Del Bianco-Borges & Franci, 2015; Lima, Ota, Cabral, Del Bianco Borges, & Franci, 2014; Pandey & Deshpande, 2015; Priyanka, Singh, Pratap, & Thyaga Rajan, 2014). However, this is not yet tested in our models using either L-NPA or 1400W. Moreover, a detailed study on estrus stage–specific expression of different isoforms of NOS is required prior to testing the drugs to target them for epileptogenesis in female animal models.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Visualization, S.S.; Writing- Review and editing, S.S. and S.P.; Writing-Original draft, S.S. and T.T.; Supervision, Project Administration and Funding Acquisition, T.T.