Ant diversity as a direct and indirect driver of pselaphine rove beetle (Coleoptera: Staphylinidae) functional diversity in tropical rainforests, Sabah, Malaysian Borneo
Abstract
Pselaphinae is a species-rich beetle subfamily found globally, with many exhibiting myrmecophily—a symbiotic association with ants. Pselaphine–ant associations vary from facultative to obligate, but direct behavioral observations still remain scarce. Pselaphines are speciose and ecologically abundant within tropical leaf litter invertebrate communities where ants dominate, implying a potentially important ecological role that may be affected by habitat disturbances that impact ants. In this study, we measured and analyzed putative functional traits of leaf litter pselaphines associated with myrmecophily through morphometric analysis. We calculated “myrmecophile functional diversity” of pselaphines at different sites and examined this measure's relationship with ant abundance, in both old growth and logged rainforest sites in Sabah, Borneo. We show that myrmecophile functional diversity of pselaphine beetles increases as ant abundance increases. Old growth rainforest sites support a high abundance of ants, which is associated with a high abundance of probable myrmecophilous pselaphines. These results suggest a potential link between adult morphological characters and the functional role these beetles play in rainforest litter as ecological interaction partners with ants.
1 INTRODUCTION
Myrmecophily, a symbiotic association with ants, is an ancient feature of ant ecology (Parker, 2016b; Parker & Grimaldi, 2014) and has arisen in many arthropod groups (Kronauer & Pierce, 2011). However, there is an evolutionary bias toward beetles (Coleoptera), where myrmecophily is most common (Parker, 2016a). Staphylinidae (rove beetles) have the most myrmecophilous lineages, and within this family of beetles, the subfamily Pselaphinae (Parker, 2016a) has many ant-associated species.
The Pselaphines are a species-rich group of predatory leaf litter beetles with 9,854 described living species in 1,247 genera worldwide (Parker, 2016a). They are generally small (∼1–3 mm in body length) and are particularly diverse and abundant in the tropics (Chandler, 2001; Chung et al., 2000; Parker & Maruyama, 2013; Nomura & Mohamed, 2008; Sakchoowong et al., 2008). In Borneo, 123 species have been recorded (Nomura & Mohamed, 2008), but many more are yet to be described. Pselaphines deviate from the typical staphylinid body plan in possessing a more compact, sclerotized integument that reduces abdominal flexibility. Although a relatively invariant body plan would appear to restrict their movements through the substrate, pselaphines have radiated successfully in the leaf litter, particularly in ant-dominated tropical forest floors (Chandler, 2001; Parker, 2016b; Sakchoowong et al., 2008).
Pselaphines have varied levels of social parasitism ranging from facultative or opportunistic nest intruders, a casual association in which the beetle is not wholly reliant on ants, through to obligate myrmecophiles, which are entirely dependent on host colonies (Geiselhardt et al., 2007; Mynhardt, 2013; Park, 1942; Parker, 2016a; Parker & Grimaldi, 2014). Direct observations of pselaphine-ant associations have been recorded (Parker & Maruyama, 2013; Sugaya et al., 2004; Yin & Li, 2013, 2015), but are mostly limited to records of beetles inside nests, with little behavioral data. Nonetheless, insights into their ecological and behavioral interactions can be gained by examining adult morphology (Mynhardt, 2013; Parker, 2016a; Parker & Grimaldi, 2014).
Functional traits associated with myrmecophily include: (a) Trichomes, thick brush-like bunches of setae conducting glandular exudates attractive to the ants; thus initiating trophallaxis, a unique liquid-feeding behavior typically occurring between worker ants or between worker ants and larvae, which the beetles exploit (Cassil & Tschinkel, 1996; Mynhardt, 2013; Park, 1942; Parker & Grimaldi, 2014). (2) Fusion of antennal and abdominal segments, which strengthens the body for protection during handling by worker ants and increases the surface area for secretions to spread (Parker & Grimaldi, 2014). In extreme cases, obligate myrmecophiles (exhibited by the tribe Clavigerini) display deep indentations in the abdomen providing grasping notches for worker ants to pick up and carry the beetle to their nest (Leschen, 1991; Parker, 2016a). (3) Body size enlargement has also been linked to inquilinous lifestyle, although the explanations for this observed trend have not been tested (Parker & Maruyama, 2013).
Pselaphines are habitat specific, occupying moist leaf litter and woody debris microhabitats (Chandler, 2001; Nomura & Mohamed, 2008), and therefore responsive to environmental change. Their ecological and functional importance have made them suitable candidates for conservation studies particularly concerning the effect of rainforest disturbances on biodiversity (Chung et al., 2000; Edwards et al., 2014; Sakchoowong et al., 2008). Ants and beetles, among other taxa, have proven to be effective bioindicators of the response of other taxa to logging and conversion to oil palm in Southeast Asia (Chung et al., 2000; Edwards et al., 2014; Sakchoowong et al., 2008). Ecosystem disturbances greatly influence the abundance and distribution of functionally important species that contribute to maintaining ecosystem functioning and biogeochemical cycles (Hooper et al., 2005; Loreau et al., 2001). Here, we measure the functional traits of pselaphines, specifically the traits putatively associated with myrmecophily, to allow us to better understand (a) how pselaphines interact with individual ants and ant nest colonies and (b) how these traits can be applied as a measure of functional diversity (FD) across varying levels of ecosystem disturbances in tropical rainforests. We use existing ant abundance data, all taken from the same plots, and samples as our pselaphine data, to examine how far our measures of putative myrmecophily match data on ant abundance.
We predict that FD of pselaphine beetles will increase in the presence of high-ant abundance.
2 MATERIALS AND METHODS
The Soil Biodiversity Group, Life Sciences Department, Natural History Museum, London provided the specimens of leaf litter Pselaphinae beetles for analysis. All sampling was conducted between September and October, 2012 in Sabah, Malaysian Borneo. Specimens were on loan from the collections of University Malaysia, Sabah (UMS).
2.1 Study site
Three study sites were selected: two old growth rainforests located in (a) the Maliau Basin Conservation Area (Belian Site, 4°49′N, 116°54′E), (b) the Danum Valley Conservation Area (4°55′N, 117°40′E), and (c) a logged rainforest area established as part of a large-scale forest fragmentation experiment: the Stability of Altered Forest Ecosystems (SAFE Project) (Ewers et al., 2011). All sampling sites located within SAFE have been through two periods of selective logging while Maliau Basin and Danum Valley Conservation Areas have never been logged (Ewers et al., 2011).
2.2 Sampling methods
A Winkler extraction method was used to sample leaf litter macrofauna (see Krell et al., 2005 for a discussion on this method). The Pselaphinae beetles (Coleoptera: Staphylinidae: Pselaphinae) inhabit forest leaf litter (Nomura & Mohamed, 2008; Sakchoowong et al., 2008), therefore Winkler sampling is appropriate. Sieved leaf litter samples were hung in Winkler bags for 3 days. A total of 15 1 m2 quadrats of leaf litter were sieved at each plot in all three sites, at 7 m intervals along a 100-m transect (as in Carpenter et al., 2012). A total of 20 plots were sampled across all three sites: eight plots were sampled in old growth forest Maliau Basin Conservation Area, six plots were sampled in old growth Danum Valley Conservation Areas) and six plots sampled in logged forest (SAFE Project).
2.3 Pselaphinae beetle identification
Specimens were identified to family and subfamily level (by SH). The leaf litter Pselaphinae beetles were separated and stored in 100% ethanol for identification and analyses. Specimens were sorted to morphospecies, based on external morphological characters of specimens (Hammond, 1994). This method has been widely adopted as a surrogate for species-level taxonomic identification in many ecological studies (Baker et al., 2004; Derraik et al., 2002; Gardner-Gee et al., 2015; Oliver & Beattie, 1996), particularly where, like here, the majority of the species are probably undescribed. The external morphological characteristics of the head, eyes, mandibles, antennae, pronotum, elytra, abdomen, and legs were carefully examined, specifically examining the shape and size of each character. Morphospecies were then identified to tribe and genus by specialist taxonomist (Joseph Parker). However, due to the tremendous species diversity of Pselaphinae beetles in Tropical Asia, much of which remains unknown (Nomura & Mohamed, 2008), not all morphospecies could be identified to genus level and some tribal identities are uncertain. A total of 42 morphospecies were recorded across all three sites (Table 1). Voucher specimens were mounted, labeled, and deposited in the Natural History Museum, London.
| Morphospecies | Tribe | Genus | Myrmecophile |
|---|---|---|---|
| 1 | Batrisini | Indet | Unknown |
| 2 | Trichonychini | Bibloporus? | Nonmyrmecophile |
| 3 | Cyathigerini | Plagiophorus | Facultative |
| 4 | Arnyliini | Harmophorus | Facultative |
| 5 | Tmesiphorini | Pseudophanias | Possible |
| 6 | Cyathigerini | Plagiophorus | Facultative |
| 7 | Proterini | Mechanicus? | Nonmyrmecophile |
| 8 | Cyathigerini | Plagiophorus | Facultative |
| 9 | Batrisini | Indet | Unknown |
| 10 | Batrisini | Batrisocenus/Batrisoplisus group | Unknown |
| 11 | Batrisini | Mnia? | Unknown |
| 12 | Trichonychini | Aphilia | Nonmyrmecophile |
| 13 | Hybocephalini | Apharinodes | Possible |
| 14 | Proterini | Mechanicus | Nonmyrmecophile |
| 15 | Batrisini | Batrisocenus/Batrisoplisus group | Unknown |
| 16 | Batrisini | Cratna | Unknown |
| 17 | Tyrini | Horniella/Hamotopsis? | Possible |
| 18 | Batrisini | Mnia? | Unknown |
| 19 | Batrisini | Cratna? | Unknown |
| 20 | Cyathigerini | Plagiophorus | Facultative |
| 21 | Tyrini | Pselaphodes? | Nonmyrmecophile |
| 22 | Cyathigerini | Plagiophorus | Possible |
| 23 | Clavigerini | Pseudacerus | Obligate |
| 24 | Batrisini | Sathytes | Unknown |
| 25 | Tyrini | Pselaphodes? | Nonmyrmecophile |
| 26 | Pselaphini | Curculionellus | Nonmyrmecophile |
| 27 | Clavigerini | Cerylambus | Obligate |
| 28 | Bythinoplectini | Indet | Nonmyrmecophile |
| 29 | Bythinoplectini | Indet | Nonmyrmecophile |
| 30 | Bythinoplectini | Indet | Nonmyrmecophile |
| 31 | Brachyglutini | Batraxis | Unknown |
| 32 | Batrisini | Cratna | Possible |
| 33 | Batrisini | Indet | Unknown |
| 34 | Proterini? | Mechanicus? | Nonmyrmecophile |
| 35 | Batrisini | Indet | Unknown |
| 36 |
Tmesiphorini/ Tyrini? |
Enantius? | Possible |
| 37 | Hybocelaphini | Apharinodes | Possible |
| 38 | Trichonychini | Aphilia? | Nonmyrmecophile |
| 39 | Brachyglutini | Batraxis | Unknown |
| 40 | Proterini | Mechanicus | Nonmyrmecophile |
| 41 |
Trichonychini/ Euplectin? |
? | Nonmyrmecophile |
| 42 | Batrisini | Diaugis? | Possible |
- a The significance of “?” indicates that some tribal identities are uncertain.
Morphospecies were assigned where possible, as facultative, obligate myrmecophile, unknown or nonmyrmecophile. Myrmecophile classification was based on current knowledge in the literature, available keys to the subfamily Pselaphinae (Chandler, 2001; Nomura & Mohamed, 2008; Park, 1942) and recorded observations from the field, and confirmed by taxonomic specialists (see Acknowledgments).
2.4 Morphological/morphometric trait analysis
The FD of Pselaphinae was assessed using morphological traits. These were selected based on an extensive literature review and are presented in Table 2. Three quantitative morphological traits were measured using a compound microscope (Wild Heerbrugg Switzerland M5–64489) at a magnification of ×25, using an ocular micrometer with a scale interval of 0.1 mm. All measurements were recorded in millimeters. Six morphological traits were also recorded as binary (present or absent) data: antennomere number, antennomere expansion, hollow cavity in terminal antennomere, trichomes, foveae, and basal elytral foveae. Antennomere expansion (size expansion of a given antennomere) was recorded by numbering individual antennomeres (antennomere 1 closest to the head and antennomere 11 furthest from the head). A single representative of each morphospecies was selected for functional trait measurements. All raw measurements are presented in Table 3 and list of functional trait abbreviations presented in Table 4. The terminology used to describe the foveal patterns follows Chandler (2001). Not all foveal positions could be recorded due to the position in which some specimens were mounted, with some body parts obstructed.
| Abbreviation | Trait | Functional link | Evidence |
|---|---|---|---|
| AL | Antennae length | Fusion of antennal segments | Parker and Grimaldi (2014) |
| TAL | Terminal antennomere length | Increased antennomere size toward the apex | Maruyama et al. (2014) |
| TAW | Terminal antennomere width | Increased antennomere size toward the apex | Maruyama et al. (2014) |
| AN | Antennomere number | Fusion of antennal segments | Geiselhardt et al. (2007); Parker (2016a) |
| AE | Antennomere expansion | Increased antennomere size toward the apex | Maruyama et al. (2014) |
| HC in TA | Hollow cavity in terminal antennomere | Grasping notches for worker ants | Leschen (1991); Parker (2016a) |
| T | Trichomes | Facilitate trophallaxis feeding by worker ants | Hill et al. (1976); Parker (2016a); Parker and Grimaldi (2014) |
| F | Foveae | Trend toward loss in myrmecophiles | Park (1942); Chandler (2001); Parker and Maruyama (2013) |
| Bef | Basal elytral foveae | Trend toward loss in myrmecophiles | Chandler (2001); Park (1942); Parker and Maruyama (2013) |
|
Morphospecies /Traits |
AL | TAL | TAW | AN | HCA | HCP | TA | TP | FP | FA | Bef2 | Bef3 | Bef1 | Bef0 | Bef4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.663 | 0.117 | 0.117 | 11 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| 2 | 0.702 | 0.039 | 0.039 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 3 | 0.702 | 0.39 | 0.195 | 7 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| 4 | 1.599 | 0.234 | 0.117 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| 5 | 1.131 | 0.234 | 0.234 | 11 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 6 | 0.507 | 0.273 | 0.234 | 7 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| 7 | 0.234 | 0.078 | 0.078 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 8 | 0.507 | 0.351 | 0.234 | 7 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| 9 | 0.624 | 0.117 | 0.078 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 10 | 0.78 | 0.156 | 0.078 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 11 | 0.624 | 0.117 | 0.078 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| 12 | 0.195 | 0.078 | 0.039 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| 13 | 1.014 | 0.546 | 0.273 | 11 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 14 | 0.702 | 0.195 | 0.117 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| 15 | 0.624 | 0.117 | 0.078 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 16 | 1.95 | 0.39 | 0.117 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 17 | 1.56 | 0.234 | 0.117 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 18 | 0.78 | 0.156 | 0.078 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| 19 | 0.936 | 0.234 | 0.117 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 20 | 0.585 | 0.273 | 0.351 | 7 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| 21 | 1.17 | 0.273 | 0.156 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 22 | 0.312 | 0.234 | 0.156 | 7 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| 23 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| 24 | 0.702 | 0.234 | 0.156 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| 25 | 1.17 | 0.273 | 1.156 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 26 | 0.858 | 0.195 | 0.195 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| 27 | 0.195 | 0.156 | 0.078 | 3 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| 28 | 0.273 | 0.117 | 0.078 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 29 | 0.195 | 0.039 | 0.039 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 30 | 0.351 | 0.156 | 0.117 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 31 | 0.78 | 0.195 | 0.117 | 11 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| 32 | 1.95 | 0.351 | 0.117 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 33 | 0.819 | 0.195 | 0.078 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 34 | 0.546 | 0.156 | 0.078 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| 35 | 0.975 | 0.195 | 0.117 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 36 | 0.858 | 0.195 | 0.117 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 37 | 0.741 | 0.234 | 0.195 | 11 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| 38 | 0.234 | 0.078 | 0.078 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 39 | 0.741 | 0.117 | 0.117 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| 40 | 0.351 | 0.117 | 0.078 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 41 | 0.312 | 0.039 | 0.039 | 11 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 42 | 1.092 | 0.234 | 0.195 | 11 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
- Note. AL, TAL, and TAW were recorded in mm, 1 = present and 0 = absent.
| AL | Antennae length |
|---|---|
| TAL | Terminal antennomere length |
| TAW | Terminal antennomere width |
| AN | Antennomere number |
| HCA | Hollow cavity absent in terminal antennomere |
| HCP | Hollow cavity present in terminal antennomere |
| TA | Trichomes absent |
| TP | Trichomes present |
| FP | Foveae present |
| FA | Foveae absent |
| Bef2 | Basal elytral foveae, two set |
| Bef3 | Basal elytral fovea, three set |
| Bef1 | Basal elytral fovea, one set |
| Bef0 | Basal elytral fovea, no set |
| Bef4 | Basal elytral fovea, four set |
2.5 Beetle imaging
Initial dorsal view images of all morphospecies were taken using an Axioskop compound light microscope and EOS 700D Canon camera (at ×10 and ×4 magnifications) in the Sackler Biodiversity Imaging lab, Natural History Museum, London. Each image was constructed by montage imaging using Helicon Focus 5.3 software (2013).
For scanning electron microscopy (SEM) analysis, specimens were examined with a LEO 1455VP SEM microscope at the Imaging and Analysis Centre, Natural History Museum, London. The mounted voucher specimen of each morphospecies was placed onto a stage and then inserted into the microscope chamber. The specimens were not coated for SEM analysis, so a low vacuum chamber setting was selected for nonconductive specimens. The micrographs obtained by this instrument were used to identify the presence or absence of the following: hollow cavity in the terminal antennomere, trichomes, sensilla, and foveae. Foveal patterns were only recorded from a dorsal or side view, because of the way that the specimens were mounted. Micrographs were edited using Adobe Photoshop CS2 version 9.0 software (2006).
2.6 Statistical analysis
Pselaphine morphological functional traits associated with myrmecophily were used to calculate FD, using Rao's quadratic diversity index (Rao, 1982) in CANOCO version 5.0 (2012). Functional diversity is therefore an estimate of myrmecophily (“putative myrmecophily”). The Rao coefficient combines measurements of species relative abundance and the pairwise morphometric differences between two individuals of a given species, identified as binomial or quantitative measurements (Rao, 2010; Ricotta, 2005; Ricotta & Moretti, 2011). The pairwise differences in this study are expressed as functional traits (both quantitative and binomial measurements were included in the analysis). FD values obtained in CANOCO were used to run general linear models (GLMs) in RStudio (version 0.98.1087—© 2009–2014 RStudio, Inc.). General linear models were used to assess the correlation of Pselaphinae beetle FD (Rao) with ant abundance across the three sites sampled. Ant abundance data was log transformed in R before GLM analysis. Ant abundance data was taken from leaf litter samples collected from the same plots and sites during the same sampling period as the beetles (i.e., exactly the same litter samples) and were calculated by Thomas Bell (unpublished Master's thesis).
3 RESULTS
A total of 613 Pselaphinae beetles were sampled, across all three sites (Table 5), representing 12 tribes and 42 morphospecies (Table 1).
| Morphospecies | SAFE 1 | SAFE 2 | SAFE 3 | SAFE 4 | SAFE 5 | SAFE 6 | Maliau 1 | Maliau 2 | Maliau 3 | Maliau 4 | Maliau 5 | Maliau 6 | Maliau 7 | Maliau 8 | Danum 1 | Danum 2 | Danum 3 | Danum 4 | Danum 5 | Danum 6 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 4 |
| 2 | 6 | 0 | 0 | 5 | 4 | 3 | 2 | 5 | 4 | 2 | 19 | 3 | 6 | 3 | 2 | 2 | 1 | 0 | 0 | 2 | 69 |
| 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 4 |
| 4 | 4 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 10 | 0 | 0 | 0 | 2 | 1 | 2 | 4 | 6 | 3 | 0 | 0 | 35 |
| 5 | 0 | 0 | 3 | 0 | 17 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 4 | 4 | 0 | 10 | 4 | 4 | 50 |
| 6 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 5 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 |
| 8 | 4 | 1 | 0 | 1 | 6 | 4 | 0 | 0 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 3 | 4 | 3 | 0 | 0 | 35 |
| 9 | 3 | 4 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 17 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 4 | 0 | 0 | 2 | 8 |
| 11 | 6 | 2 | 3 | 1 | 0 | 1 | 2 | 2 | 0 | 2 | 8 | 2 | 1 | 2 | 2 | 3 | 13 | 0 | 1 | 0 | 51 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 4 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 15 | 9 | 1 | 23 | 9 | 0 | 0 | 16 | 16 | 7 | 1 | 7 | 12 | 14 | 5 | 0 | 7 | 11 | 0 | 1 | 8 | 147 |
| 16 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 6 |
| 17 | 0 | 1 | 2 | 2 | 0 | 02 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 15 |
| 18 | 3 | 0 | 0 | 1 | 0 | 1 | 5 | 4 | 0 | 0 | 0 | 3 | 3 | 2 | 0 | 3 | 2 | 0 | 0 | 0 | 27 |
| 19 | 0 | 1 | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 10 |
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 3 |
| 21 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| 22 | 4 | 3 | 2 | 2 | 2 | 4 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 3 | 3 | 33 |
| 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 27 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 5 |
| 28 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| 29 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 30 | 0 | 1 | 1 | 2 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| 31 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| 32 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| 33 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 34 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 35 | 2 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| 36 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| 37 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| 38 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 39 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 41 | 0 | 3 | 9 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 |
| 42 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
3.1 Beetle myrmecophile traits
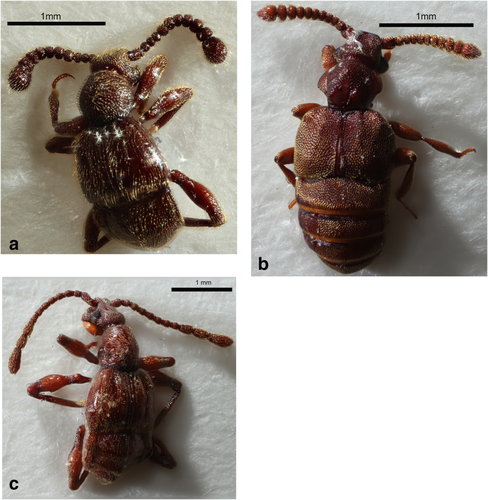
In this study, 12 morphospecies belonging to six different tribes were assigned as probable myrmecophiles, from a total of 42 total morphospecies recorded (Figure 1-3). The following groups of probable myrmecophile were found. (a) Two morphospecies were recorded from the tribe Clavigerini (Figure 1a,b). (b) Five morphospecies were recorded form the tribe Cyathigerini (Figure 2). (3) Only one morphospecies was recorded form the tribe Arnyliini (Figure 1c). (4) Two morphospecies were recorded from the tribe Tmesiphorini (Figures 1d, 3a), one morphospecies was recorded from the tribe Tyrini (Figure 3c) and one morphospecies was recorded from the tribe Batrisini (Figure 3b).

Pselaphinae beetles belonging to the tribe Clavigerini (a) morphospecies 27, (b) morphospecies 23, (c) morphospecies 4, tribe Arnyliini and (d) morphospecies 36, tribe Tmesiphorini [Color figure can be viewed at wileyonlinelibrary.com]
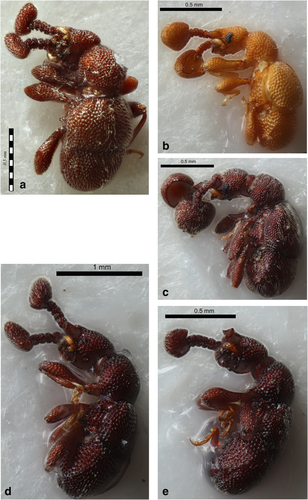
Pselaphinae beetles belonging to the tribe Cyathigerini (a) morphospecies 3, (b) morphospecies 6, (c) morphospecies 8, (d) morphospecies 22, (e) morphospecies 20 [Color figure can be viewed at wileyonlinelibrary.com]
Pselaphinae beetles belonging to the tribe Tmesiphorini (a) morphospecies 5, (b) morphospecies 42, tribe Batrisini and (c) morphospecies 17, tribe Tyrini [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Tribe Clavigerini
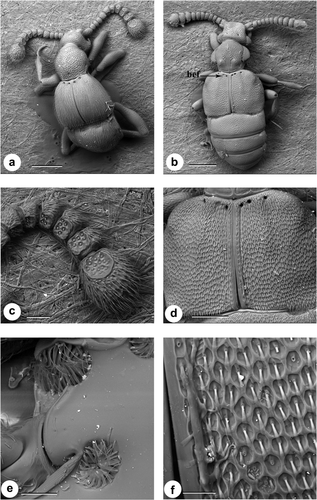
Beetles within the tribe Clavigerini are exclusively obligate myrmecophiles, exhibiting dramatic external morphological modifications for social parasitism inside ant colonies (Figure 4a,b). They are characterized by having a reduced number of antennomeres with a truncate antennal apex (Figure 4c) and are sparsely pubescent and lack any foveae. There is a distinct broad basal depression “tergal plate” in the abdomen bearing large tufts of trichomes (Figure 4d).
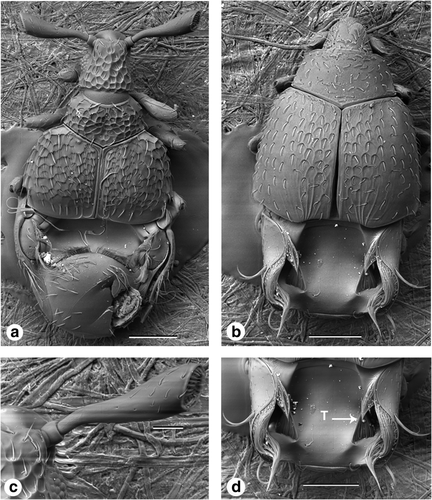
Dorsal view scanning electron micrographs of Pselaphinae tribe Clavigerini, (a) genus Cerylambus, (b) genus Pseudacerus, (c) Cerylambus antennae, (d) Pseudacerus abdomen. Label T, trichomes. Scale: a, b, d—200 μm and c—60 μm
3.3 Tribe Cyathigerini
Morphospecies belonging to the tribe Cyathigerini (Figures 5a–f, 6a,b) exhibits a reduced number of antennomeres (seven antennomeres) and are characterized by unique apical antennal club structures. The antennal clubs of morphospecies 3 (Figure 5b), 20 (Figure 6a) and 22 (Figure 6b) are enlarged, highly pubescent block-shaped terminal segments. In contrast, the antennal clubs of morphospecies 6 (Figure 5e) and morphospecies 8 (Figure 5f) are large and round and broadly excavated on the inner surface. Interestingly, trichomes are present inside the antennal club of morphospecies 6 (Figure 5e) but are absent in morphospecies 8 (Figure 5f). Morphospecies in this tribe also exhibit a reduced number of fovea, lack the presence of trichomes (excluding morphospecies 6, as previously mentioned) and dense punctures across the entire dorsal surface.
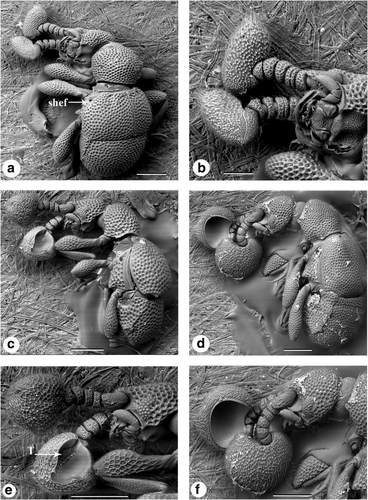
Scanning electron micrographs of Pselaphinae tribe Cyathigerini genus Plagiophorus. (a) Dorsal view, morphospecies 3, subhumeral elytral foveae (shef), (b) morphospecies 3 antennae, (c) side view, morphospecies 6, (d) side view, morphospecies 8, (e) morphospecies 6 antennae, trichomes (labeled T), (f) morphospecies 8 antennae. Scale: a, c, d, e, f—200 μm and b—100 μm
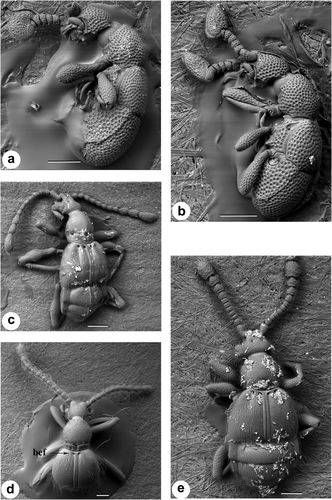
Scanning electron micrographs of the Pselaphinae beetle tribe Cyathigerini genus Plagiophorus (a) lateral view of morphospecies 20, (b) lateral view of morphospecies 22. (c) dorsal view, tribe Tyrini, morphospecies 17, (d) dorsal view, tribe Arnyliini, morphospecies 4, basal elytral foveae (bef), (e) dorsal view, tribe Tmesiphorini, morphospecies 36. Scale: a and c—400 μm and b, d, and e—200 μm
3.4 Tribe Tmesiphorini
Both morphospecies 36 (Figure 6e) and 5 (Figure 7a) have a complete set of antennomeres (11 antennomeres), which successively increase in size toward the apical antennomere and are highly pubescent across the entire body including the antenna. Both morphospecies exhibit a reduced number of basal elytral foveae (bef), with only two sets of bef. Interestingly, morphospecies 36 lacks the presence of trichomes compared to morphospecies 5. Antennomeres 6–11 of morphospecies 5 have shallow cavities embedded with impressions and setae on the ventral side (Figure 7c).
Dorsal view Scanning electron micrographs of Pselaphinae beetle tribe (a) Tmesiphorini genus Pseudophanias, morphospecies 5 and (b) Batrisini genus Diaugis, morphospecies 42, basal elytral foveae (bef). (c) Morphospecies 5 antennae (d) morphospecies 42 elytra (e) morphospecies 5, (f) morphospecies 42 elytra. Scale: a and b—400 μm, c–100 μm, d—200 μm, e—60 μm, and f—40 μm
3.5 Tribe Tyrini
Morphospecies 17 (Figure 6c) has a complete set of antennomeres (11 antennomeres), with 9–11 enlarged. Morphospecies 17 is highly pubescent across the entire body including the antenna, lacks the presence of trichomes and has a reduced number of bef (two).
3.6 Tribe Arnyliini
Morphospecies 4 (Figure 6d) has a complete set of almost symmetrical antennomeres (11 segments), lacks the presence of trichomes although is highly pubescent across the entire body, particularly along the antennae. Morphospecies 4 has a reduced number of bef (one).
3.7 Tribe Batrisini
Morphospecies 42 (Figure 7b) exhibits highly pubescent moniliform antenna (11 antennomeres), which successively increase in size toward the apical antennomere. Morphospecies 42 also has a reduced number of bef (three).
3.8 Possible myrmecophile beetles exhibiting myrmecophile traits
Both morphospecies 37 (Figure 8c) and 13 (Figure 8b) have compact antenna, partial fusion of sternites and tergites, and a reduced number of foveae. Morphospecies 32 lacks trichomes but is highly pubescent and has two sets of bef (Figure 8a). Interestingly, antennomere 6 and 7 of morphospecies 32 create a convex V-shape along the antennae, an antennal structure that has not been recorded in any other morphospecies in this study.

Scanning electron micrographs of Pselaphinae beetle (a) tribe Batrisini genus Cratna, morphospecies 32, dorsal view (b) tribe Hybocephalini genus Apharinodes, morphospecies 13, side view (c) tribe Hybocephalini genus Apharinodes, morphospecies 37, side view. Scale: a and b—400 μm and C—200 μm
3.9 Pselaphinae FD
Old growth rainforest sites Danum and Maliau recorded the highest mean ant abundances (1,193 and 911, respectively, per transect) compared with logged rainforest site SAFE (576). Pselaphinae beetle putative myrmecophile FD is significantly correlated with ant abundance across all three sites, at the plot level (t = 2.45, p = 0.0247). The presence of myrmecophile functional traits increases as ant abundance increases (Figure 9).
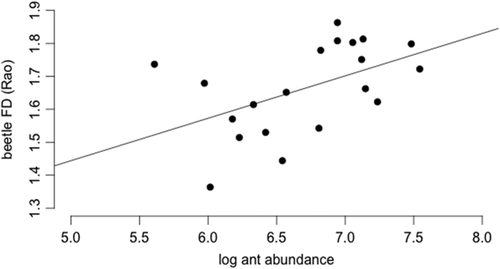
The relationship of beetle FD and ant abundance across all three sites
4 DISCUSSION
Primary rainforest sites support high abundances of leaf litter ants, which is correlated with a high abundance of probable myrmecophile pselaphine beetles. In accordance with our predictions, putative myrmecophile FD of pselaphine beetles increased as ant abundance increased.
The arrangement and presence or loss of foveae have been recognized as one of the most important morphological structures for taxonomic identification of Pselaphinae (Chandler, 2001; Park, 1942). The functional role of foveae remains unknown. Two probable foveal functions have been proposed by Chandler (2001): (a) thoracic foveae are sensory and (b) abdominal and head foveae provide structural rigidity. Moreover, the trend appears to be toward loss of foveae with basal Pselaphinae lineages exhibiting a more diverse set of foveal patterns across the entire body (Chandler, 2001). Although the functional role of foveae is unrelated to myrmecophily, there is a reduction in this trait with ant-associated Pselaphinae beetles, specifically with regards to the basal elytral foveae sets (Chandler, 2001; Parker & Maruyama, 2013). This is shown here, where nonmyrmecophile morphospecies belonging to the tribe Proterini retain a complete set of basal elytral foveae. Probable myrmecophile Pselaphinae morphospecies recorded in the tribes Tmesiphorini and Tyrini all exhibit a reduction of two sets of basal elytral foveae and tribes Arnyliini and Cyathigerini retain just one set of basal elytral foveae. Obligate myrmecophile Pselaphinae morphospecies 23 and 27 belonging to the tribe Clavigerini show a complete loss of basal elytral foveae.
The tribe Cyathigerini comprising five facultative myrmecophile morphospecies was dominant in the logged (SAFE) rainforest sites. The large excavated clubs are suggestive of morphological structures that may be associated with myrmecophily, in that they potentially provide structures for worker ants to hold onto while transporting the beetle into the ant's nest. There is no direct evidence that antennal clubs have a functional role in myrmecophily; however, pselaphine beetles in this tribe occasionally form a facultative association with ants (Sugaya et al., 2004). Moreover, the antennal clubs are known to be secondary sexual characters unique to the tribe (Chandler, 2001; Sugaya et al., 2004). Male Plagiophorus species in the tribe Cyathigerini recorded from Borneo have enlarged round antennal clubs (Nomura & Mohamed, 2008) exhibited by morphospecies 6 and 8. One female Plagiophorus species has been recorded from Borneo that has a block-like antennal club (Nomura & Mohamed, 2008) exhibited by morphospecies 3 and 22. We speculate such unique structures to be associated with sexual dimorphism and may play an essential role in beetle copulation. The tribe Cyathigerini has been documented for exhibiting remarkable sexual dimorphism in the antennal club and has been suggested that the female block-like antennal club inserts inside the excavated male antennal club, during copulation (Sugaya et al., 2004). Furthermore, internal morphological differences between males (morphospecies 6 and 8) may be an example of species variation within this particular genus. However, behavioral ecology on mating in pselaphine beetles largely remains unknown.
All members of the tribe Clavigerini are believed to be obligate myrmecophiles and are completely dependent on their ant hosts (Chandler, 2001; Park, 1942). Both morphospecies recorded in the current study exhibit morphological modifications including: reduced mouthparts for receiving trophallaxis (mouth-to-mouth liquid-feeding) from the worker ants (Park, 1942; Parker & Grimaldi, 2014), a reduction and compaction of antenna and abdominal segments that supports the beetle when handled by ant workers and provides a larger surface area for glandular secretions to spread and volatize (Cammaerts, 1992; Parker, 2016a; Parker & Grimaldi, 2014); long tufts of trichomes that ornament the base of the abdomen providing an important functional role during trophallaxis feeding by ant workers and social integration inside the ant colonies (Cammaerts, 1992; Parker & Grimaldi, 2014). Trichomes facilitate the dispersion of secretions produced by secretory Wasmann glands that are both attractive to and consumed by the worker ants thus initiating trophallaxis by the ant to the beetle (Hill et al., 1976; Parker & Grimaldi, 2014).
The presence or absence of one or more myrmecophile functional trait does not always define a myrmecophile. Morphospecies 13 and 37 (belonging to the tribe Hybocelaphini genus Apharinodes) recorded in the current study, both exhibit compact antenna and partial fusion of sternites and tergites, typical functional traits of myrmecophile Pselaphinae beetles. As previously discussed, the biology of many pselaphines is simply unknown, and therefore, it may be possible that morphospecies 13 and 37 form some kind of association with ants. Compact antenna and partial fusion of sternites and tergites are defensive modifications that allow pselaphines to withstand aggression from ants in the leaf litter (Parker, 2016a). Many facultative pselaphines also exhibit these defensive modifications indicating morphospecies 13 and 37 may exhibit some degree of myrmecophily, one that enables them to survive a high frequency of encounters with ants in the leaf litter (Parker, 2016a). Furthermore, morphospecies 13 and 37 are densely covered in squamous pubescence, not “true” trichomes, and this pubescence is thought to conduct secretions from nearby glands (Parker, 2016a). This type of pubescence also occurs in myrmecophile pselaphines belonging to the closely related tribe Ctenistini (Parker, 2016b) further suggesting some kind of association with ants. Morphospecies 32 (Batrisini) recorded in the current study, although lacks the obvious functional traits associated with myrmecophily (as previously discussed) exhibits a unique morphological antennal modification, whereby antennomere six and seven create a convex V shape. Although the functional role of such an antennal modification remains unknown, it may act as a “handle” to aid worker ants during transport of the beetle both inside and outside the ant nest, if at all this particular pselaphine morphospecies forms any association with ants. There are few if any, behavioral studies carried out on Pselaphinae beetles, given the difficulty posed by their small sizes and cryptic microhabitats, therefore it is difficult to conclude whether or not morphospecies 13, 32, and 37 form any association with ants. Behavioral studies on pselaphine beetles are needed to complement morphological studies and provide a better understanding for future ecological studies.
The current study presents a link between adult morphological characters and the functional role they possibly play in the behavior of myrmecophilous leaf litter pselaphines. Ant diversity may be a direct driver of pselaphine beetle FD, thus providing an insight into the dynamic ecological interactions pselaphines exhibit within the microhabitat of the tropical forest floor. This offers potential for exploring further the functional roles of pselaphines within leaf litter invertebrate communities and ecosystem functioning, particularly in determining changes in ecosystem processes in vulnerable habitats.
ACKNOWLEDGMENTS
We thank the University of Malaysia Sabah (UMS) for loaning the specimens used in this study. We thank Dan Carpenter, Kerry Calloway, Keiron Brown, and Kevin Ferdinand for assistance with sampling. We also thank Roger Booth and Peter Hammond (Natural History Museum, London) for examining the specimens. We are very grateful to Joseph Parker (American Museum of Natural History, New York/Columbia University, New York) for his taxonomic identification of all morphospecies recorded in this study and for valuable discussions concerning pselaphine taxonomy, morphology, and biology.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.




