Randomized phase II trial of chemoradiotherapy with S-1 versus combination chemotherapy with gemcitabine and S-1 as neoadjuvant treatment for resectable pancreatic cancer (JASPAC 04)
Abstract
Objective
The aim of the present study was to investigate which treatment, neoadjuvant chemoradiotherapy (NAC-RT) with S-1 or combination neoadjuvant chemotherapy with gemcitabine and S-1 (NAC-GS), is more promising as neoadjuvant treatment (NAT) for resectable pancreatic cancer in terms of effectiveness and safety.
Methods
In the NAC-RT with S-1 group, the patients received a total radiation dose of 50.4 Gy in 28 fractions with oral S-1. In the NAC-GS group, the patients received intravenous gemcitabine at a dose of 1000 mg/m2 with oral S-1 for two cycles. The primary endpoint was the 2-year progression-free survival (PFS) rate. The trial was registered with the UMIN Clinical Trial Registry as UMIN000014894.
Results
From April 2014 to April 2017, a total of 103 patients were enrolled. After exclusion of one patient because of ineligibility, 51 patients were included in the NAC-RT with S-1 group, and 51 patients were included in the NAC-GS group in the intention-to-treat analysis. The 2-year PFS rate was 45.0% (90% confidence interval [CI]: 33.3%–56.0%) in the NAC-RT with S-1 group and 54.9% (42.8%–65.5%) in the NAC-GS group (p = .350). The 2-year overall survival rate was 66.7% in the NAC-RT with S-1 group and 72.4% in the NAC-GS group (p = .300). Although leukopenia and neutropenia rates were significantly higher in the NAC-GS group than in the NAC-RT with S-1 group (p = .023 and p < .001), other adverse events of NAT and postoperative complications were comparable between the two groups.
Conclusion
Both NAC-RT with S-1 and NAC-GS are considered promising treatments for resectable pancreatic cancer.
1 INTRODUCTION
Pancreatic cancer (PC) is associated with a poor prognosis and an overall 5-year survival rate of <10%.1 It is the third-leading cause of cancer mortality in Japan.1 Surgery is the most effective treatment and offers the only chance for a cure of nonmetastatic PC; however, recurrence rates are high, even after curative resection. The CONKO-0012 and JASPAC 013 trials suggested that postoperative adjuvant treatment offered a good chance for prolonged survival in patients undergoing curative resection of PC. However, a certain number (30%–40%) of patients cannot receive adjuvant therapy due to postoperative morbidity or a poor general condition.4
Neoadjuvant treatment (NAT) was initially initiated for borderline resectable PC (BR-PC).5 Most reports of treatment outcomes were from retrospective studies, and there were no reports with a high level of evidence. In 2018, a multicenter randomized controlled trial (RCT) was reported from Korea that examined the efficacy of NAT against upfront surgery in BR-PC.6 In the intention-to-treat analysis, the 2-year survival rate was significantly better in the NAT group than in the upfront surgery group (40.7% vs. 26.1%). Thereafter, although the evidence is limited, the National Comprehensive Cancer Network (NCCN) and Japan Pancreas Society Guidelines recommend NAT for BR-PC.7, 8
After the promising results of NAT in BR-PC, there is increasing interest in the use of NAT for resectable PC (R-PC). NAT in R-PC would increase the proportion of patients receiving chemotherapy. Furthermore, chemotherapy delivery is more effective with an intact blood supply to tumor cells than in circumstances of impaired postoperative tissue vascularization.9 To date, various attempts, including preoperative neoadjuvant chemoradiotherapy (NAC-RT)10, 11 and preoperative chemotherapy (NAC),12-14 have been made with the aim of further improving the outcomes of patients with R-PC. In these cases, both NAC-RT and NAC were associated with relatively good results and were reported to have shown the potential to be effective treatments for R-PC.15, 16 However, at this time, there have been no trials comparing NAC-RT to NAC, and it is unclear which is more promising. Therefore, we planned a randomized phase II trial of NAC-RT versus NAC with the aim of selecting the more promising regimen as the study treatment for a future phase III trial.
2 METHODS
The JASPAC 04 trial was a multicenter phase II, randomized, open-label, parallel-group (1:1 ratio) trial conducted at 11 Japanese hospitals. The trial was completed in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies of the Ministry of Health, Labour and Welfare of Japan. All associated medical procedures were covered by the Japanese national social insurance system. The study protocol was approved by the institutional review boards of all participating centers (no. 18-15-19-3) and was registered in the University Hospital Medical Information Network (http://www.umin.ac.jp; registration number ID: UMIN000014894). All patients provided their written informed consent.
2.1 Participants
Patients with radiological R-PC based on the UICC 7th edition17 (resectability classification of stage ≤II or stage III cases with combined celiac artery resection) and Classification of Pancreatic Carcinoma 3rd English edition by The Japan Pancreas Society18 (pathological classification) were enrolled in this study. Other eligibility criteria for participants were conformity with either adenocarcinoma proven by biopsy or cytology or when—although there is no pathological evidence—cancer was evident based on imaging and a high tumor marker value (in body/tail cancer only), confirmation of a radiation oncologist that the estimated radiation field was within 10 × 10 cm in size, including the primary lesion and metastatic nodes (positron emission tomography [PET]-positive nodes considered to be metastatic) on computed tomography (CT) or magnetic resonance imaging, with no findings of gastrointestinal invasion corresponding to mucosal invasion of the stomach or duodenum by endoscopy or protrusion of the tumor into the gastric or duodenal lumen on CT. Participants also had to fulfill the following criteria: no active gastric or duodenal ulcer, age of ≥20 to ≤79 years, ECOG performance status 0 or 1, adequate oral intake, adequate organ function in measurements taken within 7 days before registration (leukocyte count ≥3000 cells/μL; hemoglobin concentration ≥9.0 g/dL; platelet count ≥100 000 cells/μL; albumin ≥3.0 g/dL; total bilirubin ≤2.0 mg/dL) (with biliary drainage) or ≤3.0 mg/dL (without biliary drainage); aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentrations ≤100 IU/L (with biliary drainage) or ≤150 IU/L (without biliary drainage); serum creatinine concentration ≤1.2 mg/dL; and creatinine clearance ≥50 mL/min/bodyweight.
2.2 Randomization
For enrollment, treatment groups were randomly assigned in a 1:1 ratio at the registration center. Random assignment was performed using a minimization method with the following adjustment factors: (1) institution, (2) primary site (head vs. body/tail), and (3) carbohydrate antigen 19-9 (CA19-9) (<100 U/mL vs. >100 U/mL) to avoid large bias. Detailed procedures for the random assignment method were not made known to the researchers at the participating centers.
2.3 Treatment
Patients assigned to the NAC-RT group received oral S-1 twice daily (80 mg/day for a body surface area [BSA] of <1.25 m2, 100 mg/day for a BSA of ≥1.25 to <1.50 m2, and 120 mg/day for a BSA of ≥1.50 m2) on the day of irradiation during radiotherapy. Radiotherapy was delivered with a linear accelerator capable of producing a ≥6-MV photon beam using a three-dimensional conformal technique. Intensity-modulated radiation therapy was not allowed. A total dose of 50.4 Gy was delivered in 28 fractions over a 5.5-week period. The gross tumor volume included the primary tumor (GTV-primary) and metastatic lymph nodes with a short-axis diameter of ≥1 cm on CT or positive on PET scan (GTV-node). The clinical target volume (CTV) was defined as the GTV-primary plus a 0.5-cm margin and the GTV-node. A CTV margin for the GTV-node was not added, and regional lymph node areas were not included in the CTV. The planning target volume included the CTV with appropriate margins depending on respiratory motion and the patient setup uncertainties: typically, 1 cm for lateral and ventral-dorsal margins and 1–2 cm for craniocaudal margins. Multifield arrangements with three or more beams were used. The isocenter set at or near the center of the PTV was used as the dose prescription point. The dose to the PTV at the isocenter plane was required to be 90% or more and 110% or less of the prescribed dose. Dose constraints for organs at risk were as follows: spinal cord, < 45 Gy; both kidneys, V18 (percentage of the organ volume receiving ≥18 Gy) < 35%; and liver, V30 < 40%, V20 < 67%.
Patients assigned to the NAC with gemcitabine and S-1 (NAC-GS) group received gemcitabine (1000 mg/m2) infusion on days 1 and 8 and oral S-1 twice daily (60 mg/day for a BSA <1.25 m2, 80 mg/day for a BSA of ≥1.25 to <1.50 m2, and 100 mg/day for a BSA of ≥1.50 m2) on days 1–14, followed by a 1-week rest. This treatment was repeated for two cycles.
If the total treatment period was >60 days, the treatment was ended at 60 days, and surgery was subsequently performed. When R0 resection was considered difficult because of the presence of distant metastasis, local disease progression, the presence of grade 4 nonhematological toxicity, or the presence of an adverse event precluding pancreatectomy within 56 days after the final day of neoadjuvant therapy, the plan for surgical resection was abandoned, and the protocol treatment was terminated.
2.4 Surgery
Patients fulfilling the criteria for surgery underwent surgery at 15–56 days after the end of preoperative treatment. Surgery was performed for curative resection. One of the following surgical techniques was selected: pancreatoduodenectomy, distal pancreatectomy, or total pancreatectomy. Resection of the portal/superior mesenteric vein or celiac axis in distal pancreatectomy was allowed when needed to achieve R0 resection. The extent of lymph node dissection was defined according to the Japan Pancreas Society classification system.18 The protocol treatment was discontinued if any of the following events occurred: distant metastasis or retroperitoneal invasion that precluded R0 or R1 resection; pathologically confirmed para-aortic lymph node metastasis; and positive peritoneal washing cytology. Patients were considered to have been treated according to the protocol if they received both NAC-RT with S-1 or NAC-GS and subsequent R0 or R1 pancreas resection.
2.5 Follow-up
After completion of the protocol treatment (R0/1 resection), postoperative adjuvant chemotherapy was given to the extent that was possible. In principle, S-1 monotherapy was recommended. However, if administration of S-1 was judged to be intolerable due to the therapeutic effect or side effects, gemcitabine monotherapy was used. Follow-up examination, including the assessment of the performance status and quality of life, abdominal CT, chest radiography or chest CT, and tumor markers (carcinoembryonic antigen and CA 19-9) was performed within 8 weeks from the date of surgery for the first time and every 12 weeks thereafter.
2.6 Outcomes
The primary endpoint was the 2-year progression-free survival (PFS) rate. The secondary endpoints were overall survival, R0 or 1 resection rate, histological response rate, rate of adverse events of NAT, surgical complication rate, rate of grade 4 nonhematological adverse events, and rate of treatment-related death. The trial was monitored by a data monitoring committee, which functioned independently of the investigators. Data were collected using the web-based clinical trial management system at the data center (Pharma Valley Center, Shizuoka, Japan). The histological response rate was classified according to the diagnostic criteria of Evans et al.19 A significant histological response was defined when degenerated cancer cells accounted for more than 50% of the total.
2.7 Statistical analysis
In the main analysis, the estimated 2-year PFS rate was obtained using the Kaplan–Meier method for all eligible cases, and the 90% confidence interval (CI) was calculated. Considering the possibility that the period from randomization to the time of surgery may differ between groups, all of the unresected cases were treated as exacerbations at 12 weeks. For each treatment group, if the lower limit of the 90% CI for the 2-year PFS rate in the main analysis exceeded 20%, that treatment group was considered a candidate for the next phase III study. On the other hand, if the lower limit of the 90% CI fell below 20%, the treatment was considered unpromising and was excluded from the next Phase III trial. If both groups exceeded 20%, the choice of NAC-RT with S-1 or NAC-GS as the more promising treatment was clinically comprehensive with safety considerations.
According to the results of JASPAC 01, the 2-year recurrence-free survival rate of patients who received adjuvant chemotherapy with S-1 was approximately 50%.3 Cases in which resection was considered to be noncurative were expected to account for approximately 20% of the study population. In addition, adjuvant chemotherapy may not be available in approximately 30% of curative resection cases. The 2-year recurrence-free survival rate of such cases is considered to be approximately 10% based on the results of CONKO-001.20 Therefore, the 2-year recurrence-free (exacerbation) survival rate of patients with pancreatic cancer who were included in this study after postoperative adjuvant therapy was as follows: 0 (noncurative resection cases) + (1–0.2) × 0.3 × 0.1 (nonadjuvant chemotherapy cases) + (1–0.2) × 0.7 × 0.5 (adjuvant chemotherapy cases) = 0.304, that is, it was estimated to be approximately 30%. Based on this hypothesis, when the threshold 2-year PFS rate was 25% and the expected 2-year PFS rate was 40%, the required number of cases per group was 45 under an α error of 5% in the one-sided test. Accordingly, a detection power of 80% could be secured. Considering that some cases would be excluded from the analysis, the study population was set to 50 cases per group, for a total of 100 cases.
3 RESULTS
3.1 Patient characteristics
Between May 2013 and April 2017, a total of 103 patients were enrolled; 52 patients were randomly assigned to the NAC-RT with S-1 group and 51 were randomly assigned to the NAC-GS group. After randomization, one patient with interstitial pneumonia was found to be ineligible and was not included in the analysis. As a result, 51 patients in the NAC-RT with S-1 group and 51 patients in the NAC-GS group constituted the per-protocol population (Figure 1).
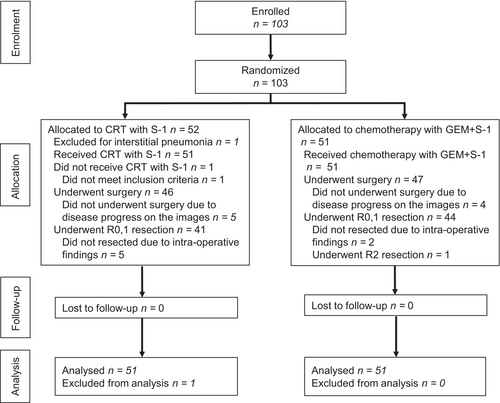
Table 1 shows the baseline characteristics of the per-protocol population. The patient demographics and tumor characteristics of the two groups were well balanced, except for the days from randomization to the initiation of neoadjuvant therapy.
| NAC-RT with S-1, n = 51 | NAC-GS, n = 51 | p-value | |
|---|---|---|---|
| Age (years) | 67 (56.5–71.5) | 68 (62.5–72.0) | .146 |
| <65 | 21 (41) | 15 (29) | .214 |
| ≧65 | 30 (59) | 36 (71) | |
| ECOG-PS | |||
| 0 | 48 (94) | 47 (92) | .695 |
| 1 | 3 (6) | 4 (8) | |
| CA19-9 (U/mL) | 76 (16.7–276.0) | 80 (22.5–205.1) | .733 |
| <37 | 21 (41) | 17 (33) | .715 |
| 37≦ <370 | 22 (43) | 25 (49) | |
| 370≦ | 8 (16) | 9 (18) | |
| Sex | |||
| Male | 33 (65) | 27 (53) | .227 |
| Female | 18 (35) | 24 (47) | |
| Primary site | |||
| Head | 34 (67) | 34 (67) | 1 |
| Body/tail | 17 (33) | 17 (33) | |
| cTa | |||
| cT1 | 2 (4) | 4 (8) | .436 |
| cT2 | 5 (10) | 8 (16) | |
| cT3 | 44 (86) | 39 (76) | |
| cNa | |||
| cN0 | 47 (92) | 45 (88) | .505 |
| cN1 | 4 (8) | 6 (12) | |
| cMa | |||
| cM0 | 51 (100) | 51 (100) | 1 |
| cStagea | |||
| cIA | 2 (4) | 4 (8) | .585 |
| cIB | 4 (8) | 6 (12) | |
| cIIA | 41 (80) | 35 (68) | |
| cIIB | 4 (8) | 6 (12) | |
| Randomization to neoadjuvant therapy (days) | 9 (7–12) | 7 (4–8) | <.001 |
- Note: Data are n (%) or median (IQR).
- a Tumors were staged according to the Union for International Cancer Control (UICC) TNM classification, seventh edition.
3.2 Treatment
In both groups, all patients received NAT. A total of 49 patients (96%) in the NAC-RT with S-1 group and 45 patients (88%) in the NAC-GS group completed NAT. A total of 40 of 51 treatment plans in the NAC-RT with S-1 group were assessed as having been completed per protocol, and 11 were assessed as having acceptable deviation. No unacceptable violations were found. All serious adverse events that occurred during NAT are summarized in Table 2. Grade 3 nonhematological toxicity was experienced by eight patients (9 episodes) in the NAC-RT with S-1 group and 12 patients (14 episodes) in the NAC-GS group. Grade 3/4 hematological toxicity was observed in 12 patients (16 episodes) in the NAC-RT with S-1 group and 34 patients (50 episodes) in the NAC-GS group. Leukocytopenia and neutropenia developed more frequently in the NAC-GS group than in the NAC-RT with S-1 group; leukocytopenia, 24% versus 6%, p = .023 and neutropenia, 59% versus 0%, p < .001. One patient in the NAC-RT with S-1 group and four patients in the NAC-GS group terminated NAT early due to toxicity, but no patients lost the chance to receive surgical treatment.
| Event | NAC-RT with S-1, n = 51 | NAC-GS, n = 51 | p-value | ||||
|---|---|---|---|---|---|---|---|
| Any | Grade 3 | Grade 4 | Any | Grade 3 | Grade 4 | ||
| Fatigue | 15 (29) | 0 (0) | 0 (0) | 19 (37) | 0 (0) | 0 (0) | 1 |
| Fever | 8 (16) | 0 (0) | 0 (0) | 7 (14) | 0 (0) | 0 (0) | 1 |
| Eruption | 3 (6) | 1 (2) | 0 (0) | 12 (24) | 4 (8) | 0 (0) | .362 |
| Urticaria | 0 (0) | 0 (0) | 0 (0) | 6 (12) | 1 (2) | 0 (0) | 1 |
| Anorexia | 27 (53) | 1 (2) | 0 (0) | 17 (33) | 1 (2) | 0 (0) | 1 |
| Diarrhea | 8 (16) | 0 (0) | 0 (0) | 10 (20) | 3 (6) | 0 (0) | .243 |
| Nausea | 6 (12) | 0 (0) | 0 (0) | 14 (27) | 1 (2) | 0 (0) | 1 |
| Stomatitis | 5 (10) | 0 (0) | 0 (0) | 10 (20) | 0 (0) | 0 (0) | 1 |
| Dysgeusia | 5 (10) | 0 (0) | 0 (0) | 4 (8) | 0 (0) | 0 (0) | 1 |
| Febrile neutropenia | 0 (0) | 0 (0) | 0 (0) | 4 (8) | 4 (8) | 0 (0) | .118 |
| Catheter-related infection | 1 (2) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Biliary tract infection | 6 (12) | 6 (12) | 0 (0) | 6 (12) | 6 (12) | 0 (0) | .741 |
| Radiation dermatitis | 6 (12) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Leukopenia | 36 (71) | 3 (6) | 0 (0) | 46 (90) | 11 (22) | 1 (2) | .023 |
| Neutropenia | 11 (22) | 0 (0) | 0 (0) | 42 (82) | 19 (37) | 11 (22) | <.001 |
| Anemia | 42 (82) | 0 (0) | 0 (0) | 42 (82) | 1 (2) | 0 (0) | 1 |
| Thrombocytopenia | 25 (49) | 1 (2) | 0 (0) | 38 (75) | 2 (4) | 0 (0) | 1 |
| Elevated bilirubin | 14 (27) | 3 (6) | 0 (0) | 14 (27) | 0 (0) | 0 (0) | .243 |
| Elevated AST | 18 (35) | 1 (2) | 2 (4) | 22 (43) | 1 (2) | 0 (0) | .617 |
| Elevated ALT | 25 (49) | 2 (4) | 0 (0) | 22 (43) | 2 (4) | 0 (0) | 1 |
| Elevated ALP | 26 (51) | 2 (4) | 0 (0) | 27 (33) | 0 (0) | 0 (0) | .495 |
| Elevated creatinine | 5 (10) | 0 (0) | 0 (0) | 5 (10) | 0 (0) | 0 (0) | 1 |
| Hypernatremia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Hyponatremia | 18 (35) | 1 (2) | 0 (0) | 9 (18) | 1 (2) | 0 (0) | 1 |
| Hyperkalemia | 11 (22) | 1 (2) | 0 (0) | 5 (10) | 0 (0) | 0 (0) | 1 |
| Hypokalemia | 8 (16) | 0 (0) | 0 (0) | 9 (18) | 1 (2) | 0 (0) | 1 |
- Note: Values in parentheses are percentages.
- ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase.
Regarding the oncological effect of NAT, 41 of 51 patients in the NAC-RT with S-1 group had decreased CA19-9 levels, with a median value decreasing from 81.8 to 35.9 U/mL, and 41 of 51 patients in the NAC-GS group had decreased CA19-9 levels, with a median value decreasing from 80.0 to 38.0 U/mL (Figure S1). The reduction rate of CA19-9 values was almost similar at 35.2% (interquartile range: 0%–74.5%) in the NAC-RT with S-1 group and 40.8% (10.0%–61.5%) in the NAC-GS group (p = .945).
3.3 Surgery
Five patients in the NAC-RT with S-1 group and four patients in the NAC-GS group did not undergo surgery due to disease progression during preoperative treatment. No patients were considered medically unfit for surgery. In the NAC-RT with S-1 group, 46 patients underwent surgery, compared with 47 in the NAC-GS group. The median time between randomization and surgery was 69 days (50–105) in the NAC-RT with S-1 group and 76 days (40–105) in the NAC-GS group. The median time between the end of NAT and surgery was 24 days (13–42) in the NAC-RT with S-1 group and 26 days (13–50) in the NAC-GS group. Five patients in the NAC-RT with S-1 group and two patients in the NAC-GS group did not undergo surgical resection due to intraoperative findings. In one patient in the NAC-GS group, distal pancreatectomy was performed but resulted in R2 resection. Finally, R0/1 resection was achieved in 41 of 51 (80.4%) patients in the NAC-RT with S-1 group and 44 of 51 (86.3%) patients in the NAC-GS group (p = .425) (Figure 1).
The type of NAT did not affect the surgical outcome (ancillary analyses) (Table S1). The postoperative complications are summarized in Table 3. No significant differences in the occurrence of complications were found between the two treatment groups. Grade 4 nonhematological toxicities were present in two patients (gastrointestinal and biliary anastomotic leakage) in the NAC-RT with S-1 group and two patients (enteritis and gastric ulcer) in the NAC-GS group. No mortality within 30 days after the final protocol treatment or treatment-related death occurred in either of the groups.
| Event | NAC-RT with S-1, n = 46 | NAC-GS, n = 47 | p-value | ||||
|---|---|---|---|---|---|---|---|
| Any | Grade 3 | Grade 4 | Any | Grade 3 | Grade 4 | ||
| Early postoperatively | |||||||
| Lung infection | 2 (4) | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | .495 |
| Postoperative hemorrhage | 1 (2) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | .495 |
| Gastrointestinal anastomotic leakage | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | .495 |
| Biliary anastomotic leakage | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | .495 |
| Pancreatic fistula | 9 (20) | 3 (7) | 0 (0) | 10 (21) | 2 (4) | 0 (0) | .677 |
| Ascites | 4 (9) | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | .495 |
| Diarrhea | 4 (9) | 0 (0) | 0 (0) | 6 (13) | 1 (2) | 0 (0) | 1 |
| Appetite loss | 1 (2) | 1 (2) | 0 (0) | 1 (2) | 1 (2) | 0 (0) | 1 |
| Decreased hepatic artery blood flow | 1 (2) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | .495 |
| Enteritis | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 1 (2) | 1 |
| Late postoperatively | |||||||
| Small bowel obstruction | 1 (2) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | .495 |
| Gastric ulcer | 0 (0) | 0 (0) | 0 (0) | 2 (4) | 1 (2) | 1 (2) | .495 |
| Biliary tract infection | 7 (15) | 3 (7) | 0 (0) | 4 (9) | 1 (2) | 0 (0) | .361 |
| Ascites | 3 (7) | 2 (4) | 0 (0) | 2 (4) | 0 (0) | 0 (0) | .242 |
| Diarrhea | 9 (20) | 1 (2) | 0 (0) | 13 (28) | 0 (0) | 0 (0) | .495 |
| Extremity edema | 2 (4) | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | .495 |
| Pancreatic fistula | 1 (2) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | .495 |
- Note: Adverse events were defined according to the Common Terminology Criteria for Adverse Events of the National Cancer Institute, version 4.0.
3.4 Survival
The median follow-up period for the censored cases was 31.4 months: 30.8 months (23.1–51.6) in the NAC-RT with S-1 group and 32.2 months (23.5–49.6) in the NAC-GS group. Postoperative adjuvant chemotherapy was conducted in 38 patients in the NAC-RT with S-1 group (all S-1) and 39 patients in the NAC-GS group (S-1 in 38 and gemcitabine in 1). Among patients who underwent R0/1 resection, 20 (48.7%) patients in the NAC-RT with S-1 group and 19 (43.2%) patients in the NAC-GS group developed recurrence. The recurrence sites (ancillary analyses) are summarized in Table S2. Locoregional recurrence was detected in three (7.3%) patients in the NAC-RT with S-1 group and seven (15.9%) patients in the NAC-GS group. In contrast, hematological metastases were observed in 12 (29.3%) patents in the NAC-RT with S-1 group and 14 (31.8%) in the NAC-GS group.
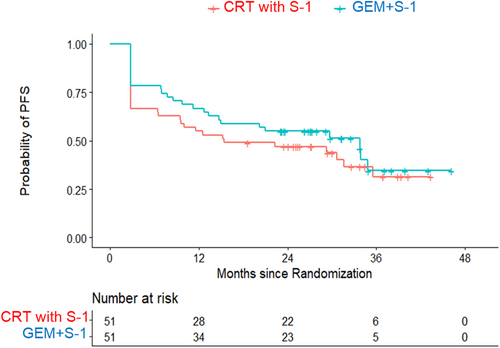
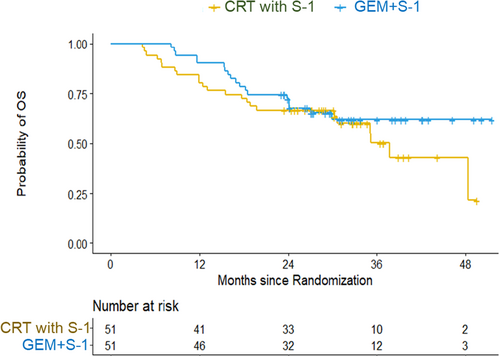
Regarding the intention-to-treat analysis, the estimated 2-year PFS rate was 45.0% (90% CI: 33.3%–56.0%) in the NAC-RT with S-1 group and 54.9% (42.8%–65.5%) in the NAC-GS group (Figure 2). The hazard ratio of the NAC-GS group to the NAC-RT with S-1 group was 0.78 (95% CI: 0.46–1.31; p = .350). The estimated 2-year overall survival rate and median survival time were 66.7% and 37.7 months in the NAC-RT with S-1 group and 72.4% and not reached in the NAC-GS group (Figure 3). The hazard ratio of the NAC-GS group to the NAC-RT with S-1 group was 0.72 (95% CI: 0.39–1.34; p = .300). A total of 38 patients in the NAC-RT with S-1 group and 39 patients in the NAC-GS group completed the per-protocol treatment (NAT-surgery-adjuvant chemotherapy). The 2-year PFS rate was 62.9% in the NAC-RT with S-1 group and 69.2% in the NAC-GS group (Figure S2). The 2-year overall survival rate was 86.8% in the NAC-RT with S-1 group and 81.9% in the NAC-GS group (Figure S3). There were no significant differences in the DFS or OS in either group (p = .701 and p = .773).
3.5 Pathological assessment
The pathological findings of R0/1 resection specimens are summarized in Table 4. Positive lymph nodes were found in 20 of 41 patients (48.8%) in the NAC-RT with S-1 group and 21 of 44 patients (47.7%) in the NAC-GS group. Destruction of more than 50% of the tumor cells (Evans classification, grade ≥ IIb) was more frequently observed in the NAC-RT with S-1 group (n = 19, 46.3%) than in the NAC-GS group (n = 10, 22.7%) (p = .022).
| NAC-RT with S-1, n = 41 | NAC-GS, n = 44 | p-value | |
|---|---|---|---|
| Tumor differentiation | |||
| Papillary | 1 (2) | 0 (0) | .616 |
| Well differentiated | 7 (17) | 9 (21) | |
| Moderately differentiated | 28 (68) | 28 (64) | |
| Poorly differentiated | 2 (5) | 4 (9) | |
| Adenosquamous | 0 (0) | 2 (2) | |
| Others | 3 (7) | 2 (5) | |
| Tumor stage | |||
| pT1 | 3 (7) | 5 (11) | .552 |
| pT2 | 4 (10) | 2 (5) | |
| pT3 | 34 (83) | 37 (84) | |
| Nodal status | |||
| pN0 | 21 (51) | 23 (52) | .923 |
| pN1 | 20 (49) | 21 (48) | |
| Margin status | |||
| pR0 | 40 (98) | 39 (89) | .204 |
| pR1 | 1 (2) | 5 (11) | |
| Pathological response (Evans classification) | |||
| I–IIa | 22 (54) | 34 (77) | .022 |
| IIb–III | 19 (46) | 10 (23) | |
4 DISCUSSION
The study was conducted with the purpose of examining the efficacy and safety of NAC-RT with S-1 and NAC-GS therapies as preoperative treatments. In the NAC-RT with S-1 group, 90% (46 of 51) of patients underwent surgery, and 80% (41 of 51) underwent R0/1 resection. In the NAC-GS group, 92% (47 of 51) of patients underwent surgery, and 86% (44 of 51) underwent R0/1 resection. These percentages indicate that the preoperative treatment regimens did not significantly change the individual chance of undergoing resection. In addition, the lower limit of the 90% CI of the 2-year PFS rate in the main analysis of both NAC-RT with S-1 therapy and NAC-GS therapy exceeded 20%, and there was no significant difference in PFS between the two treatments. Although the regimens are different, the survival time in both the NAC-RT (2-year OS: 67%, MST: 37.7 months) and NAC (2-year OS: 72%, MST: NE) groups was comparable or superior to that in previous prospective studies (MST: 14.6–22.7 months in NAC-RT10, 11 and 20.4–39.2 months in NAC12-14). However, this study was a Phase II trial and was not intended to determine the superiority or inferiority of the two treatments. Therefore, in terms of the therapeutic effect, both therapies are considered promising treatments.
The proportion of adverse events of NAT was similar in both groups, with the exception of bone marrow toxicity. The rate of bone marrow toxicity was higher in the NAC-GS group, with grade 3 and grade 4 neutropenia occurring in 37% and 22%, respectively, but many of these events were manageable. Although the rate of biliary tract infection was 12% in both groups, the incidence rates of other adverse effects were less than 10%. Early termination was required in 2% of the cases in the NAC-RT with S-1 group and 8% of the cases in the NAC-GS group; however, there were no cases that lost the chance to undergo surgery due to toxicity. Considering these results, both NAC-RT with S-1 and NAC-GS seem to be acceptably safe. In the SWOG trial, which compared FORFIRINOX therapy with gemcitabine/nab-paclitaxel therapy as preoperative treatment, among the 102 eligible patients, nine patients were unable to undergo surgery due to toxicity.21, 22 Although preoperative treatment with a strong regimen is ideal, the opportunity for surgery should not be lost due to associated toxicity in patients with R-PC. In this study, the amount of drug administered in the NAC-GS group was considered to decrease the incidence of severe adverse events and to subsequently avoid losing the chance to perform surgery due to toxicity. The dose level of S-1 used in the NAC-GS group was based on the results of a previous study.23 In a previous phase II study of NAC-GS, 1000 mg/m2 gemcitabine was combined with S-1 at doses of 80 mg/day, 100 mg/day, and 120 mg/day. In that study, the rate of treatment withdrawal due to adverse events was 41% (22 of 54 patients), the rate of grade 3 or worse neutropenia was 80%, and the dose was reduced in 56% of the patients (30 of 54 patients).24 Consequently, following a Phase III study, S-1 was administered at a 20 mg/day lower dose in the GS group than in the S-1 monotherapy group, and sufficient tolerability was achieved.23, 25 Therefore, this dose was also used in the NAC-GS group in this study. This one-step reduction in the S-1 dosage was thought to be the key to preventing withdrawal due to toxicity.
One of the concerns in performing NAT is the possibility of increased postoperative complications. In particular, we were initially concerned that preoperative NAC-RT with S-1 may increase postoperative complications due to local inflammation. Mokdad et al.26 reported that NAC-RT worsened the postoperative outcomes in comparison to NAC. In contrast, the PREOPANC study revealed that preoperative chemoradiotherapy did not increase the incidence of surgical complications or mortality.27 In the present study, there were no differences in postoperative complications between the two groups, and the incidence of serious complications was extremely low. Grade 4 complications were seen in only two patients in the NAC-RT with S-1 group and 2 patients in the NAC-GS group. No surgery-related deaths were observed. Overall, the incidence of complications is considered acceptable, as shown by a multicenter survey in comparison to the short-term outcomes.28 Therefore, in terms of adverse events, both therapies are considered promising treatments.
To date, there have been no trials comparing NAC-RT and NAC for resectable pancreatic cancer. A report that compared the results of preoperative NAC-RT and NAC for resectable pancreatic cancer using the National Cancer Database showed that preoperative NAC-RT and NAC were associated with similar OS (25 months vs. 26 months), but NAC-RT was associated with more favorable pathological features.26 A meta-analysis comparing upfront surgery to NAT in patients with R-PC or BR-PC revealed that the MST was 20.9 months for NAC and 17.8 months for NAC-RT.29 The results of this study were generally comparable to the findings of these previous studies. The pathological response was more favorable in NAC-RT with S-1 than in NAC-GS, and local control seemed to be good in NAC-RT with S-1. However, hematological metastases were comparable between both groups. Future verification is needed to determine whether local control reliably contributes to survival.
The initial consideration in determining the protocol for this study was to make the regimen simple enough to be performed at any facility. Therefore, the study regimen was based on the results of previous multicenter trials.23, 30 However, considering the relatively low regression rate in both regimens, we cannot say that such regimens were sufficient. Thus, more effective regimens should be developed in the future.
In the current study, we present a 2-year PFS based on the primary endpoint of the protocol. However, enrollment in this study ended in 2017, so we would like to consider long-term results as ancillary research in the future.
Several limitations associated with the present study warrant mention. First, S-1 is mainly used in East Asia, as the pharmacokinetics and pharmacodynamics of S-1 may differ between Westerners and East Asians.31 Therefore, whether the current results can be simply adapted to Western patients is unclear. Second, the follow-up period was relatively short, which underpowers the analysis. Third, the definition of resectability status was based on the UICC seventh edition,17 which did not meet the current NCCN definition.32 Therefore, it cannot be denied that some instances of BR-PC with bilateral involvement of the portal/superior mesenteric vein may have been included.
The initial purpose of this study was to examine the efficacy and safety of S-1 combined with radiotherapy and GS combination therapy as preoperative treatment in a randomized phase II study to find more promising treatments. The Prep02/JSAP-05 study reported that NAC-GS prolongs survival compared to surgery alone.33 Since the PFS in this study was as good as that in the Prep02/JSAP-05 study, NAT for R-PC is likely to be beneficial, and both NAC and NAC-RT can be expected to be viable treatment options to be considered in a future Phase III study.
5 CONCLUSIONS
Both NAC-RT with S-1 and NAC-GS are considered promising preoperative treatments for resectable PC.
FUNDING INFORMATION
This study was conducted by the Pharma Valley Center Clinical Research Promotion Project funded by Taiho Pharmaceutical Co., Ltd. and Yakult Co., Ltd.
CONFLICT OF INTEREST STATEMENT
The authors declare that no conflicts of interest exist regarding this study.




