Circulating microvesicles miR139-3p from bronchopulmonary dysplasia aggravates pulmonary vascular simplification by targeting 4E binding protein 1
Linchao Yu and Rui He contributed equally to this work.
Funding information: The work was supported by the National Natural Science Foundation of China (no. 82300111) and the Natural Science Foundation of Chongqing, China (no. CSTB2023NSCQ-BHX0070). The funding body played no role in the design of the study and collection, analysis and interpretation of data and in the writing of the manuscript.
Abstract
Background
Microvesicles (MVs) play a crucial role in bronchopulmonary dysplasia (BPD). There are many MVs in circulating plasma, and they are in direct contact with lung endothelial cells. However, the molecular mechanism and causative effect of circulating MVs on BPD remain unclear.
Methods
Clinical plasma samples were collected, circulating MVs were isolated, and microRNA (miRNA) sequencing was performed. The BPD model was established, and different MVs were administered. Alveoli and pulmonary vessels were examined by hematoxylin–eosin staining, and body weight and length were measured. In vitro, gene expression was disrupted by miRNA mimics, miRNA inhibitors or plasmid transfection. Cell proliferation and protein expression were detected by cell scratch assay, accurate 5-ethynyl-2-deoxyuridine test, western blotting, or immunofluorescence assay.
Results
BPD-derived MVs further aggravated pulmonary vascular simplification, while circulating MVs from control mice mitigated pulmonary vascular simplification. Micro-RNA sequencing and independent sample verification revealed that miR139-3p, but not miR6125 or miR193b-3p, was the most critical effector molecule in MVs. Mechanism studies showed that eukaryotic translation initiation factor 4E binding protein 1 was the target gene for miR139-3p. In addition, we found that supplementation of miR139-3p inhibitor partially alleviated pulmonary vascular simplification.
Conclusions
These results indicate that circulating MVs are involved in forming BPD by carrying miR139-3p molecules and support miR139-3p inhibitors as a potential therapeutic strategy for alleviating pulmonary vascular simplification in BPD.
1 INTRODUCTION
Bronchopulmonary dysplasia (BPD) is the most common chronic respiratory disease in premature infants,1 and pulmonary capillary simplification is the primary hallmark of BPD.2, 3 Epidemiologic studies have reported an estimated 48–68% incidence of “new” BPD in neonates born <32 weeks gestational age.4 Children with BPD are at an increased risk for respiratory problems,5 like lower respiratory tract infection6 and asthma,7 which undoubtedly have a profound impact on the quality of life and growth, increasing health resource utilization and household health costs.2 However, current therapeutic strategies for BPD remain limited.
Microvesicles (MVs), <1 μm in diameter, are small membrane vesicles formed by cell degranulation, which have great potential in preventing and treating BPD. Numerous studies have shown that intravenous administration of MVs from mesenchymal stem cells (MSCs) reduces alveolar simplification and improves exercise capacity.8-11 In addition to MVs derived from MSCs, adipocyte,12 amniotic epithelial13, 14 or amniotic fluid sources have also been found to have a potential therapeutic role in lung injury.14 As we know, circulating plasma also has many MVs and is in direct contact with pulmonary vessels. Circulating MVs play a crucial role in intercellular communication and the delivery of signaling molecules to proximal and distal cells. However, the regulatory relationship between circulating MVs and BPD remains unclear. Recently, Ali et al. reported that the number of vesicles in circulating plasma was significantly increased in a mouse model of BPD and that BPD murine-derived vesicles worsened alveolar simplification and endothelial cell proliferation,15 which partially explained the correlation between circulating MVs and pulmonary vessels. However, the specific mechanism by which MVs regulate alveolar simplification and endothelial cell proliferation remains unclear.
Microvesicles carry various biological molecules, such as microRNAs (miRNAs), proteins and DNAs, which regulate the biological function of target cells.16 Studies have reported that circulating vesicles inhibit endothelial cell angiogenesis through miR221-mediated repression of Angpl2.17 Eukaryotic translation initiation factor 4E binding protein 1 (4EBP1) is a translation inhibition protein family member. However, 4EBP1 could also promote the translation process of VEGF,18, 19 HIF1α and BCL-220 under hypoxia conditions. For example, Braunstein et al. reported that the hypoxia environment in cells promotes increased expression of 4EBP1 and regulates the translation of the VEGF gene, thus enabling angiogenesis.18 Inhibition of 4EBP1 down-regulates the expressions of HIF1α21 and VEGF,22 thus inhibiting the formation of blood vessels. However, the regulatory role of 4EBP1 in the pathological mechanism of BPD remains to be further explored.
In our study, we sequenced the miRNA molecules of circulating MVs and further verified the function of differential genes in vitro and in vivo. Here, we reveal that miR139-3p, rather than miR6125 or miR193b-3p, was the most differentially expressed molecule in circulating MVs in the BPD model, and miR139-3p could inhibit angiogenesis by targeting 4EBP1 molecule. In particular, supplementation of miR139-3p inhibitor partially alleviated the pulmonary vascular simplification in BPD. Therefore, the present study expands our understanding of circulating MVs in the vascular simplification of BPD.
2 RESULTS
2.1 Vesicle gene ontology terms are significantly enriched in BPD
To explore the functional changes of differential genes in BPD, we obtained datasets of human BPD samples from the Gene Expression Omnibus database. The primary screening strategies were “bronchopulmonary dysplasia[Title]” and “Homo sapiens,” and 33 datasets were retrieved. Given the interference effect of sample source, sample size and other confounding factors, only three datasets (GSE121097, GSE108754 and GSE32472) were included in this analysis (Figure S6). Samples were regrouped according to the severity of BPD, and samples with mild BPD or periventricular leukomalacia were excluded. Finally, 68 samples from the GSE121097 dataset (48 BPD samples and 20 no-BPD samples) and 10 samples from the GSE108754 dataset (five BPD samples and five no-BPD samples) were included. Sixty-three samples from different time points in the GSE32472 dataset (35 no-BPD samples and 28 BPD samples), 63 samples (39 no-BPD samples and 24 BPD samples) and 61 samples (37 no BPD samples and 24 BPD samples) were included and reanalyzed, respectively. Interestingly, Gene Ontology terms associated with vesicles were significantly enriched in different datasets of BPD (Figure S2A–C), suggesting that circulating vesicles played an essential role in the progression of BPD. However, we also found an enrichment of degranulation-related Gene Ontology terms (Figure S2C). Therefore, to exclude the effect of degranulation proteins on vesicle function, we collected plasma samples from children with clinical BPD according to the inclusion and exclusion criteria (Figure S1, Table 1, see Section 5 for details). Finally, 87 clinical samples were used for the enzyme-linked immunosorbent assay (ELISA). First, we examined neutrophil degranulocyte levels and found that only neutrophil elastase (NE) was significantly elevated in BPD plasma (Figures S1B,S3). In contrast, matrix metalloproteinase 9 (MMP9) and myeloperoxidase (MPO) did not change significantly (Figure S3). To further determine the distribution of NE, we examined the levels of NE in plasma apoptotic bodies (AB), extracellular vesicles (EV), exosomes (EXO) and supernatants, respectively, and the results showed that NE was primarily present in supernatants rather than vesicles (Figure S1C,D). These data further demonstrate the critical role of circulating membranes in BPD.
| Parameters | BPD (n = 62) | Non-BPD (n = 25) | p value |
|---|---|---|---|
| Maternal characteristics | |||
| Antenatal steroids | 58 | 22 | 0.3892 |
| Caesarean section | 35 | 11 | 0.2924 |
| Gestational diabetes | 42 | 13 | 0.1682 |
| Gestational preeclampsia | 19 | 5 | 0.3174 |
| PROM | 34 | 9 | 0.1117 |
| Infants’ characteristics | |||
| GA (week) | 28.41 + 5.4 | 30.1 + 3.5 | 0.1523 |
| BW (g) | 1337 ± 513.4 | 1542 ± 448.2 | 0.0846 |
| Gender (male) | 29 | 6 | 0.0500 |
| Apgar 1 min | 6.79 ± 1.83 | 7.1 ± 0.98 | 0.4259 |
| Apgar 5 min | 7.22 ± 1.86 | 7.55 ± 1.23 | 0.4165 |
| PDA | 48 | 15 | 0.1000 |
| IVH | 54 | 18 | 0.0916 |
| RDS | 46 | 16 | 0.3417 |
| ROP | 33 | 12 | 0.6589 |
| NEC | 4 | 0 | 0.1935 |
| LOS | 7 | 2 | 0.6484 |
| EOS | 5 | 1 | 0.4984 |
| PS treatment | 58 | 21 | 0.1631 |
| IMV (day) | 9.17 ± 3.41 | 2.57 ± 2.49 | 0.0001 |
| CPAP (day) | 11.04 ± 6.34 | 5.17 ± 4.83 | 0.0001 |
| Days with oxygen (day) | 59.40 ± 12.31 | 20.18 ± 9.98 | 0.0001 |
| Hospitalization days (day) | 67.72 ± 10.45 | 23.67 ± 8.47 | 0.0001 |
| Multiple pregnancy | 12 | 5 | 0.9452 |
- Note: p indicates statistical test efficacy.
- Abbreviations: BPD, bronchopulmonary dysplasia; BW, birth weight; CPAP, continuous positive airway pressure; EOS, early-onset sepsis; GA, gestational ages; IMV, infants mechanical ventilation; IVH, intraventricular hemorrhage; LOS, late-onset sepsis; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PROM, premature rupture of membranes; PS, pulmonary surfactant; RDS, respiratory distress syndrome; ROP, retinopathy.
2.2 Supplementation of BPD-MVs partially aggravates vascular simplification
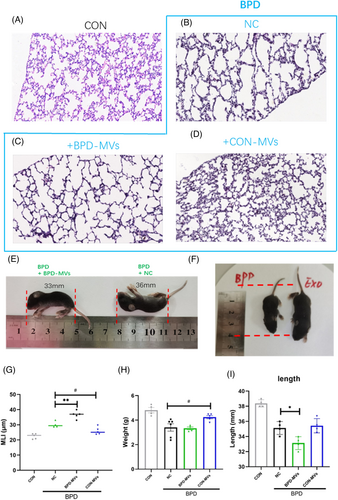
To investigate the function of MVs, circulating MVs from BPD mice (BPD-MVs) and circulating MVs from control mice (CON-MVs) were isolated and injected into the BPD mouse model separately. The results showed that BPD-MVs further aggravated the simplification of alveoli and pulmonary vessels, whereas CON-MVs partially alleviated the simplification of alveoli and pulmonary vessels (Figure 1A–D,G). In addition, body weight and length were measured in different groups of mice. The results showed that supplementation with BPD-MVs significantly reduced body length (Figure 1E) and body weight (Figure 1H) in BPD mice compared with the negative control (NC). In contrast, supplementation with CON-MVs significantly promoted body length (Figure 1F) and body weight (Figure 1H) in BPD mice, suggesting that BPD-MVs worsened lung structure and development. The above data indicate that supplementation of BPD-MVs exacerbates the severity of BPD.
2.3 MiR139-3p, miR6125 and miR193b-3p are the most significantly changed molecules in circulating MVs of BPD
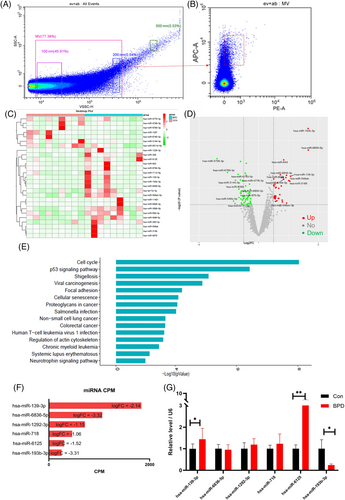
Studies showed that miRNA molecules are abundant in MV.23 To investigate the specific functional molecules of MVs, the circulating MVs were subjected to miRNA sequencing (Table 2 for detailed demographic information of included patients). After the isolation of MVs from circulating plasma by gradient centrifugation, the particle size of MVs and the CD9 marker were verified again by flow cytometry (Figure 2A,B). First, by performing second-generation sequencing of MVs, we identified 19 up-regulated miRNAs and 38 down-regulated miRNAs (Figure 2C,D). Subsequently, functional annotation of different miRNAs revealed that BPD-related pathways such as cell cycle, cellular senescence, foci adhesion and the P53 pathway were significantly enriched (Figure 2E). These data further suggest that MVs play an important role in the progression of BPD. Considering the functional characteristics of miRNAs, we screened the top 15 differential miRNAs based on the expression values (for the top six miRNAs, see Figures 2F,S4). Further, we validated these differential genes by reverse transcription polymerase chain reaction (RT-PCR) using independent samples of MVs. The PCR results agreed with the sequencing data; mir139-3p, miR6125, and miR193b-3p were the most significantly differentially expressed molecules (Figure 2G). These data suggest that miR139-3p, miR6125 and miR193b-3p are the most differentially expressed molecules of circulating MVs and play an essential role in regulating BPD by MVs.
| Parameters | BPD (n = 9) | Non-BPD (n = 9) | p-Value |
|---|---|---|---|
| Maternal characteristics | |||
| Antenatal steroids | 8 | 9 | 0.3035 |
| Caesarean section | 5 | 4 | 0.6374 |
| Gestational diabetes | 6 | 6 | 1 |
| Gestational preeclampsia | 3 | 2 | 0.5987 |
| PROM | 5 | 5 | 1 |
| Infants’ characteristics | |||
| GA (week) | 28.12 + 4.2 | 30.3 + 3.1 | 0.2283 |
| BW (g) | 1317 ± 451.7 | 1507 ± 401.3 | 0.3595 |
| Gender (male) | 4 | 4 | 1 |
| Apgar 1 min | 6.53 ± 1.42 | 7.2 ± 0.87 | 0.2450 |
| Apgar 5 min | 7.13 ± 1.56 | 7.74 ± 1.81 | 0.4549 |
| PDA | 7 | 6 | 0.5987 |
| IVH | 8 | 7 | 0.5271 |
| RDS | 7 | 7 | 1 |
| ROP | 5 | 4 | 0.6374 |
| NEC | 0 | 0 | 1 |
| LOS | 0 | 0 | 1 |
| EOS | 0 | 0 | 1 |
| PS treatment | 8 | 7 | 0.5271 |
| IMV (day) | 9.76 ± 4.15 | 2.44 ± 2.09 | 0.0002 |
| CPAP (day) | 12.37 ± 5.71 | 5.01 ± 4.34 | 0.00072 |
| Days with oxygen (day) | 60.87 ± 15.71 | 19.74 ± 9.12 | 0.0001 |
| Hospitalization days (day) | 68.73 ± 9.35 | 21.21 ± 7.41 | 0.0001 |
| Multiple pregnancy | 0 | 0 | 1 |
- Note: p indicates statistical test efficacy.
- Abbreviations: BPD, bronchopulmonary dysplasia; BW, birth weight; CPAP, continuous positive airway pressure; EOS, early-onset sepsis; GA, gestational ages; IMV, infants mechanical ventilation; IVH, intraventricular hemorrhage; LOS, late-onset sepsis; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PROM, premature rupture of membranes; PS, pulmonary surfactant; RDS, respiratory distress syndrome; ROP, retinopathy.
2.4 miR139-3p, rather than miR6125 or miR193b-3p, inhibits endothelial cell proliferation
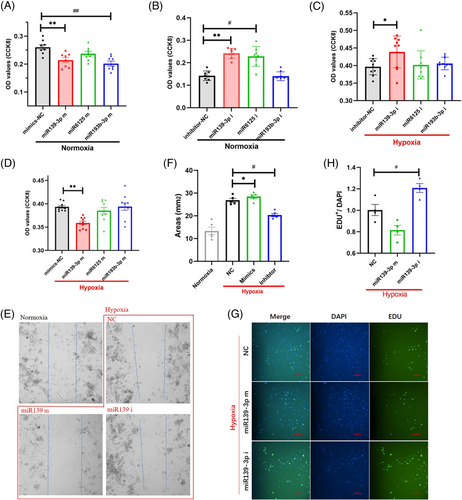
To clarify the role of miRNA molecules in endothelial cell proliferation, we examined the effects of miRNA mimics and inhibitors on cell proliferation under normoxic and hypoxic conditions, respectively. First, under normoxic conditions, we detected the proliferative ability of cells after miR139-3p, miR6125 and miR193b-3p interventions using the cell counting kit-8 (CCK8) assay, respectively. The results showed that miRNA inhibitors specifically knock down the target miRNA (Figure S5), and miR139-3p and miR193b-3p significantly inhibit the metabolism of CCK8 (Figure 3A), suggesting that cell proliferation was significantly inhibited. Then, we intervened in the cells with miR139-3p, miR6125 and miR193b-3p inhibitors and found that miR139-3p and miR6125 inhibitors significantly increased the metabolism of CCK8 (Figure 3B), suggesting that cell proliferation was promoted. Subsequently, cells were treated with miR139-3p, miR6125 and miR193b-3p mimics and inhibitors under simulated hypoxic conditions. We found that only miR139-3p mimics inhibited cell proliferation (Figure 3C), while miR139-3p inhibitors promoted cell proliferation (Figure 3D). However, miR6125 or miR193b-3p mimics and inhibitors had no significant effect on endothelial cell proliferation (Figure 3C,D). These data suggest that miR139-3p is most likely an effector of MV. To further verify the effect of miR139-3p on endothelial cell proliferation, after intervention of endothelial cells with miR139-3p mimics and inhibitors, the wound healing assay (Figure 3E) and 5-ethynyl-2-deoxyuridine (EDU) test (Figure 3G) were performed. The results were consistent with the above experimental data: miR139-3p mimics significantly inhibited the growth of healing cells and the DNA transcription process in the nucleus (Figure 3F). However, miR139-3p mimics promoted the proliferation and transcription process of guaiac cells (Figure 3H). The above data prove that miR139-3p, but neither miR6125 nor miR193b-3p, inhibits endothelial cell proliferation.
2.5 Hyperoxia down-regulates 4EBP1 expression
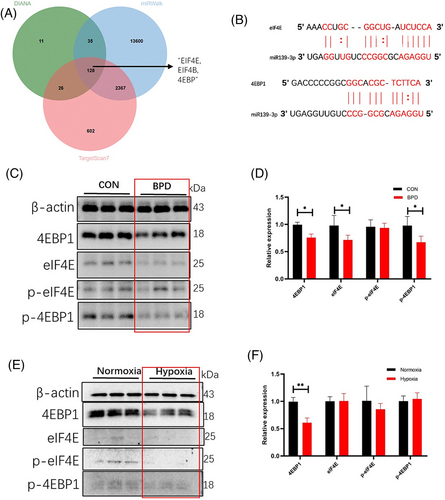
To identify the target genes of miR139-3p, potential target genes of miR139-3p were predicted using the target gene prediction database. The results showed that EIF4E, EIF4B and 4EBP were predicted in three different databases (Figure 4A, Table 3), and there was an obvious binding site between miR139-3p and EIF4E/4EBP1 (Figure 4B). Therefore, we hypothesized that EIF4E/4EBP1 might be a functional target gene of miR139-3p. To clarify the relationship between EIF4E/4EBP1 and BPD, we first examined the expression of EIF4E/4EBP1 and its phosphorylated proteins in the BPD model. We found that the expression of 4EBP1 and p-4EBP1 was significantly decreased in the BPD group compared with the CON group (Figure 4C), whereas the changes in EIF4E and p-EIF4E were not significant (Figure 4D). Subsequently, the expressions of EIF4E/4EBP1 and its phosphorylated proteins were examined again using the hypoxia cell model, and it was found that hypoxia only down-regulated the expression of 4EBP1 protein (Figure 4E) but did not affect the expression of p-4EBP1, EIF4E and p-EIF4E (Figure 4F). Overall, the above data suggest that EIF4E/4EBP1 is most likely the regulatory target of miR139-3p.
| UBR4 | BLOC1S6 | BACE1 | MPLKIP | KLHL26 | PAK3 |
| SYT2 | SP4 | RHOH | RHOG | DCX | VGLL2 |
| ELK1 | PLD5 | EIF4EBP1 | PPP2R1B | RELT | LRRTM2 |
| FAM161A | WDFY3 | PRDM9 | GRIN2B | C1orf198 | INO80D |
| EIF4E | GOLGA7 | LEAP2 | MED21 | LMTK3 | GJB1 |
| ANXA2R | ARHGDIB | APOL6 | RNF187 | RPS15A | ERVV-2 |
| GNB5 | HNRNPC | CALN1 | ZBTB37 | COL4A1 | NACC1 |
| SRSF3 | EIF4B | HK1 | ANTXR2 | CNDP1 | BRD4 |
| AGPAT3 | BAZ2B | METTL2A | SYNE3 | KCNJ6 | C2CD4A |
| RAD54B | AGMAT | ZNF624 | ERLIN2 | ST8SIA6 | MUC4 |
| TTLL6 | MDGA1 | RAB5A | CSAG1 | LDLRAP1 | SULT4A1 |
| PLP1 | DNAH3 | AMER1 | FSD2 | SLC44A4 | C21orf91 |
| RBMXL2 | PTPRT | ZZEF1 | MTRR | MMP16 | TNPO1 |
| PDE4A | FAM126A | TSPAN12 | GGA2 | ABHD2 | GAN |
| ZBTB10 | SERPINE1 | FC DXD2 | LTBP4 | SNX20 | CYSLTR2 |
| NUTM2E | ZNF879 | GAPVD1 | TMEM98 | PTPN4 | MIEF1 |
| RGS17 | STAG2 | ZNF585B | LRRC32 | BTF3L4 | IL10 |
| SERF1A | NCR3LG1 | GOLGA6L6 | KIF18B | ISG20L2 | POU2F2 |
| UVRAG | TMEM221 | SDCBP | TSHZ2 | CIT | |
| ERVV-1 | DCAF12L1 | VAPB | ITPK1 | C1D | |
| ZBTB9 | OLFML2A | IPO9 | KIAA1958 | TNR | |
| CCDC71L | GSTO2 | AGPAT1 | SRRM4 | PRRG3 |
- Note: 128 common elements in “List 1,” “List 2,” and “List 3.”
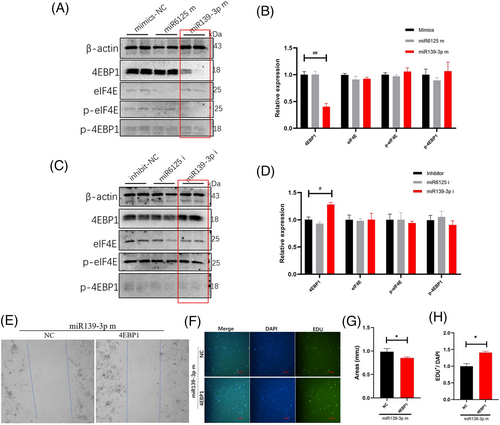
2.6 MiR139-3p inhibits endothelial cell proliferation by inhibiting 4EBP1
To clarify the target genes of miR139-3p, we examined the expression of EIF4E/4EBP1 and phosphorylated proteins after the intervention of endothelial cells with miR139-3p mimics and inhibitors. We found that miR139-3p mimics significantly inhibited the expression of 4EBP1 protein (Figure 5A) but did not affect the expression of p-4EBP1, EIF4E and p-EIF4E (Figure 5B). Moreover, we found that miR139-3p inhibitor up-regulated only 4EBP1 protein expression (Figure 5C), but not p-4EBP1, EIF4E and p-EIF4E expression (Figure 5D). These data suggested that miR139-3p regulated endothelial cell proliferation via the 4EBP1 gene. To further verify the role of 4EBP1 in miR139-3p regulation of endothelial cell proliferation, we analyzed the effect of 4EBP1 on endothelial cells using rescue experiments. After miR139-3p mimic intervention, overexpression of 4EBP1 significantly promoted endothelial cell proliferation (Figure 5E) and gene transcription (Figure 5F) compared with the NC (Figure 5G,H), suggesting that overexpression of 4EBP1 partially reversed the inhibitory effect of miR139-3p on endothelial cell proliferation. These data indicate that 4EBP1 is a major target gene of miR139-3p in regulating endothelial cell proliferation.
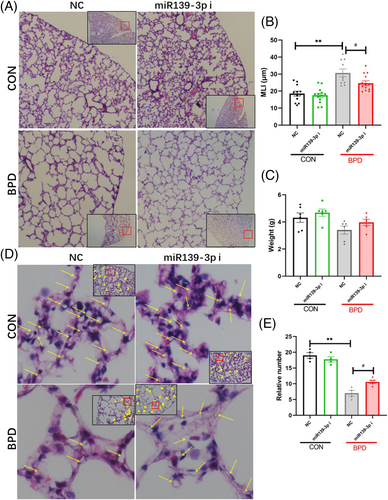
2.7 Supplementation of miR139-3p inhibitor partially alleviates pulmonary vascular simplification
MiR139-3p inhibits the proliferation of vascular endothelial cells by suppressing the 4EBP1 gene. However, it is unclear whether miR139-3p inhibitors can attenuate the simplification of the pulmonary vasculature in BPD patients. To further clarify the improvement effect of miR139-3p inhibitor on BPD, miR139-3p inhibitors were injected into mice through a caudal vein. The results showed that the miR139-3p inhibitor significantly reduced the alveolar simplification of BPD (Figure 6A) but did not significantly change the lung structure in the CON group (Figure 6B). Subsequently, we evaluated changes in pulmonary microvessels and found that the miR139-3p inhibitor significantly increased the number of pulmonary microvessels (Figure 6C,D). These data indicate that miR139-3p inhibitor significantly improves BPD's alveolar and pulmonary vascular simplification.
3 DISCUSSION
There are a large number of vesicles secreted by different cells into the circulating plasma that are closely associated with BPD. However, the regulatory relationship and mechanisms between circulating vesicles and BPD progression remain unclear. In this study, we found that BPD-derived vesicles (BPD-MVs) further exacerbated the simplification of pulmonary vasculature, whereas CON-MVs mitigated pulmonary vascular simplification. Mechanistically, miR139-3p was the major effector molecule, which inhibited vascular proliferation and formation through the miR139-4EBP1 axis. Furthermore, miR139-3p inhibitors significantly improved pulmonary vascular and alveolar simplification in BPD patients. This study provides new possibilities to explore the pathological mechanisms and therapeutic targets of BPD.
Many studies have found that mesenchymal stromal cells can improve BPD mainly through transferring vesicle particles, suggesting that vesicles are crucial in MSC therapeutic efficacy in BPD models.9, 24, 25 Many tissues and organs are capable of secreting vesicles into the circulating plasma. Although the relationship between circulating MVs and BPD has not been extensively studied, it is undeniable that many MVs are present in circulating plasma and are closely associated with BPD formation, diagnosis and prognosis. Ali et al.15 reported, using observational experiments, significant changes in MVs in the plasma of BPD mice as compared with that of CON mice and found that the presence of vesicles from BPD mice worsened alveolar simplification. Consistent with the reported results, in this study, using bioinformatics methods, we found that differential genes in children with BPD were mainly enriched around vesicles-related terms, and there were significant vesiculation phenomena with BPD. In addition, we found that circulating vesicles in control children mitigated the simplification and length development of alveoli in BPD mice. This provides us with a basis for exploring the pathomechanism of BPD from the perspective of circulating MVs.
The function of miR139-3p molecules in many diseases has been extensively studied. Studies have shown that miR139-3p inhibits the proliferation of bone marrow progenitor cells26 and tumor stem cells27-29 but promotes the apoptosis of chondrocytes.30 However, the function of miR139-3p in regulating BPD endothelial cells remains unclear. First, to clarify the specific molecules of BPD-MVs, we directly detected the changes in degranulation proteins, which showed that degranulation proteins were predominantly present in the supernatant rather than the vesicles. Therefore, we hypothesized that miRNA molecules play a vital role in regulating BPD. Then, using second-generation sequencing combined with PCR validation, we focused on miR139-3p, miR6125, and miR193b-3p as potential regulatory molecules. In children with BPD, persistent hypoxemia is the predominant clinical manifestation, suggesting that endothelial cells in vivo are in a hypoxic microenvironment, which can be attributed to lung dysfunction. Therefore, the endothelial hypoxia model was used to simulate the microenvironment of BPD. Subsequently, using miRNA mimics and inhibitors, we further confirmed that miR139-3p, rather than miR6125 or miR193b-3p, was involved in the proliferation function of endothelial cells under normoxic and hypoxic conditions. Interestingly, we also observed that miR6125 inhibitors and miR193b-3p mimics regulated endothelial cell proliferation under normoxic conditions, but these regulatory effects were absent under hypoxic conditions. This may be because the activity of some miRNA molecules is changed owing to the stress state of hypoxia.
Mechanistically, miR139-3p can inhibit the translation of target mRNAs through complementary binding to target genes, thus exerting regulatory effects on target mRNAs. As a member of the translational repressor family, 4EBP1 promotes the translation of specific proteins under hypoxic stress conditions. Under stress conditions, 4EBP1 is hypophosphorylated and biologically active, and the active 4EBP1 binds to mRNAs containing internal ribosome binding sites, thereby promoting the translation of such mRNA genes.18 This altered mode of regulation ensures that specific genes are translated correctly under hypoxic, infectious or apoptotic conditions.31 Studies have found that 70% of 4EBP1 is located in the cytoplasm, which may be related to this mode of translation regulation, such as translation of VEGF-A, HIF1α, BCL-220 and other genes. For example, the hypoxic environment in breast cancer cells promotes increased expression of 4EBP1 and translation of vascular endothelial growth factor genes, which promotes tumor angiogenesis and tumor growth.18 Many studies have reported that MMP11,29 CXCR4,32 MCPIP130 and EIF4G233 are the regulatory target genes of miR139-3p in different diseases. However, the target genes of miR139-3p in hypoxic endothelial cells remain unclear. In this study, we found that eIF4E/4EBP1 is a potential regulatory target gene of miR139-3p, and down-regulation of 4EBP1 was observed in both animal and cellular models. In contrast, miR139-3p inhibitors significantly up-regulated 4EBP1 protein expression. These data suggest that 4EBP1 is a regulatory target gene of miR139-3p. Subsequent rescue experiments showed that overexpression of 4EBP1 partially reversed the inhibitory effect of miR139-3p. These results further confirmed that 4EBP1 is a regulatory target gene of miR139-3p. Indeed, in addition to the changes in 4EBP1 expression, a decrease in p-4EBP1 expression and a slight decrease in eIF4E protein expression were also observed in animal models, suggesting that multiple complex mechanisms regulate BPD.
However, in this study, we did not use miR139-3p knockout mice to validate our results further, considering the complexity and confounding factors of miR139-3p knockout. Fortunately, we found that the use of miR139-3p inhibitor could reduce the expression level of miR139-3p by 70–90% (Figure S5), which could mimic the effect of miR139-3p knockdown and thus partially reflect the conclusion of this study. Of course, this experiment will be conducted in the follow-up study.
Overall, in this study, we found that circulating MVs derived from BPD significantly worsened pulmonary vasculature simplification, and their main effect was related to the miR139-3p molecule carried by MVs. In addition, we explored the ameliorative impact of miR139-3p inhibitor supplementation in BPD. We found that miR139-3p inhibitors partially improved alveolar simplification and pulmonary vascular simplification in BPD.
4 CONCLUSIONS
Our study indicates that circulating particles are responsible for BPD formation and identifies miR139-3p as a significant molecule responsible for pulmonary vascular simplification in BPD by targeting the miR139-4EBP axis. Furthermore, supplementation with miR139-3p inhibitors partially alleviated pulmonary vascular simplification, providing evidence for miR139-3p inhibitors as a potential therapeutic strategy for BPD.
5 MATERIALS AND METHODS
5.1 Participants
Participants (n = 113) were recruited from the neonatal department of the Children's Hospital of Chongqing Medical University (NICU level 4). Participants who provided informed consent from their parents were enrolled in the study. The detailed steps are presented in Figure S1. The diagnostic criteria of BPD were as follows: (1) gestational age <32 weeks; (2) treatment with oxygen >21% for at least 28 days; and (3) oxygen concentration ≥30% required at corrected gestational age to 36 weeks or at the time of discharge (severe BPD). The exclusion criteria were as follows: (1) gestational age ≥32 weeks; or (2) diagnosed with BPD but has other medical conditions, such as necrotizing enterocolitis, septicemia, pneumorrhagia or anoplasty; and (3) chromosome abnormality or malformation. bronchopulmonary dysplasia was diagnosed, and then venous blood was collected. Specimens were centrifuged, and the supernatant was stored immediately at −80°C. Demographic information for the included participants was collected from their medical history. All procedures were approved by the Ethics Committee of Chongqing Medical University and were performed according to ethical standards.
5.2 Animals
Eight-week-old C57BL/6J mice (20 g ± 5 g) were purchased from Chongqing Medical University (Chongqing, China). All mice were housed under pathogen-free conditions and had free access to water and food. All experimental procedures were approved by the Ethics Committee of Chongqing Medical University.
5.3 Establishment of the BPD model
The female mice were crossed with the male mouse. Given the higher incidence of BPD in boys, only male pups were used in this study (n = 22). Neonatal single male mice were divided randomly into four groups within 12 h of birth: the normoxia group (CON, n = 4); the hyperoxia group (BPD, n = 6); hyperoxia pups with BPD-MVs administration (BPD + BPD-MVs, n = 6); and hyperoxia pups with CON-MVs administration (BPD + CON-MVs, n = 6). The BPD model was established according to Aslam et al.34 with modifications. Briefly, newborn pups were exposed to 75% oxygen or room air in an enclosed chamber from birth to post-day 7. Ventilation was adjusted by a flow valve to remove CO2. Dams were exchanged between the hyperoxia and normoxia groups every other day to prevent oxygen toxicity in the adult animals. To ensure the nutrition and growth of pups, each litter consisted of fewer than eight pups. After post-day 7, the hyperoxia pups were returned to room air until the end of the experiment. Microvesicles were collected from the serum of BPD animals and injected into the caudal vein (total nucleic acid of 2 μg/mouse) after anesthesia (Phenobarbital sodium, 50 mg/kg). Normoxia mice were used as a reference, NC, and hyperoxia mice were used as a positive control. No animal was excluded.
5.4 Analysis of public RNA-seq data
The sequencing datasets of BPD infants (accession numbers GSE32472 and GSE121097) were obtained from the Gene Expression Omnibus database. The microarray data were analyzed using the GEO2R online platform (GEO2R, www.ncbi.nlm.nih.gov/geo/; versions: R, 3.2.3; Biobase, 2.30.0; GEOquery, 2.40.0; limma, 3.26.8). Functional annotations of differentially expressed genes were executed using clusterProfiler on the R platform.
5.5 ELISA
Venous blood was centrifuged and the supernatant was saved to detect the levels of NE, MMP9 and MPO using a human ELISA kit (Elabscience, E-EL-H1946c; QuantiCyto®, EHC115; Solarbio, SEKH-0262), according to the manufacturer's manuals. All samples were set up in triplicate. Briefly, the supernatant was diluted 20 times with normal saline. Samples were added to the plate and incubated for 90 min at 37°C, then biotinylated detection antibody, horseradish peroxidase conjugate working solution, substrate reagent and stop solution were added to each well. The optical density was detected using a spectrophotometer (450 nm, SpectraMax; Thermo). Each well was tested in duplicate, and the mean was used for statistics. No data point was excluded.
5.6 Vesicle isolation from plasma
Venous blood was centrifuged at 3000g for 10 min (Eppendorf, centrifuge 5810), and then the plasma was obtained to detect the concentration of degranulated proteins. Apoptotic bodies, EV and EXO were obtained using a differential centrifugation protocol with modification.35 Briefly, plasma was centrifuged at 8000g for 10 min (Thermo ST16R) to precipitate AB. The upper liquid was centrifuged at 20,000g for 60 min (Thermo ST16R) to precipitate EV. Finally, the EV-depleted supernatant was centrifuged at 140,000g for 120 min (Beckman Coulter, Allegra X-22 centrifuge) to precipitate EXO, and the EXO-depleted supernatant was saved. The protein levels in AB, EV, EXO and supernatant were detected in subsequent experiments using ELISA.
5.7 Microvesicle characterization and microRNA sequencing
Microvesicles were isolated from plasma using an exosome isolation kit (EIQ3-02001, Wayen, China) and characterized by nanoparticle tracking analysis and fluorescence-activated cell sorting. The sizes of MVs were determined by Zetasizer Nano ZSP (Malvern Instruments) and fluorescence-activated cell sorting (BD) standard nanoparticles (Sigma) and were further confirmed by labeling with a CD9 fluorescent antibody. Total RNA was isolated from 200 μL of plasma with the miRNeasy Plasma Advanced kit (Qiagen, Germany) following the manufacturer's instructions. Small RNA libraries were constructed using the QIAseq miRNA library kit from Illumina. Small RNAs were sequenced in NovaSeq 6000 and estimated to be over 50 million reads per sample. Raw data were analyzed using standardized steps. Briefly, reads were quality-checked with FastQC (version 0.11.3), and adapter sequences were trimmed with Cutadapt (version 2.7) and perl5 (version 26). Reads were then processed with bowtie (version 1.2.2) and miRDeep2 (version 0.0.7) to identify and quantify known miRNAs from the reference human genome (GRCh38).
5.8 HULEC-5a cultures
Human lung microvascular endothelial cells (HULEC-5a) were purchased from ATCC. HULEC-5a were cultured according to the operation manual. Briefly, cells were resuspended and incubated with the recommended complete medium (MCDB131 with 10 ng/mL EGF, 1 μg/mL hydrocortisone, 10 mm l-glutamine, and 10% fetal bovine serum) in an incubator with 5% CO2 at 37°C.
5.9 miRNA and plasmid transfection
Endothelial cells were seeded into six-well plates and cultured for 24 h. When the cells reached 70% coverage, miRNA mimics or inhibitors (Sangon biotech, China) were transfected into cells with Lipofectamine 3000 (Thermo Fisher, USA) according to the manufacturer's instructions. After incubating at 37°C for 48 h, mRNA or protein was extracted to detect changes. The 4EBP1 plasmid was transfected with miRNA mimics into HULEC-5a for the rescue experiment. The cells were harvested 48 h after transfection.
5.10 Wound healing, 5-ethynyl-2-deoxyuridine (EDU) and Cell counting kit-8 (CCK8) test
Wound healing, EDU and CCK8 tests were performed according to the manufacturer's instructions.36 HULEC-5a were seeded in six-well plates, and divided randomly into groups with miRNA or/and plasmid transfection. Cells were washed twice with normal saline and incubated with a complete growth medium. When HULEC-5a reached 90–100% confluence in plate wells, cells were subjected to serum-free medium for 24 h. After serum starvation, wound healing was scratched with a pipette tip. The wound healing was captured after 24 h. HULEC-5a were seeded in 24-well plates and transfected with miRNA or/and plasmid. For the EDU test, EDU reagent was added into the medium to detect the replication status of DNA, according to operating instructions, and captured after 24 h. To detect cell proliferation, a CCK8 assay was performed. Cells were seeded in 96-well plates. After miRNA or/and plasmid transfection for 48 h, CCK8 reagent was added to the medium and captured after 2 h. All samples were set up in triplicate. Negative control cells were used as a reference. No data point was excluded.
5.11 Western blotting
The protein level was detected as described previously. Briefly, animals were anesthetized with phenobarbital (50 mg/kg), and lung tissue specimens were collected. Proteins were extracted with radio immunoprecipitation assay Lysis buffer (RIPA) with 1 mm phenylmethylsulfonyl fluoride (PMSF) (Beyotime, China). Homogenates were centrifuged at 12,000g for 5 min, and subsequently, the protein concentrations of the supernatant were determined using a BCA protein assay kit (Beyotime, China). The proteins were separated by electrophoresis (Bio-Rad, CA, USA) and transferred onto 0.45 μm polyvinylidene difluoride membranes. Then, the membranes were blocked in 5% milk buffer for 1 h. After blocking, membranes were incubated with primary antibodies overnight at 4°C and secondary antibodies for 2 h at room temperature. The primary antibodies used for blotting were as follows: eIF4E (R24193, ZENBIO), Phospho-eIF4E (R24192, ZENBIO), 4EBP1 (306002, ZENBIO) and phospho-4EBP1 (R22929, ZENBIO). Finally, immunoreactive bands were detected using an ECL kit (Millipore, USA) and an ECL Imaging System (Syngene G: BOX, UK). Protein levels were normalized to β-actin using Image J analysis software. Normoxia mice or NC cells were used as a reference NC, and hyperoxia mice were used as a positive control.
5.12 Statistical analysis
DW was aware of the group allocation at the different stages of the experiment; RH conducted experiments, and LY analyzed data independently (blinding). In the BPD model, only male mice were analyzed in this study. All data are displayed as means ± SEM and statistically analyzed using GraphPad Prism (v. 8.3.0). An unpaired t-test was used to analyze the data from both groups. One-way ANOVA followed by Dunnett's post hoc tests was used to analyze the data for the three groups. The Shapiro–Wilk test was used for the normality test and Levene's test for the homogeneity test of variance. A value of p < 0.05 was considered significant.
AUTHOR CONTRIBUTIONS
Rui He conducted experiments and Linchao Yu analyzed data and wrote the manuscript. Yuan Shi and Daoxin Wang initiated the project. Linchao Yu and Chan Liu were involved in the human sample collection and analyzed the data. All authors reviewed the manuscript.
ACKNOWLEDGEMENTS
All authors acknowledge Professor Chen for facilitating the initiation of the project. We thank RH and CL for communicating with participants and collecting data.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest in this work.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.




