Genome wide analysis of dexamethasone stimulated mineralization in human dental pulp cells by RNA sequencing
Funding information: National Key Research and Development Program of China, Grant/Award Number: 2018YFE0202200; National Natural Science Foundation in China, Grant/Award Numbers: 81670962, 81271110
Abstract
Human dental pulp cells (hDPCs) contain mesenchymal stem cells and are therefore indispensible for reparative dentin formation. Here, we present a pilot study of transcriptomic profiles of mineralized hDPCs isolated from sound human maxillary third molars. We observed altered gene expression of hDPCs between control (dexamethasone free) and experimental (dexamethasone 1 nm) groups. Differential expression analysis revealed up-regulation of several inflammation and mineralization-related genes in the experimental group. After a Gene Ontology analysis for predicting genes involved in biological process, cellular component and molecular function, we found enrichment of genes related to protein binding. Based on the results of Kyoto Encylopedia of Genes and Genomes pathway analysis, it is suggested up-regulated genes in mineralized hDPCs were mostly enriched in the mitogen-activated protein kinase (MAPK) signaling pathway, fluid shear stress and the atherosclerosis signaling pathway, etc. Importantly, Gene Set Enrichment Analysis revealed dexamethasone was positively related to the Janus kinase/signal transducer and activator of transcription, MAPK and Notch signaling pathway. Moreover, it was suggested that dexamethasone regulates signaling pathway in pluripotency of stem cells. Collectively, our work highlights transcriptome level gene regulation and intercellular interactions in mineralized hDPCs. The database produced in the present study paves the way for further investigations looking to explore genes that are involved in dental pulp cells mineralization.
1 INTRODUCTION
Dental caries is an infectious disease caused by acid-producing bacteria in the oral cavity.1 In moderate caries, the microbial secretions elicit host protective responses by diffusing through the dentinal tubules, which results in the generation of reparative dentin.2 With the restoration of decayed teeth using composite resin, tissue homeostasis could be achieved. However, in the case of deep caries, cell death is rampant and the repair process starts from surviving pulp cells instead of surviving odontoblasts.3 Those cells differentiate into odontoblast-like cells that secrete reparative dentin. At this stage, when inflammation outbalances healing, the disease could manifest as irreversible pulpitis and, in some severe cases, as pulp necrosis.4
Root canal therapy has been shown to be an effective approach for maintaining the tooth structure. However, its major drawback is the loss of tooth vitality. Recently, vital pulp therapy (VPT) has gained much attention.5 VPT is a minimal invasive approach for the preservation of tooth vitality. Unfortunately, its indication is still limited to reversable pulpitis in immature permanent teeth.6 If VPT could be used in the treatment of irreversible pulpitis in either immature or mature permanent teeth, more teeth will thus be saved and the need for expensive treatments such as dental implants will consequently be sharply reduced. Toward this end, understanding the factors at a molecular level that control the dental pulp cell response to mineralization would be a critical step because progress in this field will no doubt facilitate the development of novel materials with improved efficacy.
Dexamethasone is a synthetic glucocorticoid, which was first manufactured in 1957 and was not approved for medical use until 1961.7 Dexamethasone is an anti-inflammatory and immune suppressive medication used to treat rheumatic problems, skin diseases, severe allergies, asthma and chronic obstructive lung disease. Preceding studies led to contradictory findings regarding the effects of dexamethasone in osteogenic differentiation. Some claimed dexamethasone suppresses osteoblast differentiation,8 whereas others reported the opposite.9 Indeed, dexamethasone has often been used in the osteogenic media to induce human dental pulp cells (hDPC) differentiation into hard tissue forming cells (osteoblasts/odontoblasts). Nevertheless, the concentration of dexamethasone used in those studies varies and no consensus has been achieved. In light of this, the first purpose of the present study was to specify an ideal concentration of dexamethasone in stimulating mineralization of hDPCs; after that, we endeavored to clarify some of the genes and signaling pathways that were affected by dexamethasone in the process of hDPC mineralization using RNA sequencing (RNA-seq) analysis.
To the best of our knowledge, this is the first study to perform RNA-seq analysis during long term culture of hDPCs stimulated by low dose dexamethasone. We hypothesized that the up- or down-regulated genes revealed by RNA-seq may impact osteogenic differentiation and mineralization in hDPCs.
2 MATERIALS AND METHODS
2.1 Cell culture
Permanent upper third molars were collected from patients between 20 and 35 years of age receiving dental extraction at the Oral and Maxillofacial Surgery Department, Stomatology Hospital, Tongji University. The teeth extracted from patients were complete, healthy and without any visible damage. Decayed teeth or those derived from patients with severe periodontitis were excluded from this study. Isolation of hDPCs was approved by the Ethics Committee in School of Stomatology, Tongji University. All patients who donated teeth to harvest the hDPCs gave their consent for the use of teeth in the present study. After extraction, teeth were immersed in 70% ethanol solution for around 20 min to avoid contamination of pulp tissue. Afterwards, teeth wrapped in sterile gauze were heavily punched open using a hammer, and pulp tissues were isolated and transferred to sterile phosphate-buffered saline (PBS) solution containing 1% antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin, 250 ng/ml amphotericin B; Genom Technology, Zhejiang, China), washed for three times. Finally, the pulp tissues were cut into small pieces and transferred into a sterile centrifuge tube (15 ml; NEST, Jiangsu, China). hDPCs were released from pulp tissues by type I collagenase (3 mg/ml; Sigma, St Louis, MO, USA) digestion for 3 h at 37°C with constant shaking. Cell suspensions were centrifuged after every 1 h of incubation and inoculated into a six-well tissue culture plate. Cells were grown in the maintenance media (MM) constituting α-minimal essential medium (MEM) (SH30265.01; Cytiva, Marlborough, MA, USA), 10% fetal bovine serum (FND500, Australia origin; ExCell Bio, Shanghai, China) and antibiotics (1%; Genom Technology), in a humidified atmosphere with 5% CO2 at 37°C. After 24 or 48 h, media were refreshed to remove unattached cells. Sub-cultures were performed when the cells reached 70–80% confluence until passage five.
2.2 Cell Counting Kit (CCK)-8 assay
Cell viability was determined via a CCK-8 assay (catalog. no. 40203ES80; Yeason, Shanghai, China). Briefly, hDPCs were inoculated into 96-well plates (3 × 103 per well) and cultured in MM for 3 days. On day 3, cells were starved by addition of serum free α-MEM (1% antibiotics) for another 12 h. After that, dexamethasone was added into hDPCs (four types of concentration: 0, 1 , 10 and 100 nm represented as D0, D1, D10 and D100, respectively). After cultivation for 24 h, CCK-8 reagents were added into each well and incubated for 2 h. Optical density was measured using a plate reader (SYNERGY H1; BioTek, Winooski, VT, USA) at a wavelength of 450 nm.
2.3 Osteogenic induction
hDPCs were inoculated into six-well plates (10 cm2 per well) at a density of 9.4 × 103 cm−2 and cultured in MM for 3 or 4 days until cells reached confluence; after that, MM was changed into osteogenic media (OM) containing β-glycerophosphate (10 mm, G9422; Sigma), ascorbic acid (50 μg/ml, A4544; Sigma), dexamethasone (1 , 10 and 100 nm, D2915; Sigma). OM was refreshed every 3 days until day 20 or day 22.
2.4 Alizarin Red S (ARS) staining
hDPCs were washed using PBS, fixed with 10% neutral buffered formalin (KA1418, 500 ml; Kingmorn, Shanghai, China) for 20 min, and stained with ARS solution (1%, pH 4.0, E126BA0007; Sangon Biotech, Shanghai, China) for 5 min. After that, the staining solution was aspirated and the cell monolayers were washed using distilled water until the supernatant became transparent (usually requiring 2 h). Quantitative analysis of ARS stained photographs was conducted using ImageJ (NIH, Bethesda, MD, USA).
2.5 Transcriptome analysis
2.5.1 RNA extraction and library construction
hDPCs were seeded into a six-well plate at the same density as described in osteogenic induction section. After reaching confluence on day 2, the cells were divided into two groups with each group containing three replicates. The control group represented cells cultured in OM without dexamethasone, the experimental group represented cells culture in OM containing dexamethasone (1 nm). After a total of 20 days in culture, RNA was collected from both groups using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer's instructions. In a quality control step, the RNA amount and the purity of each sample were quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA). The RNA integrity was assessed using Bioanalyzer 2,100 (Agilent, Santa Clara, CA, USA) with RIN number > 7.0, and confirmed by electrophoresis with denaturing agarose gel. Poly(A) RNA was purified from one microgram total RNA using Dynabeads Oligo (dT)250-61005 (Thermo Fisher, Waltham, MA, USA) using two rounds of purification. Then, the poly(A) RNA was fragmented into small pieces using a Magnesium RNA Fragmentation Module (catalog. no. e6150; New England Biolabs, Ipswich, MA, USA) under 94°C for 5–7 min. Then, the cleaved RNA fragments were reverse-transcribed to create the cDNA by SuperScript™ II Reverse Transcriptase (catalog. no. 1896649; Invitrogen), which were next used to synthesize U-labeled second-stranded DNAs with Escherichia coli DNA polymerase I (catalog. no. m0209; New England Biolabs), RNase H (catalog. no. m0297; New England Biolabs) and dUTP solution (catalog. no. R0133; Thermo Fisher). An A-base was then added to the blunt ends of each strand, preparing them for ligation to the indexed adapters. Each adapter contains a T-base overhang for ligating the adapter to the A-tailed fragmented DNA. Single- or dual-index adapters were ligated to the fragments, and size selection was performed with AMPureXP (Beckman Coulter, IN, USA) beads. After the heat-labile UDG enzyme (catalog. no. m0280; New England Biolabs) treatment of the U-labeled second-stranded DNAs, the ligated products were amplified by a polymerase chain reaction (PCR) with the conditions: initial denaturation at 95°C for 3 min; eight cycles of denaturation at 98°C for 15 s, annealing at 60°C for 15 s, and extension at 72°C for 30 s; and then final extension at 72°C for 5 min. The average insert size for the final cDNA library was 300 ± 50 bp. Finally, we performed 2 × 150-bp paired-end sequencing (PE150) on an Illumina Novaseq 6000 (LC-Bio Technology Co., Ltd, Hangzhou, China) in accordance with the manufacturer's instructions.
2.5.2 Bioinformatics analysis of RNA-seq data
fastp software (https://github.com/OpenGene/fastp) was used to remove the reads that contained adaptor contamination, low quality bases and undetermined bases with default parameters. Then, sequence quality was also verified using fastp. We used HISAT2 (https://ccb.jhu.edu/software/hisat2) to map reads to the reference genome of Homo sapiens GRCh38. The mapped reads of each sample were assembled using StringTie (https://ccb.jhu.edu/software/stringtie) with default parameters. Then, all transcriptomes from all samples were merged to reconstruct a comprehensive transcriptome using gffcompare (https://github.com/gpertea/gffcompare). After the final transcriptome was generated, StringTie was used to estimate the expression levels of all transcripts. StringTie was used to perform expression level for mRNAs by calculating FPKM (i.e., fragments per kilobase of exon per million mapped fragments) (FPKM = [total exon fragments/mapped reads (millions) × exon length (kB)]). The differentially expressed mRNAs were selected with fold change > 2 or fold change < 0.5 and with a parametric F test comparing nested linear models (p < 0.05) using R package edgeR (https://bioconductor.org/packages/release/bioc/html/edgeR.html).
2.6 RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was isolated using TRizol reagent (Invitrogen) in according with the manufacturer's instructions. A NanoDrop 2000 spectrophotometer (Thermo Fisher) was used for quantification of RNA and the 260/280 ratio. One microgram of RNA was used to synthesize complementary DNA (cDNA) (PrimeScript™ RT Master Mix, RR036A; TaKaRa, Shiga, Japan). A qRT-PCR was performed using Hieff Master Mix (Yeason) in a LightCycler 96 system (Roche, Basel. Switzerland). The reaction consisted of initial incubation at 50°C for 120 s and 95°C for 600 s, and a subsequent amplification reaction (95°C for 15 s, 60°C for 20 s, 72°C for 30 s). GAPDH was taken to be the internal control. The primer sequences and amplicon length are provided in the Supporting information (Table S1).
2.7 Statistical analysis
All data are expressed as the mean ± SEM. There was one variable and four independent groups; thus, one-way analysis of variance was performed to analyze the data. Tukey's test, a common and popular post-hoc analysis, was employed to perform comparisons between each specific groups. p < 0.05 was considered statistically significant.
3 RESULTS
3.1 ARS staining
The morphology of hDPCs is shown in File S1, Figure S1. The time schedule of the experiment is shown in Figure 1A. Figure 1B shows that, regardless of hDPC passage number, dexamethasone exerted biphasic effects in matrix mineralization of hDPCs; namely, a low concentration (1 nm) of dexamethasone promoted mineralization, whereas higher concentrations (10 and 100 nm) of dexamethasone inhibited it. Quantification of ARS staining denoted D1 promoted the most vigorous mineralization of hDPCs in either passage 3 or passage 4 (Figure 1C, D). In Figure B (lower), no staining is observed in D10 and D100 (passage = 3; MM: 2 days, OM: 18 days). To test the rescue effect of low concentration, we changed the media on day 18 (the same day of ARS staining) to 1 nm for both groups and cultured the cells for another 3 days (D10 18 days + D1, 3 days; D100, 18 days + D1, 3 days). In Figure 1E, ARS staining showed that the mineralization ability of cells grown in 10 nm dexamethasone could be partially rescued after alteration of concentration from 10 nm to 1 nm on day 18, whereas, in the D100 group, no similar phenomenon was observed. The quantification of ARS in Figure 1F revealed a 11-fold increase of mineralization in the D10 18 days + D1 3 days group compared to the D100 18 days + D1 3 days group.
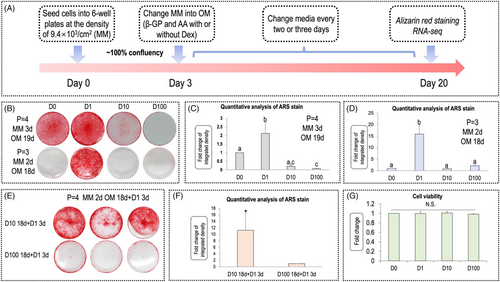
3.2 CCK-8 assay
The hDPCs were respectively treated with 0 nM (vehicle group), 1 nM, 10 nM and 100 nM dexamethasone for 24 h and a CCK-8 assay was used to detect the viability of hDPCs. As shown in Figure 1G, there was no difference in cell viability between different groups, indicating that no cytotoxicity was imposed by dexamethasone in the concentration used by this experiment. In other words, the inhibitory effects on mineralization of high concentration dexamethasone may not result from its cytotoxicity.
3.3 Gene expression profile of control and experimental groups analyzed by RNA-seq
To detect the potential key regulators associated with dexamethasone-stimulated mineralization, we analyzed the differentially expressed genes (DEGs) (fold change ≥ 2 or ≤ 0.5, q value < 0.05) between control and experimental groups. The volcano plot (Figure 2A) implicated that the whole gene expression profile of cells cultured in dexamethasone (1 nm) was dramatically different from that cultured under a dexamethasone free condition. The q value was reordered from lowest to highest and the first 100 DEGs were shown in the clustering analysis heatmap in Figure 2B. We successfully identifying 3032 up-regulated genes and 3007 down-regulated genes. Function of the DEGs was investigated using Gene Ontology (GO)-based enrichment annotation (http://geneontology.org), which was classified into three key functional categories: biological process (BP), molecular function (MF) and cellular component (CC). GO analysis revealed that genes related to protein binding were mostly enriched through dexamethasone treatment (Figure 2C).
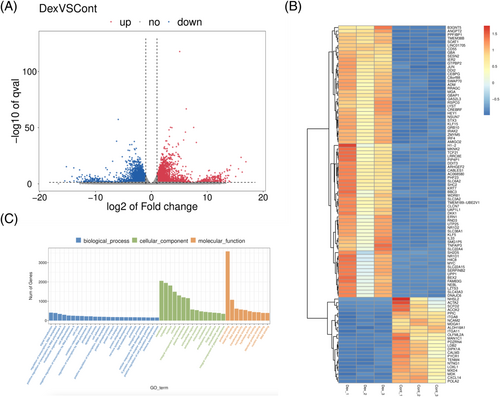
Between the control group and dexamethasone group, 11,153 DEGs had GO annotations. The majority were highly enriched in “histone demethylase activity (H3-K27 specific” (GO: 0071558), glutathione derivative biosynthesis process (GO: 1901687), glutathione transferase activity (GO: 0004364), regulation of transcription involved in G1/S transition of mitotic cell cycle (GO: 0000083), collagen-containing extracellular matrix (GO: 0062023), ciliary basal body (GO: 0036064) (Figure 3A). The GO term analysis suggested that dexamethasone treatment induces epigenetic changes and extracellular matrix remodeling. The Gene Set Enrichment Analysis (GSEA) results of the GO term are provided in the Supporting information, File S2.
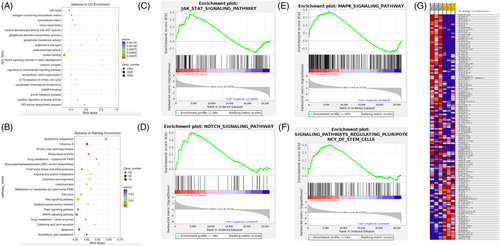
Furthermore, to explore potential key signaling pathways that had been activated, Kyoto Encylopedia of Genes and Genomes (KEGG) (https://www.genome.jp/kegg) analysis was carried out. The top 20 key signaling pathways that were enriched is shown in Figure 3B. For example, primary bile acid biosynthesis, glutathione metabolism, glycosylphosphatidylinositol-anchor biosynthesis, drug metabolism-cytochrome P450, rheumatoid arthritis, influenza A, etc. Moreover, we showed that dexamethasone was positively related to the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway (Figure 3C), Notch signaling pathway (Figure 3D) and mitogen-activated protein kinase (MAPK) signaling pathway (Figure 3E). Meanwhile, dexamethasone was also found to be positively related to the signaling pathway regulating pluripotency of stem cells (Figure 3F). The clustering analysis of DEGs involved in regulation of pluripotency of stem cells is shown in Figure 3G. The GSEA results of KEGG pathway are provided in the Supporting information, File S3.
3.4 qPCR
We excluded DEGs for which FPKM were lower than one, reordered the DEGs with q value from lowest to highest. After that, we selected the top ten up- or down- regulated genes, and the names of these genes and the fold change are given in Figure 4A. To validate the RNA-seq results, a qRT-PCR analysis was performed. It is shown in Figure 4B that, although the exact fold change number may be different in the qPCR data, the ascending trend of the genes (top 10 up-regulated genes and seven pluripotency markers) was identical between qPCR and RNA-seq analysis. Of note, the expression of MMP-3 was significantly enhanced by dexamethasone to 153.89-fold compared to the control (the fold change was 101.16-fold by RNA-seq analysis). For the other genes, the fold change was as follows: DKK-1 (99.81-fold versus 33.06-fold in RNA-seq), CXCL-8 (84.52-fold versus 39.46-fold in RNA-seq), TRIB-3 (58.28-fold versus 24.32-fold in RNA-seq), DDIT-3 (52.13-fold versus 33.51-fold in RNA-seq), GDF-15 (38.03-fold versus 19.11-fold in RNA-seq), NR1D1 (36.40-fold versus 27.67-fold in RNA-seq), FAM83G (32.12-fold versus 14.05-fold in RNA-seq), TNFAIP3 (28.56-fold versus 17.75-fold in RNA-seq) and PLIN2 (20.55-fold versus 13.65-fold in RNA-seq). To summarize, using qPCR analysis, the calculated fold change of each gene was higher than that obtained in RNA-seq.
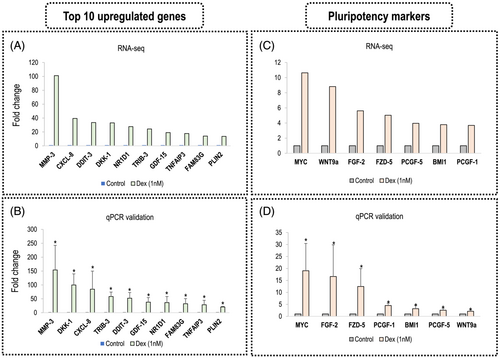
Furthermore, we showed the gene expression of seven pluripotency markers in the RNA-seq data (Figure 4C) and qPCR result (Figure 4D). The seven pluripotency markers are MYC, FGF-2, FZD-5, PCGF-1, BMI1, PCGF-5 and WNT9a. The RNA-seq data and qPCR results were as follows: MYC (RNA-seq: 10.64-fold, qPCR 19.05 ± 11.39 fold), FGF-2 (RNA-seq: 5.62-fold, qPCR 16.63 ± 13.21 fold), FZD-5 (RNA-seq: 5.03-fold, qPCR 12.48 ± 7.45 fold), PCGF-1 (RNA-seq: 3.69-fold, qPCR 4.51 ± 1.01 fold), BMI1 (RNA-seq: 3.79-fold, qPCR 3.18 ± 1.38 fold), PCGF-5 (RNA-seq: 3.96-fold, qPCR 2.56 ± 0.89 fold) and WNT9a (RNA-seq: 8.82-fold, qPCR 2.10 ± 0.58 fold). The detailed information of top ten up- or down-regulated gene names, FPKM value, p and q values, fold change, log2 (Fc) and regulation type (up or down) is provided in the Supporting information, File S1, as well as Figure S2 (comparison of log2(Fc)), Table S2 (up-regulated genes) and Table S3 (down-regulated genes). A complete list of DEGs with respect to dexamethasone-stimulated mineralization in hDPCs is also provided in the Supporting information (Figure S4). The raw data of RNA-seq was deposited in the NCBI SRA database with BioProject accession no. PRJNA815469.
3.5 Searching the results of keywords in the PubMed database
For further investigation of the 20 genes in bone and dentin, we searched those genes in combination with bone or dentin using the PubMed database (as of 21 February 2022). The results are provided in the Supporting information (Tables S4 and S5). It is shown that inflammatory factors such as MMP3, CXCL8 and CXCL12 have been investigated more often than the other genes.
4 DISCUSSION
hDPCs, a group of mesenchymal stromal cells derived from dental pulp tissue, possess the ability to form dentin, bone, periodontal ligament and a number of other tissues in vitro and in vivo.10, 11 Compared to bone marrow-dervied mesenchymal stem cells, hDPCs featured a higher proliferation capacity and a better differentiation potential.10 Therefore, hDPCs are gaining increasing interest in the dentin regeneration field. hDPC mineralization, a key step in forming reparative dentin, is a complex and dynamic process involving a plethora of genes and transcription factors.12 Uncovering the mRNA abundance and gene profiles of hDPCs during the process of mineralization is core to elucidating the underlying mechanisms of dentin regeneration.
In this preliminary experiment, the biphasic effects of dexamethasone in hDPCs were discovered and further exploration of transcriptomic profiles was conducted. Specifically, a low dose of dexamethasone promoted the most vigorous mineralization and directly impacted over 6000 genes. By contrast, the high dose groups showed inhibitory effects toward mineralization. Notably, it was found that both duration of exposure to OM and passage number could influence the mineralization. It was suggested that a longer duration in MM and OM enhanced mineralization, indicating that the timing of adding OM was another critical point to consider in the case of using dexamethasone to induce in vitro mineralization. An inhibitory effect of D10 on mineralization could be rescued by alteration of the dexamethasone concentration from 10 nm to 1 nm on day 18, whereas the inhibitory effect exerted by D100 could not be reversed. Except for our work, many other studies have already reported the pleiotropic effects exerted by glucocorticoid in cells,13 the skeleton14 and the immune system.15 It is widely accepted that a glucocorticoid such as dexamethasone binds its cytosolic receptor (glucocorticoid receptor, GR, NR3C1), which consequently undergoes conformational rearrangement and binds glucocorticoid receptor elements (GREs) in the DNA of various genes. The possible explanation for our findings may be that some GREs are hypersensitive and require only a small amount of glucocorticoid (D0) to activate,16 and a high dose of glucocorticoid (D10 and D100) may represent an excessive burst use, which is responsible for false steroid resistance in the cell.17
Among the DEGs, key genes related to osteoblast differentiation and mineralization, such as MMP-3, CXCL-8, DKK1, etc., were robustly enhanced. MMP-3 and CXCL-8 were two mostly up-regulated genes. MMP-3 encodes MMP-3 (or stromelysin-1) protein, which cleaves a series of extracellular matrix proteins such as collagens and proteoglycans.18, 19 MMP-3 also activates other MMPs such as pro-MMP1, pro-MMP8, and pro-MMP9,20-22 rendering MMP-3 pivotal in connective tissue remodeling and wound healing. Previous work reported that MMP-3 promotes dentin regeneration by exerting anti-inflammation effects; specifically, priming of MMP-3 to exposed pulp resulted in a decrease of macrophage and antigen-presenting cells and inhibition of interleukin (IL)-6 expression.23 In the present study, dexamethasone (1 nm), a type of anti-inflammation factor, stimulated MMP-3 expression by 101.16-fold compared to the control, indicating that dexamethasone exerts its anti-inflammatory function through activating MMP-3, an extracellular matrix remodeling factor. Further study is needed to explore whether MMP-3 is positively related to mineralization in hDPCs.
CXCL-8 (or chemokine ligand 8) encodes IL-8, which is a type of chemokine produced by macrophages and other cell types. The major IL-8 receptors on cell surface are G-protein-coupled serpentine receptors CXCR1 and CXCR2.24 IL-8 elicits chemotaxis in neutrophils, causing them to migrate toward the site of infection. Importantly, it stimulates phagocytosis upon arrival at the site of injury.25 When superficial caries exist, an increase of IL-8 secretion was observed. Moreover, the production of IL-8 protein could be induced by osteogenic reagents.26 Under caries conditions, hDPCs adopted a higher potential in forming a mineralized monolayer, and it is hence reasonable to hypothesize that IL-8 might participate in directing hDPCs differentiation toward mineralization phenotype. Because forming a mineralized barrier prevents further entry of bacteria, it is therefore considered that IL-8 could stop the development of caries and help a decayed tooth to heal by forming reparative dentine. The result indicates that IL-8 resists caries progression by enhancing hDPC mineralization.26 In the present study, we found that CXCL-8, the encoding gene for IL-8, was up-regulated by 39.46-fold compared to the control, indicating that dexamethasone (1 nm) might be able to induce the up-regulation of this pro-inflammatory gene to indirectly promote dentin regeneration. Further research is warranted to confirm our hypothesis.
DNA damage inducible transcript 3 (DDIT3, also known as CHOP) encodes a pro-apoptotic transcription factor DDIT3, which is a member of the CCAAT/enhancer-binding protein (C/EBP) family of DNA-binding transcription factors. The protein is activated in response to stress. Furthermore, DDIT3 was found to mediates apoptosis signaling, for which the downstream effectors are yet to be discovered.27 Ongoing research established that DDIT3 inhibits hDPC proliferation but enhances its differentiation into an odontoblast-like phenotype (Wu et al, 2014).28 Meanwhile, it was demonstrated that DDIT3 leads to osteogenic differentiation without alteration of proliferation in murine ST-2 stromal cells (Pereira et al, 2004).29 The present study revealed DDIT3 was promoted by dexamethasone at the concentration of 1 nm, which is different from a previous work reporting the decrease of DDIT3 expression by the use of dexamethasone at 0.1 μm (100 nm).30 It is therefore assumed that the effects of dexamethasone are largely dependent on the dose and cell line type.
Dickkopf-related protein 1 (DKK1) encodes DKK1 protein, a type of secreted protein, which contains two cysteine rich regions and regulates embryonic development through its inhibition of the Wnt signaling pathway. The Wnt pathway is an essential signaling cascade in bone and tooth biology, governing hard tissue development and remodeling. DKK1 is an antagonist of the Wnt/β-catenin signaling pathway that acts by: (1) binding LRP6 co-receptor to prevent its interaction with Wnt-Frizzled complexes and (2) binding to cell surface receptor Kremen 1 or Kremen 2 to induce internalization of LRP5/6.31 Activation of the Wnt pathway results in up-regulation of DKK1, a downstream negative regulator of Wnt pathway.32 DKK1 plays a key role in embryo and tooth development.33, 34 More importantly, it participates in directing differentiation of pre-odontoblast into odontoblast, denoting its key role as a mesenchymal cell differentiation regulator.35 A recent study found inhibition of DKK1 during osteoblast differentiation leads to reduced mineralization without impacting its ALP activity.36 Here, we observed an augmentation of DKK1 expression in dexamethasone-stimulated hDPCs. The time we selected for RNA-seq was 20 days, when mineralized matrix was already formed in dexamethasone group. It is therefore reasonable to assume that the Wnt pathway was activated prior to the significant up-regulation of DKK1. DKK1, a target gene of Wnt pathway, acted as a stop signal to avoid constant activation of Wnt, thereby constituting a regulation system in hDPCs to fine-tune mineralization.
Nuclear receptor subfamily 1 group D member 1 (NR1D1, also known as Rev-ErbA) is a transcriptional repressor and plays a vital role in inflammatory reactions, it is one of the members of core circadian clock genes (Bmal-1, Per-2, Nr1d1 and Dbp). In the present study, there were no differences in the expression of Bma-1 and Dbp between control and dexamethasone groups (see Supporting information, Figure S1). Regarding Per-2, a slight but not significant increase (1.29-fold) in its expression was observed (see Supporting information, Figure S1). Among four core circadian clock genes, only NR1D1 displayed an increase over 20-fold (36.40-fold by qPCR) compared to the control. This result promoted us to hypothesize that NR1D1 may positively regulate mineralization. However, other studies revealed that NR1D1 inhibits mineralization in osteoblasts and cementoblasts.37, 38 We attributed this inconsistency to a different cell type used, and further studies are needed to elucidate the detailed role of NR1D1 in hDPCs. NR1D1 up-regulates mitochondrial-related genes by activating AMPK signaling pathway. It hence enhances mitochondrial function, protects tissues from oxidative stress and inflammation.39, 40
Tribbles homolog 3 (TRIB3), a mammalian homolog of Drosophila tribbles, is a pseudo-kinase with a kinase domain that lacks enzyme activity. TRIB3 mRNA and protein levels increase during osteogenic differentiation, its expression reached its peak on day 14. Knockdown of TRIB3 resulted in the inhibition of osteogenesis manifested by decrease of ALP activity and the delay of mineralization.41 Local delivery of TRIB3 using a PLGA-based scaffold stimulated robust bone regeneration and inhibited cyst formation in rodent non-healing mandibular defect models, indicating TRIB3 favors osteoblastogenesis.42 In the present study, it was found TRIB3 was promoted by dexamethasone to 24.32-fold compared to the control, implying that dexamethasone might induce the mineralization and differentiation of hDPCs by enhancing the expression of TRIB3.
Growth differentiation factor 15 (GDF15), belongs to transforming growth factor-β and bone morphogenetic protein superfamily. Here, it was found the expression of GDF15 was elevated by dexamethasone to 19.11-fold compared to the control. GDF15 dose-dependently increased the number of multinucleated TRAP-positive cells in the presence of RANKL, whereas it inhibited osteoblast differentiation by decreasing the expression of osteoblastic markers such as Runx-2, COL1A1 and osteocalcin (OCN).43 However, another study using GDF15 in human primary osteoblasts reveals that GDF15 (5 ng/ml) promoted the expression of several osteogenic markers (Runx-2, OCN and ALP).44 The contradictory findings may be a result of differences in the cells or doses. In the present study, it is hypothesized that GDF15 is positively associated with hDPC mineralization stimulated by dexamethasone.
Tumor necrosis factor alpha-induced protein 3 (TNFAIP3 or A20) is a gene for which the expression is rapidly induced by the tumor necrosis factor. It is an innate immune regulator, which is associated with a variety of autoimmune, inflammatory and hematologic malignancies. A gene knockout study showed TNFAIP3 was indispensable for limiting inflammation by terminating endotoxin- and TNF-induced nuclear factor-kappa B responses.45 Furthermore, TNFAIP3 protects osteoprogenitors from undergoing apoptosis stimulated by TNF-α.46 More recently, this gene was found to maintain a young state in hematopoietic stem and progenitor cells (HSPC): namely, the expression level of TNFAIP3 was lower in aged HSPC than that in young HSPC, and partial deletion of TNFAIP3 in young HSPC results in typical features of aging.47 Here, the expression of TNFAIP3 was promoted by dexamethasone, suggesting that it exerted immune-regulating effect through activating TNFAIP3. For example, a genetic disease caused by haploinsufficiency of TNFAIP3 or A20 (HA20) could be treated by application of corticosteroids such as dexamethasone, which aids in the secretion of extra TNFAIP3 in patients.48
Family with sequence similarity 83 member G (FAM83G) was reported to be a tumor-promoting factor. FAM83G was reported to be involved in hair morphogenesis, hair follicle differentiation and cycling.49 Except for this, most of the studies regarding FAM83G were related to its involvement in tumor metastasis and prognosis. For example, FAM83G promotes the proliferation, invasion and migration of hepatocellular carcinoma (HCC) cells; hence, its high expression may signify the poor prognosis of HCC patients.50 Experiments about the function of FAM83G in bone and dentin are quite rare (there were only three literatures displayed as searched using the keywords “FAM83G and Bone”). Here, we show that FAM83G was promoted to 14.05-fold under the treatment of dexamethasone in hDPCs, indicating that dexamethasone might stimulate the mineralization of hDPCs partially through activating FAM83G. However, further studies are needed to clarify the hypothesis.
Adipose differentiation-related protein, also known as perilipin 2, ADRP or adipophilin, is a protein encoded by PLIN2 gene. PLIN2 was up-regulated by dexamethasone to 13.65-fold compared to the control. PLIN2 is a cytoplasmic lipid droplet scaffolding protein expressed in many organs, including the liver, adipose tissue and skeletal muscle. Whole body knockout of PLIN2 in mice leads to obesity resistance when chronically exposed to a western diet or a high-fat diet. Treatment of wild-type mice with antisense oligonucleotides against Plin2 mRNA leads to a significant reduction in adiposity, improvement in liver steatosis and attenuation of insulin resistance.51 Total PLIN2 deletion protected western style food-fed mice from obesity, insulin resistance and liver fibrosis found in wild-type counterparts.52 Searching using “PLIN2 and Bone” in PubMed resulted in only 13 articles and search with “PLIN2 and dentin” resulted in zero articles, indicating that research on PLIN2 in the bone and dentin field remains rare. Further studies are needed to clarify its role in bone and dentin development.
Pertaining to the top ten down-regulated genes induced by dexamethasone, we found two frequently studied genes: CXCL-12 and COL1A1. By searching them in combination with “Bone” in PubMed, we found 2538 and 1432 articles, respectively. Stromal cell-derived factor 1 (SDF-1 or CXCL-12), belonging to the CXC family, is a type of chemokine protein encoded by CXCL-12 on chromosome 10. CXCL-12 is ubiquitously expressed in many tissues and cell types and is known for its role in recruiting and activating leukocyte precursors. It specifically binds to seven-transmembrane G-coupled protein receptor CXCR-4. Mice lacking CXCL-12 are lethal because of abnormalities and a variety of defects in cardiovascular systems and neuronal systems. CXCL-12 induces hDPSCs migration via the FAK/PI3K/Akt and GSK3β/β-catenin pathways.53 It is shown in the Supporting information (Table S2) that the expression of CXCL-12 was decreased to negligible level by dexamethasone, suggesting that cell migration was inhibited during the late stage mineralization of hDPCs.
COL1A1 encodes the α1 chain of type I collagen, an extracellular matrix protein that can be found in most connective tissues including bone, dentin and cartilage. This gene plays a key role in maintaining homeostasis in bone development and remodeling. The mutation of COL1A1 is one of the major causes for osteogenesis imperfecta,54 a genetic disease characterized by fragile bones, skeletal deformities, bone fractures and prenatal death. In COL1A1 mutated patients, it was found that the type I collagen has increased D-periodic spacing, with variable enlarged collagen fibrils coated with fewer minerals. Furthermore, the mutated collagen leads to a rougher dentin surface and a decreased Young's modulus in proband's dentin,55 indicating that COL1A1 is indispensable in maintaining a sound and complete dentin nanostructure. Indeed, secretion of type I collagen is the first step in forming mineralized cell monolayers, which was achieved by addition of ascorbic acid into the culture media. We observed a sharp decrease of COL1A1 expression in dexamethasone group, denoting cells entered the late mineralization stage, in which the formation of type I collagen matrix was already finished. By contrast, in the control group where cells just started to mineralize, they need to build up a type I collagen network where calcium could precipitate. The above may be the possible reason for a sharp decrease of COL1A1 expression in the dexamethasone group.
We searched PubMed using above 20 genes in combination with bone or dentin. The results revealed that MMP3 had been studied more often in dentin than the other genes, whereas DKK1 had been mostly studied in the bone field. CXCL8 was the third most studied target gene in both the bone and dentin fields. Five genes (NR1D1, TRIB3, TNFAIP3, FAM38G and PLIN2) have not been studied in the dentin field; therefore, the search results were zero. It is concluded that inflammation related factors such as MMP3 and CXCL8 have been studied quite considerably compared to other genes. DKK1, a Wnt signaling pathway related gene, was studied frequently as well. In futurethe, it is suggested that attention be directed to other less studied genes in the bone and dentin fields, such as TNFAIP3 and PLIN2 etc.
Based on the pathways involved or the functions performed, DEGs were categorized using the KEGG database. The presence of unique pathways at each developmental stage with respect to the control suggests their potential novel roles in dexamethasone-stimulated mineralization. Furthermore, GSEA analysis was conducted and the relationships between dexamethasone with several important signaling pathways in tooth development were uncovered. Dexamethasone was positively involved in the JAK/STAT signaling pathway, MAPK signaling pathway and Notch signaling pathway.
The JAK/STAT signaling pathway is a central communication spot in cells with respect to regulating cancer and inflammation (see the analysis result of correlation between MAPK signaling pathway and Dexamethasone in hDPCs at File S5). The JAK family has four members (JAK1, JAK2, TYK2 and JAK3), whereas the STAT family (STAT1–4, STAT5a, STAT5b and STAT6) has seven distinct proteins.56 It was reported GR forms a complex with Stat5, which binds to DNA independently of glucocorticoid receptor elements, thus regulating the expression of genes.57 Another study found that dexamethasone inhibits immune evasion by facilitating nuclear translocation of the GR/STAT3 complex, a process responsible for down-regulation of two immune inhibitory molecules (PD-L1 and IDO1).58
The MAPK signaling pathway regulates a number of cellular activities such as inflammation, apoptosis, proliferation, differentiation and mineralization (see the analysis result of correlation between MAPK signaling pathway and Dexamethasone in hDPCs at File S6). The MAPK family is composed of ERK, JNK and p38. Dexamethasone mediated the mineralization of odontoblasts via p38.59 Our experiment indicated down-regulation of p38 (MAPK14) by the treatment of dexamethasone (see Supporting information, File S2). Meanwhile, glucocorticoids suppress the JNK pathway by activating glucocorticoid receptor-JNK interaction.60 Furthermore, a more recent study revealed that a high dose of dexamethasone inhibited chick embryo myogenesis via downregulation of the FGF-ERK signaling pathway.61 Based on the above findings, dexamethasone appears to inhibit MAPK pathways and act as a negative regulator of cells.
The Notch pathway is responsible in regulating a wide range of biological activities including embryo development and cell differentiation. One distinct characteristic of the Notch pathway is that its ligands are transmembrane proteins instead of soluble molecules.62 The RNA-seq data revealed that Hey1, a target gene of Notch pathway, was enhanced by dexamethasone by 8.92-fold (see Supporting information, File S7). However, a previous study by Zanotti et al.63 reported that glucocorticoids oppress the expression of Hey1, Hey2 and HeyL in osteoblasts. It was suggested that both chromatin accessibility and GRE sensitivity appear to regulate the effects of glucocorticoids.16 Therefore, the inconsistent findings between the prresent study and previous studies may be a result of the different cell types and doses used.
In addition, dexamethasone was also found to be positively related to signaling pathway in regulating pluripotency of stem cells. We quantified the expression of several pluripotency related genes including MYC, FGF-2 and FZD-5, etc. Notably, FGF-2 was found to be enhanced by dexamethasone by over 16-fold compared to the control. Indeed, FGF-2, a growth factor governing organogenesis and tissue homeostasis, plays an essential role in stemness maintenance and regulates dental pulp cells in a stage-specific manner.64, 65 Another study further explored its role in odontoblast differentiation in αSMACre-tdTomato mice model and concluded that early and pulsed exposure to FGF-2 facilitated the differentiation of odontoblasts and promoted the formation of reparative dentin devoid of osteoblasts.66 In physiological conditions, stem cells differentiate and replace dead odontoblasts to maintain the function of pulp over an individual's lifetime. Odontoblasts are terminally differentiated cells and not capable of proliferating to generate daughter cells. The replacement of cells for dead odontoblasts originates from dental pulp stem cells, which can be induced to undergo odontoblastic differentiation upon demand. As such, stem cell self-renewal is indispensable for the long-term function and viability of pulp. BMI1 (B lymphoma Mo-MLV insertion region 1 homolog) is a polycomb ring finger oncogene regulating stem cell self-renewal. BMI1 and FGF-2 are both important stemness retainers. The ability to enhance the expression of these genes strongly suggested the potential of dexamethasone in maintaining the stemness of dental pulp stem cells and indirectly aiding in dentin regeneration.
Here, we present an in-depth profiling of hDPC mineralization. We established an in vitro model of mineralization using dexamethasone, and sequenced the genes in the control and dexamethasone groups. Top 10 up- or down-regulated genes were identified. Despite the findings of the present study, it is necessary to note that a major drawback of bulk transcriptomes is that only the average gene expression levels are measured from different cell types in the tissues, which are dominated by highly abundant cell types. Hence, the results from bulk RNA-seq mask the information from minor cell types. For example, immune cells constitute only a fraction of DPCs, but they play significant roles in the resistance to foreign pathogens invading the dental pulp. Another sequencing technology called single-cell RNA-seq (scRNA-seq) empowers researchers to investigate the function of each specific cell type within a tissue. It is suggested that, in the future, scRNA-seq to be used to better characterize the behavior of hDPCs in reparative dentine formation in vivo. Moreover, it is known that cell mineralization is a consecutive process starting from cell proliferation, differentiation and final mineralization. Therefore, multi-time point (e.g., 7 days, 14 days and 21 days, etc.) transcriptomic analysis may enable a more comprehensive deciphering of key molecular biological changes during hDPC mineralization.
5 CONCLUSIONS
The present study demonstrates that exogenous dexamethasone exhibited biphasic effects toward hDPC mineralization. A lower concentration (1 nm) of dexamethasone enhanced mineralization, whereas high concentrations (10 and 100 nm) inhibited it. To further explore potential genes that were activated in hDPC mineralization, the concentration 1 nm was used to analyze the transcriptional changes that occur during the mineralization process of hDPCs after dexamethasone treatment. The expression of certain inflammatory factors was significantly increased upon dexamethasone treatment; moreover, 1000s of differentially expressed genes were identified and further analyzed. In total, 3032 DEGs were significantly up-regulated and involved in a wide range of signaling pathways. In sum, although there are limitations to the present study, the results offer novel insights into the regulatory mechanism of dexamethasone-stimulated mineralization in hDPCs.
ACKNOWLEDGEMENTS
The present study was supported by the grant from the National Key Research and Development Program of China (No. 2018YFE0202200), the National Natural Science Foundation in China (No. 81271110 & No. 81670962).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
ZLW and JT contributed to conception and design of the study. JT performed the experiments, statistical analysis and drafted the manuscript. ZLW revised the manuscript. All authors approved the final version of the manuscript submitted for publication.
Open Research
DATA AVAILABILITY STATEMENT
All data generated are available from the corresponding author upon reasonable request.




