Histidine-enhanced gene delivery systems: The state of the art
Makkieh Jahanpeimay Sabet, Akbar Hasanzadeh, Mahtab Kamrani Mousavi and Niloofar Haeri Moghadam contributed equally to this work.
Funding information: Iran University of Medical Sciences, Grant/Award Number: 97-3-75-13318
Abstract
Gene therapy has emerged as a promising tool for treating different intractable diseases, particularly cancer or even viral diseases such as COVID-19 (coronavirus disease 2019). In this context, various non-viral gene carriers are being explored to transfer DNA or RNA sequences into target cells. Here, we review the applications of the naturally occurring amino acid histidine in the delivery of nucleic acids into cells. The biocompatibility of histidine-enhanced gene delivery systems has encouraged their wider use in gene therapy. Histidine-based gene carriers can involve the modification of peptides, dendrimers, lipids or nanocomposites. Several linear polymers, such as polyethylenimine, poly-l-lysine (synthetic) or dextran and chitosan (natural), have been conjugated with histidine residues to form complexes with nucleic acids for intracellular delivery. The challenges, opportunities and future research trends of histidine-based gene deliveries are investigated.
1 INTRODUCTION
In recent years, several approaches to treat diseases using nucleic acids have been proposed under the broad umbrella of ‘gene therapy’. These diseases include genetic disorders, cancer and other serious conditions.1-3 In addition, the world is currently beset by the pandemic of COVID-19 (coronavirus disease 2019) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).4 Gene-based vaccine platforms based on DNA, RNA or viral vectors have revealed promising outcome in not only humoral, but also cell-mediated immune responses in recent investigations, supporting their deploying for SARS-CoV-2 vaccine enhancement. More importantly, the US Food and Drug Administration have recently authorized the emergency implement of two effective RNA-centered SARS-CoV-2 vaccines.5 The concept initially faced some challenges, including low selectivity for specific genetic alterations, and the possibility of errors in targeting the wider genome.6 The development of improved viral and non-viral vectors has increased the likelihood of targeting the desired gene, as well as reducing the likelihood of negative side effects.7-9 It should be noted that the most successful approach and the nearest to clinical approval comprises the use of viral vectors.10 However, viral vectors can themselves lead to genetic alterations and mutations and can even cause cancer. These genetic changes, mutations and interactions are not readily detectable in the short term; therefore, there are still safety concerns concerning gene therapy using viral vectors. Therefore, the discovery of efficient non-viral vectors has been a hot topic in recent years because they do not show unwanted interactions with genetic material or lead to the development of harmful mutations.11-15 Non-viral vectors have often been designed based on biodegradable polymers, which can be optimized to form complexes with nucleic acids, can be stable in different environments and can escape from endosomes after cellular uptake.16-19 Additional factors, such as cytotoxicity, controlled release of nucleic acids and interactions with cells and tissues, all require physical and chemical optimization in the laboratory phase.20, 21 Therefore, the size of the vectors, zeta potential, surface and bulk functional groups, and loading capacity must be carefully studied. Non-viral vectors can also be based on synthetic polymers, biopolymers, inorganic nanomaterials,22, 23 layered double hydroxides,23 gold nanoparticles (NPs),24, 25 MXenes26 or metal–organic frameworks.27 In the case of polymeric vectors, whether natural or synthetic, the parameters can be optimized according to the principles of organic chemistry.28 One desirable goal is the design of a versatile multifunctional non-viral vector, which could be used to deliver different kinds of therapeutic nucleic acids, namely plasmid DNA, antisense oligonucleotides, short-interfering RNA or clustered regularly interspaced short palindromic repeats (CRISPR) gene editing platforms.29, 30 If this goal was achieved, it could be described as ‘multifunctional gene therapy’.31
In this regard, the use of well-designed gene delivery vectors with a high degree of chemical and physical tunability can help to achieve the goals of green chemistry.32 The amino acid histidine is a naturally occurring bioorganic compound that can be used in a green chemistry approach in biomedical applications such as drug delivery,33, 34 imaging,35 photodynamic therapy36 and particularly gene delivery.37-39 In gene delivery realm, the role of histidine as an imperative segment in gene carriers has been suitably reported during past two decades.39-54 Relatively simple modification of polymers with histidine residues (Figure 1) can easily control the size, zeta potential, targeting groups and molecular interactions. In recent years, histidine-centered cyclodextrin,55 mesoporous silica,56 curdlan,57 polyethylene glycol58 and also zeolitic imidazole framework-8 (ZIF-8)59 film have been successfully deployed in gene delivery systems. In the present review article, cutting-edge histidine-based gene carriers, including peptides, polymers, lipids and nanocomposites, published from 2015 to present are described in detail. The section on linear polymers, includes synthetic polymers such as polyethyleneimine (PEI) and poly-l-lysine (PLL), as well as natural biopolymers such as dextran and chitosan. Moreover, histidine-based dendrimer-polymer NPs including polyamidoamine (PAMAM) are included.
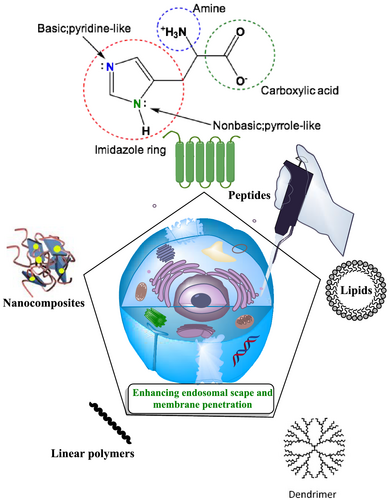
1.1 Histidine: Chemistry and biological role
The amino acid histidine behaves in a similar manner to other organic salts. Histidine has a large dipole moment and is to some extent soluble in water; however, it is insoluble in hydrocarbons. Furthermore, histidine is amphiprotic, which means that it could simultaneously react as an acid or a base, depending on the conditions. In acidic solution, the amino acid zwitterion behaves as a base that can accept a proton to render a cation, whereas, in basic solution, it loses a proton to generate an anion. Among amino acids, only histidine, lysine and arginine have a basic amino group in their side chains. At the physiological pH of 7.3, the heterocyclic imidazole ring of histidine is not sufficiently basic to be protonated. More importantly, in histidine, just the pyridine-like nitrogen atom is basic. The singly bonded nitrogen atom is not considered as a basic nitrogen because its lone pair of electrons is part of the six-p-electron aromatic imidazole structure.60 In the basic solution, the histidine can lose a proton and behave as an anion, whereas, in acid solution, it gains a proton and consider as a cation. At a moderate pH, the amino acid is completely balanced between anionic and cationic forms and exists as a neutral, dipolar zwitterion. This pH is known as the amino acid isoelectric point (pI) and has a value of 7.59 for histidine.61 The pKa of protonated form of the imidazole segment of histidine is almost 6.0.62 Therefore, the imidazole ring is quiet often protonated below a pH of 6 (as demonstrated by the Henderson–Hasselbalch equation). The resulting imidazolium ring bears two NH bonds and possess a positive charge. The positive charge is equally distributed between these two nitrogens and could be represented with two equally critical resonance structures. Beyond pH 6, one of the two protons is lost. The remaining proton of the imidazole ring could reside on either nitrogen, giving rise to the N1-H or N3-H tautomers. At all pH values, the imidazole/imidazolium ring of histidine is aromatic.63
Regarding the biological role of histidine, it is clear that the crucial act of these amino acid residues is to enhance the endosomal escape polyplexes by protonation in the acidic pH of the endosome, as well as the proton sponge mechanism, which was evidently shown by Kawakami et al.,64 where bafilomycin, as an inhibitor of vacuolar H+-ATPase (V-ATPase) with the ability to bind to the V0 sector subunit c of the V-ATPase complex and inhibit H + translocation, causing an accumulation of H + in the cytoplasm of treated cells and preventing endosomal acidification, was used for the assessment of endosomal pH influences on the effect of Gal-His-C4-Chol and Gal-C4-Chol lipoplexes. Interestingly, gene expression levels by Gal-His-C4-Chol lipoplexes and Gal-C4-Chol lipoplexes were significantly inhibited in the presence of bafilomycin A1, confirming that histidine moieties enhance the endosomal escape of vectors based on pH-buffering mechanism. Aside from that, histidine could boost the increased extracellular stability and enhance intracellular release of the nucleic acids.55 That is to say, histidine is capable of protecting polyplexes from the disruptive effects of serum. The profound cause of this claim would be the hydrogen-bond networks between the imidazole ring and nucleic acids as hydrogen donors.65, 66 Lysis of endosomes by histidines/imidazoles in carriers emanating from a combination mechanism was also demonstrated. In addition to the proton sponge effect, membrane destabilization and polymer swelling could influence the endosomal escape of polyplexes.67
2 HISTIDINE-MODIFIED PEPTIDES
There are both intracellular and extracellular barriers to be overcome for efficient gene delivery.68 Therefore an ideal gene delivery vector must be able to preserve the chemical and physical integrity of nucleic acids in the extracellular space, avoid unwanted interactions with serum proteins or non-target cells, promote good cellular uptake, allow escape from endosomal compartments, as well as cytosolic transport, and finally encourage nuclear localization to overcome these barriers.69 One of the most promising approaches to fulfill these goals is the use of functionalized peptides, histidine-rich peptides in particular, because they not only enhance the cellular uptake and nuclear localization as well, but also improve specific cell binding and escape from endosomes.70, 71 Some of these peptides are discussed below.
Cell-penetrating peptides (CPPs) were originally derived from the HIV virus, and are less than 30 amino acids in length. CPPs have attracted a growing body of research interest for their potential as delivery vehicles. They have shown outstanding potential for intracellular delivery of various nucleic acid-based cargos, such as plasmid DNA, oligonucleotides and small interfering RNAs (siRNA).72 Interestingly, histidine-rich CPPs can improve the permeability of cargoes through the cell membrane by increasing the net hydrophobicity, thereby boosting direct membrane translocation.48 When the histidine moieties are positioned at the C-terminus of the polypeptide, the secondary structure can be guaranteed and the proton influx into the endosomes can be facilitated, culminating in endosomal rupture.73, 74
For example, Liu et al.75 improved the intracellular delivery of quantum dots (QDs) through direct membrane translocation mechanism by conjugating the QDs with histidine-rich and arginine-rich CPPs (HR9 peptides). The position of the histidine residues is crucial in the design of CPPs; to allow endosomal escape and the proton sponge effect, they are best positioned at the end of the peptide sequence.76 Although various types of proteins and polypeptides have been developed for gene delivery applications, one of the most attractive groups are those inspired by nature.77 For example, protamine and histone are nuclear proteins that can act as safe gene delivery vehicles,78 but the low endosomal escape capacity is a major limitation. These natural gene vectors can be improved by incorporation of histidine residues to facilitate endosomal escape. Zarei et al.79 devised a multifunctional delivery system based on low molecular weight protamine conjugated with histidine tags and a nuclear localization signal peptide, which could transfer plasmid DNA into adenocarcinomic human alveolar basal epithelial cells (A549) cells with high efficiency.
Artificial histidine-rich proteins can also be engineered to become vectors for efficient gene transfection in vitro and in vivo. For example, suckerin proteins are derived from squid sucker ring teeth, and possess His-rich domains with the ability to form supramolecular networks stabilized by nanoconfined beta-sheets. Suckerins can efficiently transfer DNA into several cell lines, as well as tumor-bearing nude mice. In addition to providing water solubility, His-rich domains in suckerin provide a positive charge for DNA complexation and allow passive chloride entry and water influx, leading to swelling and rupture of endosomes, because they are protonated at the endolysosomal pH.80
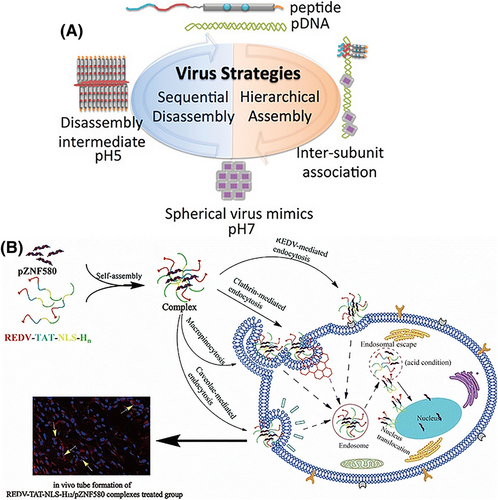
Furthermore, TAT peptides can be further modified by attachment of His residues to produce improved CPPs to deliver drugs, proteins and nucleic acids into cells. His residues can be added onto polycationic dendrimers and polymers as well. This functionalization offers multiple advantages, such as escape from endolysosomal vesicles by the proton sponge effect. Kamaruzaman et al.81 introduced an oligonucleotide delivery system by functionalization of poly-l-lysine dendrons with TAT peptide and His residues. Moreover, a His-modified-star-shaped polymer carrier with enhanced endosomal escape ability was described by Ren et al.82 and Hao et al.83 They used this vector to transfer the ZNF580 gene into endothelial cells to enhance their proliferation and migration, but inhibit smooth muscle cells. Additionally, Wang et al.84 designed a novel delivery system based on polyethylenimine and a TAT peptide modified with His and cysteine residues for transfection of the gWiz-GFP plasmid into umbilical cord blood-derived mesenchymal stem cells for bone regeneration and delivery of therapeutic proteins.
The introduction of redox-responsive cross-linkers, such as disulfide bonds, into the structure of His-rich peptides employed for gene delivery may provide improved cellular uptake and transfection efficiency. Yao et al.85 prepared a highly-efficient DNA delivery system based on a disulfide-crosslinked stearylated polyarginine peptide functionalized with His residues. The transfection efficiency of the resulting peptide was improved by two mechanisms. First, the disulfide bonds can be cleaved by a thiol-disulfide exchange reaction with intracellular glutathione, leading to DNA release into the cytoplasm and hence to the nucleus, thereby improving transfection efficiency. Second, the stearyl lipophilic chain enhanced the cellular uptake and endosomal escape ability of resulting peptide by improving the buffering capacity of the His residues.85 Likewise, a disulfide-cross linked chimeric polypeptide based on octa-d-arginine and tetra-l-His peptides was described by Wang et al.86 as an efficient gene delivery system for cancer therapy.
Another advantage of His-rich peptides is their potential to act as smart or stimulus-responsive delivery vehicles. The His residues within the structure of CPPs can serve as pH-responsive compounds. In this regard, Ni and Chau87 designed and fabricated a pH-responsive, self-assembled nanodisc using His amino acids and aromatic moieties, which formed a viral capsid-like structure to incorporate DNA based on the inter-nanodisc interactions. These nanostructure acted as functional artificial viruses enclosing DNA in the interior, which became disassembled in response to the acidic pH condition, allowing pH-dependent membrane permeation and/or endosomal buffering as well as DNA release into the cytoplasm (Figure 2A). Similarly, a potent pH-responsive, bioreducible cell penetrating foldamer was constructed via covalent dimerization of short amphipathic oligourea sequences bearing His units for efficient delivery of DNA into cells as described by Bechinger et al.89 Moreover, Xiang et al.90 reported a pH-responsive activatable CPP-functionalized liposomal system for siRNA-based cancer therapy.
Recently, RNA-based therapeutic approaches have been investigated for immunotherapy, protein replacement therapy and treatment of genetic disorders. His-rich peptides have shown great potential for the cellular delivery of various cargos, including RNA transfection. In this context, many investigators have developed various types of His-based peptides, including a His-containing amphipathic amino acid pairing peptide,91 and a series of multivalent peptide-modified bioreducible polymers.92 Novel CPPs based on oligohistidine, stearyl moieties and oligoarginine93 have been used for highly-efficient delivery of various kinds of RNA in vitro, as well as in vivo.
It is difficult to deliver nucleic acids to the correct tissues and organs, and this remains a major challenge to be overcome for gene therapy approaches. To this end, targeted delivery is an emerging strategy to enhance the therapeutic efficacy, at the same time as reducing undesired side effects on normal tissues and organs by delivering cargoes to the right place and avoiding off-target effects. A substantial number of targeted gene delivery systems based on His-containing peptides have been described. These include, a His peptide with stearyl moieties and a laminin receptor targeting segment,94 lauroylated His-enriched S413-PV peptide,95 Arg-Glu-Asp-Val peptide modified with TAT-nuclear localization signal (NLS)-oligohistidine88 (Figure 2B), an interleukin-6 receptor binding succinoyl tetraethylene pentamine-histidine oligomer peptide with blood–brain barrier-crossing ability96 (Figure 2B) and transferrin receptor-targeted oligoaminoamides.97 Hepatocyte growth factor receptor/c-Met binding His-rich polyplexes were designed to target tumor cells with overexpression of c-Met on their surface.38, 98 A bioreducible aptamer-decorated peptide was used for the co-delivery of fasudil and mi-RNA-195 to target hepatocellular carcinoma, where using this type of peptide vector resulted in delivering fasudil and mi-RNA-195 to target cancer cells with more efficiency, leading to the more increase in cancer cell apoptosis and reduction in tumor growth, compared to control vector.99 An arginine-His-rich peptide conjugated with an iRGD ligand was employed to deliver nucleic acids to targeted cancer cells expressing αvβ3 integrins.100
3 HISTIDINE-BASED POLYMERS
3.1 Histidine-modified linear polymers
Recently genome editing using CRISPR has become a promising strategy to tackle a wide range of diseases.6, 101, 102 However, the lack of suitable delivery systems for the components of CRISPR systems remains a challenge. Over the past decade, reduction-sensitive and pH-sensitive cationic polymers have been tested as versatile nanocarriers for triggered intracellular release of cargos in biomedical applications (gene delivery, drug delivery, etc.).102 To improve the targeted delivery, and enhance the expression efficiency various, complexes between DNA and cationic polymers have been functionalized by surface modification.
Linear cationic polymers can be categorized into those with a synthetic origin (e.g., polyethylenimine, poly-l-lysine) or a natural origin (e.g., chitosan, dextran). The potential of linear polymer-based nanocarriers for efficient gene delivery has been investigated in previous studies, in which PEI showed the best performance as a result of the high density of amine groups that can exert the proton sponge effect, leading to endosomal escape of vector/pDNA complexes and high cellular uptake efficiency.103 Moreover, the pronounced positive charge of PEI not only allows effective condensation of pDNA, but also can protect it against enzymatic digestion. In addition, the synthetic polymers are safe and cheap and can comply with administration standards. Of course, the endosomal escape activity of PEI is dependent on its molecular weight. In low molecular weight PEIs (such as PEI10kDa), the endosomolytic effect is not sufficient to release all the polymer/DNA polyplex, but PEI10kDa has a significantly lower cell toxicity compared to PEI25kDa.104 Therefore, higher efficiency gene transfection and lower cytotoxity must be balanced against each other for human gene therapy applications. Therefore, various chemical modifications have been tested to overcome this limitation.105-107 For example, the effect of hydrophobic modification of low molecular weight (LMW) PEIs is to increase the transfection efficiency, whereas cross-linking of the LMW PEIs using biodegradable cross-links such as amide bonds, could allow gene transfer with minimal toxicity.105 Another approach is the use of a PEI core conjugated with peptides containing specific sequences, such as TAT, RGD or KALA to enhance both penetration and endosomolytic activity. Polymer modification of LMW PEIs could improve the transfection efficiency at the same time as retaining the low cytotoxicity.104 Some studies have reported the structure of a series of alkyl-oligoamine derivatives of PEI10KDa with increased lipophilicity providing low toxicity and high transfection efficiency. His residues can be used to modify the backbone of linear polymers such as PEI to increase the efficiency at the same time as maintaining low toxicity.
In this section, the use of histidine to modify gene therapy nanovectors such as PEI and chitosan for elevated transfection with improved biocompatibility are discussed. For example, LMW PEI chains could be connected together by degradable bonds such as a disulfide, ester or B-aminoester to improve transfection efficiency and lower toxicity.108 Also, the use of polyethylene glycol (PEG) and other hydrophilic chains to reduce aggregation and prolong the circulation time are very common. Notably, histidylated linear polymers are based on the imidazole ring as an effective endosomal escape mechanism, which can acquire a cationic charge when the pH drops below 6, allowing it to destabilize the membrane and induce permeability. As the permeabilization increases, the proton sponge effect also intensifies and the transfection efficiency improves.109, 110
3.1.1 Histidine-modified polyethylenimine
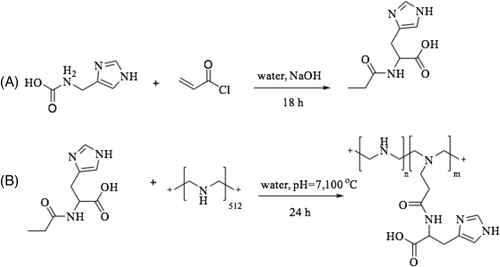
Bertrand et al.53 reported that linear polyethylenimine (lPEI) polymers modified with histidine residue increased buffering capacity, lowered toxicity and enhanced transfection efficiency compared to unmodified lPEI. Hence, different numbers of histidine residues were attached to a series of linear polyethylenimine polymers (His-lPEI) using the Michael addition reaction (Figure 3). The structure of the product was confirmed by 1H NMR. Protons of lPEI appeared at 3.04 ppm and the signals at 7.06 and 7.8 ppm were assigned to histidine. It was found that the transfection of HEK293-T7, HeLa and ΣCFTE9o- cells with His16−lPEI polyplexes was improved compared to lPEI polyplexes. Moreover, the cytotoxicity of His16-lPEI polyplexes was lower (only 10%) compared to lPEI polyplexes, which was reported to be 76% with C2C12 cells. Cell uptake of the polyplexes was measured by flow cytometry, and it was shown that 61% and 97% of HEK293 T7 cells were transfected using lPEI and His16-lPEI polyplexes.53
In 2020, Wu et al.108 constructed two novel polycations based on the low molecular weight polyethylenimine backbone (600 Da) with amino acid-containing bridges through a Michael addition reaction. Histidine and lysine were able to improve DNA binding, condense nucleic acids into nano sized complexes, and enhance the pH buffering capacity. In comparison with PEI25kDa, these new cationic polymers showed improvements, such as an up to 10.2 times higher transfection efficiency, as well as lower cytotoxicity and serum compatibility, which ultimately resulted in a promising candidate for non-viral gene delivery. Compared with LysP, HisP revealed relatively lower toxicity, despite the higher substitution ratio. This might be because of its overall lower molecular weight. In HeLa cells, the cell viability was < 20% and 84% at a w/w of 32 for LysP and HisP, respectively. In other cell lines, as expected, HisP also gave higher cell viability than LysP at w/w of 16 and 32. In summary, this result suggested that these two novel polycations could be excellent contenders for non-viral gene delivery.
3.1.2 Histidine-modified poly-l-lysine
Kodama et al.111 synthesized a modified nanocomplex consisting of pDNA and poly-l-lysine with the addition of poly-l-histidine and/or γ-polyglutamic acid to optimize the gene delivery performance, reduce the cytotoxicity, and enhance the pH-buffering effect. These composite complexes showed a small average particle size (52–76 nm) with cationic surface charge both in binary and ternary combinations. The gene transfection efficiency using ternary complexes with PLL replaced by PLH was about 25% for PLL6-PLH2 and 50% for PLL4-PLH4 complexes as a result of the increased pH-buffering capacity. Anionic γ-poly-l-glutamic acid (PGA) was used to lower the observed partial cytotoxicity. The quaternary combination showed higher gene expression and no cytotoxicity. Also, the in vivo transgene efficiency of the quaternary complexes showed higher luciferase activity in the spleen after their intravenous administration.
3.1.3 Histidine-modified dextran
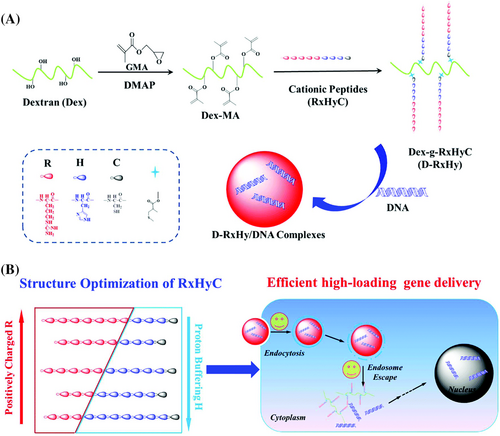
Hu et al.112 synthesized five different dextran-peptide vectors (D-RxHy) based on the dextran backbone (Figure 4). D-R3H7 demonstrated the best potential with the highest DNA-binding ability, lowest toxicity, the highest luciferase expression and the highest buffering capacity compared to the others with respect to gene delivery. Also, D-R5H5 showed the highest cell uptake at the low N/P ratio of 2, similar to D-R3H7. D-R3H7 had the highest buffering capacity as a result of having the highest number of ionizable groups.
3.1.4 Histidine-modified chitosan
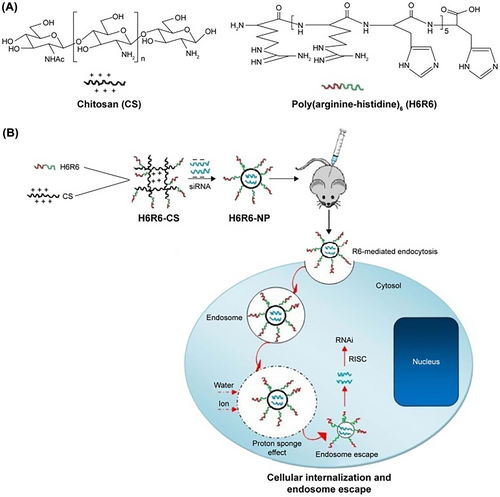
Chitosan and its derivatives are biodegradable and biocompatible cationic polysaccharides composed of glucosamine and N-acetyl-glucosamine, with the potential to be used a gene carrier. Additionally, its polycationic character allows the condensation of nucleic acids and protection of naked DNA against serum nucleases, along with its nontoxicity, have attracted much attention.113 However, the optimized formulation failed to produce a high transfection efficiency because of the poor cell membrane penetration capacity and slow endosomal escape rate, resulting in low nucleic acid uptake by the target cell. Moreover, the amino group of chitosan should allow endosomal escape because it could evoke the proton sponge effect similar to PEI. The modification of chitosan by attaching a variety of biological and chemical moieties has been investigated to overcome these drawbacks. Many studies have shown that CPPs, which could be natural or artificially synthesized polypeptides, can be used as efficient candidates in cell-penetrating biomaterials.114 For example, studies have reported that artificially synthesized CPP sequences containing arginine-rich peptides have good membrane-permeability as a result of their strong positive charges. With this in mind, Sun et al.114 designed poly(histidine-arginine)6 peptides including chitosan as a novel vector for siRNA delivery and breast cancer therapy. These were designed to have good cell membrane-penetrating capability and a swift endosomal escape rate (Figure 5). Histidine was used to modify the siRNA carriers to improve its buffering capability. These H6R6-chitosan/siRNA NPs showed improved properties compared to chitosan alone. Moreover, the poly(histidine-arginine)6chitosan (H6R6-Cs) copolymer could inhibit tumor cell growth and metastasis both in vitro and in vivo.
Mao et al.115 reviewed the factors involved in the expression efficiency of chitosan-mediated siRNA/DNA and chitosan-derived backbone nanovectors. They suggested that the transfection efficiency of chitosan-modified NPs and their cellular uptake depended strongly on the particle size, N/P ratio of chitosan to DNA or siRNA, degree of deacetylation, ambient pH and siRNA/DNA concentration, and could be optimized by varying these parameters.
In another study, Zheng et al.116 constructed three different chitosan-peptide vectors by grafting cationic peptides (arginine, histidine or cysteine) onto trimethyl chitosan-polymers to improve gene transfection efficiency. Then, the vectors were condensed with pDNA to form nanocomplexes, and the cellular uptake, endosomal escape, nuclear distribution, release behavior, and in vitro and in vivo gene expression efficiencies were evaluated. In TH/pDNA nanocomplex, despite the high ability of endosomal escape, good transfection results were not observed because of the lack of efficient pDNA condensation, poor structural stability and insufficient cellular uptake. On the other hand, TCNC (TC/pDNA nanocomplexes) showed excellent potential for gene transfection because of good stability, desirable uptake and suitable release. The drawbacks, including insufficient stability and poor pDNA release from TRNC (TR/pDNA nanocomplexes), led to a lower transfection efficiency. However, the addition of sodium tripolyphosphate could overcome the disadvantages of TRNC, resulting in improved gene expression.
In another study, Veilleux et al.117 identified formulations that preserve chitosan/DNA polyplex physicochemical properties and biological activity after lyophilization using minimal amounts of both lyoprotectant and buffer. Therefore, 3.5 mm histidine buffer (pH 6.5) combined with 5 kDa trehalose or dextran combined with 0.5% wt/vol sucrose were used to prevent NP lyophilized aggregation during freeze-drying. The results shown that in this situation NPs lyophilized could be rehydrated to a concentration up to 20-fold higher (Rh20X) at the same time as maintaining isotonicity, overtaking clinically relevant concentrations of approximately 1 mg of DNA/ml. These formed spherical nanocomplexes with a zeta potential of 19 mV, PDI below 0.32 and diameter below 200 nm. The cytotoxicity and transfection efficiency were evaluated in human embryonic kidney 293 cells. The polyplexes showed transfection efficiencies of approximately 65% without an effect on the viability and metabolic activity of named cells.
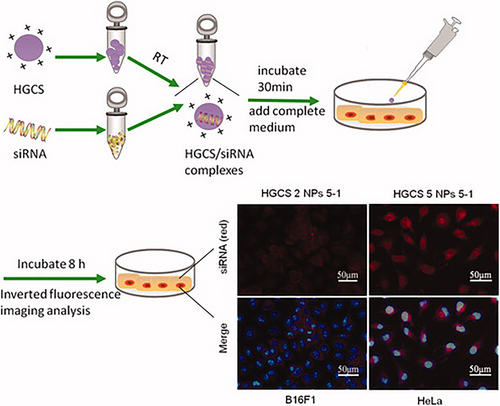
Liu et al.118 successfully fabricated a chitosan-based polymer conjugated to histidine and used it to achieve high expression efficiency and good biocompatibility for siRNA delivery (Figure 6). In their study, histidine was grafted onto chitosan via a Michael addition to produce histidine grafted on chitosan (HGCS)/siRNA nanocomplexes. These synthetic polymers demonstrated high buffering capacity, sufficient stability and high siRNA-binding. The cytotoxicity of the polymers was examined in RSC96 cells. They found that the HGCS polymers achieved efficient siRNA transfection in HeLa and B16F1 cell lines.
3.1.5 Miscellaneous linear polymers
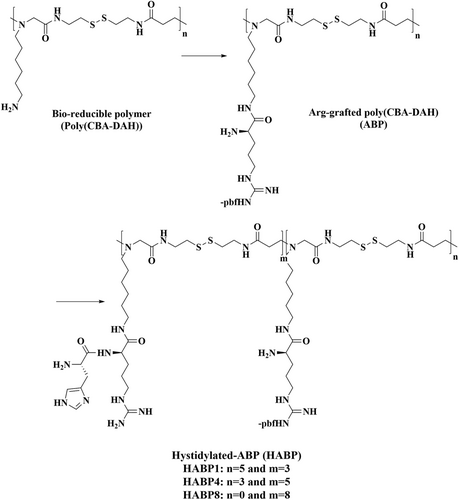
Nam et al.119 designed a histidinylated arginine-grafted bio-reducible polymer as a non-viral vector to improve cellular uptake and endosomal escape efficacy (Figure 7). This polymer consisted of an arginine-grafted bioreducible polymer containing a disulfide bond with different histidinylation ratios [histiylated arginine grafted bio-reducible polymer (HABP)]. The use of arginine provided cell-penetrating functionality and improved the cell uptake. The pDNA could be easily dissociated from the polyplexes by taking advantage of the bio-reducible functionality of the disulfide bond backbone. Moreover, histidine provided increased endosomal buffering capacity via its ‘proton sponge effect’ to facilitate the endosomal escape of the polyplexes into the cytosol. The particle diameter was estimated to be 90 nm. The optimal histidylation (HABP4) showed the highest transfection efficiency. These NPs were a good candidate for gene delivery with no significant cytotoxicity, in HeLa CT26, and H9C2 cells comparedw to the arginine-bio-reducible polymer without histidine grafts (arginine-grafted bio-reducible polymer), PEI25kDa, or lipofectamine.
Li et al.120 investigated the modification of poly(ω-aminohexyl methacrylamide (PAHMAA) with l-arginine and l-histidine to produce cationic polymers with high buffering capacity, improved gene transfection efficiency and decreased cytotoxicity. In the company of histidine, the optimized transfection efficiency can reach 10-fold higher than PEI25kDa (as a reference) because histidine plays a significant role in reducing cell membrane damage and cytotoxicity, and also provides high gene transfection efficiency both in the absence or presence of 10% fetal bovine serum. The concluded that PAHMAA-R18-H16 was a promising transfection agent with good transfection efficiency, improved serum tolerance and low toxicity for practical applications.
In recent years, there has been growing interest in antifouling materials that can respond to environmental stimuli (such as temperature, or pH) for many usages, including coating, drug and gene delivery vectors, as well as protein purifications. Accordingly, in an elegant study, Wang et al.121 prepared an amino acid-based zwitterionic and pH-responsive polymer, poly(histidine methacrylamide) to better understand the effects of zwitterionic materials. They investigated PHisMA materials under different pH conditions. They showed good antifouling properties that resisted protein attachment by the brush surface, and could form chelates with multivalent metal ions, such as Ca2+, Ni2+, Mg2+, Cu2+, or Fe3+, PhisMA could be a candidate for an extensive range of applications in drug and gene delivery, material coatings, bioseparation and even wound healing.
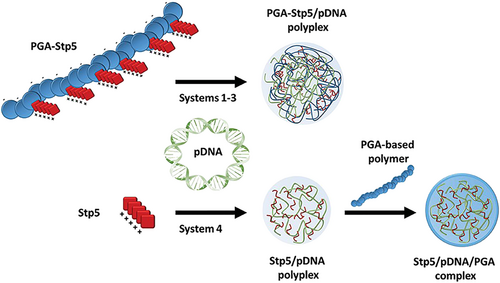
Niño-Pariente et al.122 investigated negatively charged polypeptides as effective non-viral vectors. They designed PGA derivatives and modified them with different pentameric succinyl tetraethylene pentamines (Stp5), plus histidine or cysteine moieties to improve pDNA condensation, endosomal buffering capacity, and the stability of pDNA complexes (Figure 8). These complexes were tested in N2a cells and 4 T1 breast cancer cells as shown by a luciferase expression assay and a GFP expression microscopy study, but no toxicity was reported.
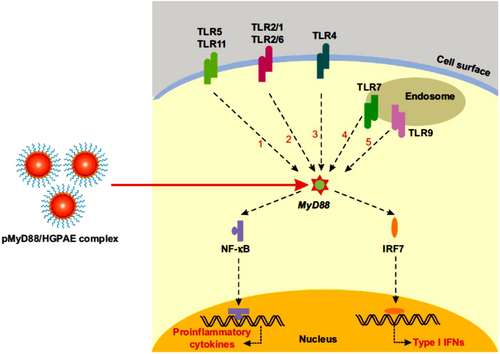
Toll-like receptors (TLRs), as an activator of biochemical pathways, through dendritic cell maturity and initiation of adaptive immune responses, result in provoke allograft rejection. Accordingly, Hu et al.,123 aiming to prolong allograft survival time by selectively prohibiting the expression of myeloid differentiation factor 88 (MyD88) as an essential adaptor in TLR signaling, designed a new histidine-grafted poly(β-amino ester) nanocomplex in a gene delivery system with safe and efficient properties (Figure 9). The resulting HGPAE nanovector with plasmid containing shRNA targeting MyD88 (pMyD88/HGPAE) had good biocompatibility and high transfection efficiency in the delivery and release of the pMyD88 both in vitro and in vivo. They also reported that the pMyD88/HGPAE complexes mediated remarkable inhibition of MyD88 expression in rat liver in vivo, resulting in pMyD88/HGPAE nanocomplexes that efficiently prevented MyD88 action with the therapeutic potential to prevent allograft rejection and graft refiusal of the donor liver, and serum levels of IL-2 and IFN-γ in the recipient were also significantly decreasd.
3.2 Histidine-modified branched polymers
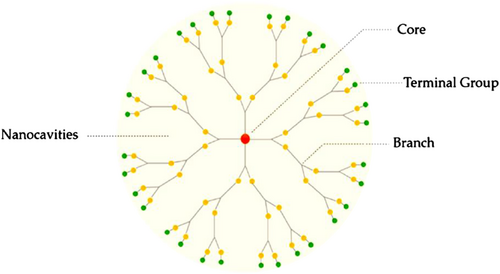
Dendrimers are a unique group of synthetic polymers with a highly branched, monodisperse, globular structure. Dendrimers consist of three main components: the core, the inner part and the end groups, often adopting a three-dimensional spherical structure (Figure 10). The core is in the center and the dendrons are connected to the core, and they are finally capped by the end groups. Dendrimers could be prepared using two major approaches: divergent and convergent. The size of the dendrimer pertains to the number of monomer layers that have been built up, each comprising another generation. Therefore, their structure, molecular weight and size (in the range of 1 to 100 nm) can be precisely controlled by the generation number.125 It should be noted that the unique dense architecture of dendrimers arises from their monomeric composition and arrangement. Their properties (e.g., well-defined size, positive charge, molecular weight, shape and monodispersity) make them suitable candidate for clinical application, especially as gene delivery systems. Depending on the nature and functionality of the end groups (e.g., the use of amino acids such as histidine), the physicochemical properties of dendrimers can be optimized, based on structure, chirality, shape and branching, etc. Dendrimers can be classified several types, including simple, hybrid, peptide, multilingual, amphiphilic, PAMAM, PAMAMOS, chiral, poly-(propylenimine) (PPI), metallo, micellar and liquid crystalline dendrimers.126 Among them, PAMAM and PPI both possess cationic primary amine groups at their surface, and have been extensively studied as gene vectors to treat a wide range of disorders.127 So far, as a result of their unique structure, several medicinal and practical applications have been investigated, such as gene delivery, vaccines and drug delivery systems for transdermal, oral, central nervous system, pulmonary, intravenous, nasal and ocular administration routes.124
3.2.1 Histidine-modified polyamidoamine (PAMAM) dendrimers
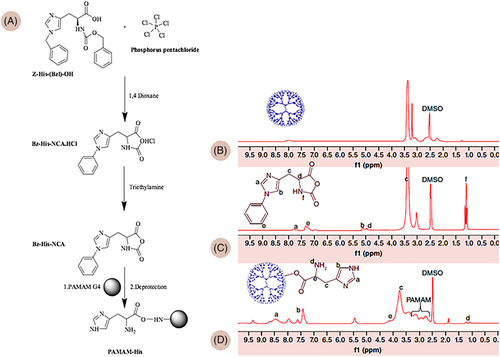
PAMAM dendrimers are hyperbranched, constructed from subunits of amide and amine groups, with spheroidal or ellipsoidal shapes, and a divergent method is employed to synthesize them, starting with ethylenediamine or ammonia as the core material. The high solubility and reactivity of these dendrimers is because of the presence of many functional end groups along with empty internal cavities. PAMAM dendrimers have a higher amine functional groups density than other commonly used macromolecules. Therefore, they have gained tremendous attention for gene therapy applications. The addition of other functional groups, such as histidine (His) to dendrimers, can increase their pH buffering capacity and provide excellent transfection efficiency with low cytotoxicity.128, 129 Each amino acid has different functions in the gene delivery process; therefore, gene transfection efficacy depends on the conjugation ratio and the type of amino acid.128, 130 The cargo is delivered to the target cells mainly through clathrin-independent endocytosis and, in some cases, micropinocytosis.131The structure of histidine grafted PAMAM G4.0 is shown in Figure 11.129
Wang et al.130 reported that the modification of dendrimers with His residues facilitated the endosomal escape of the polyplex, and achieved relatively high transfection efficacy as well as low cytotoxicity in the HEK293 cell line. They proposed that dendrimers modified with cationic or hydrophobic amino acids provided higher transfection and viability compared to anionic or hydrophilic amino acids. Chemical modification with the amino acids, arginine, phenylalanine and histidine of the various generations of PAMAM dendrimers (G4.0 and G5.0) revealed significantly increased efficiency with less cytotoxicity using PAMAM dendrimers conjugated with histidine (PAMAM-His) compared to the PAMAM-Arg, PAMAM-Phe, PAMAM-Arg-Phe or native PAMAM, However, the best efficiency was seen when the NPs contained all three amino acids.128, 132 Furthermore, various configurations of histidine conjugated to PAMAM dendrimers were evaluated. They found that PAMAM G4.0-HFR demonstrated improved transfection efficiency but increased cytotoxicity compared to PAMAM G4.0-FHR.132
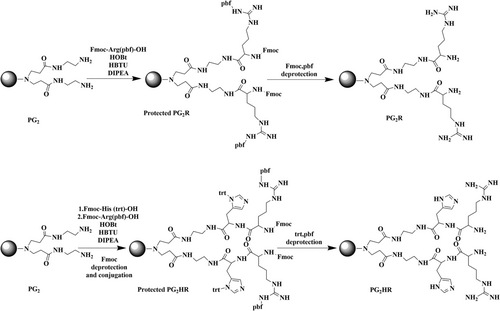
Different generations of dendrimer-based nanosystems have been investigated for the treatment of cancer, myocardial infarction and ischemic stroke. Lee et al.131 designed and fabricated a PAMAM generation 2 dendrimer conjugated with histidine and arginine (PG2HR) as a nanostructure to deliver a heme oxygenase-1 (HO-1) plasmid for treatment of ischemic stroke, as a result of its anti-inflammatory and anti-apoptotic properties (Figure 12). Local injection of the PG2HR nanocarrier into the brain of middle cerebral artery occlusion (MCAO)-reperfusion stroke animal model showed that the infarct size was reduced >10% and apoptotic cells were decreased approximately 30% by the pHO-1/PG2HR compared to MCAO control group and another HO-1/polymer complex. The pHO-1/PG2HR had higher gene delivery efficiency compared to PG2, PG2R and PEI25k as a result of the capability of histidine to facilitate endosomal escape.
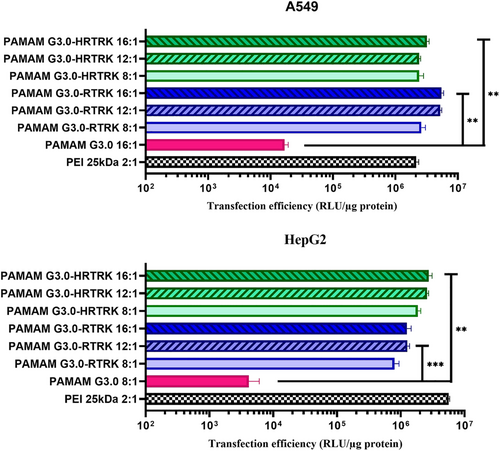
To enhance the gene expression efficiency of a 3rd generation PAMAM, surface modification was performed with influenza B virus nucleoprotein lysine-arginine-threonine-arginine peptide as a nuclear localization signal. Lee et al.133 synthesized PAMAM-G3.0-RTRK and PAMAM-G3.0-histidine-arginine-threonine-arginine-lysine (HRTRK) peptides to evaluate the influence of the histidine residue on cytotoxicity. They found that histidine residues could reduce cytotoxicity and allow prolonged gene expression. In HepG2 cells, the transfection efficiency of PAMAM-G3.0-RTRK and PAMAM-G3.0-HRTRK was similar to that of PEI 25 kDa, but, in A549 cells, the gene expression efficiency of PAMAM-G3.0-HRTRK and G3.0-RTRK was much higher than PEI25 kDa (Figure 13).
A polyplex composed of PAMAM-FHR/pJDK-apoptin for inducing apoptosis in a human glioma cell line was described by Bae et al134 They showed that this nanocomplex increased the transfection efficiency at the same time as retaining the cell viability in comparison to the native PAMAM in a weight-ratio-dependent manner, even with high polymer concentrations. Analysis of the cell cycle phase distribution, annexin V staining and tetramethylrhodamine ethyl ester staining showed that apoptin caused apoptosis in the GBL-14 cell line, but not in normal fibroblasts.
Amongst all the PAMAM generations, the fourth generation has been the most widely investigated for histidine modification. Histidine-grafted PAMAM-G4 provided higher transfection efficiency and lower cytotoxicity in HeLa and mouse neuroblastoma 2a cells (Neuro 2A) compared to PEI or native PAMAM.135, 136
In addition, in U87-MG glioblastoma cells, which are considered highly vulnerable to exogenous gene transfection, Bae et al.137 demonstrated excellent transfection efficiency and high cellular uptake using PAMAM-G4.0-H-R/pJDK apoptin, resulting in decreased cell viability in U87-MG cells. By contrast, cell death was not observed in a newborn human dermal fibroblast cell line. The histidine residues increased the biocompatibility of the polymers, and decreased the cationic charge density, leading to less destructive interactions with the cells compared to PEI25KD and native PAMAM. In an attempt to prevent cardiomyocyte apoptosis post-hypoxia/reperfusion injury during myocardial infarction, a nanoplatform consisting of PAMAM G4.0-His and miR-194 was prepared and assessed in cardiomyocytes (H9c2) and primary rat ventricular cardiomyocytes. PAMAM G4.0-His/miR-194 increased the expression of anti-apoptotic genes and decreased pro-apoptotic genes so that H9c2 cells and primary rat ventricular cardiomyocytes had survival rates more than 80% and 50%, respectively, compared to antimycin A.129
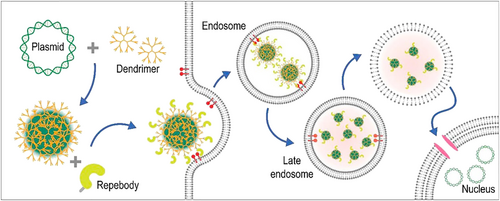
Previous reports have shown the rapid clearance of positively charged particles from the bloodstream through non-specific uptake by phagocytic cells. Therefore, researchers have investigated a negatively charged dendrimer complex to increase blood circulation time, which was assembled via electrostatic interactions between the HR-G4.0 dendrimer, PAP (polyanionic peptide)-repebody and pDNA. A ‘repebody’ is an artificial protein containing repeat units that can recognize a specific target. This nanocomplex delivered pDNA with high efficiency in a receptor-specific manner (Figure 14).138
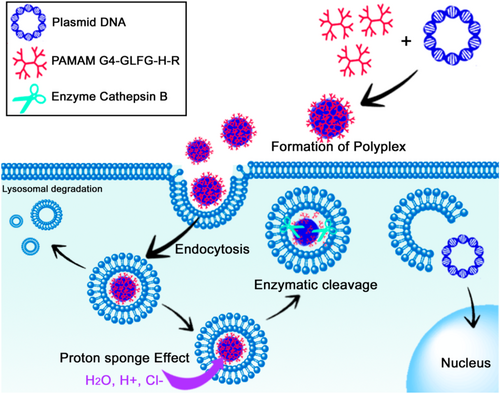
The differences between the environment of normal and cancer cells can be taken advantage of to prepare smart or stimulus-responsive NPs, to reduce off-target effects. For example, cathepsin-B, a lysosomal cysteine protease, is up-regulated in some cancers; therefore, using a cathepsin-B sensitive sequence (glycine-phenylalanine-leucine-glycine, GFLG) in the design of nanostructures can allow smart targeted delivery of the cargo. This was demonstrated in a report by Lee et al.,135 where the GFLG sequence was degraded by tumor cathepsin B and promoted the instability of a polyplex based on G4-GLFG-H-R/pDNA. The addition of histidine and arginine increased the proton sponge effect and the cellular uptake of the polyplex, respectively (Figure 15). G4-GLFG-H-R displayed higher transfection efficiency compared to PEI 25Kd and PAMAM G4 for HeLa and L929 mouse normal cells. The cytotoxicity was different in these two cell lines. In HeLa cells, all polymers were non-toxic, whereas, in L929 cells, PAMAM G4.0 showed low toxicity at high concentration, whereas the G4-GLFG-R and G4-GLFG-HR showed significant and dose-dependent cytotoxicity at high concentrations (> 50 μg/ml).
Another difference between cancer cells and normal cells is the acidic pH, caused by the Warburg effect. Based on this difference, a pH-sensitive hybrid gene vector [PAMAM/SBTS/LAH4-L1 (PSL) complex] was fabricated by self-assembly of poly(amidoamine) (PAMAM) dendrimers, the short cationic histidine-rich amphipathic peptide from the LAH4 peptide family, LAH4-L1, and the sleeping beauty transposon system (SB transposon system, a plasmid system capable of efficient and precise genomic insertion). The effects on HeLa and Madin–Darby canine kidney (MDCK) cells (a difficult cell line to transfect) were investigated. At acidic pH, four histidine residues were protonated, thus altering the conformation of the PSL complex and releasing the LAH4-L1 peptides. These nanocomplexes showed good serum tolerance (even in a medium containing 50% serum), and gave more than 90% and 30% transfection efficacy in HeLa and MDCK cells, respectively. In addition, > 30% of long-term gene expression was observed in the HeLa cell line.139
3.2.2 Other histidine-based dendrimers
Histidine-conjugated PAMAM dendrimers demonstrated better behavior compared to non-conjugated PAMAM dendrimers, confirming the crucial role of histidine. The same approach has been applied to other types of dendrimers for gene delivery, such as peptide dendrimers (e.g., PLL) and polyglycerol dendrimers.
Peptide dendrimers are radial or branched polymers consisting of a peptide-based core to which amino acid units are attached as dendrons by covalent bonding. The well-defined composition and ease of synthesis render these dendrimers good candidates for clinical applications such as vaccine delivery, diagnostic purposes and contrast agents for magnetic resonance angiography or magnetic resonance imaging, etc.140
Furthermore, PLL dendrimers are peptide dendrimers with the ability to condense oligonucleotides, whereas their biodegradability, biocompatibility, and water solubility are similar to other dendrimers.141
One study reported the preparation of three kinds of PLL dendrimers (containing lysine, arginine or histidine residues in their structure), their complex formation with siRNA molecules, transfection efficiency in myeloid cell lines and their cytotoxicity in vitro. The dendriplexes consisted of D3K2, containing two additional lysine residues (Lys-Lys) between each pair of neighboring branch points of the standard third generation lysine dendrimer, as well as D3R2, and D3H2 containing two additional arginines (Arg-Arg) or histidine (His-His) residues at the branch points. These differed in the strength of the dendrimer–siRNA interaction and binding stoichiometry. All of them showed > 90% uptake of the complexes regardless of the dendrimer structure, but had varying degrees of cytotoxicity in THP-1 and U937 cell lines. The D3R2 dendrimer had the highest surface elec0074rostatic potential, but the most cytotoxicity.142
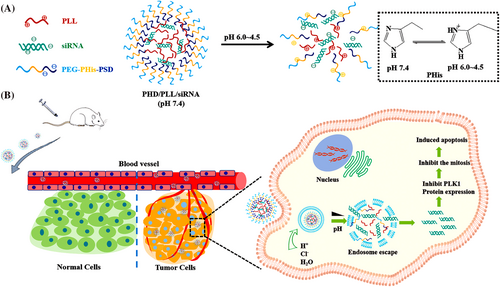
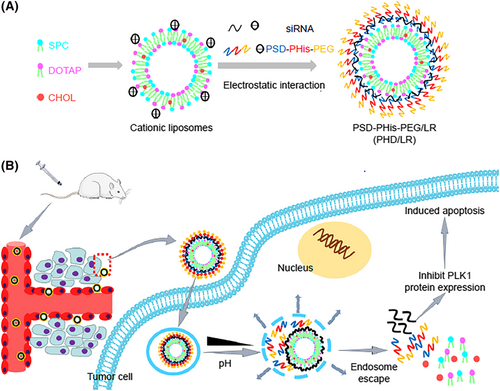
A nanoplatform composed of a polymer hybrid NP was enhanced for multistage siRNA delivery based on both pH-responsive and endolysosomal escape properties. It was prepared through a combination of electrostatic interactions between the PLK1-siRNA complex (siPLK1) and the copolymer methoxy poly(ethylene glycol)-poly(l-histidine)-poly(sulfadimethoxine) (mPEG-PHis-PSD, shortened to PHD), dendritic PLL for the treatment of non-small cell lung cancer (NSCLC) (Figure 16). The in vitro results showed that the PHD/PLL/siRNA NPs showed a good encapsulation efficiency, serum stability and endolysosomal escape ability. The PHD/PLL/siRNA NPs has a higher cellular uptake than PD/PLL/siRNA NPs on account of the proton sponge effect of the histidine chain, with > 85% viability in MCF-7 and A549 cell lines, even at high concentrations of NPs. The polyplexes were evaluated in vivo in A549 solid tumor xenografts in nude mice. The results showed that the PHD/PLL/siRNA NPs gave a 67 ± 4.68% tumor growth inhibition rate with a favorable safety profile compared to both PLL/PLK1 siRNA, PD/PLL/PLK1 siRNA polyplexes and the control group.143
In a study by Hashemi et al.136 PAMAM and PPI dendrimers with the same surface modification were compared. To increase the DNA transfection activity of these dendrimers, the third and fourth generation PAMAM dendrimers, and the fourth and fifdth generation PPI dendrimers were modified by partial replacement of surface primary amines with histidine, piperazine or pyridine, which have buffering capacity properties. The highest percentage of transfection was provided by higher generation dendrimers and higher grafting percentages (30% and 50% primary amines). In addition, the results of cell culture studies using murine neuroblastoma (Neuro-2a) cells showed that, when the number of primary amines was the same, PPI dendrimers gave a higher transfection rate than PAMAM dendrimers. It is worth noting that none of these vectors was noticeably toxic, and the most effective substituents for PPI and PAMAM were pyridine and piperazine.
Regarding the beginning of this section, polyglycerol dendrimers are considered in gene delivery. Polyglycerol dendrimers contain a glycerol core (polyglycerol dendrimer), and are attractive for the design of gene delivery vectors as a result of their blood biocompatibility.144
Zeng et al.145 reported a dendritic polyglycerol system (dPG) modified with a combination of histidine and aromatic amino acids, which was tested for safe and efficient siRNA delivery. The synergistic effect of the amino acid combination, the effects of the functionalization ratio, molecular weight of the dPG core and ligand density were studied in detail. The histidine-tryptophan-functionalized dPGs at a 3:1 molar ratio and 50% functionalization ratio (14kD-50%-75H25W) showed the best transfection efficiency with the lowest toxicity compared to other groups and non-functionalized amine-terminated dPGs.
A dendritic peptide containing bolaamphiphiles consisting of two l-lysine dendrons connected to a fluorocarbon core through disulfide linkages, and functionalized with l-histidine and l-tryptophan to increase endosomal escape and cellular uptake (Bola/mf10-PEG[2K]-siRNA) was described,146 This gave over 70% knockdown of GAPDH gene in differentiated adipocyte cells which are primary adipocytes and hepatocytes.
A summary of the different types of dendrimer/histidine polyplexes is provided in Table 1.
| Assessed nanostructures | Histidine-based carrier | Investigated cargo format | Diameter of polyplex | Reported stage of development | Effectiveness | Reference |
|---|---|---|---|---|---|---|
| PAMAM-G5.0 dendrimer modified with cationic amino acids | PAMAM-G5.0-Histidine65a/pDNA | plasmid DNA (luciferase) | ~200 nm | In vitro (HEK293 cells) | Gene transfection efficacy was ~100% (with 0 μM Nigericin) | 130 |
| PAMAM-G5.0 dendrimer modified with cationic amino acids (F,R,H) | PAMAM-G5.0-Arg47Phe24His25/pDNA | plasmid DNA (luciferase) | ~200 nm | In vivo reporter model on a HeLa tumor xenograft mice | Gene transfection efficacy in triple-functionalized dendrimer was higher than single-functionalized dendrimer | 128 |
| PAMAM-G4.0-FR PAMAM-G4.0-FHR PAMAM-G4.0-HFR | PAMAM-G4.0-HFR/pDNA | plasmid DNA (luciferase) | < 263.67 nm | In vitro (HeLa and HepG2 cells) | Gene transfection efficacy in PAMAM G4.0-FHR was lower than PAMAM G4.0-HFR | 132 |
|
PAMAM-G2.0 PAMAM-G2.0-R PAMAM-G2.0-HR |
PAMAM-G2.0-HR/HO-1 pDNA | plasmid DNA Heme oxygenase-1 (HO-1) | 50.1 ± 5.3 nm | In vivo with local injection into (MCAO)b- reperfusion stroke animal model | The PAMAM-G2.0-HR/HO-1 pDNA reduced the size of the infarct by 10% | 131 |
| PAMAM-G3.0 PAMAM-G3.0-RTRK PAMAM-G3.0-HRTRK (as NLS peptides from the influenza B virus nucleoprotein) | PAMAM-G3.0-HRTRK/pDNA | plasmid DNA (luciferase) | G3.0-HRTRK < G3.0-RTRK < PAMAM-G3.0 | In vitro (1. HepG2 cells 2. A549 cells) |
1. Gene transfection efficacy by PAMAM-G3.0-HRTRK/pDNA in HepG2 cells was similar to PEI 2. Gene transfection efficacy by PAMAM-G3.0-HRTRK/pDNA in A549 cells was much higher than of PEI |
133 |
|
PAMAM/pJDK PAMAM- FHR/pJDK PAMAM/pJDK-apoptin PAMAM-FHR/pJDK-apoptin |
PAMAM-FHR/pJDK-apoptin | plasmid DNA (pJDK or pJDK-apoptin) | 178.7 ± 1.2 nm | In vitro (GBL-14 cells) | compared to other complexes investigated, histidine complex has shown the highest percentage of transfection | 134 |
|
PAMAM-G4.0 PAMAM-G4.0-R PAMAM-G4.0-H-R |
PAMAM-G4.0-H-R/pJDK apoptin | Plasmid DNA (pJDK or pJDK-apoptin) | – | In vitro (U87-MG cells) | compared to other complexes investigated, histidine complex showed the highest percentage of transfection | 137 |
| PAMAM-G4.0-His | miR/antagomiR–PAMAM-G4.0-His | miRNA | 60 nm | In vivo (adult rat ventricular cardiomyocytes) | > 50% viability with miR/antagomiR–PAMAM-G4.0-His as compared with the antimycin A-treated control cells | 129 |
| PAMAM-G4.0-H-R/PAP- repebodyc | PAMAM G4.0-H-R/PAP- repebody/pDNA | plasmid DNA (luciferase) | 185.5 nm | In vitro (MDA-MB-231, HeLa and HepG2 cells) | EGFR expression level in MDA-MB-231 was higher than HeLa and HepG2 cells | 138 |
|
PAMAM-G4.0 PAMAM-G4.0-R PAMAM-G4.0-H-R containing the cathepsin B-enzyme sensitive sequence |
PAMAM-G4.0-GLFG4-H-R/pDNA | plasmid DNA (luciferase) | 177.23 ± 2.6 nm | In vitro (HeLa and L929 mouse primary cells) | The order of transfection efficiency: PAMAM-G4.0 and PAMAM-G4.0-R < PAMAM-G4.0-H-R in both cell lines | 135 |
| pH-sensitive PSL complex (PAMAM-G5.0/SBTS/LAH4-L1) | PAMAM-G5.0/SBTS/LAH4-L1 | plasmid DNA (sleeping beauty transposon system: SBTS) | 60 nm | In vitro (1. HeLa cells 2. MDCK cells) |
1. Gene transfection efficacy by PAMAM-G5.0/SBTS/LAH4-L1 in HeLa cells was more than 90% 2. Gene transfection efficacy by PAMAM-G5.0/SBTS/LAH4-L1 in MDCK cells was more than 30% |
139 |
| the third generation of PLL dendrimers (containing K or H or R) | D3H2 dendrimers/siRNA | siRNA | 249.67 ± 14.02 nm | In vitro (THP-1 and U937 cells) | Cellular uptake of dendrimer-siRNA complexes was shown exceeding 90% regardless of the dendrimer structure | 142 |
| mPEG-PHis-PSD, shortened to PHD/PLL and PD/PLL dendrimers | PHD/PLL/PLK1 siRNA | siRNA | 102.44 ± 3.6 nm | in vivo reporter model on A549 solid tumor nude mice | Tumor inhibition ratio by PHD/PLL/siRNA group was 67 ± 4.68% | 143 |
| PAMAM-G3.0/G4.0 and PLL-G4.0/G5.0 dendrimers modified with histidine, piperazine, and pyridine | PPI-G5.0-His50 and PAMAM-G4.0-His50/pDNA5 | plasmid DNA (luciferase) |
PPI-G5.0-His50: 253.75 ± 7.28 nm PAMAM-G4.0-His50: 126.53 ± 6.85 nm |
In vitro (murine neuroblastoma (Neuro-2a) cells) | The order of transfection efficiency when the number of primary amines is equal: PAMAM dendrimers < PLL dendrimers | 136 |
| dendritic polyglycerol modified with histidine and tryptophan | dPG-H/W6 (14kD-50%-75H25W) /siRNA | siRNA | – | In vitro (NIH 3 T3 fibroblast cells) | > 70% knockdown of GFP gene (at 75% serum concentration) | 145 |
| Lysine dendrons connected to a fluorocarbon core modified with histidine and tryptophan | Bola/mf10-PEG(2K) siRNA | siRNA | < 150 nm | In vivo reporter model on mice primary adipocytes and hepatocytes | > 70% knockdown of GAPDH gene | 146 |
- a G5-His65 shows much higher efficacy than G5-His113.
- b MCAO, brains of middle cerebral artery occlusion.
- c PAP- repebody, polyanionic peptide (epidermal growth factor receptor) – repebody.
- (a repebody has much smaller molecular size [30 kDa] than an antibody).
- 4 GLFG, glycine-phenylalanine-leucine-glycine.
- 5 The highest percentage of transfection was associated with higher generations of dendrimer and higher grafting percentages.
- 6 These dPG-AAs (dPG-H/W) were named by the molecular weight of the dPG core, the amine density, the single-letter amino acid code and its corresponding functionalization ratio. For example, 14kD-50%-75H25W refers to a dPG molecule with a 14 kDa dPG core and 50% amine functionalization, with 75 mol % of the amine conjugated to histidine and 25 mol % conjugated to tryptophan.
4 HISTIDINE-BASED NANOCOMPOSITES
Yan et al.147 designed tumor-targeted redox-responsive Au NPs conjugated with three types of multifunctional polypeptides to produce (AuNPPs). They used the targeting peptide GE11, the cell-penetrating peptide octaarginine (R8) and polyhistidine (Figure 17A). In comparison with the commercially available reagent BPEI 25 K, these AuNPPs showed better cancer cellular internalization, along with targeted gene transfection. The various relative positions of GE11 and R8 could affect the gene transfection efficiency and the targeting ability. The synthetic peptide GE11 was confirmed to effectively target the epidermal growth factor receptor (EGFR) that is overexpressed in various cancer cells. The best relative positions of R8 and GE11 were able to show synergistic effects. Because the short peptides were not able to efficiently condense the nucleic acids as a result of their low molecular weight, when the peptides were conjugated onto AuNPs via Au−S bonds, the nucleic acid loading was increased significantly. Moreover, AuNPs have suitable biocompatibility along with an adjustable size,148 making them useful as gene delivery vectors. The eco-friendly Au−S bonds can be cleaved in the high glutathione intracellular environment to allow redox-responsive cargo release (Figure 17B).149, 150 Among these nanocomposites, AuNPP6-1 showed the best efficiency, which was 5.6-fold higher than PEI 25 K at the optimal N/P ratio in lung cancer cells. The best-performing AuNPP6-1 was selected to transport anti-EGFR-shRNA into A549 tumor-bearing BALB/c nude mice, and in vivo fluorescence imaging illustrated that AuNPP6-1 specifically accumulated in the tumor sites to allow targeted gene therapy.
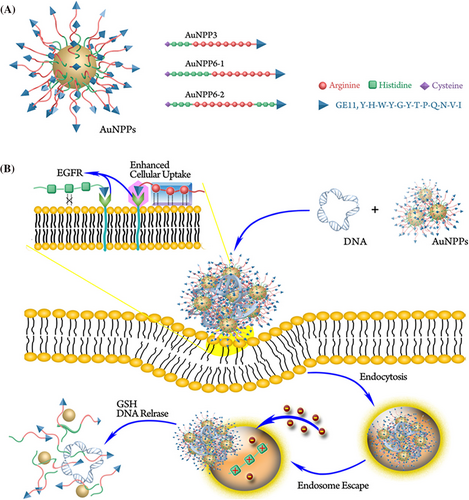
AuNPs possess a high surface area along with unique physical and chemical properties, making them a promising and efficient nonviral gene delivery platform for cancer therapy. Dhanya et al.151 prepared AuNPs using starch-PEI, where PEI acts as a reducing agent and the starch biopolymer as a stabilizing agent. Cytocompatibility investigation showed that C6 cells retained good viability even at higher polymer concentrations. Regardless of the high cellular internalization, the transfection efficiency of a p53 plasmid was compromised by modification of with the AuNPs. To achieve more efficient transfection, the starch-PEI was further modified with l-histidine or l-arginine and the corresponding Au NPs were prepared. Although starch-PEI AuNPs showed only low transfection efficiency, the amino acid-modified counterparts showed both good transfection efficiency and low cytotoxicity. The improved transfection could be a result of the presence of guanidinium functional group in the arginine moiety or the imidazole ring in the histidine moiety. Both amino acids assist in the endosomal escape once the nanoplex get internalized via diverse mechanisms along with proton sponge properties accelerated by amino groups of PEI. Qi et al.152 report that nanoplex including guanidino segments swiftly escaped from the endosomal compartment not via a proton sponge mechanism but via membrane penetration by interacting with a phosphate group. This observation demonstrated why the transfection efficiency of arginine-modified cationic polymer had no impact on the reduction of protonable amino groups by gold modification.
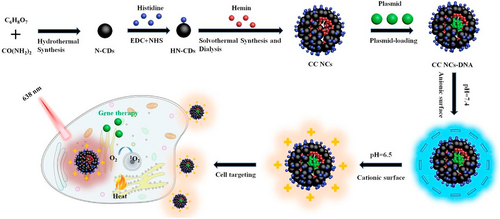
Photodynamic therapy (PDT) is a novel non-invasive treatment for cancer and other diseases, with high selectivity as well as low systemic toxicity.153, 154 Nevertheless, many photosensitizer agents that have been tested for tumor therapy have limitations such as poor targeting to tumors and insufficient water solubility.155 To tackle these problem, Yang et al.156 developed a tumor extracellular microenvironment responsive and photosensitive nanocomposite based on a combination of histidine and hemin along with nitrogen doped carbon dots (N-CDs) for improved PDT of tumors (Figure 18). The histidine grafted nanocomposites was able to undergo charge conversion, from a negatively-charged surface to a positive one in the moderately acidic tumor extracellular microenvironment (pH ∼6.5). This increased the affinity of the NPs to the negatively charged cancer cell membrane, thus boosting the targeting capability. At the same time, the photothermal activity of N-CDs and the photodynamic activity of hemin were improved through the fluorescence resonance energy transfer effect from the charge-convertible nanocomposites, which produced multifunctional nanocomposites that could deliver a plasmid into tumor cells for gene therapy. The transfection efficiency of this nanocomposite and DNA condensation ability with various mass ratios of 5:1, 10:1 and 20:1 at pH 6.5 were approximately 11.9%, 12.3% and 14.2% respectively.
5 HISTIDINE-MODIFIED LIPIDS
Cationic lipids are mostly applied as a transfection agent for packaging and condensing the DNA by ionic interactions. These lipids can increase the uptake of cargo into cells by binding to negatively charged mammalian cell membranes in vitro; nevertheless, the in vivo results have been relatively limited. The limitations are a result of immune reactivity and the increased uptake by cells of the reticuloendothelial system after binding to blood components. To tackle this problem, cationic lipids can be modified with additional moieties.157 Lipids generally have three component parts, including a hydrophobic tail, a linker and a polar head group.
Positively charged amino acids can be incorporated into the head group in cationic lipids.158 Histidine has the advantages of biocompatibility, amphoteric nature,159 antioxidant properties and cytoprotection.160 The imidazole ring in this amino acid provides a pH-responsive characteristic leading to endolysosomal escape.159 In several studies, histidine has been used as the head group of amphiphilic lipid molecules. The difference between these types of lipids is their hydrophobic tail and the modification route. The hydrophobic tail could be cholesterol,158 α-tocopherol,161, 162 1,2-dioleoyl-sn-glycero-3-phosphatidyl ethanolamine (DOPE),163 octadecyl amine (ODA),160 monolein glycerol164 or dioleoyl-glutamate.165 To produce a cationic liposome formulation, neutral lipids such as DOPE are sometimes used as the co-lipid.158
Although histidine alone has not shown the best results compared to other amino acid residues such as lysine or arginine,158, 165 the use of α-tocopherol as the hydrophobic tail attached to histidine by a disulfide linker showed a better transfection efficiency than lysine.161 These lipids were both pH sensitive and reduction sensitive. The disulfide bond was degraded in the cytoplasm in response to the high levels of the reducing tripeptide glutathione. Another tocopherol-based dipeptide cationic lipid was developed based on this dual-responsive system. Liu et al.162 compared six different natural amino acids, namely serine, lysine, phenylalanine, tyrosine, tyrptophan and arginine, including histidine as a pH-sensitive moiety in all of them. The materials were prepared from histidine, a disulfide linker, and tocopherol coupled with tert-butyloxycarbonyl-protected amino acids. In previous studies, positively charged liposomes showed the highest efficiency with an equal molar ratio of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), thus the liposomes were constructed using DOPC as a co-lipid along with the dipeptide lipids at a molar ratio of 1:1. All of the studied compounds showed better DNA-binding ability than the histidine containing base structure. The cytotoxicity of the lipopolexes were investigated in HEK293 (human embryonic kidney cell lines) cells using the MTT assay, with Lipofectamine 3000 used as the positive control. The outcome showed that the different polar head-groups significantly affected the toxicity. Serine or basic amino acids, such as arginine and lysine increased cell viability in comparison to aromatic amino acids. Examination of the cytotoxicity of the lysine and arginine compounds in human hepatocellular carcinoma and HeLa cell lines showed that lysine gave the lowest cytotoxicity to both cell lines, whereas arginine indicated higher cytotoxicity to HeLa cells. Among these six residues, the dipeptide lipid based on Arg-Hist gave the highest transfection efficiency. The transfection efficiency in HEK293 cells, HeLa cells and HepG2 cells was 18.1 times, 52.1 times and 3.4 times higher than Lipofectamine 3000, respectively.
The incorporation of histidine in the C3(C16His)2/DOPE nucleic acid delivery vector gave superior transfection efficiency and biocompatibility in COS-7 and HeLa cells, with results comparable or superior to Lipo2k.163
The in vitro transfection efficacy is often not representative of that seen in vivo because the positive surface charge increases binding between lipid vector and serum proteins, which increases the recognition by the reticuloendothelial system and decreases the distribution of particles throughout the body. Decoration of such systems by PEG159, 160 or PEI166 have been tested to overcome these problems and to increase circulation time. Although PEGylation can protect the positive charge of the structure from the serum proteins, they can also make the system too stable to release the cargo, thus decreasing the overall efficacy. To improve body distribution and on-demand cargo release, researchers have designed various PEG derivatives more suited to their environment. First, a cationic lipid core including the soybean phospholipids, N-[1-(2, 3-di-oleyloxy) propyl]-N,N,N-trimethylammonium chloride, and cholesterol (4:8:2, w/w) was bound to a copolymer shell (PEG-block-Phis-block-poly sulfadimethoxine [PSD]) triblock copolymer, to construct a multifunctional delivery system with pH-sensivity and the proton sponge effect for delivering siPLK1 into the cytoplasm of lung cancer cells (Figure 19). The constructed NPs could efficiently deliver siRNA into the cells and knock down PLK1 expression.159
Recently, a lipid NP formulation was developed for effective delivery of the BMP-9 gene to bone marrow mesenchymal stem cells using histidinylated ODA to reduce the toxicity and to boost the transfection rate. In this formulation, compounds with a C18 tail and an ionizable headgroup were synthesized (Boc-His-ODA, BHODA and His-ODA, HODA) and characterized. Lipid NPs were used to encapsulated the bone morphogenetic protein-9 gene (BHODALNP, HODA-LNP) and the bone-homing peptide targeted (HODA-LNP, HODA-LNPT). Boc-histidine was coupled to ODA using a day reaction time at a Boc-His to ODA molar ratio of 1 to 0.5. The size of the HODA-LNPs was below 100 nm, whereas the size of BHODA-LNPs was 107 nm. Moreover, BHODA-LNP showed a somewhat more positive zeta potential than HODA-LNP. All the lipid NPs showed good in vitro transfection efficiency. These particles could induce the differentiation of C2C12 cells into osteogenic cells shown by deposition of calcium phosphate in vitro. These 100 nm size particles can passively accumulate in the bone. Cytotoxicity analysis showed they were non-toxic to C2C12 cells. They carried out in vivo studies using a OVX (ovariectomized) rat model of osteoporosis, showing bone resorption and reduced bone density in the spinal and femoral bones, similar to the human condition. Both lipid nanostructure effectively delivered BMP9 to increase bone formation in OVX rats.160
In 2019, a study described the preparation of a structure composed of mTat (HIV-1 Tat peptide modified with histidine and cysteine) plus INTERFERin, a cationic amphiphilic lipid-based reagent. This vector achieved 100% siRNA-mediated knockdown of β-actin expression in HSC-3 human oral squamous carcinoma cells compared to control cells.166
If histidine is incorporated in a peptide to potentiate its endosomal escape, the location of the histidine-rich domain will be critical for increasing the transfection efficiency. One investigation studied the effects of the histidine domain position in CPPs and the length of the fatty acids that were attached to this peptide. After selecting the best vector, this was used that to efficiently knockdown luciferase gene expression in U87-Luc cells.167
6 COMPUTATIONAL APPROACHES FOR GENE DELIVERY: ROOM FOR IMPROVEMENT
Computational chemistry along with bioinformatics has a long-standing record in the drug and gene delivery area. Computational approach and computer modelling are considered versatile tools for investigating nanomedicine-related dynamics at molecular and sub-molecular phases.168 Deploying an in silico modelling method alongside expensive experimentation could alleviate the costs and enlarge the scope of the investigation. Moreover, computer simulations have a great wealth of potential to lead the rational design of delivery systems.169
To perform quantitative investigation for interaction between media, carrier and drug or gene, a broad spectrum of precise computational strategies have been launched: docking, QSAR, quantum mechanics,170 chemoinformatic modelling171 coarse-grained,172 Monte Carlo172 and molecular dynamics (MD).172 Some methods are deployed in an odd assortment of software packages in MD, encompassing LAMPPS, GROMACS, CHARMM, AMBER and NAMD.173
Apart from those, other NP-based continuum models, such as the finite element method and the finite volume method, are used to determine the motion of drugs or genes via the vascular system.174, 175 These computational models could enhance drug and gene delivery efficacy as well as the therapeutic efficiency of bionanomedicine.176
Nowadays, artificial intelligence (i.e., AI) is considered as a promising and privilege tool that could be applied to tackle the current difficulties in medicine and pharmaceutics.177
One noticeable application of AI is the machine learning (ML) strategy, which deploys the machine accompanied by algorithms to automatically interpret and learn from an avalanche data to estimate the outcome.178 ML is surely appropriate to help scientists in drug and gene delivery because this approach enables the extraction of the efficient features in massive datasets not only to render an accurate prognostication devoid of any brute force of repetitive biological examines, but also to introduce novel rules, distinguish unexpected patterns and even reveal hidden knowledge from the big data.
ML models are universally divided to the supervised and the unsupervised models. A supervised ML model usually needs both of the input and the corresponding labelled output to investigate the relevance between the input and the output. Conversely, an unsupervised ML model often runs without any pre-assigned data labels, which could extract the data structure and subsequently cluster the input data into various groups with no labels.168
One of the well-known ML algorithms in the delivery systems field is support vector machines (SVMs). For instance, it is reported that SVM algorithms could recognize CPPs and prepare other potential sequences, which revealed developed cell-penetration capabilities.179
Another critical algorithm employed in biological neural networks and generally deployed to tackle delivery system problems that possess data with a non-linear relation is the artificial neural network.180, 181
The advent of powerful hardware with respect to graphics processing units and tensor processing units has culminated in the enhancement of the deep neural learning (DNNs) approach. DNNs have been established as a cutting-edge strategy for various types of data, namely text, voice, image and even biological data.182 Very recently, a new multimodality deep learning technique was used in COVID-19 drug repurposing183 and drug discovery.184
Recently, a deep learning long short-term memory neural network model was reported to predict anticancer peptides, which was named ACP-DL.185 The proposed ACP-DL is more appropriate than prevailing machine-learning models for an anticancer prediction mission with a critical comparison.
It is undeniable that mixing ML and experimental methods result in better delivery system outcomes.186, 187 Accordingly, in an elegant study in 2021, Pentelute et al.188 devised an approach to effectively sample the enormous space of functional peptides by deploying deep learning as well as standardized experimentation. This model was used to design abiotic miniproteins that could deliver an antisense PMO to the nucleus with considerable efficacy for a polypeptide-based variant and be applied as privileged intracellular carriers to deliver other types of biomacromolecules (e.g. fluorescent protein, enzymes and antisense PNA) to the nucleus and cytosol.
7 FUTURE TRENDS
Nowadays, research based on the use of histidine along with different functional groups and cross-linking to various organic groups has led to a gradual but limited increase in this stability, as well as fewer undesirable interactions. These histidine-based carriers have not yet been sufficient to make this material suitable for human testing. Nevertheless, there are several promising and particularly important reports. Undoubtedly, computational chemistry, bioinformatics and universally computer-based methods such as docking, QSAR, molecular dynamic simulation, machine learning and deep learning could revolutionize the discovery of more effective carriers and biomaterials. Future efforts may focus on preparing nanocomposites containing histidine and inorganic compounds, including NPs, metal–organic frameworks, layered double hydroxides and even MXenes. All of these inorganic nanomaterials have been used both alone and combined with organic structures for gene delivery, but, so far, very little research has been conducted with respect to combining these materials with histidine. No systematic research based on different derivatives of histidine with this group of inorganic nanomaterials has been performed. It is possible that, by using this approach, both the stability and the interactions will significantly improve. Furthermore, multicomponent reactions (MCRs) are one-pot reactions encompassing three or more readily accessible starting materials. They can allow the preparation of compounds with high molecular diversity, greater economy of atoms and a better E-factor (environmental factor, a measure of how ‘green’ a reaction is). More sustainable synthetic routes could not only improve green chemistry and sustainability, but also be used as flexible synthetic tools to design advanced macromolecules as gene carriers. Because MCRs can incorporate different reagents such as amino acids, polymers, fatty acids, carbohydrates and inorganic materials into a single platform, this approach may allow the preparation of highly practical and versatile gene carriers.
8 CONCLUSIONS
In the present review, the recent and significant research progress on non-viral vectors based on histidine has been summarized. Different aspects of physics, chemistry and biological mechanisms have been covered. The studies that have been outlined can give a clearer view into future research approaches for gene therapy. The green chemistry approach can be combined with gene therapy to prevent any unwanted genetic mutations, reduce harmful interactions and lower cytotoxicity. Biomedical scientists throughout the world should pay more attention to the trend in favor of green chemistry with the aim of preventing multidimensional problems. The specific chemical structure of histidine has led to the design of many synthetic approaches and the construction of non-viral gene delivery vectors that have been tested both in vitro and in vivo. The low stability of some of these structures along with the possibility of weak interactions have been considered as limitations. These disadvantages have led to limited numbers of clinical trials being undertaken. Notwithstanding, we anticipate that this review article could help scientists to design and generate more sustainable and efficient carriers.
ACKNOWLEDGEMENTS
Funding support was received from Iran University of Medical Science (Grnat 97-3-75-13318).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
SEH was responsible for writing sections 4 and 8, as well as study conceptualization. SEH and NR were responsible for writing section 1. AH was responsible for writing section 2. MJS and NHM were responsible for writing section 3. SMKM was responsible for writing section 5. SAH was responsible for writing section 6. SHE, SAH and NR were responsible for writing section 7. SEH, AH, YL, MRH and MK were responsible for reviewing and editing the whole manuscript. MRH and MK provided special advice.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




