Thoracic complications of pancreatitis
Abstract
Acute pancreatitis in its severe form may lead to systemic inflammatory response syndrome and multisystem organ dysfunction. Acute lung injury is an important cause of mortality in the setting of severe acute pancreatitis. Besides lung involvement, acute and chronic pancreatitis may also lead to the involvement of other thoracic compartments, including mediastinum, pleura, and vascular structures. These manifestations are an important cause of morbidity and may pose diagnostic and therapeutic challenges. These manifestations have not been discussed in detail in the available literature. In this review, we discuss the thoracic complications of pancreatitis, including lung, pleural, mediastinal, and vascular manifestations.
Introduction
Acute pancreatitis is an inflammatory process caused by varying etiologies that involve the pancreas and extrapancreatic tissue. It is triggered by the autodigestion of the pancreas and extrapancreatic tissue by the inappropriately activated pancreatic enzymes. This leads to necrosis of pancreatic as well as peripancreatic tissue. This necrotic and inflammatory process may also involve remote organs, leading to multiple organ failure.1
In the 1992 Atlanta Symposium, acute pancreatitis was divided into two groups, “mild” and “severe,” based on the clinical and biochemical findings.2 In 2008, this classification was revised by the “Acute Pancreatitis Classification Working Group,” developing a new morphological classification based on imaging findings. Acute pancreatitis was divided into two groups as “interstitial edematous pancreatitis” and “necrotizing pancreatitis.” This revised classification identifies two phases of the disease—early and late—and severity of the disease has been classified as mild, moderate, or severe depending on the absence or presence of organ failure, fluid collections, and comorbid conditions.3-5
The complications of acute pancreatitis may be local or distant and immediate or delayed. Factors that can increase the likelihood of complications include old age, gallstones, the presence of organ failure, and pancreatic parenchymal necrosis.6
Pancreatic necrosis is considered to be one of the most important determinants of disease complications and severity.7 The severity of the pancreatic inflammation is classified based on the radiological appearances on computed tomography (CT) using the modified CT severity index.8 This score is based on the percentage of necrosis, the extent of fluid collections, and extrapancreatic involvement. It has been shown to have prognostic accuracy for the development of complications.8-10
Acute pancreatitis in its severe form can be complicated by systemic inflammatory response syndrome (SIRS) and multiorgan dysfunction syndrome (MODS). Acute lung injury (ALI) is one of the most important forms of organ dysfunction. In 22–29% of the deaths due to pancreatitis, intrathoracic complications have been implicated as the major factor, and are the contributing factor in a further 29–39%.11, 12 Intrathoracic complications can involve the pleura, lung, heart, pericardium, or the mediastinum, including the mediastinal vasculature.11-13
A total of 60% of deaths occur in the first week of hospital admission, and in these, the pleuropulmonary complication rate is up to 94%.14 Pulmonary complications include arterial hypoxia, atelectasis, pneumonia, and acute respiratory distress syndrome (ARDS). ARDS and respiratory failure are considered to be the most serious consequences of pulmonary involvement in acute pancreatitis.15, 16 Other intrathoracic complications include pleural effusion, empyema, pericardial effusion, cardiac arrhythmias, mediastinal collections, or pseudocyst.6, 17 Involvement of pulmonary vasculature may manifest as a pulmonary thromboembolism.18 Pseudoaneurysms involving the thoracic aorta and, rarely, the pulmonary artery have also been reported.19, 20 The overview of these complications is provided in Table 1. Tables 2 and 3 summarize the imaging features and management, respectively.
| Pulmonary | ALI ARDS Pulmonary edema Infections |
| Pleural | Pleural effusion Empyema Pancreaticopleural fistula |
| Mediastinal | Pseudocysts Mediastinitis |
| Cardiac | Pericardial effusion Congestive cardiac failure |
| Vascular | Pulmonary embolism Thoracic aortic aneurysm Pulmonary artery pseudoaneurysms |
- ALI, acute lung injury; ARDS, acute respiratory distress syndrome.
| Pulmonary | ALI/ARDS: Diffuse bilateral coalescent opacities nonresolving on diuretics with increased density posteriorly |
| Pulmonary edema: Diffuse bilateral alveolar opacities with peripheral sparing that resolves on administration of diuretic | |
| Infections: Lobar or broncholobar consolidations with air bronchograms, GGOs | |
| Pleural | Pleural effusion: Blunting of costophrenic/cardiophrenic angle Sensitivity: CT > USG > CXR (USG is the most practical method) |
| Empyema | |
| CT: Fluid bounded by thick and enhancing pleura-split pleura sign | |
| USG: Fluid with septations and internal echoes | |
| Pancreaticopleural fistula | |
| Xray: Massive pleural effusions | |
CT: Massive effusion with or without mediastinal collections Communication with abdominal collections can be seen |
|
| MRI/MRCP: Better demonstrates the fistulous tract | |
| ERCP: Invasive. Highly sensitive in demonstrating fistulous communication with the pancreatic duct | |
| Mediastinal | Pseudocyst |
| X-ray: Retrocardiac or paracardiac opacity-posterior mediastinal | |
| CT: Highly sensitive for detection, anatomic delineation, and extent. Thin- or thick-walled homogenous peripheral-enhancing hypodense lesion | |
| MRI: T2 hyperintense cystic lesion in the posterior mediastinum with or without fistulous communication with the abdominal collections | |
| EUS: Anechoic to hypoechoic cystic structure in the posterior mediastinum | |
| Cardiac | Pericardial effusion |
| Echocardiography/CT: Fluid in pericardial cavity | |
| Congestive cardiac failure | |
| Echocardiography: Cardiomegaly with dilated IVC | |
| Vascular | Pulmonary thromboembolism |
| CTPA: Hypodense filling defect within pulmonary arteries | |
| Thoracic aortic aneurysm | |
| CTA: Saccular outpouching, usually associated with mediastinal collections |
- ALI, acute lung injury; ARDS, acute respiratory distress syndrome; CT, computed tomography; CTA, CT angiography; CTPA, CT pulmonary angiography; CXR, Chest Xray; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; GGO, ground glass opacity; IVC, inferior vena cava; MRCP, magnetic resonance cholangiopancreatography; MRI, magnetic resonance imaging; USG, ultrasound.
| Pulmonary | ALI/ARDS: Respiratory support/mechanical ventilation with low tidal volumes/fluid management and treatment of underlying acute pancreatitis |
| Pulmonary edema: Administration of diuretics/fluid management | |
| Infection: Antibiotics | |
| Pleural | Pleural effusion |
| Mild to moderate with normal SpO2-conservative, fluid management | |
| Massive effusion or moderate effusion with suboptimal SpO2-Percutaneous catheter drainage | |
| Empyema: Percutaneous catheter drainage | |
| Pancreaticopleural fistula | |
| Drainage ± somatostatin analogs | |
| ERCP—assisted pancreatic duct stenting is the definitive procedure | |
| Surgery—second-line option | |
| Mediastinal | Pseudocyst |
| EUS-assisted transesophageal or transgastric drainage | |
| ERCP-assisted transpapillary pancreatic duct stenting | |
| Surgery, only if above measures fail | |
| Cardiac | Pericardial effusion |
| Mild/moderate—conservative | |
| Moderate/gross (with tamponade)—catheter drainage | |
| Vascular | Pulmonary thromboembolism: Thrombolysis/thrombectomy |
| Thoracic aortic aneurysm | |
| Endovascular repair | |
| Surgery |
- ALI, acute lung injury; ARDS, acute respiratory distress syndrome; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound.
Although there are detailed reviews on various complications of acute pancreatitis, thoracic complications find little mention in the literature. Therefore, in this review, we discuss in detail the various thoracic complications of acute pancreatitis, with emphasis on imaging findings.
Pulmonary complications
In the initial phase of inflammation, acinar cell injury occurs due to intrapancreatic digestive enzyme activation. The second phase is characterized by an intrapancreatic inflammatory reaction and varying degrees of acinar cell necrosis. The last phase is characterized by further progression of the pancreatic injury and the appearance of extrapancreatic changes, including SIRS and ARDS.21, 22 Pancreatic proteolytic enzymes as well as inflammatory mediators released as a result of pancreatic injury play a key role in the pulmonary complications. The pathophysiology of ARDS in acute pancreatitis has not been clearly understood and may involve leakage of protein-rich transudate into the alveolar spaces and decreased lung compliance, manifested clinically as refractory hypoxemia and radiologically as diffuse infiltration in the lungs.23-27 Activated trypsin can lead to increased vascular permeability and damage to the pulmonary vasculature. The main etiological factor for ARDS in acute pancreatitis is phospholipase A2, which causes the destruction of the surfactant.28-32
Diffuse involvement of the lung parenchyma with impairment in the exchange capacity at the alveolocapillary level is classified under the common term, called alveolar-interstitial syndrome. This is usually seen in critically ill patients with respiratory compromise.31
Alveolar-interstitial syndrome comprises many conditions such as acute pulmonary edema, ALI/ARDS, interstitial pneumonia, exacerbation of chronic interstitial lung disease, and other miscellaneous conditions.33-36
Pancreatic proteolytic enzymes as well as inflammatory mediators released as a result of pancreatic injury play a key role in the pulmonary complications.13 The pathophysiology of ARDS in acute pancreatitis has not been clearly understood and may involve leakage of protein-rich transudate into the alveolar spaces and decreased lung compliance, manifested clinically as refractory hypoxemia and radiologically as diffuse infiltration in the lungs.23, 37, 38
Acute lung injury
ALI in patients with acute pancreatitis occurs in three stages. In the first stage, patients have arterial hypoxia without any noticeable radiological changes followed by the second stage, characterized by mild radiological changes, and the third stage is synonymous with ARDS. Hypoxemia may occur without radiological abnormalities in 75% of cases.21
Early arterial hypoxia
A reduction in the oxygen saturation of blood is a frequent respiratory complication encountered in cases of acute pancreatitis, more frequent in the initial few days and in patients who are having their first attack of pancreatitis.23 This subset of patients has tachypnea and hyperventilation, leading to mild respiratory alkalosis. Clinical evidence of respiratory failure may not be obvious in these patients, and only 11% of these patients have radiological evidence of respiratory complications.15, 16 The radiological changes are in the form of patchy infiltrates on chest radiography and as patchy areas of consolidations and atelectasis on CT.37-39
Basal atelectasis
Nonspecific plate- and band-like atelectasis in the lower lobes of lungs is due to impaired surfactant activity and limited expansion of the lung bases.23 In addition, passive atelectasis can be seen due to the compressive effect of pleural effusions. On chest radiography, an area of increased density with associated features of volume loss in cases of segmental atelectasis can be observed.37 Linear or band atelectasis will be seen as a linear line or thick band-like opacity, mostly seen parallel to the diaphragmatic surface. CT can also demonstrate these atelectatic changes very well.40, 41
ALI/ARDS
In patients with acute pancreatitis, up to 20% of deaths occur prior to admission to hospital, the majority of them being caused by ALI and consequent respiratory failure.27
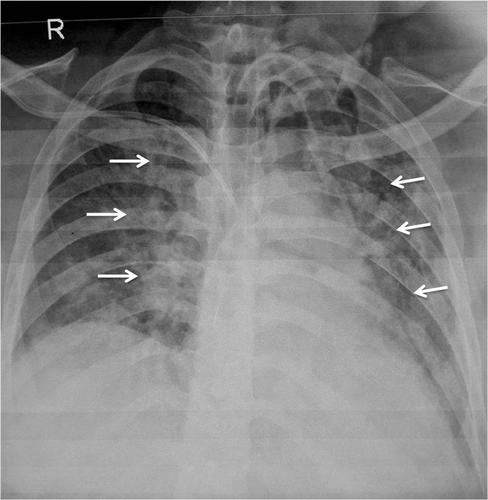
The presence of pleural effusion at the initial presentation correlates with the development of respiratory failure and mortality better than atelectasis and consolidation.28 ARDS usually manifests itself between 2 and 7 days following the onset of pancreatic inflammation. It is the most sinister complication of acute pancreatitis, with a reported mortality of up to 50%.29-31 Radiological manifestations are similar to ARDS of other causes and present as multilobar pulmonary infiltrates in patients with previously normal radiographs (Fig. 1).40, 42 The findings are, however, nonspecific. The time course of ARDS may help in differentiating it from pulmonary edema. In contrast to pulmonary edema, which clears in response to diuretic therapy, ARDS persists for days to weeks. In addition, as the initial radiographic findings of ARDS clear, the underlying lung appears to have a reticular pattern secondary to type 2 pneumocyte proliferation and fibrosis.40 Lung ultrasound can differentiate between edema and ARDS.41
No specific drug or therapy has proven beneficial in the prevention or management of ALI/ARDS. Although treatment of the underlying acute pancreatitis and the ongoing SIRS and MODS is essential along with supportive care, noninvasive ventilation, or mechanical ventilation using low tidal volumes, and conservative fluid management play an important role in recovery.25, 31
Pulmonary edema
Pulmonary edema may be partially due to generalized fluid overload because of excessive administration of fluids, diminished urine output, or both.30, 31 Imaging features are similar to ARDS and can be differentiated by the clearance of radiographic opacities following the administration of diuretics.37
Recently, transthoracic lung sonography has been proposed and used as a noninvasive modality for diagnosis of the alveolar-interstitial syndrome.33-36 It is primarily based on the detection and quantification of comet tail artifacts produced due to the reverberation of the sonographic beam. The comet tails or the B-lines are described as vertical artifacts fanning out from the lung wall interface and spreading up to the edge of the screen. Ground-glass opacities and thickened interlobular septa can produce this artifact. Increased extravascular water in pulmonary edema also can cause this artifact.33
Presence of intervening air foci or calcifications can limit the acoustic window for evaluation of the comet tail artifacts. Pneumothorax, chest wall emphysema, chest tubes, and pleural calcifications are obvious acoustic barriers to lung ultrasonography.33
Lung ultrasound can also help in predicting disease severity in the early stages of acute pancreatitis. A greater number of comet tail artifacts are described in patients with respiratory dysfunction, severe disease, and with elevated C-reactive protein (CRP). It was found to be particularly useful when only ultrasound of the nondependent lung (lower lateral and upper quadrants) was performed.39, 43
In pulmonary edema, interstitial fluid progress in a centripetal fashion to affect the peribronchial interstitium initially, and alveoli are affected in the later stages. In ALI/ARDS, the alveolar membrane integrity is compromised early, leading to early flooding of alveolar spaces resulting in heterogeneous areas of ground-glass opacities or consolidations.41
In ALI/ARDS, the comet tail artifacts are heterogeneous in distribution and are usually associated with the presence of pleural line abnormalities. Pleural line abnormalities include pleural thickening greater than 2 mm, coarse appearance of the pleural line, small subpleural consolidations, and reduction/ absence of pleural gliding. The presence of posterior dependent consolidations with air bronchogram is typical of ALI/ARDS. On the contrary, in pulmonary edema, comet artifacts show a homogeneous distribution with the regular pleural line, and normal lung sliding and lung consolidations are not characteristic.41
Fluid therapy in pancreatitis: Association with pulmonary complications
Supportive management, including aggressive fluid resuscitation, remains one of the important pillars of management of acute pancreatitis.43 Endothelial dysfunction secondary to cytokine storm leads to vasodilation and capillary leakage, which along with vomiting or ileus causes hypovolemia and perfusion failure.44 The aggressive fluid resuscitation leads to correction of this hypovolemia and thus maintains pancreatic perfusion as well as prevents systemic circulatory dysfunction.43 The aggressive fluid resuscitation involves the administration of 250–300 mL of intravenous fluid (saline or ringer lactate) per hour, with proposed end-points for guiding fluid therapy being clinical parameters, such as arterial blood pressure, heart rate, and urinary output; or laboratory parameters, such as blood urea nitrogen, or hematocrit; or invasively monitored parameters, such as central venous pressure (CVP).45 However, the evidence supporting aggressive fluid resuscitation is not so robust, and there is also evidence to suggest that overzealous fluid resuscitation can lead to various pulmonary complications (pleural effusion, atelectases, and pneumonia) and that rapid and massive hemodilution can lead to tissue hypoxia and consequent multiple organ dysfunctions.46, 47 Moreover, increased intestinal wall edema as well as increased intra-abdominal pressure because of overzealous fluid resuscitation can lead to paradoxical perfusion failure.48, 49 Hence, as uncontrolled aggressive fluid resuscitation can be associated with serious adverse effects, adequate fluids should be cautiously administered and tailored according to goal-directed therapy. However, pressure-based parameters like CVP have been reported to be unreliable because of factors like intra-abdominal pressure, mechanical ventilation, chest wall as well as mediastinal edema, and pleural effusions, confounding the CVP readings.49 The various goals for fluid resuscitation can be either noninvasive clinical targets, such as heart rate <120/min, mean arterial pressure between 65 and 85 mmHg (8.7–11.3 kPa), and urinary output >0.5–1 mL/kg/h, or invasive clinical targets of stroke volume variation and intrathoracic blood volume determination or biochemical targets of hematocrit 35–44% or trends in blood urea nitrogen levels. However, none of these parameters are ideal, and we need newer tools for optimizing fluid resuscitation in acute pancreatitis.
Pulmonary infections
Pulmonary infections can occur in the setting of acute pancreatitis secondary to generalized sepsis due to infected pancreatic necrosis, hypoproteinemia, and malnourishment and nosocomial infections due to prolonged hospitalization as well as mechanical ventilation and interventional procedures.
Imaging manifestations are similar to pulmonary infections due to any other causes.
Pleural complications
Pleural effusion
There are several mechanisms of pleural effusion in pancreatitis. One of the mechanisms is the transdiaphragmatic lymphatic blockage. There may be a disruption of pancreatic duct, leading to the leakage of pancreatic enzymes and the formation of a pancreaticopleural fistula. The latter is more likely to occur if the duct disruption is posteriorly into the retroperitoneum. Exudation of fluid into the pleural cavity from the subpleural diaphragmatic vessels may also cause pleural effusion.13
The incidence of pleural effusion in acute pancreatitis is reported to be about 3–17% in older literature, but recent reports show that the incidence is up to 50% based on detection by CT.42 Usually, effusions are mild to moderate and are left sided. These left-sided effusions are usually chemically induced or sympathetic in nature, with normal fluid amylase levels. In chronic pancreatitis, pleural effusion is quite rare and is usually due to the formation of pancreaticopleural fistulas.50, 51 Chest radiograph is the primary imaging modality for evaluating pleural effusion in the setting of pancreatitis. However, typically, the radiographs are taken at the bedside (portable), and due to the inadequate positioning and supine nature, mild to moderate effusions may be missed. Ultrasonography is the most sensitive method for detecting even a mild pleural effusion in the intensive care setting and has the advantage of being easily available at the bedside. Septations and internal echoes seen in ultrasonography can indicate infection. Chest CT is extremely sensitive in detecting even minimal amounts of fluid in the pleural cavity; however, it is rarely used for this indication. Infected effusions on CT are seen bounded by a thick and enhancing pleural wall on either side (“split pleura sign”).
In acute pancreatitis, the pleural effusion usually resolves as the inflammation subsides. If the effusion persists for longer than 2 weeks or if there is right-sided massive pleural effusion, the possibility of a pancreatic pseudocyst or pancreaticopleural fistula should be considered.51, 52 Overall, only 1% of the pleural effusions in the setting of pancreatitis are secondary to pancreaticopleural fistula.53
In case of massive pleural effusion or infected effusions/empyema, drainage of the same can be performed through an intercostal drainage tube or a pigtail catheter.
Pancreaticopleural fistula
Internal pancreatic fistulas are abnormal communications between the pancreatic duct and viscera or third-space cavities of the thorax or the abdomen. They can be direct or indirect through pancreatic fluid collections. They are seen in 0.4% of cases of pancreatitis and are more commonly seen in alcoholics.53 Recurrent effusions in the clinical background of pancreatitis or rapidly accumulating effusions should raise a suspicion of pancreaticopleural fistula. These effusions are also refractory to drainage procedures. The pancreatic secretions secondary to duct disruption dissect through the fascial planes, forming collections in the retroperitoneum, which then tracks and ascends superiorly into pleural cavity, forming pancreaticopleural fistula.53
The confirmation of the fistula is by demonstration of high pleural fluid amylase levels. Amylase-rich fluid can also be seen in conditions such as parapneumonic effusion; pulmonary tuberculosis; esophageal perforation; liver cirrhosis; hydronephrosis; leukemia/lymphoma; and malignancies of the lung, pancreas, rectum, and the gynecological system. To differentiate these conditions from pancreaticopleural fistula, pleural fluid amylase levels are helpful. Levels above 50 000 U/L favor fistula.53 Magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP) is the imaging modality of choice to visualize the fistula as it is superior to both CT and endoscopic retrograde cholangiopancreatography (ERCP) in delineating the tract within the pancreatic region.53, 54 MRCP is noninvasive and is also able to demonstrate parenchymal abnormalities of pancreas and collections in addition to the fistula (Fig. 2a). T2-weighted fast MRI sequences, such as the half-fourier acquisition single shot turbo spin echo (HASTE) sequence, can demonstrate the fistulous tract and its communication with the dilated pancreatic duct and pleural effusion. Heavily T2-weighted sequences, such as MRCP, allow a better demonstration of the fistula with the biliary and pancreatic ductal systems.52 ERCP allows therapeutic procedures and is rarely performed for diagnosis alone (Fig. 2b). CT scan has also been successful in making a diagnosis of pancreaticopleural fistulas, although it is less sensitive (43%) compared to ERCP (79%).55 Although less sensitive in delineating fistula, CT is useful to demonstrate pancreatic parenchymal atrophy, calcification, duct dilatation, and pseudocysts. CT scan performed immediately after ERCP has been found to increase the sensitivity of fistula detection because of the retained iodinated contrast in the ductal system and fistulous tract.56 Transabdominal ultrasound has also been reported to demonstrate pancreaticopleural fistulas, but the bowel gas and body habitus preclude adequate visualization in most of the patients.
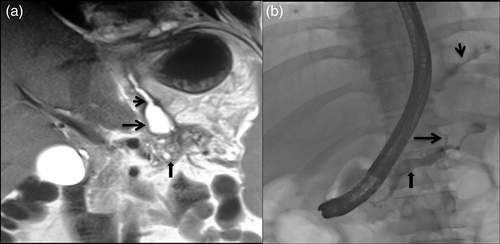
Rarely, the ascending pancreatic collections may also communicate with the pericardium, tracheobronchial tree, or the esophagus, forming pancreaticopericardial, pancreaticobronchial, or pancreaticoesophageal fistulas, respectively.
No standard guidelines have been established for the treatment of pancreaticopleural fistulas. Both conservative medical management and surgery have been used for the treatment of pancreatic fistulas. The aim of medical management is to reduce the pancreatic secretions and reduce the input to the fistulous tract. Multiple measures that were used in the past, including reducing the oral intake, insertion of the nasogastric tube, and total parenteral nutrition, are no longer recommended.57 Other measures such as pigtail or intercostal tube drainage combined with administration of somatostatin analogs like octreotide in order to reduce the exocrine pancreatic secretions have also been used.58-60 However, restoration of the pancreatic ductal anatomy is more important for the permanent resolution of the fistula.61 Recent advances in the management of pancreaticopleural fistula include the endoscopic therapies. Endoscopic transpapillary pancreatic duct stenting or nasopancreatic drain placement has been developed as an effective and minimally invasive alternative to surgery for the management of pancreatic duct disruptions and consequent pancreatic fistulae.62, 63 Transpapillary endoprosthesis facilitate the healing of pancreatic fistulae by obliterating the transpapillary pressure gradient and facilitating the drainage of pancreatic juice through papilla.64 Endoscopic drainage has the best results in patients with partial pancreatic duct disruption that has been bridged by endoprosthesis and is usually not effective in patients with complete duct disruption.64, 65 Therefore, during pancreatic endotherapy, it is important to bridge the disruption along with the stricture/stones in pancreatic duct by endoprosthesis. ERCP can demonstrate the fistulous track and simultaneously enable placement of a stent in the pancreatic duct. ERCP with transpapillary stenting has become the standard of management in several centers and has significantly reduced the need for surgical intervention. The main aim of treatment with a stent is to achieve drainage of ducts and fistulae (drainage into the duodenum) in the short term and drainage of the stenosed pancreatic duct in the long term.53 ERCP with stent procedures were reported to have a success rate of 96%.53 Surgery should be the second-line therapy. Indications for surgery are a failure of medical and endoscopic therapy, large-volume pseudocyst, persistent or recurrent effusions, and multiple strictures or complete duct disruption.53
Mediastinal and vascular complications
Mediastinal pseudocyst
A mediastinal pseudocyst occurs due to the extension of the pancreatic collections through the esophageal or aortic hiatus. It occurs secondary to the rupture or disruption of the pancreatic duct posteriorly and communication with the mediastinum. Less frequently, the collection can track and ascend anteriorly into the mediastinum through the foramen of Morgagni, the inferior vena cava hiatus, or into the retrocrural space by penetrating the left diaphragm.66 Anterior mediastinal pseudocysts can occur from an extension through the foramen of Morgagni, while middle mediastinal pseudocysts can occur through the diaphragmatic erosion or an inferior vena cava hiatus.67, 68 Usually, the pancreatic secretions will spread preferentially into the posterior pararenal space and pelvic extraperitoneal space in acute pancreatitis. Unusual drainage pathways can extend into the mediastinum. In patients with mediastinal collections, history of previous upper abdominal trauma, surgery, alcoholism, or previous hospitalization for pancreatitis were reported to be present in most of the case studies.69, 70 In these cases, the usual pathways undergo secondary inflammatory fibrosis from previous episodes, causing greater resistance to the spread of fluid collections. The mediastinum may represent the pathway of least resistance, leading to ascending pancreatic pseudocysts.71, 72
The patient may present with features of adjacent visceral compression-like dysphagia, odynophagia, dyspnea, or chest pain.69 A mediastinal mass or widening in the retrocardiac or paracardiac region, with or without associated pleural effusion, may be seen on chest radiograph. Ultrasonography allows for the accurate visualization of peripancreatic pseudocysts, but it is less helpful in a mediastinal pseudocyst owing to its location. CT is very sensitive and is the modality of choice in demonstrating pancreatic abnormalities and fluid collections (Fig. 3a, b).67, 68 It should be the first imaging study in cases suspected to have mediastinal pseudocysts. In these patients, the chest CT can be combined with the abdominal evaluation. CT establishes the location, extension, and relation of the pseudocyst with the surrounding structures. A mediastinal pseudocyst appears as a thin-walled, peripherally enhancing homogenous cystic lesion. Communication with the peripancreatic collection can also be demonstrated in some cases. Air may be seen in the case of infected collections.73-75 MRI with MRCP can demonstrate fistulous communications with the pancreatic duct or the peripancreatic collections. In addition, ductal morphology, such as disruption, communication with pseudocyst, stricture, and dilatation, are best defined by MRCP.68 Endoscopic ultrasound (EUS) is an important diagnostic modality for the evaluation of mediastinal pseudocysts (Fig. 3c). In addition to its diagnostic values, it can help in planning optimal therapy and allows for aspiration and drainage of the cysts (Fig. 3d). However, EUS is limited by its availability and expertise.74, 75
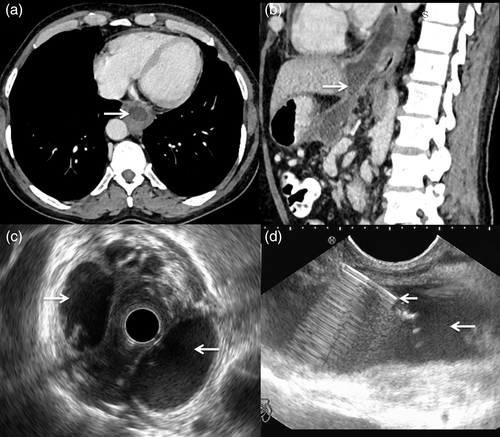
Life-threatening complications secondary to mediastinal pseudocysts can occur.76 A case of mediastinal pseudocyst eroding into the pericardial sac and presenting as life-threatening tamponade has also been reported in the literature.74 Spontaneous regression of mediastinal pseudocysts is rare.71
Management depends on the clinical symptoms, underlying etiology, ductal anatomy, size of the pseudocyst, and availability of expertise. Spontaneous resolution with conservative management is rarely reported. Recent advancements in endoscopic procedures have significantly improved the management of mediastinal pseudocysts. EUS-assisted endoscopic drainage through either a transesophageal or transgastric approach has been described, with immediate technical success in 90–95% of patients and long-term success in 85–90% patients.77 ERCP-assisted transpapillary stenting of the main pancreatic duct has been described for the management of mediastinal pseudocysts and has resulted in the successful resolution of mediastinal pseudocysts.71, 76-81 Complications of endoscopy-guided cyst aspiration include perforation of the esophagus, infection, and stricture formation.76-80 The complication rate of endoscopic procedures for pancreatic pseudocysts is approximately 5%, and pancreatic pseudocysts recur in approximately 15% of patients. Surgical treatment has often been used for the management of patients with mediastinal pseudocyst, and these can vary from pancreatic resections to external or internal drainages.17
Acute mediastinitis
Mediastinitis is usually secondary to esophageal perforation or surgery. Descending mediastinitis from the infections of the oral cavity and pharynx is less common. Pancreatitis is an extremely uncommon etiology for acute mediastinitis, and only a few case reports have described this rare complication.82-84 The underlying mechanism is similar to that leading to internal fistulae with the extension of pancreatic secretions into the mediastinum. It is seen as extensive mediastinal fat stranding on CT. Management is similar to that of internal pancreatic fistula. Stimac et al. reported mediastinal necrosis in a patient with alcohol-related acute pancreatitis and massive right pleural effusion.82 They managed the patient with octreotide; however, the patient succumbed to his illness. Chong et al. reported a patient of alcohol-related acute pancreatitis who had clinical worsening and progressive mediastinal fat necrosis on CT.83 This patient also had progressive intraluminal thrombus within the ascending aorta. He was managed surgically with debridement of mediastinal fat and removal of aortic thrombus. In the case reported by Choe et al., the diagnosis was based on an extension of the pseudocyst into the mediastinum.84 This patient was successfully managed by ERCP stenting.
Pulmonary embolism
Pulmonary embolism is a rare complication of pancreatitis, and there are only a few case reports in the literature.18 Multiple mechanisms have been postulated for vascular thrombosis and subsequent pulmonary embolization, such as cyst rupture into vessels, the release of proteolytic enzymes causing direct vasculitis, hypercoagulability secondary to pancreatitis, and hepatic dysfunction.18 The thromboembolic event may be suspected clinically but requires confirmation prior to starting definitive management. Doppler ultrasound of the lower extremity is commonly used to detect deep venous thrombosis. In patients presenting with acute shortness of breath, CT pulmonary angiography allows the detection or exclusion of pulmonary embolism with a high degree of sensitivity and specificity.85
Others
Pancreatitis can also cause pathological changes in cardiac chambers and the pericardium. Pericardial effusion has been found in some cases and is usually mild. Generalized cardiomegaly and features of congestive heart failure have also been reported to be associated with pancreatitis.38 Thoracic aortic pseudoaneurysm and dissection secondary to intrathoracic pseudocyst have also been rarely reported.19, 86, 87 Pulmonary artery pseudoaneurysm secondary to necrotizing pneumonia in a patient with acute pancreatitis has been reported.20




