Function and Regulation of Age-Associated B Cells in Diseases
Zi Geng, Yejin Cao, and Longhao Zhao contributed equally to this work as co-first author.
ABSTRACT
The aging process often leads to immune-related diseases, including infections, tumors, and autoimmune disorders. Recently, researchers identified a special subpopulation of B cells in elderly female mice that increases with age and accumulates prematurely in mouse models of autoimmune diseases or viral infections; these B cells are known as age-related B cells (ABCs). These cells possess distinctive cell surface phenotypes and transcriptional characteristics, and the cell population is widely recognized as CD11c+CD11b+T-bet+CD21-CD23- cells. Research has shown that ABCs are a heterogeneous group of B cells that originate independently of the germinal center and are insensitive to B-cell receptor (BCR) and CD40 stimulation, differentiating and proliferating in response to toll-like receptor 7 (TLR7) and IL-21 stimulation. Additionally, they secrete self-antibodies and cytokines to regulate the immune response. These issues have aroused widespread interest among researchers in this field. This review summarizes recent research progress on ABCs, including the functions and regulation of ABCs in aging, viral infection, autoimmune diseases, and organ transplantation.
1 Introduction
As the body ages, humoral immunity and cellular immunity gradually weaken, leading to an increase in inflammation levels in older individuals (Franceschi et al. 2000). During the aging process of the immune system, immune cells also change. First, atrophy of the thymus is a characteristic feature of immune aging, which leads to a decrease in the number of activated T cells (Douek et al. 1998; Liang et al. 2022), thereby affecting the regulatory effect of activated T lymphocytes on the development of bone marrow B cells and thus reducing the process of producing and maturing B cells (Ben-Yehuda et al. 1994). However, studies have shown that the number of peripheral B cells is relatively stable during aging because peripheral B cells can self-renew, and when new B cells stop producing, their lifespan is extended (Derhovanessian et al. 2010; Sprent et al. 1991; Ricker et al. 2021). Researchers first identified a special subset of B cells in aged female mice that increased with age and accumulated prematurely in mouse models of autoimmune diseases or viral infections; these B cells are referred to as age-related B cells (ABCs). The characteristic markers of ABCs include T-bet, CD11c, and CD11b, which are deficient in CD21 and CD23. The combination of these markers is conducive to differentiating ABCs from other B-cell populations (Hao et al. 2011; Rubtsov et al. 2011; Rubtsova et al. 2013). In a normal, healthy body environment, ABCs are almost undetectable in the lymph nodes and tonsils. In contrast, trace amounts of ABCs can be detected in the spleen, peripheral blood, and bone marrow, especially in mice with autoimmune diseases or viral infections, in which the proportion of this subpopulation increases and subsequent to the clearance of the infection, its quantity is predominantly maintained in the spleen (Mouat and Horwitz 2022).
ABCs exhibit heterogeneity, meaning that ABCs can be further subdivided into different subgroups with diverse functions and characteristics. Hence, clarifying the differentiation mechanism and immune effect of ABCs is not only conducive to understanding alterations in the immune system of elderly individuals, offering novel ideas for the development of treatment strategies for immune-related diseases in elderly individuals, but also provides new therapeutic modalities in the domains of autoimmune diseases and cancer, thereby increasing the quality of life of patients. This review summarizes the discovery process, main characteristics, and differentiation process of the ABC subset in humoral immunity, as well as the research progress in aging, autoimmune diseases, viral infections, and organ transplantation.
2 Discovery of ABCs
ABCs were first described by Hao et al. (2011) and Rubtsov et al. (2011) in infected elderly female mouse models. In 2011, Hao et al. (2011) used aged female BALB/c (BALB/c and C57BL/6) F1 and DBA/2 mice and reported that ABCs are neither a strain-specific trait nor the result of extensive homozygosity, suggesting that their accumulation is a universal and age-related process. These ABCs are responsive to intrinsic stimulation by toll-like receptor 7/9 (TLR7/9) signaling and preferentially produce interleukin (IL)-4 and IL-10 upon stimulation, thereby promoting polarization toward the Th17 phenotype. Rubtsov et al. (2011) used male BXSB mice to confirm the crucial role of TLR7 signaling in the accumulation of ABCs that form spontaneously; this also explains why the proportion of ABCs is greater in elderly female mice than in males, as the TLR7 gene is located on the X chromosome. Research has also revealed that elderly women (over 60 years old) with rheumatoid arthritis (RA) or systemic sclerosis (SSc) have abundant ABCs in their peripheral blood, but these ABCs are completely different from those in the spleen (Figure 1).
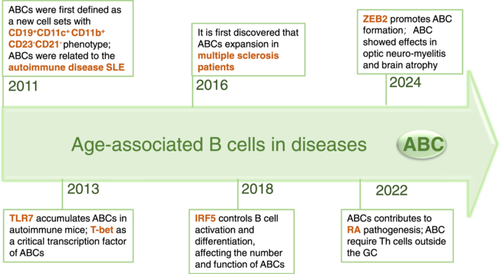
The number of ABCs in the spleens of young mice was almost absent, the proportion of ABCs in the spleens of mice from 12 to 24 weeks was very small, and the number and proportion of ABCs in the spleens of old mice from 48 to 72 weeks were the greatest (Cancro 2020). Moreover, ABCs were almost impossible to detect in the lymph nodes of both young and aged mice. ABCs are a group of B-cell subsets with unique characteristics, most of which are memory B cells (Cancro 2020). Although their definitions of ABCs are not exactly the same, there is a large overlap, which is of reference significance.
Although the markers employed for defining the ABC subpopulation remain unclear at present, researchers commonly believe that the cell subpopulation expressing CD19+CD21-CD23-CD11c+CD11b+T-bet+ is ABCs. CD21 (also referred to as CR2) is a complement receptor (Gorelik et al. 2004). CD23 is a B-cell-specific molecule that can function both as a receptor for IgE (FceR2) and as an adhesion molecule (Bonnefoy et al. 1995). CD21 and CD23 are typically expressed in mature B cells and significantly influence the activation process of B cells (Hardy and Hayakawa 2001). A significant reduction in the expression of CD21 and CD23 may have an impact on the function and survival of B cells. Hence, their absence might suggest that ABCs are at different developmental stages or functional states, thereby differentiating them from typical mature B cells such as follicular (FO) B cells and marginal zone (MZ) B cells. CD11b is expressed in murine myeloid cells, such as monocytes and macrophages. With respect to B cells, CD11b is related to the migratory potential of the cells. CD11c is highly expressed in murine dendritic cells (DCs) and is frequently employed for the identification of these cells. Research has indicated that ligand binding to CD11c can trigger the proliferation of B cells and prevent their adhesion to fibrin (Erdei et al. 2019; Nagy-Baló et al. 2021). Both CD11b and CD11c are associated with the adhesion and migration capabilities of cells, which might influence the localization and function of ABCs in the immune system. In 2013, Rubstsov et al. used anti-BCR and TLR7 agonists to stimulate most FO B cells in the spleen, which promoted the expression of the B-cell transcription factor T-bet and simultaneously drove the expression of CD11c and CD11b on B cells (Rubtsova et al. 2013; Yang et al. 2022). It has also been confirmed that T-bet within B cells can coordinate the formation of long-lived antibody-secreting cells induced by IFN-γ in a mouse model of viral infection (Stone et al. 2019). T-bet is an important transcription factor known as the T-box transcription factor, which plays a key role in regulating the activation, proliferation, differentiation, lifespan and effector function of T cells (Lazarevic, Glimcher, and Lord 2013). T-bet is an important factor in the conversion of the IgG2a class in B cells mediated by IFN-γ (Peng, Szabo, and Glimcher 2002). Therefore, ABCs are B cells that secrete antibodies but are not fully mature plasma cells. The definition of ABCs has been further refined as CD11c+CD11b+CD21-T-bet+Ig2a/c+. Subsequently, further in-depth studies were conducted on ABCs, as well as the gradual refinement of their phenotypic definition and function. Some ABC-like B cells (Wang et al. 2018) and double-negative (DN) B cells (Jenks et al. 2018) are referred to as ABCs.
3 Differentiation Mechanism of ABCs
Studies have demonstrated that the development of ABCs is not dependent on the germ center (GC), which is a separate pathway of development, and that the emergence of T-bet+CD11c+ B cells precedes the response of GC-B cells to lymphocytic choriomeningitis virus (LCMV) infection (Song et al. 2022).
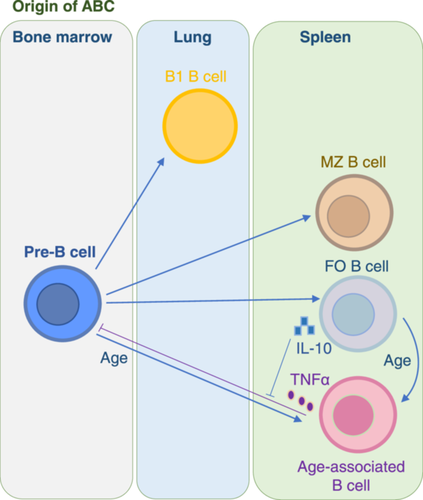
3.1 Origin of ABCs
In the lymphoid lineage, mature B cells are divided into different developmental subgroups by applying phenotypic definitions (Figure 2). By using the same method, mature B cells can be further divided into the B lymphoid FO subgroup, which is characterized by B220+CD19+CD11b-CD21intCD1dint; the MZ B-cell subgroup is defined as B220+CD19+CD11b-CD21highCD11dhigh; and the B1 B-cell subgroup, which is defined as CD5+B220lowCD19+CD11blow. ABCs are a newly formed B-cell pool that is distinct from FO B cells, MZ B cells, and B1 B-cell subsets in terms of phenotypic criteria, function, and other aspects (Table 1). First, they have specialized anatomical sites. FO B cells are predominantly present in the lymphoid follicles of the lymph nodes, while the marginal region of the spleen is the main habitat of MZ B cells. B1 B cells, as one of the peripheral B-cell subsets, are confined mainly to the abdominal cavity and mucosal tissues. In contrast to FO B cells, ABCs are not present in most lymph nodes or lymphatic vessels, which allows them to be effectively distinguished. Second, ABCs respond to TLR9 and TLR7 ligands but not to BCR or CD40, which are important factors for stimulating FO and MZ cell proliferation and survival. Third, the survival rates of FO B cells and MZ cells are affected by B lymphocyte stimulators (Blys), whereas those of ABCs are not (Hao et al. 2011). Fourth, the secretion of antichromatin IgG is exclusive to ABCs, whereas other B-cell populations predominantly secrete IgM (Rubtsov et al. 2011). Fifth, ABCs express histocompatibility complex-II (MHC-II) and some costimulatory molecules on their surfaces and may act as antigen-presenting cells (APCs) for T-cell differentiation (Hao et al. 2011; Rubtsov et al. 2015).
| ABCs | FO B cells | MZ B cells | B1 B cells | |
|---|---|---|---|---|
| Cell marker | CD11c+CD11b+ CD21- T-bet+ |
++- B220+CD19+ CD11b- CD21int CD1dint |
++- B220+ CD19+ CD11b- CD21highCD11dhigh |
+low+ CD5+ B220low CD19+ CD11blow |
| Anatomical sites | Spleen and peripheral blood | Lymph nodes | Marginal region of the spleen | Abdominal cavity and mucosal tissues |
| BCR and CD40 Response | × | √ | √ | √ |
| Blys stimulation | × | √ | √ | √ |
| TLR7/9 response | √ | × | × | × |
Secretion of antichromatin IgG |
√ | × | × | × |
Expression of MHC-II |
√ | × | × | × |
- Abbreviations: ABC, age-associated B cell; BCR, B-cell receptor; BLys, B-lymphocyte stimulator; FO, follicular; MZ, marginal zone.
ABCs constitute a previously uncharacterized new cell subpopulation that is somewhat similar to exhausted B cells but differs from FO B cells, MZ B cells, and naïve B cells in that the number of cells increases with age (Moir et al. 2008). Hao et al. (2011) studied peripheral B-cell subsets after autoreconstruction in aging mice irradiated at sublethal doses. The results showed that the aging B-cell progenitor population did not skew toward ABC generation in terms of internal and microenvironment characteristics, but the FO B-cell or MZ B-cell subset was restored. Whereas ABCs are similar to FO B cells, they used flow cytometric analyses of cell-surface Ags, concluding that they express similar levels of IgM and CD40. Additionally, they employed various in vivo and in vitro techniques, including adoptive B-cell transfer and sublethal irradiation, to confirm that under the synergistic stimulation of TLRs and BCR, FO B cells can acquire the ABC phenotype. ABCs may constitute a subpopulation that has undergone FO B-cell stimulation and differentiation.
Furthermore, as the proportion of ABCs increases, the number of FO-like B cells decreases in aged mice. However, ABCs induced the apoptosis of pre-B cells through the direct action of TNF-α (Frasca et al. 2012; Ratliff et al. 2013). FO-like B cells can “block” the effects of TNF-α on B-cell precursors by secreting IL-1028 (Figure 2). ABCs in aged mice suppress the growth of B-cell precursors through multiple mechanisms. Therefore, the pool of naïve B cells remains unchanged, whereas ABCs gradually accumulate and replace FO B cells in their niche.
3.2 Differentiation Signals of ABCs
3.2.1 Participation via TLR7 and TLR9 Signals
Research findings of Hao et al (Hao et al. 2011). suggest that ABCs are insensitive to stimulation related to BCR and CD40 costimulation in adaptive immunity. Additionally, qPCR analysis revealed that ABCs expressed TLR7 and were enriched in the expression of TLR9. Furthermore, in in vitro experiments, ABCs exhibited a special response to TLR7/9 ligands. Interestingly, they showed the maximum degree of cell division in response to combined BCR and TLR stimulation; this may be because some studies have shown that mild self-reactivity of BCR plays a certain role in promoting B-cell development (Zikherman, Parameswaran, and Weiss 2012). Rubtsov demonstrated for the first time that the TLR7 signaling pathway directly regulates ABCs to produce anti-chromatin IgG antibodies in a mouse model of autoimmune disease (Rubtsov et al. 2013). TLR9 is a receptor that senses endogenous DNA and is associated with autoimmune diseases. Previous studies have shown that when BCRs bind to TLR9 agonists, they trigger primary proliferation of B cells (Leadbetter et al. 2002), followed by cell apoptosis. The activation of TLR9 leads to the activation of p38 MAPK, which subsequently causes cell cycle arrest and mitochondrial apoptosis, such as when DNA-bound antigens bind to BCRs to trigger B-cell proliferation and cell cycle arrest. However, B cells can be saved during this process; how they are saved determines their subsequent fate of being split in half. Researchers have concluded through in vitro experiments that if BLyS is added to save B cells, they will differentiate into cells that secrete antibodies. However, if CD40 costimulation and IFN-γ or IL-21 are used to save B cells, they will express the T-bet+ phenotype (Sindhava et al. 2017). Leibler et al. subsequently reported through studies on lupus-prone mice with TLR9 point mutations that TLR9 can negatively regulate the pro-inflammatory signal transduction of TLR7 by controlling the endoplasmic reticulum localization of TLR7. Additionally, TLR9 has anti-inflammatory effects that are dependent on ligands that are independent of MyD88 signaling (Leibler et al. 2022) (Figure 3).

In addition to the classical regulation of ABCs by TLR7/9, recent studies have shown that in the cells of lupus patients, the mammalian target of rapamycin complex 1 (mTORC1) pathway is significantly activated (Wu et al. 2019). By blocking the effect of rapamycin on mTORC1 signaling, the differentiation of T-bet+ B cells can be inhibited (Figure 3).
3.2.2 Many Transcription Factors Promote the Differentiation of ABCs
The differentiation of ABCs requires the participation of various factors, such as IRF5, IRF8, and ZEB2. The transcription factor T-bet plays a role in promoting category switching during ABC differentiation and providing protective immune subtypes, but its role in this process is not essential.
3.2.2.1 IRF5 Promotes ABC Differentiation
According to the findings of Manni et al. in the absence of the SWEF protein, B cells exhibit a stronger ABC formation response to IL-17 and IL-21 because of the increased activity of the specific interferon regulatory factor (IRF) family member IRF4. Subsequently, IRF4 can interact with T-bet at selected ABC-specific regulatory regions to perform regulatory functions (Manni, Ricker, and Pernis 2017). They further discovered that IRF5 regulates ABCs by modulating the formation of IL-21-mediated ABCs. In the Michela Manni (Manni et al. 2018) study, IRF5 deletion significantly reduced the ability of IL-21-driven double knockout (DKO) B cells (lacking SWAP-70 and DEF6) to produce ABCs and attenuated their ability to produce IL-6, CXCL10 and IgG2c. In addition, IRF5 also regulates the binding of ABC-specific sites by interacting with T-bet, further reducing the activation level of age-related B cells. Therefore, IRF5 plays an important role in the regulation of ABCs. IRF8 and IRF5 synergistically promote the generation and differentiation of ABCs. In ABCs, IRF5 and IRF8 are highly expressed (Ricker et al. 2021; Manni et al. 2018). Hui Shen et al. reported that knockout of estrogen receptor-α (ERα) in spleen cells resulted in relatively low Irf5 mRNA expression, suggesting that Irf5 gene expression is associated with the sex of mice (Shen et al. 2010) (Figure 3).
3.2.2.2 ZEB2 Promotes ABC Differentiation
The latest study by Dai and colleagues revealed that the transcription factor zinc finger E-box binding homeobox 2 (ZEB2) promotes the formation of ABCs (Figure 3). ZEB2 binds to the intronic enhancer of muscle cell enhancer factor 2b (Mef2b), which is a key transcription factor for GC development and is specific to humans and mice. Therefore, the ability of activated B cells to deviate from the GC and differentiate into ABCs outside the follicle determines the fate of ABCs. ZEB2 may also work together with other transcription factors, such as IRF5, T-bet, and metabolic regulator factors, to form a regulatory complex in ABCs, thereby controlling ABC differentiation through the regulation of gene expression. This study also used RNA sequencing technology to analyze B cells lacking ZEB2 and reported that ZEB2 deficiency leads to an inverse expression pattern of characteristic ABC genes, thereby verifying these findings (Dai et al. 2024).
3.2.2.3 Bcl-6 Is Not Essential During the ABC Differentiation Process
There is controversy as to whether ABC differentiation requires the presence of Bcl-6. First, this study revealed that the early development of CD11c+T-bet+ B cells in a mouse Ehrlichia infection model is dependent on endogenous Bcl-6 expression in B cells. Experimental data revealed that these cells failed to develop in the absence of endogenous Bcl-6. Moreover, while Bcl-6 expression was detected during the early stages of CD11c+T-bet+ B-cell development, it significantly decreased during the later stages of infection (Levack et al. 2020). However, the opposite conclusion was subsequently drawn (Laidlaw and Cyster 2021). These findings suggest that the development of CD11c+T-bet+ B cells may not depend on the typical germinal center pathway but may still require the expression of Bcl-6. Researchers subsequently discovered that by crossing KIKA mice with Bcl6flox/floxcd23 cre mice, which are unable to form GCs, although the number of GC B cells decreased, the proportion of ABCs and plasma cells in the mice was significantly greater than that in the control group, and self-antibody production was detected, suggesting that the development of ABCs is not dependent on Bcl-6 (Brown et al. 2022). A mixed bone marrow chimerism experiment conducted by Song w et al. also revealed that the development of T-bet+CD11c+ B cells in a competitive environment does not require the intracellular expression of Bcl-6 (Song et al. 2022).
3.2.3 Multiple Cytokines Promote the Differentiation of ABCs
Naïve B cells must receive direct stimulation from TLR7/9 to differentiate into ABCs (Figures 3, 4). However, only these innate signals are insufficient; adaptive signals, such as IL-21 and IFN-γ, are also required (Wang et al. 2018; Naradikian et al. 2016).
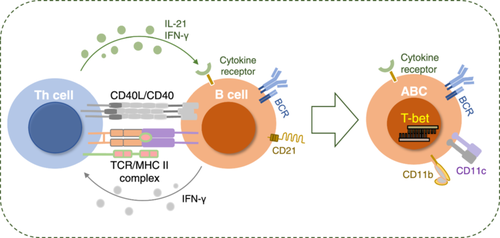
Naradikian et al. (2016) discovered through an H. polygyrus infection model and a Helicobacter pylori infection model that type 1 helper T cells (Th1 cells) secrete IL-21 and that IFN-γ can directly promote the expression of T-bet in B cells, whereas IL-4 has an inhibitory effect on the induction of T-bet. In other words, IL-4 blocked the upregulation of T-bet driven by IL-21 but enhanced the upregulation of T-bet mediated by IFN-γ. Furthermore, while IL-21 can induce the expression of CD11c in B cells, IFN-γ does not perform this function. The interactions between these cytokines and their regulation of the process of T-bet and CD11c+ cell formation in B cells stimulated by TLRs were demonstrated in a previous study (Naradikian et al. 2016). In 2017, Michela Manni et al. used gene knockout technology to produce mice lacking the SWEF protein (a member of the Rho GTPase regulating protein family, including Def6 and Swap-70) and reported that the SWEF protein controls the proliferation of ABCs by regulating the IL-21‒IL-21R signaling pathway (Manni, Ricker, and Pernis 2017). Levack et al. subsequently used the E. muris mouse model to demonstrate that early postinfection, the cytokines IL-21 and IFN-γ produced by follicular helper T cells (Tfh cells) drive the generation of CD11c+T-bet+ B cells. In contrast, in the later stages of infection, this process is mainly dependent on the Tfh costimulatory signal CD40:CD40L (Levack et al. 2020). Studies have shown that Th1 cells stimulate B cells to promote the generation of CXCL9/10-producing T-bet+ effector B cells (Nakayama et al. 2021).
3.2.4 Helper T Cells Cooperate With ABC Differentiation
Current studies suggest that Bcl-6 performs a core function in the formation process of Tfh cells. Tfh cells, through interaction with B cells, promote the activation and differentiation of B cells (Johnston et al. 2009). Song et al. reported that in acute infections, T helper cells drive the production of T-bet+CD11c+ B cells by providing close-range assistance (Figure 4). T-bet+CD11c+ B cells begin to develop before GC formation and have distinct phenotypic and transcriptional profiles from those of GC B cells (Song et al. 2022). In addition to Tfh cells, PD-1hiCXCR5- T peripheral helper cells enhance B-cell function by secreting MAF and IL-21 and promote B-cell differentiation into CD11c+ B cells, thereby further driving the progression of lupus (Bocharnikov et al. 2019). In addition, Th1 cells and Tfh cells are both CD4+ T-cell subpopulations. Since Th1 cells can also produce IFN-γ, researchers have discovered that B cells can form a new subpopulation of T-bet+ B cells under the stimulation of IFN-γ secreted by Th1 cells (Nakayama et al. 2021). Helper T cells facilitate the differentiation of ABCs, allowing them to generate high-affinity antibodies that participate in immune regulation and play a significant role in combating viral and certain bacterial infections. This finding offers a novel perspective for understanding the role of B cells in various immune responses. However, the role of other helper T-cell subtypes (such as Th2 and Th17) in promoting or inhibiting ABC differentiation has not been studied.
4 Immunological Functions of ABCs
4.1 Autoantibody Production
Analysis of the transcripts of ABCs revealed that they highly expressed the plasma cell markers CD80 and CD138 and highly expressed CXCR6 and CXCR3, which are involved in lymphocyte homing to inflammatory sites (Rakhmanov et al. 2009; Tipton et al. 2015). They also secrete cellular factors that promote plasma cell differentiation, such as Prdm1, Irf4, and Xbp1 (Rubtsov et al. 2011). These data suggest that ABCs may be precursors of plasma cells.
Researchers have demonstrated through in vitro culture and preparation of B-cell hybridoma experiments that ABCs are under differentiated plasma cells (Figure 5). Only when stimulated (e.g., by a TLR7 agonist) can they secrete anti-chromatin IgG autoantibodies (Rubtsov et al. 2011; Jenks et al. 2018). Through ELISPOT experiments and autoantibody microarray tests, Song et al. reported that T-bet+CD11c+ B cells can secrete virus-specific antibodies and autoantibodies after LCMV infection (Song et al. 2022). In female DKO mice with an autoimmune propensity, the absence of B-cell T-bet significantly reduces the production of anti-dsDNA IgG2c but does not correspondingly increase the production of anti-dsDNA IgG1. Therefore, B-cell T-bet is not essential for ABC generation or differentiation but has a specific requirement for the production of IgG2c autoantibodies (Ricker et al. 2021; Rubtsova et al. 2013; Stone et al. 2019; Peng, Szabo, and Glimcher 2002).
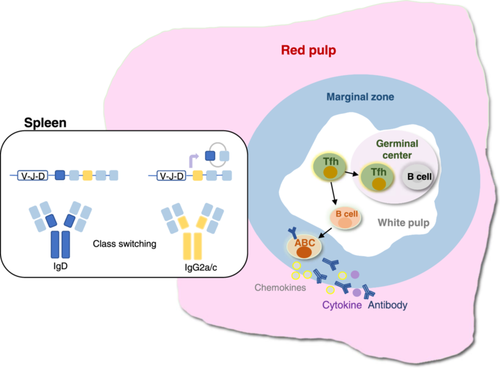
4.2 Antigen Presentation
The anatomical location of ABCs is located at the boundary of the spleen, thus providing conditions for their interaction with Tfh cells (Figure 5). Research has shown that ABCs can act as antigen-presenting cells, helping activate naïve T cells to favor Th17 differentiation (Hao et al. 2011; Rubtsov et al. 2015).
By sequencing the VH and Vk genes of unimmunized 22-month-old C57BL/6 mice, it was found that ABCs expressed diverse recombination sequences in their VH and Vκ genes, suggesting that their high mutation rate may enable them to recognize and respond to a variety of different antigens (Russell Knode et al. 2017). T-bet+CD11c+ B cells can undergo Ig somatic hypermutation and class switch recombination, both of which occur outside the germinal centers (Song et al. 2022; Karnell et al. 2017; Knox, Myles, and Cancro 2019). Ramirez De Oleo et al. (2023) have shown that ABCs express a variety of receptors related to phagocytosis, such as CD36 and AXL. These receptors help ABCs effectively phagocytose antigens. Additionally, ABCs express high levels of the antigen-presenting molecule MHC II, which can present the ingested antigens to T cells. By using metformin (a drug that can block the process of B-cell terminal differentiation into plasma cells) to reduce antigen uptake and thus inhibit the presentation process in ABCs, Tfh differentiation can be reduced (Lee et al. 2017). These findings demonstrate that ABCs can act as antigen-presenting cells (APCs) to present ingested antigens to T cells and initiate an immune response. Similarly, the proliferation of Tfh cells requires CD11c+T-bet+ ABCs (Zhang et al. 2019). The experimental data revealed that when cocultured with OVA protein, both ABCs and FO B cells could induce T-cell proliferation and activation (Ramirez De Oleo et al. 2023). In the present study, ABCs together with chicken egg albumin (OVA) were cultured with naïve CD4+ T cells, resulting in stronger T-cell receptor (TCR) signal induction. ABCs promote the differentiation of Tfh cells by inducing the production of IL-21 and the expression of Tfh cell markers such as CXCR5, PD-1, and Bcl-6 (Ramirez De Oleo et al. 2023).
4.3 Cytokine/Chemokine Production
ABCs are activated B cells that have been shown in ex vivo experiments to preferentially secrete IL-4, IL-10, and TNF-α after TLR7 or TLR9 agonist stimulation (Hao et al. 2011; Ratliff et al. 2013) (Figure 5). Furthermore, ABCs produce more IL-1β and IL-6 than FO B cells (Ramirez De Oleo et al. 2023). Additionally, Th1 cells stimulate the BCR/CD40 and IFN-γ signaling pathways of B cells, thereby promoting the secretion of CXCL9-11 by T-bet effector B cells (Nakayama et al. 2021; Long, Kleiner, and Looney 2023). When CXCL9-11 binds to CXCR3 on the surface of Th1 cells, it can attract them to inflamed tissues and further induce an increase in the release of IFN-γ, thus further intensifying the inflammatory response (Long, Kleiner, and Looney 2023). Additionally, a study revealed that the proliferation of Th1 cells is associated with ABCs (Unger et al. 2018).
4.4 Migration to End Organs and Associated Tissue Damage
ABCs can be detected in both viral infection models and autoimmune disease models at corresponding validation sites. This is because ABCs express CD11c (an integrin involved in cell adhesion and migration) on their surface and highly express CCR7 (Rubtsov et al. 2015) (which regulates the migration and localization of lymphocytes), CXCR6 and CXCR3 (Rakhmanov et al. 2009; Tipton et al. 2015) (which participate in homing to inflammatory sites); thus, after being guided by inflammation signals, ABCs can specifically migrate to the corresponding inflammatory sites and accumulate.
4.5 Inhibiting the Survival of B-Cell Precursors
During coculture of ABCs and FO B cells, ABCs did not inhibit the growth of other B cells (Hao et al. 2011). Frasca et al. discovered through experiments such as an IL-7 colony formation assay, that ABCs induce various biological mediators and cytokines, such as PGE2 and IL-1, in macrophages and adipocytes by secreting TNF-α, thereby altering the bone marrow microenvironment and inhibiting the survival of B-cell precursors (Berg and Scherer 2005). In 2015, researchers reported that these cells did not alter the level of pro-B cells in the bone marrow when ABCs or FO B cells were transplanted from aged mice to young mice. Instead, there is an interaction between age-related B cells in aged mice and alternative light chains λ5 (SLCs), which may lead to the apoptosis of pre-B cells in the bone marrow and the generation of autoimmune B cells (Ratliff et al. 2015).
5 ABCs Mediate Disease via Humoral Immunity
ABCs can express various cytokines, such as IL-4, IL-10, IL-17, IFNγ, and TNF-α (Hao et al. 2011; Ratliff et al. 2013; Frasca et al. 2021), which enable ABCs to play different roles in various diseases.
5.1 ABCs in Cell Aging
ABCs can promote inflammatory responses. B cells play an important role in producing antibodies in humoral immunity. With age, the differentiation and function of B cells may change, leading to a decline in humoral immunity. In addition, aging can lead to the redistribution of B-cell subsets, increasing the frequency and number of inflammatory B cells. Moreover, aging can reduce the expression of immunoglobulin class switching and recombination involved in high-affinity antibody production and the expression of highly mutated molecules in somatic cells, as well as reduce germinal center formation and the diversity of immune pools (Frasca et al. 2020).
Studies have shown that the number and proportion of ABCs increase in older individuals and that they also exhibit an inflammatory response. ABCs secrete TNF-α, which can promote an inflammatory microenvironment in the bone marrow and inhibit the growth or survival of pre-B cells and common lymphoid progenitor cells (Ratliff et al. 2013; Riley, Khomtchouk, and Blomberg 2017). In this study, CFSE-labeled OTII T cells were cultured under different polarization conditions in the presence of aged FO or ABCs. The results showed that ABCs were able to effectively present antigens to T cells and promote T-cell proliferation under all polarization conditions (Hao et al. 2011). Since ABCs express high levels of MHC II and costimulatory molecules, they may promote the differentiation of Th17 cells by presenting self-antigens to T cells (Rubtsov et al. 2011). Th17 cells mainly produce inflammatory cytokines such as IL-17 and IL-22, thereby promoting inflammatory responses (Miossec and Kolls 2012). Older people have a weaker antibody response to the flu vaccine. The diminished antibody response of elderly individuals to the influenza vaccine is closely related to the age-related expansion of B-cell populations and decreased levels of PAX5 (Nipper et al. 2018). The enhancement of B-cell function in elderly individuals may therefore be a necessary strategy to optimize the efficacy of vaccines that rely on humoral immune protection.
5.2 ABCs in Autoimmune Disease
B cells play important roles in autoimmune diseases, including producing autoantibodies, presenting antigens, providing costimulatory signals, and producing cytokines. Some autoimmune diseases are closely related to B cells. Rubtsov was the first to link ABCs with autoimmune diseases (Rubtsov et al. 2011). The ABC subgroup was initially identified in a mouse model of autoimmune disease. In clinical studies, researchers have reported that ABCs tend to accumulate in various autoimmune diseases, such as systemic lupus erythematosus (SLE), primary Sjögren's syndrome (pSS), systemic sclerosis, and rheumatoid arthritis (RA) (Figure 6).
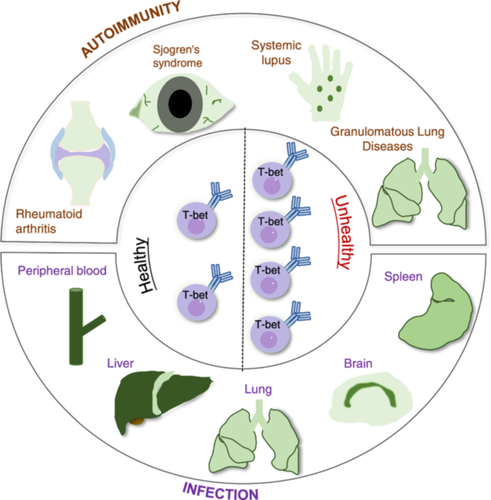
5.2.1 Systemic Lupus Erythematosus (SLE)
SLE is a typical autoimmune disease characterized by abnormal autotolerance and nuclear autoantigen production, leading to inflammation in multiple organs. Furthermore, studies have shown that the risk of developing SLE is greater in women than in men (Durcan, O'Dwyer, and Petri 2019). Disease progression can be divided into active and quiescent phases. During the active phase, the B-cell population, including ABCs, tends to increase, whereas during the quiescent phase, the number of helper T cells increases (Manion et al. 2024). The features of SLE coincide with those of ABCs, such as age and sex dependence and the production of anti-chromatin antigens. Therefore, a correlation can be established between the two (Brown et al. 2022). ABC accumulation has been observed in both spontaneous SLE mouse models and induced SLE mouse models.
Previous studies have shown that ABCs can secrete IgG2a (Rubtsova et al. 2013; Peng, Szabo, and Glimcher 2002). Interestingly, IgG2a and IgG2b are pathogenic subtypes of SLE (Ehlers et al. 2006). TLR7 and TLR9 are believed to be closely related to the pathogenesis of SLE (Ni et al. 2024). Studies have shown that mice with a TLR7 gene deletion are unable to form spontaneous GCs in B cells (Fan, Ren, and Hou 2018), but the TLR7-mediated pathogenesis of SLE is not related to GCs but rather to ABCs (Brown et al. 2022). The proportion of ABCs in the peripheral blood of SLE patients is greater than that in the peripheral blood of healthy individuals. Most patients with active disease have increased expression of IFN-I-induced transcripts. Moreover, the peripheral blood B-cell pool in SLE patients changes, such as a reduction in the IgM memory B-cell group and an increase in ABCs, suggesting that their functions are dysregulated (Becker et al. 2013; Wehr et al. 2004; Mouat, Goldberg, and Horwitz 2022a).
ABCs, as APCs, interact with T cells and promotes their differentiation into Th17 cells (Hao et al. 2011). Abnormal T-cell metabolism in SLE patients can affect and reprogram the balance of Th17 and Treg cells (Rubtsov et al. 2015). Therefore, ABCs may play a certain role in the pathogenesis of SLE by promoting Th17 cell differentiation. Recent studies have revealed that the absence of IL-13Rα1 leads to the upregulation of inflammatory markers and pro-inflammatory mediators in ABCs. Additionally, IL-13Rα1 is an X-linked receptor associated with SLE. Therefore, IL-13Rα1 plays an important role in the generation and differentiation of ABCs as well as in the pathogenesis of SLE (Chen et al. 2022). The prognosis of patients with SLE after recovery is also closely related to the clearance of ABCs. However, there is no definitive evidence that ABC expansion is the direct cause of autoimmune diseases, and the causal relationship still needs further research.
5.2.2 Rheumatoid Arthritis
RA is a common autoimmune disease characterized by erroneous attack of the articular cartilage by the immune system (Imboden 2009). Studies have also shown that in some patients with RA, an abnormal population of B cells downregulates the complement receptor CR2/CD21 (Isnardi et al. 2010). Mouat et al. used Tbx21fl/fl and Cd19-cre-/+ mouse models (a mouse model of γ herpesvirus 68 (gHV68) that mimics latent infection with EBV) to demonstrate that latent gamma herpes virus infection leads to an increase and activation of age-related B cells, which subsequently promote inflammatory responses and the development of arthritis (Mouat et al. 2021). The findings from these studies offer novel insights for advancing the comprehension and management of arthritis.
Yi Qin et al. detected increased levels of ABCs in collagen-induced arthritis (CIA) mice and RA patients using flow cytometry. Specifically, they reported increased levels of ABCs in the blood, spleen, and inflamed joints of CIA mice and in the blood, synovial fluid, and synovial tissue of RA patients. Furthermore, by blocking related signaling pathways, ABCs induce fibroblast-like synoviocyte (FLS) activation through the TNF-α-mediated ERK1/2 and JAK-STAT1 pathways and participate in the pathogenesis of RA (Qin et al. 2022).
5.2.3 Other Autoimmune Diseases
Other autoimmune diseases are associated with ABCs. Multiple sclerosis (MS) is an inflammatory disease. In 2016, Nele Claes and others conducted a phenotypic and functional analysis of B cells in the peripheral blood and cerebrospinal fluid of MS patients. The results revealed that in MS patients aged younger than 60 years, the proportions of peripherally expanded DN (IgD-CD27-) and CD21- (CD21-CD11c+) B cells were greater than those in age-matched healthy controls. Additionally, the frequency of ABCs in the cerebrospinal fluid of MS patients is increased, indicating their ability to migrate to the central nervous system (Claes et al. 2016). These cells promote inflammation by inducing T-cell responses and producing pro-inflammatory cytokines (Bar-Or et al. 2010; Fraussen et al. 2014).
Sjogren's syndrome is a typical B-cell-related autoimmune disease characterized by chronic inflammation of the salivary and lacrimal glands. Researchers have reported that CD21- B-cell amplification occurs in patients with Sjogren's syndrome (Saadoun et al. 2013). Additionally, in axial spondyloarthritis (axSpA) patients, the proportion of CD27-CD38-CD21- B cells also tends to increase (Wilbrink et al. 2021). There are also increased numbers of ABC-like cells in granulomatous lung diseases (Phalke et al. 2020; Polverino and Curtis 2020). There are also articles reporting that the intestines and peripheral blood of ulcerative colitis patients were examined and that the number and proportion of plasma cells increased during the active period (Uzzan et al. 2022). Since ABCs can be considered precursors of plasma cells, ABCs may also be associated with the development of ulcerative colitis.
5.3 ABCs in Viral Infection
Some studies have demonstrated that ABCs are found in the marginal zone of Ehrlichia muris infection models (Trivedi et al. 2019). In a viral infection model, ABCs proliferate in the spleen and the infected site, and the spleen becomes the main storage depot for ABCs (Mouat and Horwitz 2022; Johnson et al. 2020; Mouat, Shanina, and Horwitz 2022b). Following the clearance of viral infection in mice, the quantity and proportion of ABCs in their spleens and infection sites (such as the lungs and mediastinal lymph nodes) decline, and the majority of ABCs merely reside in the spleen (Johnson et al. 2020).
During acute infection, ABCs produce antibodies specific to the virus (Song et al. 2022). Patients with common variable immunodeficiency (CVID) are often susceptible to recurrent bacterial infections, especially respiratory infections (Quinti et al. 2007). A study by Mirzokhid Rakhmanov et al. indicated that in CVID patients, B cells with low expression of CD21 show signs of activation and proliferation and have limited calcium signaling and proliferation ability in response to B-cell receptor stimulation (Rakhmanov et al. 2009). Recent studies have shown that in patients with CVID-ILD (CVID-related interstitial lung disease), Th1-CD21low B cells interact with their bronchoalveolar lavage fluid (BALF), with the number of ABCs (CD21low B cells) markedly increasing in the BALF (Friedmann et al. 2021). In other pulmonary diseases, such as sarcoidosis (SarcP), similar findings have been reported in patient populations (Phalke et al. 2020). Moreover, the frequency of T-bet+ B cells increased in chronic HCV-infected patients.
Kugler-Umana et al. (2022) discovered that immunizing elderly mice with an inactivated vaccine, known as iABCs, could elicit an infection-induced antibody-mediated immune response, which closely resembled the immune response triggered by live influenza virus infection. Nucleic acid-based vaccines for COVID-19, such as mRNA vaccines, can stimulate innate immunity by activating TLR7/8 or TLR9, thereby inducing ABC generation and triggering autoimmune responses (Mahroum and Shoenfeld 2022). Furthermore, ABCs express high levels of inhibitory FcγRIIB receptors, and the Fc region of antigen‒antibody complexes can bind to FcγRIIB receptors to form immune complexes, thereby reducing vaccine responses (Yam-Puc et al. 2023). Steuten et al. reported that after secondary immunization, ABCs rapidly proliferate, but their numbers gradually decrease over time (Steuten et al. 2023). Researchers have analyzed the regulation of endogenous retroviruses (ERVs) by T-bet+ B cells through the control of TLR signaling-dependent spontaneous germinal centers and IFNγ-dependent regulation by infecting Moloney-Murine leukemia virus-marked GFP (ERV-GFP) in mouse GC-derived cell lines (Rauch et al. 2024). ABCs begin to accumulate antiviral humoral responses during the peak of the antiviral response (Rubtsova et al. 2013). These cells are maintained during infection and can significantly reduce their frequency after antiviral treatment (Chang, Li, and Kaplan 2017). In addition, there are other infectious diseases, such as LCMV (Rubtsova et al. 2013) and HIV infection (Knox et al. 2017), which are most closely related to ABCs.
5.4 ABCs in Organ Transplant
Lei Wang and others reported that the number and percentage of ABCs significantly increased in patients with end-stage renal disease before and after kidney transplantation, especially in patients who experienced viral or bacterial infections after the transplant operation (Wang et al. 2021). The immune system of elderly individuals is compromised, namely, immunosenescence, which leads to impairment of the development and antibody-producing capacity of B cells (Franceschi et al. 2000). In elderly patients undergoing kidney transplantation, the B-cell population in their body transforms more mature memory cells, especially with an increase in ABCs, which might influence the immune response of the patients (Wang et al. 2021). Consequently, an increase in the proportion of ABCs in transplant patients might be related to the adaptability of the immune response and the response to infections, but the specific benefits and mechanisms still await further research.
6 Future Research Prospects
According to a recent study, the extent of adipose tissue increases with age, which promotes inflammation and dysfunction of B cells (Frasca et al. 2020; Khan, Tsai, and Winer 2019; Bharath, Ip, and Nikolajczyk 2017; Carey et al. 2024). Adipose tissue secretes leptin, a fat cell factor that is able to activate B cells to secrete pro-inflammatory cytokines, such as IL-6 and TNF-α. Additionally, the inflammatory state in adipose tissue also causes changes in B-cell subsets, including an increase in pro-inflammatory ABCs and a decrease in FO B cells (Frasca and Blomberg 2017). Furthermore, the inflammatory state of adipose tissue is closely related to the development of metabolic diseases. Therefore, these findings are crucial for understanding the relationship between the immune system function of adipose tissue and metabolic health.
Research has shown that PEGylated gold nanoparticles (GNPs) can bind to the CD22 receptor on the surface of B cells and be absorbed by ABCs. High concentrations of GNPs have a certain inhibitory effect on the function of ABCs, thus enabling the selective targeting of B cells. This discovery has the potential to alleviate symptoms of related diseases such as Alzheimer's disease (Hočevar et al. 2022). The mechanism of interaction between ABCs and other B-cell subpopulations (such as conventional B cells) is not yet clear, and further research is needed to elucidate whether ABCs cooperate or interact with each other in the development of this disease. We know that as people age, their immune system function gradually declines, and their memory function weakens, leading to a reduced antibody response to vaccines (Nipper et al. 2018). In young people, the peripheral blood consists mainly of unprimed B cells and memory B cells, whereas in elderly people, the peripheral blood consists mainly of plasma cells (Blanco et al. 2018). Therefore, improving B-cell function in elderly individuals may be one of the necessary ways to improve the effectiveness of vaccines that rely on humoral immunity protection, such as reversing the differentiation of ABCs. B-cell depletion therapies, such as rituximab, are used to eliminate B cells and reduce the number of ABCs. Some studies have shown that ABCs express higher levels of CD20 than other B-cell subpopulations in some patients with RA or SLE, which may suggest that ABCs have greater potential for targeted therapy. In recent years, immunotherapy has become a hot topic. It has already been proven that anti-CD19 CAR-T-cell therapy can effectively deplete B cells and reduce the number of CD21-CD11c+ B cells (Mackensen et al. 2022). Various inflammatory diseases, such as SLE, can be effectively managed. Although there have been no reports on the relevant role of ABCs in tumors, it has been reported that in tumors, the expression of CD11b and CD11c can facilitate the adhesion of B cells to stromal cells and increase the viability of B cells (Erdei et al. 2019). This finding implies that ABCs with high expression of CD11b and CD11c might possess high migratory capacity, aggregate in tumors, and exert antitumor immune effects. Nevertheless, experimental validation is still needed, but these findings offer a new perspective for cancer treatment.
Author Contributions
Zi Geng and Guangwei Liu summarized the references and wrote the manuscript. Zi Geng, Yejin Cao, Longhao Zhao, Likun Wang, Yingjie Dong, and Yujing Bi participated in discussions.
Acknowledgments
The authors' research is supported by grants from the National Natural Science Foundation for General Programs of China (32370924, G.L.; 32170911, G.L., and 31970863, Y.B.) and Beijing Municipal Natural Science Foundation of China (5202013, G.L.).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.




