Single-Cell Transcriptomic Analysis Reveals Biomechanical Loading-Induced Imbalance in Bone and Fat, Leading to Ossification in Lumbar Intervertebral Disc Nucleus Pulposus Degeneration
Ping Zhang and Yuan Wang contributed equally to this study.
ABSTRACT
In this study, we explored the impact of different biomechanical loadings on lumbar spine motion segments, particularly concerning intervertebral disc degeneration (IVDD). We aimed to uncover the cellular milieu and mechanisms driving ossification in the nucleus pulposus (NP) during IVDD, a process whose underlying mechanisms have remained elusive. The study involved the examination of fresh NP tissue from the L3-S1 segment of five individuals, either with IVDD or healthy. The analysis consisted of histopathological evaluation and single-cell RNA sequencing. To further validate the impact of biomechanical loading on IVDD, particularly on the CITED4 + METRN + NP chondrocytes and the bone-fat balance mechanism, a retrospective analysis was conducted using paraffin-embedded NP samples from patients. A distinct subset of CITED4 + METRN+ chondrocytes in the degenerated NP that were influenced by biomechanical loading was identified. These cells were evaluated for their potential as diagnostic biomarkers. Pseudotemporal analysis indicated that inflammation and repair processes were integral to NP ossification. Notably, the L4/5 and L5/S1 segments with severe IVDD showed pronounced ossification and heightened lipogenic metabolism. Cell communication analysis sheds light on the roles of bone-fat balance proteins and various ossification genes. Additionally, immunohistochemistry and immunofluorescence confirmed that biomechanical loading intensified IVDD by fostering osteogenic differentiation, mediated by macrophage migration inhibitory factor (MIF)-regulated bone-fat balance. This research reveals the microenvironmental factors of IVDD NP ossification under biomechanical loading, highlighting the role of bone-fat imbalance. These insights significantly enhance the understanding of IVDD pathogenesis and pave the way for innovative therapeutic approaches.
Abbreviations
-
- AOD
-
- average optical density
-
- DEGs
-
- differential gene expression
-
- ECs
-
- effector chondrocytes
-
- FC
-
- fibrocytes
-
- FibroCs1
-
- fibrochondrocytes
-
- GO BP
-
- Gene Ontology Biological Process
-
- HomCs
-
- homeostatic chondrocytes
-
- HTCs
-
- hypertrophic chondrocytes
-
- IOD
-
- integrated option density
-
- IVDD
-
- intervertebral disc degeneration
-
- NP
-
- nucleus pulposus
-
- RegCs
-
- regulatory chondrocytes
-
- scRNA-seq
-
- single-cell RNA sequencing
1 Introduction
Lower back pain, a prevalent global health concern, affects approximately 80% of individuals at least once in their lives, posing a substantial economic burden (Blanquer, Grijpma, and Poot 2015). A leading cause of this ailment is intervertebral disc degeneration (IVDD), which manifests with varying incidence and severity across different lumbar spine motion segments.
In a study utilizing magnetic resonance imaging (MRI) on a sample of 200 healthy individuals, the incidence of IVDD was 7.0% at L1/2, 12.0% at L2/3, 15.5% at L3/4, 49.5% at L4/5, and 53.0% at L5/S1 (Kanayama et al. 2009). This study noted that the severity of IVDD was significantly lower at the L3/4 segment compared to the L4/5 and L5/S1 segments (Fujiwara et al. 1999). These segments exhibit different biomechanical characteristics, with the L4/L5 segment contributing the most to total extension motion from L2 to S1 (Aiyangar et al. 2015). At the L5/S1 segment, both the shear force during flexion-extension and the rotational angle during the transition from a bent to an upright position is maximal among the lumbar spine segments (Iorio, Jakoi, and Singla 2016; Byrne, Aiyangar, and Zhang 2019). Furthermore, during the lifting of weights from a 45° forward flexion to the maximum extension position, the L4/5 and L5/S1 segments exhibit the largest anterior-posterior and proximal-distal translations in the lumbar spine, respectively (Wu et al. 2014). These observations suggest a correlation between biomechanical loading and the incidence of IVDD in specific lumbar spine segments.
Disparities in mechanical stress across lumbar spine segments exert a profound influence on the pathological phenotype of IVDD. Excessive mechanical loading and instability can lead to disc collapse and osteophyte formation (Zehra et al. 2022). Despite notable advances in multi-omics and single-cell RNA-sequencing (scRNA-seq) methodologies for investigating IVDD (Tu et al. 2022; Gan et al. 2021), the precise mechanisms by which mechanical stress promotes abnormal nucleus pulposus (NP) ossification remain unclear.
This study employed an innovative methodology to elucidate the cellular microenvironment within distinct biomechanically loaded segments during the NP ossification process. This investigation took into account the variations in pathological phenotypes to provide a comprehensive understanding of the cellular dynamics associated with NP ossification. A key finding was the identification of a subet of CITED4 + METRN+ chondrocytes that is preferentially enriched to regulate mitotic exit and 1-phosphatidylinositol 4-kinase activity function. The presence of this subset was positively correlated with both degeneration severity and mechanical loading and is a potential marker of IVDD. This study is moreover highlighting the role of lipogenic metabolism in IVDD NP ossification. Notably, it was observed that biomechanical loading in the IVDD process skewed the bone-fat balance towards ossification by upregulating macrophage migration inhibitory factor (MIF) expression. These findings offer fresh perspectives that can inform the development of targeted therapies and effective interventions for addressing NP ossification in human IVDD.
2 Materials and Methods
2.1 Human Intervertebral Disc NP Tissue Samples
In this study, we examined gel-like NP tissue that is the herniated or bulging NP derived from human intervertebral discs by transforaminal lumbar interbody fusion. Fresh NP samples were prospectively isolated from five donors for scRNA-seq. Additionally, retrospective analyses were performed utilizing 65 and 30 human IVDD NP paraffin samples for the identification of CITED + METRN+ chondrocytes and mechanism verification, respectively. All samples were classified as per the Pfirrmann grading system (Gan et al. 2021). For scRNA-seq, we used one nearly non-degenerated Pfirrmann grade I NP-L (L5/S1) as a healthy control, a Pfirrmann grade III NP-M (L4-5), and three Pfirrmann grade IV NP-S samples (L5/S1, L4/5, L3/4) (refer to Material S1, Figure S1A). All five NPs were collected according to inclusion and exclusion criteria. Inclusion criteria: (1) Patients with a precise diagnosis of degenerative lumbar spinal stenosis and treated with transforaminal lumbar interbody fusion were included in the test group. (2) Patients with burst fracture or lumbar spine trauma and lumbar spine MRI showing no obvious lumbar degenerative disease were included in the control group. (3) Patients who have been ineffective in conservative treatment for more than 3 months and have clear indications for surgery must undergo conventional debridement surgery. (4) Aged 40–75 years old. (5) Patients who voluntarily participated and signed the informed consent. Exclusion criteria: (1) Pregnant or breastfeeding women, patients with other spinal disorders such as ankylosing spondylitis, vertebral compression fracture, and tumors. (2) Patients with definite lumbar spondylolisthesis of II degree or higher. (3) Patients who are proposed to undergo lumbar internal fixation revision surgery. (4) Patients with ossification of the ligamentum flavum. (5) Patients with diabetes mellitus and other diseases that have a potential impact on the ligamentum flavum. (6) Special groups of people who are not suitable to participate in the clinical study (blind, deaf, dumb, intellectual or mental disabilities, etc. who cannot cooperate with the completion). (7) Patients with serious primary diseases such as heart, brain, liver, kidney, hematopoietic system, or other contraindications to surgery. In retrospective studies, Pfirrmann grade I–II NP was considered mild IVDD, while grades III and IV–V were classified as moderate and severe IVDD, respectively (Cherif et al. 2022). The gelatinous NP from discs with different levels of degeneration is isolated by transforaminal lumbar interbody fusion, removed from other tissues, and washed clean with PBS. Hematoxylin, Eosin O/solid green staining, and ACAN immunohistochemical staining were used for tissue identification. ScRNA-seq was then performed.
2.2 ScRNA-Seq
For the single-cell RNA sequencing process, we employed the Single Cell 3′ Library & Gel Bead Kit V3.1 (10x Genomics, 1000075) in conjunction with the Chromium Single Cell B Chip Kit (10x Genomics, 1000074). The cell suspension, containing 300–600 live cells per microliter, was prepared in PBS with 0.04% BSA and processed using the Chromium Single Cell Controller (10x Genomics). After cell lysis and RNA release, barcoded reverse transcription was performed. cDNA synthesis and amplification were carried out using a Bio-Rad S1000TM Touch Thermal Cycler, with quality assessment done using the Agilent 4200. Final library sequencing was executed on an Illumina NovaSeq. 6000 sequencer, targeting a minimum of 100,000 reads per cell with a PE150 reading strategy.
2.3 Data Processing
Data clustering in this study was performed using Seurat 3.0 (R package). Cells with fewer than 200 genes, those in the top 1% by gene count, and those with a mitochondrial gene ratio exceeding 25% were systematically excluded as outliers. Dimensionality reduction involved principal component analysis (PCA), and uniform manifold approximation and projection for dimension reduction (UMAP) were used for visualization. The annotation of cell types was achieved through SingleR, an unbiased recognition approach, by referencing human cells against Blueprint_Encode or HPCA databases.
Enrichment analysis for subgroup marker genes was conducted utilizing the KEGG Orthology-Based Annotation System (KOBAS) software for Gene Ontology (GO) enrichment. Single-cell trajectories were constructed using Monocle2 (R package) and incorporated pseudotime. Gene selection criteria included expression in more than 10 cells, an average expression value above 0.1, and a Q-value below 0.01 in different analyses. CellPhoneDB software was used for analyzing cell communication, making use of cell expression matrices and clusters to determine the average expression and significance of ligand-receptor complexes.
2.4 Pathology Tissue Staining
Paraffin-embedded NP tissue sections were subjected to various staining techniques, such as Safranin O-Fast Green, Alcian Blue, immunohistochemistry, and immunofluorescence. The primary antibodies used were CITED4 (Abcam, ab134072), METRN (Invitrogen, PA5-62335), BMP2 (Proteintech, 66383-1-Ig; Abcam, ab284387), FABP4 (Abcam, ab92501), MIF (Proteintech, 20415-1-AP), NR3C1 (Proteintech, 24050-1-AP), RUNX2 (SANTA, sc-390351), and ACAN (Boster, BA2967-1).
The extent of ossification was assessed by measuring the calcification area after Alizarin Red S staining (Yan et al. 2022). Quantification of immunohistochemistry and immunofluorescence involved calculating the number of positive cells, integrated optical density (IOD), or average optical density (AOD) (Nakamichi et al. 2016). Each slide was analyzed in three random fields.
Pathology interpretations were independently conducted by two pathologists, with consensus results considered final. Discrepancies were resolved by consulting a third pathologist.
2.5 Statistical Analysis
Statistical analysis was performed using SPSS v.18.0. Quantitative data conforming to a normal distribution were presented as the mean ± standard deviation. One-way analysis of variance (ANOVA) is used to compare whether there is a significant difference in the means between multiple groups where the data are independent, the data follow a normal distribution, and the overall variance of the samples from each group is the same. Mann–Whitney U-test is used to compare whether there is a significant difference between the medians of two independent groups that do not satisfy normal distribution or homogeneity of variance. “Post hoc comparisons” were made when the ANOVA table for comparisons between multiple groups showed rejection of H0. For homogeneity of variance, Least-Significance-Difference (LSD) was used, and LSD was compared between two groups using a t-test. A non-parametric test, the Mann–Whitney U-test, was used for heterogeneity of variance. A p value of < 0.05 was considered statistically significant.
3 Results
3.1 Characteristics of IVDD NP as Per Pathology and scRNA-Seq Analyses
The workflow is depicted in Figure 1A. Safranin O-Fast Green and immunohistochemistry staining revealed NP as a tissue characterized by sulfated glycosaminoglycan deposition and ACAN+ expression. In NP-S samples (L5/S1, L4/5, and L3/4), reduced chondrocyte count and partial hypertrophy were observed (Figure S1). NP-S samples from L5/S1 and L4/5 showed diffuse calcification in cartilage tissue, whereas there was no significant ossification in the L3/4 NP-S sample (Figure 1B).

After quality control procedures, 50,796 NP cells were retained for scRNA-seq analysis. Nineteen subgroups were identified, and these included homeostatic chondrocytes 1 and 2 (HomCs1-2), effector chondrocytes 1 and 2 (ECs1-2), fibrochondrocytes (FibroCs1-2), hypertrophic chondrocytes (HTCs), regulatory chondrocytes (RegCs1-5), nucleus pulposus progenitor cells (CPCs), endothelial cells, neutrophils, monocytes/macrophages, T cells, and a small number of fibroblasts (FCs1) and erythrocytes (Figure 1C,E).
COL2A1, a cartilage marker, was predominantly expressed in NP chondrocyte subgroups (Figure 1F). Classic subgroups, such as CPCs, HTCs, and FibroCs, were identified through known markers (Figure S1E) and new characteristic genes (Figure 1F). High expression of HAPLN1 in FibroCs1 and ASPN and TNC in FibroCs2 was noted. Elevated levels of ARC, KRT16, FGF2, BMP2, and SLPI were found in different RegCs, including the newly reported RegCs4 (CITED4 and METRN). CILP was predominant in HomCs1 and EGR1 and ATF3 in HomCs2. GPX3, CTSL in ECs1, and MT-ATP6 in ECs2 exhibited high expression levels. Immune cells were marked by PTPRC overexpression. These subgroups highlighted the heterogeneity between healthy and degenerative ossified NP, providing a comprehensive array of markers for NP subgroup identification.
3.2 Heterogeneity of NP Cells Across Ossification Stages
The functional roles of various NP cell subgroups were elucidated using GO Biological Process (BP) analysis (details are provided in Material S2). Consistent with previous findings (Gan et al. 2021), CPCs showed enrichment in nuclear division, HTCs in ossification processes, and homeostatic chondrocytes (HomCs) in circadian rhythm and developmental processes. Effector chondrocytes (ECs1 and ECs2) demonstrated enrichment in carbohydrate metabolism and mitochondrial metabolism, respectively.
Fibrochondrocytes (FibroCs), known for their multidirectional differentiation ability that is crucial for stability (Zhang et al. 2021), showed ossification and cartilage development predominance in FibroCs1 and acidic substance and mechanical response focus in FibroCs2. The novel RegCs4 subgroup, primarily enriched in hypoxic response and mitotic exit, constituted the highest cell proportion in NP-S (L5/S1) at 11.36% (Figure 1E).
Extracellular matrix functionalities varied among chondrocyte subgroups, with FibroCs1 and FibroCs2 expressing fibrous matrix (FN1, SPARC) (Li et al. 2022; Pan et al. 2020) and bone differentiation matrix (TNC, POSTN) (Pan et al. 2020; Lin et al. 2022) genes, while HTCs expressed mineralization matrix genes (IBSP, SPP1) (Figure S1D).
HomCs1, FibroCs1, FibroCs2, HTCs, CPCs, and RegCs1 all showed enrichment in ossification function, with HomCs1 (12.06%), FibroCs1 (12.71%), and RegCs1 (4.28%) having the lowest proportions in NP-S (L3/4) (Figure 1E), suggesting segmental differences in NP subgroups under varying biomechanical loadings.
3.3 Divergent Pseudotime Trajectories in IVDD NP Subgroups Facilitate Ossification
By remodeling the pseudotime evolutionary trajectories of all NP subgroups, we identified CPCs positioned at one end as the root. Ossification-active subgroups such as HomCs1, FibroCs1, FibroCs2, and HTCs were distributed along the entire trajectory. RegCs1 was located in the later part of the trajectory, with GO BP enrichment in metal ion homeostasis regulation, a function that promotes bone/cartilage repair (Yang et al. 2021; Qiao et al. 2022) (Figure 2A,B). Interestingly, RegCs3, enriched in lipid synthesis metabolism, was located at the tail end of the trajectory (Material S2 and Figure S2 in Material S1).
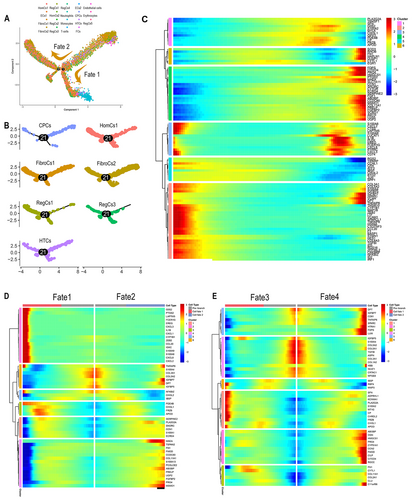
Analysis of the top 100 differentially expressed genes (DGEs) along the pseudotime for co-expression module GO BP enrichment revealed that Cluster 2 was predominantly involved in osteogenic and chondrogenic differentiation. This included fibrocartilage matrix genes (COL3A1 and COL5A2) (Han et al. 2022), bone metabolism regulators (IGFBP5 and IGFBP7) (Conover 2008), osteogenic markers (SPARC) (Zhang et al. 2023a), and the vitamin D receptor downstream target DPT (Li et al. 2009), which exhibited an upregulated expression trend along the trajectory, contrasting with most genes in the module (Material S2 and Figure 2C).
Two discrete trajectory branches denoted Fate1 and Fate2, were identified in the analysis (Figure 2A). Fate1, termed as the inflammation ossification branch, demonstrated heightened expression levels of inflammation factors (IL1B, PTGS2, CXCL2, and CXCL8) (Haimerl et al. 2021) along with ossification-related genes (CHI3L1 and FRZB) (Park et al. 2022; Thysen, Luyten, and Lories 2015). Conversely, Fate2, termed the repair ossification branch, was characterized by a high expression of cartilage repair (SNORC, CCN1, and FN1) (Hara et al. 2016; Zhao et al. 2020) and matrix protein regulation genes (FMOD, COL11A1, PRELP, PRG4, and ABI3BP) (Li et al. 2022; Das et al. 2023) (Figure 2D). In the subsequent trajectory branches, Fates 3 and 4, identified at the second branching point, genes associated with tissue repair (FN1, LUM, CCN2, CILP, and FMOD) (Li et al. 2022; Zhao et al. 2020) and osteogenic differentiation (HTRA1, OGN, SPARC, FBZB, and CHI3L1) (Zehra et al. 2022; Park et al. 2022; Thysen, Luyten, and Lories 2015) were upregulated (Figure 2E and Figure S2B).
Notably, lipogenic metabolism genes at branching points 1 (APOD, HMGCS1, FDPS-1, and CITED4) and 2 (PLA2G2A, APOD, HMGCS1, and CYP51A1) showed increased expression along the trajectory (Figure 2D,E). This suggests that ossification in IVDD NP is intricately linked with ongoing inflammation, repair processes, and a notable shift in lipogenic metabolism pathways.
3.4 Influence of Biomechanical Loading on Lipid Metabolic Imbalance in Ossification Involving NP Chondrocytes
The pseudotime trajectories of chondrocytes were reconstructed based on three progressively degenerated IVDD samples [NP-L (L5/S1), NP-M (L4-5), and NP-S (L4-5)] as well as three IVDD samples with severe degeneration under different biomechanical loading conditions [NP-S (L5/S1, L4/5, L3/4)], resulting in Trajectory 1 and Trajectory 2.
NP-M (L4/5) and NP-S (L4/5) were primarily situated at the end and start of Trajectory 1, respectively, suggesting a dedifferentiation process in degenerated NP chondrocytes (Figure 3A,B). RegCs3, known for lipid synthesis, predominantly originated from NP-S (L4/5) and NP-S (L5/S1) (Figure 3C,D and Figure S2B).
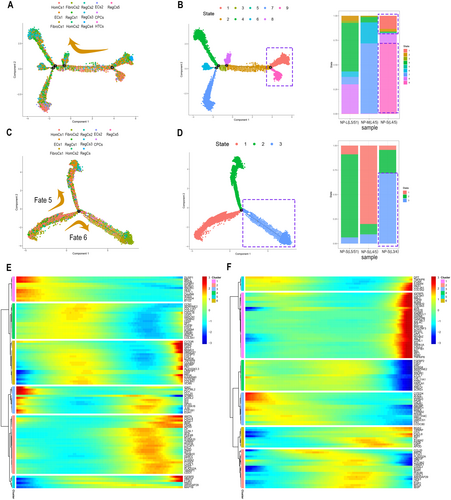
Cholesterol and steroid synthesis metabolic pathways were enriched in both trajectories, displaying distinct expression patterns: genes such as HMGCS1, FDPS, MSMO1, and FGF1 were upregulated in Trajectory 1, while HMGCS1, FDPS, MSMO1, and IGFBP7 were downregulated in Trajectory 2 (Figure 3E,F). This suggests that lipid synthesis metabolism is more pronounced in biomechanically burdened, ossified NP chondrocytes, particularly in the L4/5 and L5/S1 segments during severe degeneration.
3.5 Cell Communication Analysis Highlights the Role of Signaling Networks Driving Ossification in NP Subgroups
In this study, a total of 1182 active ligand-receptor pairs were identified between NP subgroups, with the most frequent cell interactions occurring between CPCs and FibroCs (Figure 4A,B). CPCs are known for their role in chondrogenesis and osteogenic differentiation in NP (Gan et al. 2021), while FibroCs are predisposed to calcification in IVDD (Hawellek et al. 2017). Notably, interactions involving proteins related to lipogenic metabolism and ossification (MIF-TNFRSF10D, CD44-HBEGF, and GRN-SORT1) were among the top 20 interaction relationships (Figure 4C).
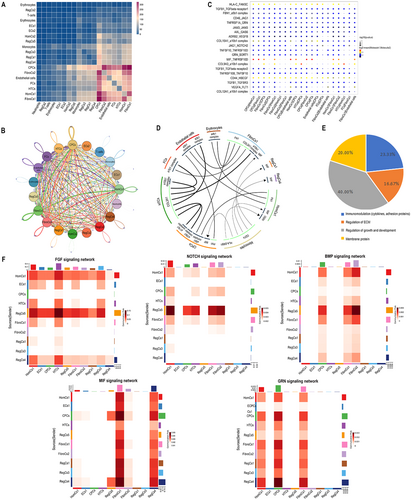
MIF is implicated in regulating IVDD inflammation via the NF-KB pathway (Ling et al. 2022) and cartilage synthesis/degradation metabolism through CD74 (Xiong et al. 2017). Significant interactions of MIF with TNFRSF10D and CD74 were observed in NP subgroups (Figure 4C,D). Additionally, GRN—a lipid factor with pro-inflammatory effects and a role in insulin resistance metabolism (Liu et al. 2023)—and SORT1, associated with calcific aortic valve disease (Iqbal et al. 2023), were identified. CD44 was involved in adipocyte precursor differentiation and lipid metabolism (Jiang et al. 2020), with HBEGF overexpression linked to increased chondrocyte numbers (Wei et al. 2021).
Using “Calcification,” “Ossification,” and “Mineralization” as keywords in the Human Phenotype Ontology (HPO) and DisGeNET databases, 58 receptor-ligand pairs and 100,458 interaction relationships related to ossification genes were extracted. These ligands were categorized into secretory and membrane proteins, revealing four types of ossification ligands: immune regulation, growth regulation, extracellular matrix regulation, and membrane proteins (Figure 4E and Figure S2D–G). Pathways such as BMP, FGF, NOTCH, MIF, and GRN exhibited close communication with CPCs (Figure 4F).
3.6 Role of MIF in Biomechanical Loading-Induced Bone-Fat Balance and NP Ossification
Immunohistochemistry was utilized to detect BMP2 and FABP4, markers of osteogenic and adipogenic differentiation, respectively. Increased ossification and decreased adipogenesis were observed in severe IVDD at L4/5 and L5/S1 compared to L3/4 (Figure 5A). MIF, known to regulate bone-fat balance, exerts bone-protective effects by opposing glucocorticoids (GC) and their receptor NR3C1 (Hoes et al. 2011), and can elevate RUNX2 levels to initiate osteogenic differentiation (Yuan et al. 2016). However, conflicting reports exist (Yao et al. 2016). MIF also plays a role in IVDD progression, influencing mitochondrial autophagy, oxidative stress, and immune responses (Ling et al. 2022; Wang et al. 2021), but its specific connection to IVDD NP ossification has not been previously explored.
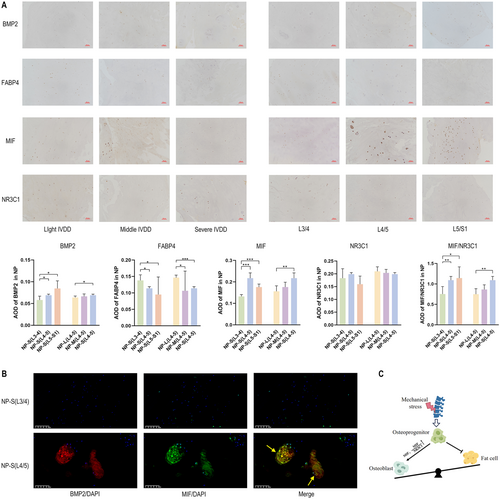
scRNA-seq results indicated higher MIF and MIF/NR3C1 mRNA levels in NP-S (L4/5) and NP-S (L5/S1) compared to NP-S (L3/4), corroborated by immunohistochemistry findings (Figure 5A and Figure S2H). Immunofluorescence analyses of MIF and BMP2 expression in Pfirrmann IV L3/4 and L4/5 NP suggested that biomechanical loading aggravates degenerative ossification by upregulating MIF, thereby altering the bone-fat balance (Figure 5B). This mechanism is illustrated in Figure 5C.
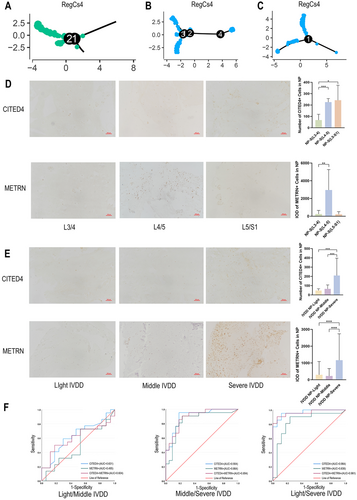
3.7 RegCs4 as a Marker of Degeneration in Biomechanically Loaded NP Segments
In this study, the identification of the CITED4 + METRN + RegCs4 chondrocyte subgroup marks a novel discovery (Figure 1F). RegCs4, predominantly located at the end of the pseudotime trajectory (Figure 6A–C), is linked to the progression of IVDD. Immunohistochemistry analysis of CITED4 and METRN revealed an increased presence of RegCs4 in severely degenerated NP at L4/5 or L5/S1 (Figure 6D), suggesting an association with biomechanically induced NP degeneration. The diagnostic efficacy of these markers for different IVDD stages was evaluated in 60 NP cases from L4/5 or L5/S1. These patients consisted of 32 males and 28 females with an age range of 25–80 years and a mean age of 51.28 ± 12.85. Notably, CITED4 demonstrated the highest area under the curve (AUC) value for differentiating between moderate-to-severe and mild-to-severe IVDD NP (Figure 6E,F).
4 Discussion
It is well established that the L4/5 and L5/S1 segments are more susceptible to IVDD than other lumbar spine segments. Abnormal mechanical stress can result in disc cell metabolic disintegration and tissue remodeling (Peng and Li 2022), with microcalcifications in NP and annulus fibrosus being pivotal in advancing IVDD (Karamouzian et al. 2010). Yet, the specific mechanisms underlying the pathological ossification of NP under mechanical stress remain poorly understood.
In our study, the application of scRNA-seq facilitated a distinctive characterization of the cellular microenvironment involved in IVDD NP ossification under mechanical loading. Notably, our study unveiled novel cell markers within the scRNA-seq, including CITED4, METRN, HAPLN1, ASPN, ARC, KRT16, CILP, EGR1, ATF3, GPX3, CTSL, ABI3BP, CCDC80, SOD3, ANGPTL4, and UBE2S, thereby expanding the gene pool of cell subgroup markers within the degenerative NP microenvironment. Moreover, our study revealed the presence of ossification-regulating genes such as DPT, CCN1, CCN2, ABI3BP, and HTRA1 that promote NP ossification and genes like NBL1, ECM1, and CRABP2 that inhibit ossification, contributing to cell differentiation in degenerative NP at L3/4 (Figure S2I).
This research highlights the prevalence of the METRN + CITED4 + RegCs4 cell subgroup in severe IVDD NP of the L4/5 and L5/S1 segments. Notably, CITED4 emerged as a key diagnostic marker in discerning the degeneration levels in L4/5 and L5/S1 IVDD NP. CITED4 is involved in the coordination of lipid metabolism and impedes HIF-1α transactivation by hindering the binding of HIF-1α to CBP/p300 (Ng et al. 2010). The diminished expression of HIF-1α in advanced stages of IVDD NP can obstruct endogenous stem cell repair processes (He et al. 2021) explaining the correlation between elevated CITED4 levels and aggravated IVDD. METRN, implicated in glial cell differentiation and axonal network formation during neurogenesis, has a homolog, METRNL, associated with various carbohydrate and lipid metabolism disorders (Zheng et al. 2016). Currently, clinical diagnosis of IVDD relies mainly on imaging diagnostics with reasonable specificity and noninvasive safety, and there is a relative lack of pathologic diagnostic markers. Because of their slow change, tissue imaging features remain limited as objective indicators for early diagnosis and efficacy assessment. Abnormal changes in the molecular biological features of diseases are usually accompanied or even preceded by histomorphologic changes. The identification of a CITED4 + METRN+ chondrocyte subset in NP associated with increased degeneration and aggravated biomechanical loading based on the NPs of clinical IVDD patients using scRNA-seq provides a strategy for potential diagnostic biomarkers and therapeutic targets at the molecular level of the cell nucleus.
Inflammatory cytokines are known to induce microcalcification (Zehra et al. 2022). The findings derived from the pseudotemporal analysis conducted in this study consistently highlight an association between the onset and progression of IVDD and inflammatory responses. Cell communication analysis underscored the regulatory role of immune-type ossification genes (Figure 5E). Previous studies have illustrated significantly higher expression levels of IL-6, TNF-α, and IL-1β in degenerated discs compared to healthy discs. After treatment, TNF-α and IL-6 decreased, and anti-inflammatory markers such as IL-4 increased (Puerto Valencia, He, and Wippert 2024). IL-1β, CTX-1, IL-10, and CCR6 may be associated with a decrease in pain scores of lower back pain after treatment, and MCP-1, IL-6, and IL-8 are associated with the clinical and functional assessment of lower back pain (Sima, Chen, and Diwan 2024; Hiyama et al. 2023, 2022). Pro-inflammatory cytokines such as IL-1β and TNF may induce an increase in CaSR expression in the degenerated discs. TNF can affect IVDD calcification by stimulating BMP2 release (Zehra et al. 2022). Additionally, our investigation revealed an enrichment of matrix repair, cartilage development, and osteogenic regulatory genes along the pseudotime trajectory. The presence of active osteogenic growth factors in cell communication suggests that abnormal injury repair and tissue remodeling in NP contribute to ossification (Figures S2D–G).
In healthy intervertebral discs with a Pfirrmann grade of I, PROCR+ resident CPCs NP demonstrate tri-lineage differentiation potential, including osteogenic, adipogenic, and fibrogenic lineages (Gan et al. 2021). However, the exact role of progenitor cells in degenerative NP ossification, particularly under mechanical stress, is still not fully understood. The differentiation of mesenchymal stem cells or progenitor cells into adipogenic and osteogenic lineages involves a “bone-fat balance,” a metabolic equilibrium extensively studied in conditions such as bone aging and osteoporosis (Deng et al. 2021). In the study, each patient for the scRNA-seq assay had no self-reported history of hyperlipidemia, but lipid levels were checked before the procedure. Patients with Pfirrmann grade I NP-L (L5/S1) and those with Pfirrmann grade IV NP-S (L3/4) had no significant abnormalities in their lipids. Patients with Pfirrmann grade III NP-M (L4-5) had triglycerides of 2.36 nmol/L (reference range: 0–2.3 nmol/L) and HDL cholesterol 0.74 nmol/L (reference range: 1.05–1.68 nmol/L). Similar Pfirrmann grade IV NP-S (L4/5) patients had triglycerides 2.31 nmol/L (reference range: 0–2.3 nmol/L) and HDL cholesterol 0.99 nmol/L (reference range: 1.05–1.68 nmol/L). Pfirrmann grade IV NP-S (L5/S1) patients showed no abnormalities in other parameters. However, the homocysteine test value of 18.27 μmol/L was higher than the 0–15 μmol/L reference range. Although limited by the number of samples examined and the number of tests performed, the reduced HDL and mildly elevated triglycerides or increased homocysteine in patients with L4/5 and L5/S1 IVDD somewhat corroborate the potential value of lipid synthesis to promote IVDD. Interestingly, our findings indicate that lipid metabolism, particularly cholesterol and steroid synthesis, is accentuated in the IVDD pseudotime trajectory. This implies that a disruption in the “bone-fat balance” plays a significant role in the degenerative ossification process of IVDD NP.
The differentiation patterns evident in chondrocyte Trajectory 1 and Trajectory 2 emphasize that under biomechanical stress, the activation of lipogenic metabolism genes contributes significantly to the exacerbation of degenerative NP ossification. This process occurs through the disruption of the “bone-fat imbalance” within chondrocytes. The spatial arrangement of lipogenic metabolism genes, including HMGCS1, FDPS, and MSMO1, corresponds to the pseudotemporal arrangement of chondrocytes from L4/5 and L5/S1 segments, indicating their correlation with increased biomechanical loading. HMGCS1, a crucial enzyme in cholesterol synthesis, plays a role in the PPAR signaling pathway (Tang et al. 2023) and, along with FDPS, regulates key enzymes in the mevalonate pathway (Ramos et al. 2023). HMGCS1 and MSMO1 are also involved in steroid biosynthesis and are markers for metabolically active CPCs, especially in Pfirrmann grades III and IV discs (Tu et al. 2022).
MIF is recognized for its role in regulating bone-fat balance. In MIF-deficient mice, activated NR3C1 was found to boost fructose-mediated lipogenesis, while MIF counteracted GC and its receptor, NR3C1 (Gligorovska et al. 2021). Moreover, MIF has been shown to regulate ossification in a bidirectional manner. Biomechanical overload triggers the activation of ossification growth factors in NP (Zehra et al. 2022) and elevates MIF expression (Wang et al. 2021), though the interplay between these factors is not yet fully understood. Our immunohistochemistry and immunofluorescence findings reveal that biomechanical loading shifts the bone-fat balance towards osteogenesis, marked by increased MIF expression in ossification-involved chondrocytes. Moreover, our observations indicate that MIF, expressed in macrophages, actively engages in NF-κB-mediated inflammation, thereby contributing to the progression of IVDD (Ling et al. 2022). MIF is widely involved in inflammatory responses as well as immune regulation. Specifically, increased MIF expression can be caused by activating the NF-κB signaling pathway in mouse models, which triggers a range of pro-inflammatory responses. These responses include prompting macrophages to migrate toward damaged areas and enhancing their inflammatory activity, thereby exacerbating IVDD (Zhang et al. 2023b). MIF regulates several inflammatory markers, and it binds to CD74, CXCR2, and CXCR4 on the surface of inflammatory chondrocytes in knee osteoarthritis. MIF-CD74 and MIF-CXCR2/4 protein interactions are critical for transcribing pro-inflammatory cytokines and complement systems in knee osteoarthritis (Fan et al. 2024). By promoting the acquisition of a Th17 cell-like phenotype in Treg, MIF can modulate type 3 immune responses in spondyloarthritis. In addition, MIF could activate MAPK, TLR, PI3K/AKT, NF-κB, and NLRP3 inflammatory vesicle pathways and inhibit the P53 pathway (Zeng et al. 2024). Therapeutically, small molecule inhibitors targeting MIF, such as CPSI-1306, have been shown to attenuate disc degeneration in mouse models, where the inhibitor reduces apoptosis and inflammatory responses in the NP by lowering MIF levels, thereby protecting the structural integrity of the disc. The diverse impact of MIF across different cell types in IVDD NP suggests intricate regulatory mechanisms that warrant further investigation.
5 Conclusions
In conclusion, we comprehensively mapped out the cellular milieu of ossification in human lumbar spine IVDD NP under biomechanical loading. The identification of the CITED4 + METRN+ chondrocyte subgroup, along with the understanding of the bone-fat balance regulatory mechanism, contributes deeper insights into the nuances of mechanical loading-induced human IVDD. These findings pave the way for the exploration of new avenues for developing preventive and therapeutic strategies against pathological ossification in IVDD.
Author Contributions
Ping Zhang conceptualized the study, analyzed the data, obtained the funds, and drafted the manuscript. Yuan Wang collected and analyzed the data and drafted the manuscript. Jianqi Bai, Jingru Zhang, Shimin Zhang, and Jiawen Zhan collected, interpreted, and analyzed the data. Xiaofei Guo analyzed the data and drafted the manuscript. Liguo Zhu conceptualized the study, obtained the funds, and edited the manuscript. All authors read and approved the final draft.
Acknowledgments
Thanks to colleagues from Wangjing Hospital and the China Academy of Chinese Medical Sciences for their contributions to single-cell RNA sequencing, immunohistochemistry, and immunofluorescence experiments. Xiaoxi Deng, Jiaqi Kang, Yimeng Zhang, and Xin Bai assisted in data collection and statistical analysis. Hongmei Su participated in the pathological diagnosis. Jiao Jin, Peiyan Hu, and Yan Li assisted in all sample collection and imaging diagnosis. Jianpeng Wang and Huiyang Yu assisted in the data analysis and production of the manuscript figures. Jie Yu and Yongli Dong provided suggestions for the revision of the manuscript. This study was funded by the Science and Technology Innovation project of the China Academy of Chinese Medical Sciences (CI2021A05054) and the National Natural Science Foundation of China (81930118; 82174415).
Ethics Statement
This study was conducted with approval from the Ethics Committee of Wangjing Hospital of China Academy of Chinese Medical Sciences (Approval Number: WJEC-KT-2021-052-P001). This study was conducted in accordance with the declaration of Helsinki.
Consent
Written informed consent was obtained from all participants.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




