Integrated transcriptomics and metabolomics reveal protective effects on heart of hibernating Daurian ground squirrels
Abstract
Hibernating mammals are natural models of resistance to ischemia, hypoxia-reperfusion injury, and hypothermia. Daurian ground squirrels (spermophilus dauricus) can adapt to endure multiple torpor-arousal cycles without sustaining cardiac damage. However, the molecular regulatory mechanisms that underlie this adaptive response are not yet fully understood. This study investigates morphological, functional, genetic, and metabolic changes that occur in the heart of ground squirrels in three groups: summer active (SA), late torpor (LT), and interbout arousal (IBA). Morphological and functional changes in the heart were measured using hematoxylin-eosin (HE) staining, Masson staining, echocardiography, and enzyme-linked immunosorbent assay (ELISA). Results showed significant changes in cardiac function in the LT group as compared with SA or IBA groups, but no irreversible damage occurred. To understand the molecular mechanisms underlying these phenotypic changes, transcriptomic and metabolomic analyses were conducted to assess differential changes in gene expression and metabolite levels in the three groups of ground squirrels, with a focus on GO and KEGG pathway analysis. Transcriptomic analysis showed that differentially expressed genes were involved in the remodeling of cytoskeletal proteins, reduction in protein synthesis, and downregulation of the ubiquitin-proteasome pathway during hibernation (including LT and IBA groups), as compared with the SA group. Metabolomic analysis revealed increased free amino acids, activation of the glutathione antioxidant system, altered cardiac fatty acid metabolic preferences, and enhanced pentose phosphate pathway activity during hibernation as compared with the SA group. Combining the transcriptomic and metabolomic data, active mitochondrial oxidative phosphorylation and creatine-phosphocreatine energy shuttle systems were observed, as well as inhibition of ferroptosis signaling pathways during hibernation as compared with the SA group. In conclusion, these results provide new insights into cardio-protection in hibernators from the perspective of gene and metabolite changes and deepen our understanding of adaptive cardio-protection mechanisms in mammalian hibernators.
1 INTRODUCTION
Hibernation is a remarkable survival strategy employed by many mammals to withstand seasonal food shortages, prolonged low temperatures, and other environment extremes (Andrews, 2019). During hibernation, small mammalian hibernators such as ground squirrels exhibit a remarkable drop in body temperature (often to just 2–10°C), reduced metabolic rate, slower heartbeat, and significantly lower organ perfusion rates (often less than 10% of normal) (Han et al., 2022; Horii et al., 2018). Despite these extreme physiological changes, ground squirrels experience brief interbout arousal (IBA) periods every few days or weeks that are characterized by a rapid return to euthermic body temperature and normal organ perfusion rates, before sinking into another torpor bout within ~24 h. Such torpor-arousal cycles are repeated multiple times over the hibernation season (Miao et al., 2022). Hence, over the annual hibernation cycle, tissues and organs undergo repeated bouts ischemia-reperfusion (I/R) and major changes in body temperature, but ground squirrels have evolved mechanisms to resist the damage that often arises from such conditions in non-hibernators, including humans (Andrews, 2007; Han et al., 2022; Otis et al., 2017)
Compared with non-hibernators, the heart of hibernating species is highly protected, showing a strong resistance to myocardial ischemia, allowing animals to be protected from I/R injury, which is similar to protection against ischemia that can be achieved with preconditioning (Yan et al., 2015). For instance, Hong Li and colleagues employed proteomic analysis to investigate the myocardium of Arctic ground squirrels (Spermophilus undulatus) versus rats following in vivo coronary artery ligation, and showed that markers of apoptosis (TUNEL and caspase-3) are lower in ground squirrels than in rats, whereas upregulation of sirtuin-3 and MnSOD, that are known to protect against ischemic injury, played a cardio-protective role (Yan et al., 2015). Furthermore, another study showed that a decrease in electron transport chain (ETC) protein abundance and increased lipid metabolism in ground squirrels contributed to cardio-protection (Quinones et al., 2016). Similarly, during an ex-vivo cardiac I/R operation, the expression of myocardial infarction markers was found to be lower in Arctic ground squirrels than in rats, demonstrating the superior ischemia tolerance of ground squirrel hearts (Salzman et al., 2017). Furthermore, in a study by Zhao et al., a coronary artery occlusion experiment was conducted on the hearts of summer versus winter woodchucks (Marmota monax), that showed that the arrhythmia fraction and mortality rate were significantly lower in winter woodchucks (5%) as compared with summer woodchucks. This indicated that the hearts of the hibernation group were more resilient to ischemia-induced arrhythmias and mortality compared with those in the summer (Zhao et al., 2018).
The hearts of hibernators exhibit a remarkable ability to withstand a low partial pressure of oxygen, that helps them resist pathological arrhythmias. For instance, hibernating hedgehogs (Erinaceus europaeus) can survive in pure N2 or CO2 for 1–2 h without experiencing any mortality, whereas non-hibernators can only survive for 3–5 min in such conditions (Biorck et al., 1956). When exposed to hypoxia (4.5% oxygen) hypoxia, rats exhibited a mortality rate of 80% within 1 h, whereas all 13-lined ground squirrels (Spermophilus tridecemlineatus) survived, indicating a higher tolerance to hypoxia in hibernators (D'Alecy et al., 1990). Furthermore, in non-hibernators, the dysregulation of cardiomyocyte calcium homeostasis at body temperatures below 20°C is likely to cause arrhythmias or fatal cardiac arrest but, by contrast, hibernators can regulate calcium homeostasis within cardiomyocytes to ensure survival under hypothermia (Egorov et al., 2012; Ivanov, 2000). Moreover, hibernators can maintain blood supply to vital organs such as the heart and brain by redistributing blood flow while in hibernation (Sjöquist et al., 1986). Taken together, numerous experiments from various perspectives have demonstrated that the hearts of hibernators exhibit resistance to ischemia, hypoxia, and hypothermia, rendering them a good natural model for investigating the actions that can provide protection against ischemia/hypoxia-reperfusion injury. Furthermore, information gleaned from hibernator studies can potentially provide solutions to extend the time that human organs explants can survive under hypothermic conditions or perhaps even during cryopreservation (Tessier & Storey, 2016).
High-throughput technologies have opened up new possibilities for investigating how hibernators adapt to extreme environmental stress in which pathway analysis and identification of functional groups of coregulatory genes are now preferred over traditional studies based on physiology or the selection of “star” molecules (Grabek et al., 2015). A number of studies have been conducted to apply omics to hibernation. For example, a previous cardiac transcriptomics study reported that differentially expressed genes (DEGs) in the heart are involved in metabolic, contractile, calcium handling, and cryogenic catalytic processes during hibernation as compared with active 13-lined ground squirrels (Hampton et al., 2011). Fedorov et al. also analyzed the hearts of black bears (Ursus americanus) in both winter and summer groups, and reported that 24 genes were significantly upregulated in the heart during hibernation, and that these genes were mainly associated with lipolytic metabolism and protein biosynthesis (Hampton et al., 2011). These genes supported adaptive mechanisms that reduce cardiac atrophy during prolonged hypometabolism and quiescence in bears during hibernation (Fedorov et al., 2011). Modulating metabolism is a key mechanism by which hibernators achieve tolerance to ischemia, hypoxia, and hypothermia. Other studies have demonstrated that Arctic ground squirrels are resistant to organ damage and inflammation following systemic I/R, which may arise primarily from altered metabolic responses, and is crucial to hibernation (Bogren et al., 2014). In a study of 13-lined ground squirrels, upregulation of recombinant pyruvate dehydrogenase kinase 4 in the heart was found to phosphorylate pyruvate dehydrogenase during hibernation, thereby inhibiting pyruvate entry into the TCA cycle and preserving carbohydrate resources, while enabling lipids to serve as the primary fuel for ATP production (Andrews et al., 1998). Furthermore, enzymatic processes related to metabolism were found to change during hibernation, with the heart primarily utilizing ketone bodies for energy supply, ensuring an adequate ATP supply without producing harmful metabolites (Andrews et al., 2009).
These metabolic changes are ultimately reflected at the level of metabolites. Metabolomics can provide insight into underlying physiological mechanisms by analyzing all small molecular weight metabolites of cells or organisms simultaneously in a specific physiological group, thereby revealing different metabolic patterns by analyzing differentially expressed metabolites (DEMs; Lai et al., 2020). Barbas et al. conducted a metabolomics study on Syrian hamster (Mesocricetus auratus) brain tissue and found significant changes in 337 metabolites during hibernation, which confirmed that altered amino acid metabolism and its derivatives, reduced glycolysis, diminished tricarboxylic acid cycle, and changes in phosphorylation levels of tau proteins play a role in neuroprotection during hibernation (Gonzalez-Riano et al., 2019). Furthermore, a metabolomic analysis of plasma from 13-lined ground squirrels, showed a novel pattern of seasonal variation in metabolites, and reported that clearance of potential oxidative stress-related metabolites is facilitated during IBA, whereas metabolic markers associated with I/R injury, including succinate and ferredoxin, remained relatively stable over the torpor-arousal cycle (D'Alessandro et al., 2017). Thus, metabolic studies can provide further evidence of resistance to I/R injury during hibernation.
In summary, hibernators possess remarkable metabolic regulation and tissue protection capabilities that enable them to withstand the drastic changes in physiological fluctuations that occur during hibernation and, thereby, lead to minimal damage. The complex and integrated nature of hibernation makes it difficult for a single omics approach to fully uncover its underlying mechanisms. However, a multiomics approach offers unique advantages in this regard. For instance, Yizhao Luan et al. used comprehensive transcriptomic and metabolomic analyses to examine the retina of 13-lined ground squirrels and revealed that the retinal energy source is altered during hibernation (Luan et al., 2018). Similarly, Yonggang Niu et al. performed transcriptomic and metabolomic analyses of hibernating Tibetan frogs (Nanorana parkeri) and discovered the energy strategy of liver metabolism (Niu et al., 2023). Hence, a multiomics approach is effective for assessing biochemical and molecular regulatory mechanisms. In the present study, heart tissue of ground squirrels from summer active (SA), late torpor (LT), and IBA groups were selected for transcriptomic and metabolomic analyses, to identify genes and small molecule metabolites that are associated with resistance to ischemia, hypoxia, and hypothermia in hibernators. In addition, hematoxylin-eosin (HE) staining, Masson staining, and echocardiography were performed on heart tissue, and protein levels of CK-MB, cTnI, and BNP were measured in serum of ground squirrels. The present study aims to deepen our understanding of the mechanisms of cardio-protective adaptation in hibernators.
2 MATERIALS AND METHODS
2.1 Chemical reagents
The 4% neutral paraformaldehyde solution was purchased from ZZHC Technology CO., Ltd. (p1015-5). HE staining kit (G1003) and Masson staining kit (G1006) were obtained from Wuhan Servicebio Technology Co., Ltd. creatine kinase isoenzyme (CK-MB) (JM-01833R2), Cardiac troponin I (cTnI) (JM-02031R2) and b-type natriuretic peptide (BNP) (JM-01474R2) ELISA kits were obtained from Jiangsu Jingmei Biotechnology Co., Ltd. Total RNA extraction reagent (AG RNAex Pro Reagent) was from Accurate Biotechnology Co., Ltd (AG21102). The Uni ALL-in-One First-Strand cDNA Sythesis SuperMix kit (AU341) and the Green QPCR SuperMix kit (AQ061) were from TransGen Biotech Co, LTD.
2.2 Animals and tissue extraction
2.2.1 Animal acquisition and grouping
All procedures were in accordance with the guidelines of the Experimental Animal Management Committee of the Ministry of Health of the People's Republic of China (approval number: SL2012-42). Daurian ground squirrels were housed and treated as previously described by our laboratory (Han et al., 2022; Miao et al., 2022). Two groups of ground squirrels were studied. SA animals were captured from Weinan, Shaanxi Province, China, and placed in an 18–25°C environment with a 12–12 h light–dark cycle and fed rodent food with the addition of peanuts and water until they were euthanized in July. A second batch ground squirrels were captured in November and were held from November to March in a hibernation room at environment temperature (about 0–12°C)and in darkness throughout the day. Confirmation of continuous torpor was determined by (a) placing sawdust on the back of each animal (that was shaken off if IBA occurred), and (b) by monitoring body temperatures twice a day by thermography in combination with an infrared thermometer (Fluke VT04 Visual IR Thermometer) to distinguish and record torpor and arousal bouts.
Ground squirrels were divided into three groups: (1) SA, active squirrels sampled in July with a Tb of 36–38°C, (2) LT, animals were allowed to cycle through torpor-arousal bouts for 60 days and sampled when they had re-entered torpor with body temperature stabilized at 5–8°C for ≥5 days, and (3) IBA animals were allowed to cycle through torpor-arousal bouts for 60 days and were sampled when fully aroused and showing a body temperature 34–37°C; sampling occurred within 12 h while animals were still in euthermia. The average length of torpor was 7.4 ± 3.7 days, and accounted for about 89.9% of the whole hibernation period, and the average length of IBA was about 1.4 days, which accounted for about 10.1% of the whole hibernation period. The average length of a torpor-arousal cycle was about 8.8 days (Miao et al., 2022).
2.2.2 Sample collection and preparation
Ground squirrels were anesthetized by intraperitoneal injection of 100 mg/kg sodium pentobarbital. Blood was collected from the main abdominal vein and was centrifuged at 10,000 rpm for 5 min after being placed at room temperature for 30 min. The supernatant was carefully aspirated, centrifuged at 3000 rpm for 5 min, and then the serum with clean supernatant was carefully aspirated. Supernatant was collected and dispensed into 200 μL centrifuge tubes of 150 μL each. Serum samples were quickly frozen in liquid nitrogen and stored in a −80°C freezer for later use. After blood collection, heart samples were collected. To avoid RNA degradation and any postmortem metabolic processes, hearts were snap frozen in liquid nitrogen immediately after collection, and then transferred to a −80°C freezer.
2.3 Histological staining
Fresh myocardial tissue samples were fixed in 4% paraformaldehyde for more than 24 h. Paraffin sections were cut into approximately 4 µm sections and dewaxed to water, followed by use of an HE staining kit and Masson staining according to the instructions. Dehydrated sections were sealed according to the instructions provided, and sections were observed with a light microscope (Nikon Eclipse E100; Nikon).
2.4 ELISA
Protein expression levels of cTnI, CK-MB, and BNP were measured in 10 µL of serum using the kits (as described above) according to the instructions provided, and absorbance at 450 nm was estimated using a Rayto RT-6100 microplate reader (Shenzhen Radu Life Science Co., Ltd.).
2.5 Echocardiography
Ground squirrels were anesthetized with 2% isoflurane and held on a 37°C thermostatic plate. The left thorax was exposed and cardiac ultrasound M-mode images were recorded using a small animal ultrasound system (Fujifilm Visual Sonics Vevo3100) with a 30 MHz ultrasound probe, selected with the parasternal short axis as the cut surface. Measurements included: (1) Ultrasound structure: left ventricular (LV) posterior wall thickening rate (ΔT% = LVPW;s-LVPW;d/LVPW;d), septal thickening rate (ΔIVST% = IVS;s-IVS;d/IVS;d), and LV systolic/diastolic internal diameter (LVID;s/LVID;d). (2) Ultrasound function: ejection fraction (EF), shortening fraction (FS) of the short axis of the left ventricle, stroke volume/body weight (SV/BM), heart rate (HR), and cardiac output (CO). Myocardial contractility parameters were acquired according to the same horizontal short-axis section, and two images were recorded for each section, with three consecutive heart beats recorded for each parameter.
2.6 Real-time quantitative PCR (RT- qPCR)
Three groups of ground squirrel hearts (SA, LT, IBA, n = 6 each) were analyzed. Samples of 50–100 mg of myocardial tissue were flash frozen in liquid nitrogen and then ground into powder, followed by extraction of RNA with RNAex Pro Reagent, adapting the system according to the instructions of the reverse CDNA kit and qPCR kit, followed by performing qPCR. Data were processed using the method, with 18s rRNA as an internal reference gene (Xiao et al., 2020). Primer sequences for 18s rRNA were Forward primer: TCAAGAACGAAAGTCGGAGG and Reverse primers: GGACATCTAAGGGCATCACA. Primer sequences for other genes are listed in Supporting Information: Table S1. The correlation between RNA-Seq and qPCR was then determined using correlation analysis and linear regression analysis based on R2 and p value.
2.7 Transcriptomic analysis
2.7.1 RNA quantification and qualification
RNA integrity was assessed using the RNA Nano 6000 Assay Kit on a Bioanalyzer 2100 system (Agilent Technologies).
2.7.2 Library preparation and Illumina sequencing
Total RNA was isolated, and mRNA with poly A tails was enriched by use of Oligo(dT) magnetic beads, followed by random interruption of the obtained mRNA with divalent cations in Fragmentation Buffer. Fragmented mRNA was used as a template and random oligonucleotides as primers to synthesize first strand cDNA in an M-MuLV reverse transcriptase system, followed by degradation of RNA strands with RNaseH and synthesis of the second strand of cDNA with dNTPs in a DNA polymerase I system. The purified double-stranded cDNA was end-repaired, A-tailed and connected to the sequencing junction, and cDNA of 370~420 bp was screened by AMPure XP beads followed by PCR amplification. PCR products were then purified again using AMPure XP beads to obtain the final library. After construction, the library was initially quantified using a Qubit 2.0 Fluorometer and diluted to 1.5 ng/uL, and then the insert size of the library was detected using an Agilent 2100 bioanalyzer, and the effective concentration of the library was accurately quantified by RT-qPCR after the insert size met expectations. Next, the effective concentration of the library was accurately quantified by RT-qPCR and showed a value that was higher than 2 nM), ensuring the quality of the library.
2.7.3 Data quality control
The image data of sequenced fragments by a high-throughput sequencer were converted into sequence data (reads) by CASAVA base identification, and the raw data were filtered. The main purpose of this was to remove reads with adapters, reads with N (N means base information cannot be determined), and low-quality reads (reads with Qphred ≤20 bases accounting for more than 50% of the entire read length). Meanwhile, Q20, Q30 and GC contents were calculated for clean data. All subsequent analyses were performed based on clean data for high quality analysis.
2.7.4 Sequence alignment and differential gene analysis
A reference genome (ftp://ftp.ensembl.org/pub/release 105/fasta/Spermophilus dauricus/) was downloaded along with the gene model annotation file (ftp://ftp.ensembl.org/pub/) from the ensembl database website. Since the gene annotation file of Daurian ground squirrels is incomplete, we used the Swissport database for comparison (http://us.expasy.org/sprot/). The index of the reference genome was constructed using HISAT2 v2.0.5 and paired endclean reads were compared with the reference genome using HISAT2 v2.0.5. feature counts (1.5.0-p3) and used to calculate the number of reads mapped to each gene. The FPKM of each gene was then calculated based on the length of the gene (FPKM refers to the expected number of transcript sequence fragments per kilobase fragment per million base pairs sequenced). Our biological replicates (n = 3) were used for differential expression analysis between the two comparative combinations using DESeq. 2 software (1.20.0). The resulting p values were adjusted to control for false discovery rates using the method of Benjamini and Hochberg. Genes found to have adjusted p values ≤ 0.05 by DESeq. 2 were assigned as differentially expressed. After identification as DEGs, gene ontology (GO) enrichment analysis of DEGs and statistical enrichment of DEGs in the KEGG pathway were achieved by cluster profiler (3.8.1) software and were corrected for gene length bias. GO terms with corrected p values less than 0.05 were considered to be significantly enriched by DEGs.
2.8 Untargeted metabolomic analysis
2.8.1 Metabolite extraction
Heart tissue (100 mg) was removed from the −80°C freezer, transferred to a mortar precooled with liquid nitrogen and ground to a powder under liquid nitrogen. The powder was placed in an EP tube and 500 μL of 80% aqueous methanol was added followed by vortexing, placing on ice for 5 min, and then centrifugation at 15,000g for 20 min at 4°C. The supernatant was removed and diluted with mass spectrometry grade water to 53% methanol and subsequently centrifuged at 15,000g for 5 min at 4°C. Supernatant was collected and injected into the LC-MS for analysis (an equal volume of each sample was mixed from each experimental sample as QC samples).
2.8.2 UHPLC-MS/MS analysis
UHPLC-MS/MS analyses were performed using a Vanquish UHPLC system (Thermo Fisher) coupled with an Orbitrap Q Exactive TMHF-X mass spectrometer (Thermo Fisher) from Novogene Co., Ltd. Samples were injected onto a Hypesil Gold column (100 × 2.1 mm, 1.9 μm) using a 17-min linear gradient at a flow rate of 0.2 mL/min. The eluents for the positive polarity mode were eluent A (0.1% FA in water) and eluent B (methanol). The eluents for the negative polarity mode were eluent A (5 mM ammonium acetate, pH 9.0) and eluent B (methanol). The solvent gradient was set as follows: 2% B for 1.5 min, 2-100% B for 3 min, 100% B for 10 min, 100-2% B for 10.1 min, and 2% B for 12 min. The Q Exactive™ HF-X mass spectrometer was operated in positive/negative polarity mode with spray voltage of 3.5 kV, capillary temperature of 320°C, sheath gas flow rate of 35 psi, and aux gas flow rate of 10 L/min, S-lens RF level of 60, Aux gas heater temperature of 350°C.
2.8.3 Metabolomics data preprocessing, metabolite identification, and data analysis
The raw data files generated by UHPLC-MS/MS were processed using Compound Discoverer 3.1 (CD3.1, Thermo Fisher) to perform peak alignment, peak picking, and quantification for each metabolite. Peak intensities were normalized to the total spectral intensity. Normalized data were used for additive ions, molecular ion peaks, and fragment ions. Peaks were then matched to the mzCloud (https://www.mzcloud.org/), mzVault and MassList databases for accurate qualitative and relative quantitative results. Background ions were removed with blank samples (53% methanol aqueous solution instead of experimental samples), and the raw quantification results were normalized. Final identification and relative quantification of the metabolites were performed based on the Linux operating system (CentOS version 6.6) and the software programs R and Python.
Transformations were performed based on the metabolome processing software metaX, that in turn yielded VIP values for each metabolite. In the univariate analysis part, statistical significance (p value) was calculated for each metabolite between the two groups based on t test and the fold change (FC value) of the different metabolites between the two groups was calculated. The default criteria for differential metabolite screening were VIP > 1, p value < 0.05, and FC ≥ 2 or FC ≤ 0.5. The KEGG database (https://www.genome.jp/kegg/pathway.html), the HMDB database (https://hmdb.ca/metabolites), and LIPID Maps database (http://www.lipidmaps.org/) were used to annotate the identified DEMs and differential metabolite volcanoes were plotted using the R package ggplot2. A clustering heat map was plotted using the R package pheatmap and the metabolite data were standardized using z-score. The bubble map was plotted with the R package ggplot2, and the KEGG database was used to investigate the functions and metabolic pathways of metabolites that were considered enriched when x/n > y/n and significantly enriched when the p value of the metabolic pathway was <0.05.
2.9 Integrative transcriptomic and metabolomic data
We performed correlation analysis based on Pearson's correlation coefficient for the DEGs and DEMs that had p values were significantly ranked in the top 50 to measure the degree of association between DEGs and DEMs. Then we mapped all DEGs and DEMs obtained from transcriptomic and metabolomic analyses to the KEGG pathway database at the same time to obtain their common pathway information. This allowed determination of the main biochemical pathways and signal transduction pathways in which DEGs and DEMs participated together.
2.10 Statistical analysis
Statistical analysis was performed using SPSS 22.0 software, and data are reported as mean ± SEM, with differences between groups analyzed using one-way analysis of variance. A homogeneity of variances test was performed within groups, with variance chi-significance >0.05 identified as no significant difference. An LSD test was used when homogeneity was not detected, and Tamhane's T2 was used for testing. Statistically significant differences are reported as p < 0.05 or p < 0.01.
3 RESULTS
3.1 Morphological changes in the heart
HE staining showed that the morphology of cardiac tissue was not abnormal in the three groups, with clear myocardial fiber structure, regular arrangement, visible intercalated disc structure, intact cytosolic membrane, clear transverse lines, clear boundaries, and uniform neat cytoplasm and nucleus coloring (Figure 1a–f). Masson staining showed clear staining of collagen fibers (blue) and cardiomyocytes (red), and showed no significant collagen deposition in the perivascular area and myocardial interstitium of the three groups (Figure 1g–i).
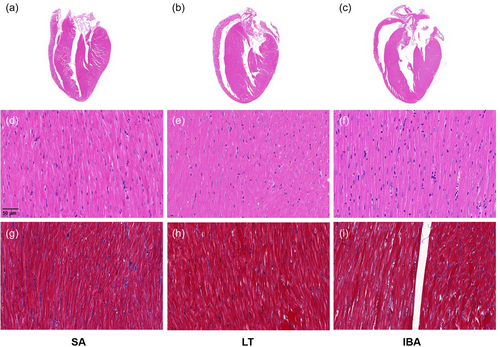
3.2 Cardiac function assessment, protein levels of CK-MB, cTnI, and BNP in serum
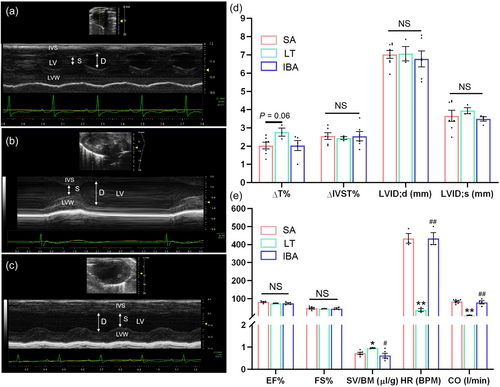
Echocardiographic tests were performed on the hearts of ground squirrels in three groups, that were divided into cardiac structure and function for statistical analysis (Figure 2 and Supporting Information: Table S2). As shown in Figure 2d, the ΔT% in the LT group increased by 37% (p = 0.06) as compared with the SA group. The rate of ΔIVST% and LVID;d, LVID;s did not show significant changes in the three groups. As shown in Figure 2e, EF% and FS% did not show any significant differences among the three groups and, as compared with the SA group, SV/BD was significantly increased by 33% in the LT group. However, compared with the LT group, the IBA group showed a significant decrease. HR and CO were significantly reduced by 92% (p < 0.01) in the LT group as compared with the SA group and returned to SA level in the IBA group.
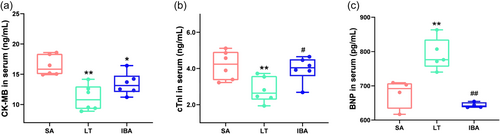
Changes in protein levels of serum markers CK-MB, cTnI, and BNP were also detected (Figure 3). The results showed that the expression of both CK-MB and cTnI were significantly decreased by ~33% in the LT group as compared with the SA group (p < 0.01). The protein level of CK-MB was significantly reduced by 19% in the IBA group as compared with the SA group (p < 0.05). In addition, cTnI level in serum was significantly increased by 41% in the IBA group as compared with the LT group (p < 0.05). BNP expression level was significantly increased by 17% in the LT group as compared with the SA group (p < 0.01), but returned to a value not significantly different from the SA expression level in the IBA group.
3.3 Changes in mRNA of ground squirrel heart
3.3.1 Identification of DEGs and related functional analysis
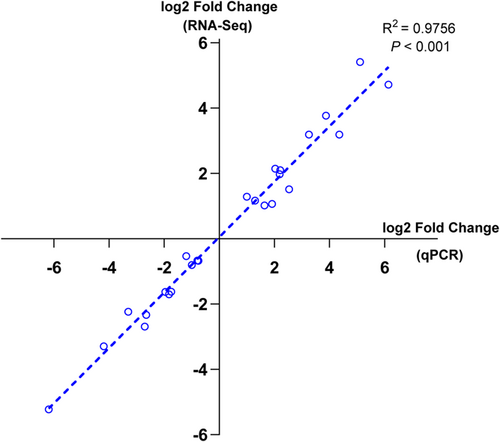
After sequencing the samples, the data were tested for quality control, and each sample contained between 4.14 and 4.58 million clean reads after removing the reads with sequencing junctions or low sequencing quality. Clean reads accounted for more than 90% of raw reads. Sequencing error rates were all less than 0.2%, and the GC content percentage was between 40% and 50%. These QC results all showed strict data quality control (Supporting Information: Table S3). When the clean reads were compared with the reference genome (ftp://ftp.ensembl.org/pub/release-105/fasta/spermophilus dauricus/), the total map value of each sample was greater than 60%, which met the basic requirements for nonmodel organisms. To further validate the reliability of our transcriptomic data, 25 mRNA types (that encode key proteins) that have important roles during hibernation were selected for RT-qPCR validation. These were involved in biological pathways and molecular functions (MFs) such as protein processing, amino acid metabolism, maintenance of cytoskeleton, cell growth and development. RT-qPCR results were tested with correlation analysis of the transcriptomic data and showed R2 > 0.9, p < 0.01, demonstrating that our data were ready for the next step of analysis (Figure 4).
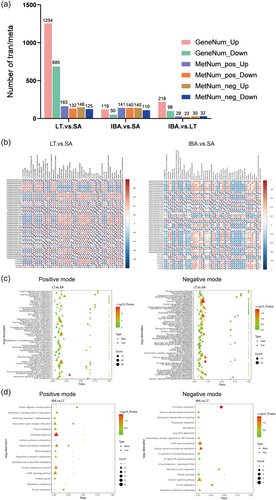
A total of 31,159 genes were identified in heart tissue of different groups. The DEGs between different groups are listed in Table 1, and the screening threshold for selecting DEGs was log2|(fold change)| ≥1, padj ≤ 0.05. According to the screening criteria for DEGs, 1939 genes were classified as DEGs between LT and SA groups with 685 (35.3%) genes being significantly upregulated and 1254 (64.6%) genes being downregulated in the LT group. The strongest change in gene expression was for nicotinamide nucleoside kinase 2 (NMRK2), that was significantly upregulated by 14.2-fold (log2 fold change = 3.9). By contrast, the expression of prostaglandin G/H synthase 2 (PTGS2) showed the greatest decrease by approximately 98.6% (log2 fold change = −6.2).
| Compare | All | Up | Down | Threshold |
|---|---|---|---|---|
| LT versus SA | 1939 | 685 | 1254 | DESeq2 padj ≤ 0.05 |log2FoldChange | ≥ 1.0 |
| IBA versus SA | 169 | 50 | 119 | DESeq2 padj ≤ 0.05 |log2FoldChange | ≥ 11.0 |
| IBA versus LT | 316 | 218 | 98 | DESeq2 padj ≤ 0.05 |log2FoldChange | ≥ 11.0 |
- Abbreviations: IBA, interbout arousal; LT, late torpor; SA, summer active.
In a comparison of IBA versus SA groups 169 DEGs were identified, of which 50 (29.5%) genes were significantly upregulated and 119 (70.4%) were downregulated. Notably, 35 of the 50 significantly upregulated DEGS were consistent with those that were upregulated during LT versus SA, and most of the downregulated genes were similar to the LT group, which suggests that most of the genes associated with torpor were not fully reversed in the IBA group. There were 218 (68.9%) significantly upregulated genes and 98 (31%) downregulated genes in the IBA group as compared with the LT group. DEGs can be seen in Supporting Information: Table S4. DEGs were then analyzed by hierarchical clustering analysis to visualize the changes in gene expression. The results showed that individuals from three groups were clustered together separately, with good clustering within the group and significant differences between the groups (Figure 5).
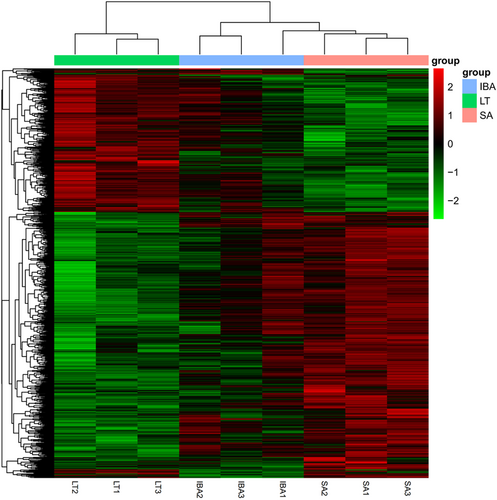
3.3.2 Functional classification annotation of DEGs
To further clarify the biological functions and classification of DEGs, GO analysis of DEGs was performed with the different groups, and DEGs showed a broad functional distribution, in which the LT group was enriched in GO terms as compared with the SA group. The results showed that 37 (34%) of the upregulated DEGs mapped to cellular components (CC) that were mainly present in mitochondria and mitochondrial membrane, and four genes were enriched in the proteasome core complex, which might enhance the proteasome pathway. In terms of MF, genes in this category were mainly associated with oxidoreductase activity. Downregulated genes were strongly and significantly enriched in protein phosphorylation in biological processes (BP), and protein kinase activity in MF.
DEGs between IBA and SA were enriched in GO terms, including oxidoreductase activity in MF, oxidation–reduction process in BP, the actin cytoskeleton, cytoskeletal part, and cytoskeleton in CC. DEGs between IBA and LT groups were enriched in protein serine/threonine kinase activity in MF, lipid transport and lipid localization in BP (Table 2).
| GO Category | Gene Ratio | p value | Count | Up | Down | |
|---|---|---|---|---|---|---|
| Biological process | ||||||
| LT versus SA | ||||||
| protein phosphorylation(GO:0006468) | 60/380 | 0.00000195 | 60 | 0 | 60 | |
| IBA versus SA | ||||||
| oxidation-reduction process(GO:0055114) | 9/48 | 0.0054824 | 9 | 1 | 8 | |
| IBA versus LT | ||||||
| lipid transport(GO:0006869) | 4/93 | 0.019720308 | 4 | 4 | 0 | |
| lipid localization(GO:0010876) | 2/93 | 0.03212831 | 2 | 2 | 0 | |
| Protein tyrosine/serine/threonine phosphatase activity(GO:0008138) | 3/152 | 0.025079022 | 3 | 2 | 1 | |
| Cellular component | ||||||
| LT versus SA | ||||||
| mitochondrion(GO:0005739) | 11/109 | 0.00000554 | 11 | 11 | 0 | |
| mitochondrial membrane(GO:0031966) | 7/109 | 0.000105979 | 7 | 7 | 0 | |
| mitochondrial part(GO:0044429) | 8/109 | 0.000107042 | 8 | 8 | 0 | |
| mitochondrial outer membrane(GO:0005741) | 4/109 | 0.000791931 | 4 | 4 | 0 | |
| proteasome core complex(GO:0005839) | 4/109 | 0.007487644 | 4 | 4 | 0 | |
| IBA versus SA | ||||||
| actin cytoskeleton(GO:0015629) | 3/26 | 0.01272764 | 3 | 0 | 3 | |
| cytoskeletal part(GO:0044430) | 4/26 | 0.013027165 | 4 | 0 | 4 | |
| cytoskeleton(GO:0005856) | 4/26 | 0.022513119 | 4 | 0 | 4 | |
| Molecular function | ||||||
| LT versus SA | ||||||
| protein kinase activity(GO:0004672) | 61/666 | 0.0000218 | 61 | 0 | 61 | |
| oxidoreductase activity(GO:0016491) | 24/260 | 0.001813768 | 24 | 24 | 0 | |
| IBA versus SA | ||||||
| oxidoreductase activity(GO:0016491) | 11/76 | 0.001081748 | 11 | 3 | 8 |
3.4 Differential metabolic patterns in the heart of the ground squirrels
3.4.1 Total metabolite principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA)
A total of 936 metabolites were identified in positive and negative mode using a nontargeted metabolomics technique based on LC-MS/MS technology. Better Pearson's correlation coefficients were obtained (Supporting Information: Figure S1). PCA of peaks obtained from all experimental and QC samples extracted showed differences in metabolic profiles between the groups, with satisfactory separation between the SA and hibernating groups (LT and IBA groups), but the LT group and IBA group were not well separated during torpor-arousal cycles, which might indicate that seasonal differences were greater than thermal metabolic differences (Supporting Information: Figure S2). The results of PLS-DA (Supporting Information: Figure S3) showed that both R2 and Q2 were close to 1, indicating a stable and reliable model. The model was ranked to detect that R2 was greater than Q2 and the intercept of Q2 with the y-axis was less than 0, indicating that the model was not overfitted (Supporting Information: Figure S4).
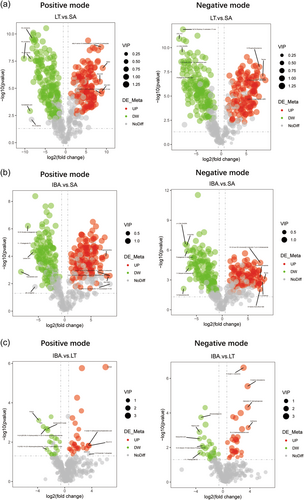
3.4.2 Volcano plots of differential metabolite expression
Metabolites that were significantly different between groups were screened with VIP > 1.0, FC > 1.5 or FC < 0.667 and p value < 0.05, (Delcayre & Swynghedauw, 1975; Herron & McDonald, 2002; Lowes et al., 2002) and the overall distribution of DEMs is presented visually in a volcano map (Figure 6). The data shows 566 DEMs in LT versus SA, 531 in IBA versus SA, and only 112 in IBA versus LT groups. As compared with the SA group, 257 metabolites were significantly upregulated and 309 downregulated in the LT group. Comparing IBA versus SA, 250 metabolites were significantly upregulated and 281 downregulated in the IBA group. In addition, there were 58 metabolites significantly upregulated and 54 downregulated in IBA versus LT. The data showed more DEMs between LT versus SA and IBA versus SA than between LT versus IBA, indicating an overall reduction in metabolite changes during hibernation. The DEMs distinguished are listed in Supporting Information: Table S5.
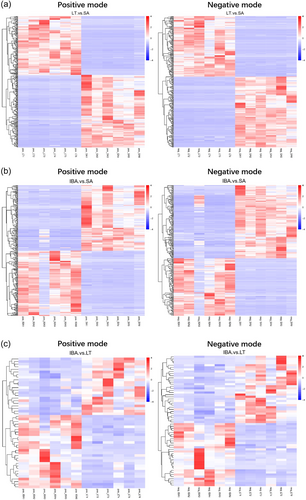
3.4.3 Metabolite heat map of DEMs
Clustering analysis of different metabolites between groups (Figure 7 and Supporting Information: Figure S5), showed good clustering between samples within groups and significant metabolic differences between groups.
3.4.4 KEGG enrichment pathway analysis of DEMs
KEGG pathway enrichment identified the most significant biochemical metabolic pathways and signal transduction pathways involved in DEMs in different groups. The major enriched metabolic pathways in LT versus SA groups were glutathione metabolism, pentose phosphate pathway, fatty acid biosynthesis, including the upregulation of L-pyroglutamic acid, L-ornithine, spermidine, L-glutamic acid, D-gluconic acid, D-ribulose 5-phosphate, D-erythrose 4-phosphate, D-sedoheptulose 7-phosphate, lauric acid, decanoic acid as well as downregulation of palmitic acid and stearic acid. Higher levels of L-glutamine, indole, various amino acids and purine metabolites were observed in a comparison of IBA versus SA, and were mainly involved in the pathways of protein digestion and absorption, aminoacyl-tRNA biosynthesis and purine metabolism. In addition, some metabolites showed differences not only between LT versus SA, but also between IBA versus SA. These included increased expression levels of creatine, phosphocreatine, D-proline, taurine, and sulfoacetic acid that indicates enriched arginine and proline metabolism, taurine and hypotaurine metabolism, and nitrogen metabolism pathways, suggesting that these pathways might play key roles in hibernation. As compared with the LT group, vitamin digestion and absorption, pyrimidine metabolism and phenylalanine metabolism were enriched in the IBA group, that included upregulated levels of nicotinamide 2-deoxyuridine, methylmalonic acid and thymidine, and downregulated levels of pantothenic acid, hippuric acid, and phenylacetyl glycine. The main KEGG pathways based on the DEMs in each group are shown in Table 3.
| Pathway | Groups | p Value | x | y | n | N | EnrichDirect | MetaIDs | Mode |
|---|---|---|---|---|---|---|---|---|---|
| Glutathione metabolism | LT versus SA | 0.3955 | 4 | 5 | 49 | 84 | Over | L-Pyroglutamic acid; L-Ornithine; Spermidine; L-Glutamic acid | Positive |
| Pentose phosphate pathway | LT versus SA | 0.2947 | 4 | 4 | 64 | 98 | Over | D-Gluconic acid; D-Ribulose 5-phosphate; D-Erythrose 4-phosphate; D-Sedoheptulose 7-phosphate | Negative |
| Fatty acid biosynthesis | LT versus SA | 0.2947 | 4 | 4 | 64 | 98 | Over | Lauric acid; Decanoic acid; Palmitic acid; Stearic acid | Negative |
| Protein digestion and absorption | IBA versus SA | 0.2589 | 7 | 8 | 56 | 84 | Over | L-Glutamine; Indole; Methionine; Histamine; L-Phenylalanine; L-Lysine; L-Histidine | Positive |
| Aminoacyl-tRNA biosynthesis | IBA versus SA | 0.2604 | 8 | 9 | 56 | 84 | Over | L-Glutamine; Methionine; L-Phenylalanine; L-Aspartic acid; L-Lysine; L-Histidine; L-Tyrosine; L-Glutamic acid | Positive |
| Purine metabolism | IBA versus SA | 0.3429 | 8 | 11 | 56 | 98 | Over | Adenosine diphosphate ribose; 5’-Adenylic acid; Adenylosuccinic acid; Adenine; Adenosine 5’-monophosphate; Uric acid; Xanthine; Adenosine 5’-Diphosphate | Negative |
| Arginine and proline metabolism | LT versus SA | 0.4592 | 6 | 8 | 49 | 84 | Over | Creatine; L-Ornithine; Creatine phosphate; S-Adenosylmethionine; Spermidine; L-Glutamic acid | Positive |
| IBA versus SA | 0.7129 | 6 | 8 | 56 | 84 | Over | Creatine; Creatine phosphate; S-Adenosylmethionine; N-Methylhydantoin; Spermidine; L-Glutamic acid | Positive | |
| IBA versus SA | 0.5051 | 2 | 2 | 56 | 98 | Over | D-Proline; 4-Oxoproline | Negative | |
| Taurine and hypotaurine metabolism | LT versus SA | 0.2947 | 4 | 4 | 64 | 98 | Over | Taurine; acetyl phosphate; Sulfoacetic acid; L-Cysteinesulfinic acid | Negative |
| IBA versus SA | 0.1326 | 4 | 4 | 56 | 98 | Over | Negative | ||
| Nitrogen metabolism | LT versus SA | 0.5080 | 2 | 2 | 49 | 84 | Over | L-Glutamine; L-Glutamic acid | Positive |
| IBA versus SA | 0.5502 | 2 | 2 | 56 | 84 | Over | Positive | ||
| Vitamin digestion and absorption | IBA versus LT | 0.1256 | 2 | 5 | 11 | 84 | Over | Pantothenic acid; Nicotinamide | Positive |
| Pyrimidine metabolism | IBA versus LT | 0.0368 | 4 | 9 | 16 | 98 | Over | 2-Deoxyuridine; dCDP; Methylmalonic acid; Thymidine | Negative |
| Phenylalanine metabolism | IBA versus LT | 0.1235 | 2 | 4 | 16 | 98 | Over | Hippuric acid; Phenylacetylglycine | Negative |
3.5 Integrative transcriptomic and metabolomic analysis
To reveal the biological pathways of cardiac alterations at different periods of ground squirrel torpor/arousal, the results of transcriptomics and metabolomics were integrated. The number of DEGs and DEMs are presented in a uniform manner in Figure 8a. The integrative analysis focused on LT and IBA groups, and the DEGs and DEMs with significant p value rankings in the top 50 were correlated based on Pearson's correlation coefficient to measure the degree of association between DEGs and DEMs (Figure 8b). Then, the KEGG database was used to analyze the integrative pathway of DEGs and DEMs obtained from transcriptomic and metabolomic analysis (Figure 8c,d). The specific KEGG integrated pathway analysis is shown in Supporting Information: Table S6. The main pathways that were significantly enriched in LT versus SA were ferroptosis, oxidative phosphorylation, glycine, serine and threonine metabolism, HIF-1 signaling pathway, calcium signaling pathway, tryptophan metabolism, and regulation of the actin cytoskeleton. In IBA versus LT, metabolic pathways were enriched in pyrimidine metabolism, amino acid metabolism including glycine, serine and threonine metabolism, and arginine and proline metabolism. Gene and metabolite interactions played an important role in the cardio-protection of ground squirrels during hibernation, that provided more information than a single omic study could.
4 DISCUSSION
Ground squirrel hibernation is characterized by a significant decrease in HR and CO, without any significant morphological changes in the heart, as revealed from the morphological staining results (Figure 1). This suggests that hibernating ground squirrels are a natural model of resistance to I/R without causing any pathological damage. A significant decrease in HR was observed in the LT group as compared with the SA group by echocardiographic analysis (Figure 2), which was a distinct feature of hibernators (Nelson & Robbins, 2015). Stroke volume divided by body weight was used to account for the differences in individual body weight, and our results showed a significant increase in SV/BW and a significant decrease in CO in the LT group, which was consistent with a previous study on golden-mantled ground squirrels (Callospermophilus lateralis) (Nelson & Rourke, 2013). A small decrease in EF% in the LT group was observed, indicating a slight weakening of cardiac systolic function. However, the ΔT% increase in the LT group (p = 0.061), that might help to normalize cardiac contraction and maintain ideal contractile performance (Lorell & Carabello, 2000). Therefore, ground squirrels might have developed a compensatory response to increase the stroke volume and LV wall thickness to maintain a certain CO during hibernation.
CK-MB and cTnI are classic markers of cardiac injury that are mainly distributed in the myocardium and rapidly released in large amounts in the blood when myocardial injury occurs (Yildiz et al., 2014). The results of the Elisa assay for CK-MB and cTnI in serum showed that both were significantly lower in the LT group than in the SA group (Figure 3), and cTnI levels were significantly higher in the IBA group as compared with the LT group. Consistent with our findings, a decrease in CK isoenzymes during hibernation was found in the left ventricle of grizzly bears (Ursus arctos horribilis) and in muscle of Richardson's ground squirrels (S. richardsonii). CK-MB plays an important role in cellular energy metabolism, and therefore a decrease in CK-MB was consistent with reduced energy requirements of the heart and muscle during hibernation (Abnous & Storey, 2007; Barrows et al., 2011). The finding that cTnI was significantly higher in 13-lined ground squirrel plasma in the IBA group as compared with the LT group supported our findings (Bonis et al., 2019). Therefore, we assumed that the dynamic changes in cTnI seen over torpor-arousal cycles indicated the adaptive response of the myocardium, in which the myocardial cells were able to repair themselves and gradually restore their normal function after repeated I/R injury. The difference between the two markers of myocardial ischemic injury might be due to the earlier release and clearance of cTnI (Mythili & Malathi, 2015). In conclusion, the dynamic changes in protein levels of CK-MB and cTnI in serum reflected the absence of persistent I/R injury in the heart of ground squirrels during hibernation.
Furthermore, assessment of cardiac function in ground squirrels has relied on measuring serum BNP levels, as shown in Figure 3. Elevated level of BNP is typically associated with cardiac insufficiency, indicating changes in ventricular pressure and volume load (Sabatine et al., 2004). A significant increased BNP levels in the LT group might actually serve as a protective mechanism for the heart. While hibernating, the heart undergoes drastic physiological changes and reducing the load that it bears by elevating BNP might be one such protective mechanism. Moreover, a previous study showed that both plasma BNP levels and cardiac gene expression of BNP were significantly increased in mice kept at 4°C compared with mice kept at room temperature, (Bordicchia et al., 2012) suggesting a potential relationship between elevated BNP level and the low temperatures experienced during hibernation.
4.1 Cytoskeletal regulation
To compensate for reduced HR and EF% during hibernation, ground squirrels require effective molecular motors to maintain CO. Through transcriptomic analysis, myosin-6 (MYH6) was shown to significantly increase in the network associated with cytoskeleton maintenance in the LT group as compared with the SA group. By contrast, myosin-7 (MYH7), myosin light chain kinase family member 4 (MLCK4), myosin regulatory light chain 2 (MLC2), rough transcriptomic analysis (ROCK), myosin light chain phosphatase (MLCP), and myosin light chains (MLC) were all significantly downregulated in LT. However, expression of these genes was restored in the IBA group to the level of the SA group. Myosin, a cytoskeletal and muscle contraction-associated protein, is composed of two heavy chains and four light chains in the heart (Delcayre & Swynghedauw, 1975). Among them, upregulation of MYH6 and downregulation of MYH7 have been shown to play a protective role in myocardial diseases and functional regulation of the heart. For instance, MYH6 level was significantly reduced before treatment in patients with heart failure or dilated cardiomyopathy (DCM), whereas upregulation of MYH6 levels were observed along with improved cardiac function after treatment (Herron & McDonald, 2002). Similarly, in patients with DCM treated with β-blockers, upregulation of MYH6, downregulation of MYH7, and increased MYHC-α mRNA were found after treatment, leading to significant improvements in cardiac function (Lowes et al., 2002). The MYH6 and MYH7 genes encode α and β heavy chain subunits of cardiac myosin (MYHC-α and MYHC-β), respectively, and are located adjacent to chromosome 14 although their expression is independent (Yamauchi-Takihara et al., 1989). An adaptation of interconversion between MYHC-α and MYHC-β has been observed in hibernators exposed to stressful environments, which helps to maintain cardiac contraction and energy supply and was consistent with previous studies on golden-mantled ground squirrels and European hamsters (Cricetus cricetus L.) (Morano et al., 1992; Nelson & Rourke, 2013). In non-hibernators, a decrease in MYHC-α might result from myocardial pathological hypertrophy, restrictive diets, or hypoxia (Haddad et al., 1993). Therefore, upregulation of MYHC-α during hibernation might imitate the cardio-protective effects observed in non-hibernators, such as maintaining sarcomere stability and providing protection to heart in cases of HR failure. In addition, in MYHC Isozyme of rat ventricle, - MYHC-α High proportion of myocardial ATPase activity (Pope et al., 1980). This suggests that the upregulation of MYHC-α during hibernation may provide sufficient ATP for the next arousal.
Cardiac myosin plays a crucial role in regulating cytoskeletal and cellular function by controlling the phosphorylation levels of MLC2 (Yuan et al., 2002). MLC2 phosphorylation is mainly regulated by the Ca2+/calmodulin (Cam) dependent kinase MLCK and the rho effector molecule-ROCK mediated pathway (Jin & Blikslager, 2020). The transcriptomic results of the present study suggest that a significant downregulation of ROCK and MLCK may coregulate reduced MLC2 mRNA levels. Previous studies have shown that an increase in the MLCK inhibitor, ML-7, significantly reduced I/R injury in rat hearts. Therefore downregulation of MLCK during hibernation might contribute to the protection of ischemic myocardium and correlate with decreased levels of MLC2 phosphorylation (Lin et al., 2012). Ca2+triggers MLCK activation and some research on hibernating Daurian ground squirrels and hedgehogs have shown that intracellular calcium stores, particularly in the sarcoplasmic reticulum, are enhanced for calcium reuptake during hibernation (Li et al., 2011; Wang et al., 2002). Although Ca2+ storage increases during hibernation, MLCK reduction does not conflict with this observation since MLCK's slow kinase response is not suitable as a [Ca2+]i receptor in transverse muscle (Tsukamoto & Kitakaze, 2013). Therefore, a reduction of MLCK and MLC2 might be interpreted as a self-protective action of hibernating ground squirrels to avoid unnecessary high-intensity contractions caused by high intracellular Ca2+ concentrations.
4.2 Protein homeostasis
Changes in gene expression related to cardiac protein synthesis during hibernation in ground squirrels align with previous physiological findings in liver of golden-mantled ground squirrels that indicated that protein synthesis was suppressed during torpor and restored during arousal (van Breukelen & Martin, 2001). Given the constant contraction and metabolic demands of the heart, tight regulation of protein folding, turnover, synthesis, and degradation is necessary. Transcriptomic analysis revealed that the heart of the ground squirrels in the LT group had 64 genes involved in the BP of protein phosphorylation (GO:0006468), with 60 of them significantly downregulated, the reduction in phosphorylation suggesting increased protein stability.
Protein misfolding and oxidative stress are potential threats to cardiac function. Molecular chaperones, such as the heat shock protein 70 (HSP70) family, play a crucial role in protecting the heart from these threats by regulating protein synthesis and degradation, assisting in the refolding of misfolded proteins, and inducing cell death under stress or pathological conditions (Tarone & Brancaccio, 2014). Previous studies have shown that increasing HSP70 can prevent acute I/R injury but increasing HSP70 in transgenic animals can lead to pressure overload or accelerate heart failure in DCM. Furthermore, inhibiting the transcription of HSP70 can protect animals from cardiac fibrotic injury (Ranek et al., 2018). The expression of the HSP70 family, including HSPH1, HSPA1L, HSPA4L, HSPA6, and HSP70, was significantly reduced in the LT group, but HSP70 returned to SA levels in IBA group. In response to hypoxic immersion and aerobic recovery in turtles (Trachemys scripta elegans), five heat shock proteins (HSP25, Hsp40, Hsp70, Hsc70, and Hsp90) were not upregulated in heart, but were significantly upregulated in skeletal muscle, and furthermore, some heat shock proteins were differentially upregulated in the kidney and liver (Krivoruchko & Storey, 2010). The expression of HSPs in the heart of hibernating gray mouse lemurs (Microcebus murinus) were minimally affected, with only a 1.15–1.49-fold upregulation of HSP40, HSP70, and HSP90 observed in liver and brown fat, (Wu et al., 2015) which suggested that the expression of HSPs might be tissue-specific. In the current study, heart exhibited an alternative expression pattern in response to hibernation, and the downregulation of HSPs might have reduced the process of protein synthesis to preserve ATP levels, thus playing an important role in protein synthesis in the endoplasmic reticulum.
The ubiquitin proteasome system (UPS) and autophagy play crucial roles in the degradation of misfolded and dysfunctional proteins (Vidar Hansen & Serhan, 2022). Ubiquitin is comprised of an activating enzyme (E1), a binding enzyme (E2), and a ligase (E3). E3 recognizes the degraded protein, carries the ubiquitin molecule to E2, and attaches it to the degraded protein under the guidance of E3. When there are enough ubiquitin molecules, the degraded protein is transported to the proteasome for degradation (Su & Wang, 2010). Transcriptomic results showed that most genes in the ubiquitin-mediated protein hydrolysis pathway (KEGG: rno04120) were downregulated in the LT group, suggesting that protein degradation associated with the ubiquitin-proteasome was reduced during hibernation. The mRNA levels of 8 E3 ubiquitin ligases including BIRC3, Cbl, Nedd4, XIAP, PIAS1, Smurf1, SMURF2, CBLB, as well as the E2-binding enzyme UBE2H, MAPK3 and BIRC3 were all significantly lower in the LT group than in the SA group. However, the expression levels of BIRC3 and MAPK3 were significantly upregulated in the IBA group as compared with the LT group, whereas the other genes returned to the levels found in the SA group. MAPK3 was the most significantly reduced of the LT group (log2 fold change = −3.34401). MAPK3 is highly specialized for myohistogenesis and myogenic cell differentiation and proliferation (Bonni et al., 1999). BIRC3 possesses E3 activity that regulates caspase protein degradation, and it has been found that NF-KB can up-regulate BIRC3, MAPK, and PI3-Kinase in the regulation of XIAP under oxidative stress, which in turn is involved in the inhibition of apoptosis (Philip & Shivakumar, 2013). Thus, activation of oxidative stress over torpor-arousal cycles can lead to cyclic changes in BIRC3 and MAPK3 expression levels that in turn inhibit cell death to maintain cardiac homeostasis. We also investigated changes in the ubiquitin-proteasome protein hydrolysis pathway during hibernation but the results were inconsistent. A reduction in the degree of ubiquitin-proteasome pathway hydrolysis was found, that was consistent with the decrease of E3 Ubiquitin ligase (Atrogin1 and MuRF1) gene expression levels during hibernation of Asian black bear (Ursus thibetanus japonicus) (Miyazaki et al., 2022). By contrast, the ubiquitin-proteasome pathway was activated and ubiquitin conjugate concentrations increased during torpor in the skeletal muscle of Daurian ground squirrels, (Chang et al., 2018) the gut of 13-lined ground squirrels and liver of golden-mantled ground squirrels, (Breukelen & H., 2002) and the heart of 13-lined ground squirrels (Zhang et al., 2016). However, all studies concluded that the rate of protein hydrolysis was reduced during hibernation, indicating that hibernating animals might maintain proteostasis via autophagy or other pathways without muscle atrophy or damage.
4.3 Antioxidant damage defense mechanism
Prostaglandins are metabolites of arachidonic acid and have proinflammatory actions (Zhu et al., 2020). In the metabolomic analysis, a significant downregulation of arachidonic acid was observed in the LT group compared with the SA group, but returned to SA levels during IBA. In addition, transcriptomic analysis revealed a significant downregulation of prostaglandin G/H synthase 2 (PG/H2), the rate-limiting enzyme for prostaglandin production in mammals, that converts arachidonic acid to prostaglandins (Zhu et al., 2020). Our findings are consistent with a study on hibernating Syrian hamsters (Mesocricetus auratus) that also showed upregulation of arachidonic acid in the brain during torpor-arousal cycles, as compared with the SA group (Gonzalez-Riano et al., 2019). The downregulation of PG/H2 in the LT group might inhibit the inflammatory response by limiting the conversion of arachidonic acid to prostaglandins, thereby reducing myocardial injury. By contrast, the upregulation of arachidonic acid in the IBA group might exert anti-inflammatory and protective effects.
This study found that several free amino acids, including L-glutamic acid, threonine, L-ornithine, methionine, L-lysine, L-glutamine, taurine, arginine, L-histidine, and L-tyrosine, were elevated in cycles as compared with the SA group. These amino acids may play a cardioprotective role during torpor-arousal cycles by serving as raw materials for energy production (Kugler, 2004). Glutamine, a crucial amino acid produced from glutamate and NH3, is an important nitrogen carrier in the body and plays a protective role in the heart response to I/R injury (Durante, 2019). At least two important actions are mediated by glutamine. First, increased glutamine during hibernation may serve as a precursor to L-arginine (upregulated in LT vs. SA) by providing additional substrate to nitric oxide synthase (NOS) and driving NO production, that can improve blood flow, diastole, and increase ischemic organ perfusion rate. Second, glutamine and its metabolites also exert potent antioxidant, anti-inflammatory, and antiapoptotic effects in the circulation by stimulating heme oxygenase 1 (HO-1) and inducing HSP and GSH production (Korthuis & Durante, 2005; Wang et al., 2015). Furthermore, a study of hibernating garden dormice revealed a significant increase in glutamine in the brain that is believed to be linked to the production of ammonia (Mandel et al., 1966), the accumulation of free ammonia potentially triggering arousal of hibernators from torpor.
Over torpor-arousal cycles, hibernators experience reperfusion and reoxygenation, leading to increased metabolism and oxygen levels, as well as oxidative stress that can result in increased production of reactive oxygen species (ROS) (Xie et al., 2021). The glutathione system plays a crucial role in the nonenzymatic antioxidant defense system and helps to detoxify ROS. Glutathione is a low molecular weight antioxidant and its reduced form (GSH) can be converted to its oxidized form (GSSG) to help break down peroxides (Sáez et al., 2017). Metabolomic analysis based on KEGG enrichment results showed a significant upregulation of GSH and the glutathione metabolism pathway (KEGG: map00480) over torpor-arousal cycles as compared with the SA group (Table 3). This suggests that the total antioxidant capacity of ground squirrel heart was enhanced during hibernation. Glutathione sulfotransferase class (GST) catalysis plays a crucial role in binding glutathione to chemicals or their metabolic reaction products, reducing their toxicity and protecting cells from damage (Hayes et al., 2005). Transcriptomic analysis revealed that glutathione S-transferase mu 1 (GSTM1) and glutathione S-transferase mu 2 (GSTM2) were significantly downregulated in the LT group as compared with the SA group, whereas GSTM1 was significantly upregulated in the IBA group. Similarly, mRNA levels of various isoforms of glutathione S-transferase in Chinese soft-shelled turtles (Pelodiscus sinensis) and ground squirrels (Citellus citellus now known as Spermophilus citellus) were shown to remain stable or decrease during hibernation but increase in during IBA (Blagojević et al., 1998; Tang et al., 2021; Zhang et al., 2017). This supports our findings that increased binding of GSTM to glutathione may be necessary to remove metabolic wastes during the arousal period, potentially to avoid metabolic waste accumulation from oxidative stress over repeated torpor-arousal cycles.
Taurine has a protective role in oxidant-induced injury (Schuller-Levis & Park, 2003). Metabolomic results showed that the metabolic pathways of taurine and hypotaurine (KEGG: map00430) (Table 3) were upregulated throughout the torpor-arousal cycle as compared with the SA group. For example, the expression levels of taurine, a downstream product of carbon-sulfur metabolism, and hypotaurine, a precursor of taurine, were significantly increased over the torpor-arousal cycles as compared with the SA group, but there was no significant difference between the LT group and the IBA group. Similarly, plasma metabolomics of 13-lined ground squirrels showed that the level of taurine decreased from torpor into the arousal period, which suggested that hibernators regulate the levels of taurine-binding bile acids to prevent excessive inflammation (D'Alessandro et al., 2017). Cysteine sulfinic acid decarboxylase (CSAD) is the rate-limiting enzyme of taurine biosynthesis in mammals (Jong et al., 2021). Compared with the SA group, elevated gene expression of CSAD during torpor-arousal cycles is consistent with the metabolic level of taurine expression. Taurine can also alleviate myocardial cell damage caused by ischemia and hypoxia by inhibiting lipid peroxidation under oxidative stress, regulating Ca2+ homeostasis, and inhibiting apoptosis (Yang et al., 2019). Hence, the increased taurine expression levels during hibernation may act as a cytoprotective agent, and it has been suggested that the antioxidant effect of taurine is attributed to the promotion of intracellular GSH production. Thus, taurine and GSH may work together to reduce oxidative stress damage in hibernating ground squirrels.
4.4 Altered fatty acid metabolic preferences of heart
The heart is an organ that requires a significant amount of energy, and one of the most notable adaptations during hibernation is a shift in fuel source from carbohydrates to lipids. Previous work has shown selective fatty acid mobilization in adipose tissue during fasting-induced energy expenditure in vivo (Raclot & Groscolas, 1996). In non-hibernators, the mobilization of unsaturated fatty acids is greater than that of saturated fatty acids (Raclot & Groscolas, 1996; Raclot, 2003). However, in the fatty acid biosynthesis pathway (KEGG: map00061), stearic acid and palmitic acid, that are saturated fatty acids, were significantly downregulated during torpor. This indicated that hibernators have unique metabolic mechanisms at the level of fatty acid metabolism that preferentially mobilize saturated fatty acids. In addition, during hibernation, Arctic ground squirrels showed an increase in polyunsaturated fatty acid (PUFA) content that is required to maintain the fluidity of the fat pool at low body temperatures (Frank et al., 2008). This pattern of fatty acid metabolism has also been observed in isolated adipocytes from 13-lined ground squirrels and alpine marmots (Marmota marmota), (Cochet et al., 1999; Price et al., 2013) suggesting that it might be a common pattern of fatty acid metabolism in hibernators. The mobilization of saturated fatty acids may result in the retention of unsaturated fatty acids, which can also play a protective role in maintaining calcium homeostasis in the heart and prevent arrhythmias.
4.5 Energy metabolism
Various reports have found a low capacity of the pentose phosphate pathway in myocardium of different animals (Zimmer et al., 1984). In non-hibernators, it takes several days for myocardial ATP levels to return to normal after I/R (Reimer, 1981). Metabolomic analysis has revealed that the pentose phosphate pathway (KEGG: map00030) was enriched with upregulated metabolites such as D-gluconic acid, D-ribulose 5-phosphate, D-erythrose 4-phosphate, and D-sedoheptulose 7-phosphate in an LT group of ground squirrels as compared with the SA group. We proposed that ground squirrels might rapidly restore the ATP metabolic pool by upregulating the conversion of ribulose-5-phosphate (R5P) to ribulose-5-phosphate pyrophosphate (PRPP) for energy replenishment (Stincone et al., 2015). Furthermore, during hibernation, xanthine and uric acid, downstream metabolites of adenine ribonucleotide phosphate (AMP), were elevated as compared with the SA group, and purine degradation intermediates reflected the energy status of cardiomyocytes (Zucchi et al., 1992). This increased energy expenditure seems inconsistent with the low metabolism during torpor. However, over the torpor-arousal cycle, their levels decreased significantly during torpor, which suggests that the myocardium conserves energy in the LT group for the restoration of ATP during IBA.
Adenosine 5′-monophosphate (5-AMP) and adenosine have received considerable attention as molecules with hibernation-inducing potential (Andrews, 2019). In previous studies, 5-AMP was found to induce a hypometabolic state in non-hibernators, as well as cause a torpor-like state and a decrease in body temperature in thermostatic Syrian hamsters in the summer (Dugbartey et al., 2015; Zhao et al., 2014). In the current study, metabolomic analysis revealed a significant increase in 5-AMP and adenosine levels in the IBA group as compared with the SA group. This suggests that these molecules may reduce metabolism and protect the heart from I/R injury.
Mitochondria play a crucial role in regulating cardio-protection and manipulating mitochondrial energy metabolism that can aid in overcoming I/R injury (Stancic et al., 2018). During hibernation, there is a significant decrease in oxygen consumption, with over 90% of the body's O2 being consumed in the mitochondria (Rolfe & Brown, 1997). Studies have reported varying degrees of inhibition or even no significant change in mitochondrial respiration in the skeletal muscle and liver of hibernators (Brown et al., 2012; Chang et al., 2018). However, studies of the inhibition of mitochondrial respiration in the heart of hibernators are inconclusive. Brown et al. found a decrease in the rate of mitochondrial respiration in the myocardium of 13-lined ground squirrels over torpor-arousal cycles (Brown & Staples, 2014). By contrast, other studies have shown that mitochondrial respiration rate in the myocardium of hibernators either increases or remains unchanged during hibernation in hamsters and 13-lined ground squirrels (Gallagher & Staples, 2013; South, 1960). Tissue specificity and respiratory substrates may account for the differing direction of changes in mitochondrial respiration rate of different tissues and organs. In the present study, integrative transcriptomic and metabolomic KEGG pathway analysis revealed that oxidative phosphorylation (KEGG: ko00190) was enriched by upregulated genes and metabolites. In the LT group, compared with the SA group, 22 of the 23 DEGs associated with the ETC were upregulated, including various subunits of mitochondrial NADH dehydrogenase (Complex I: Ndufs4, Ndufs7, Ndufv2, Ndufa5, Ndufa9, Ndufab1, Ndufa12, Ndufb8, Ndufb10, Ndufb11), as well as several subunits of cytochrome C oxidase (Complex III: COX7A, COX6B, COX6C, cytochrome C1, cyc1), and the E, F, H, and c subunits of v-ATPase (Complex V). In addition, transcriptomic and metabolomic analyses of 13-lined ground squirrel retina showed that several genes associated with the respiratory chain exhibited active transcriptional upregulation (Luan et al., 2018). ATP synthase-coupling factor 6 (ATP5PF), a crucial component of F-ATPase, was significantly downregulated, whereas various metabolites, such as NADH, fumaric acid, and NAD+, showed upregulation (Figure 9). The ATP synthases, that are coupled to the ETC (Complex V), are divided into three primary types: F-ATPase, V-ATPase, and A-ATPase (Cross & Müller, 2004). Among them, F-ATPase synthesizes ATP driven by a proton concentration gradient to provide energy to the organism, whereas V-ATPase relies on the energy generated by hydrolysis of ATP to transfer protons, thereby generating an electrochemical gradient across a membrane and regulating the pH inside and outside the cell (Wilkens et al., 2005). During mitochondrial energy metabolism, oxidative phosphorylation and ETCs are coupled. In the present study, the mRNA levels of the E, F, H, and c subunits of V-ATPase were significantly upregulated in the LT group, whereas mRNA levels of ATP5PF, an important component of F-ATPase, was downregulated, indicating a decrease in total ATP synthesis consistent with hypometabolism during hibernation. Therefore, the transcriptional upregulation of ETC-related genes and downregulation of ATP5PF was not in conflict. Two points need to be considered. First, an active respiratory chain implies an increase in oxygen consumption but it has been noted that the heart only accounts for ~0.5% of the basal metabolic rate in small rodents, whereas the oxygen consumption of an animal is the sum of the metabolic rates of all tissues and organs (Martin & Fuhrman, 1955). Second, unlike other organs, the heart needs to continue functioning even when HR is reduced during hibernation, and mitochondria in the myocardium may need to remain active to consume enough oxygen and maintain oxygen partial pressure to prevent excessive ROS production from damaging the myocardium.
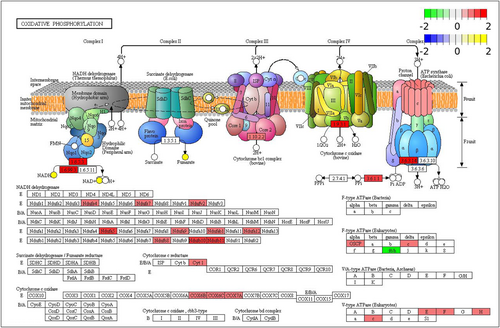
Oxidative phosphorylation is responsible for generating ATP as the respiratory signal, whereas phosphocreatine acts as the energy reservoir and transmitter outside the mitochondria. Phosphocreatine production can be coupled to the ETC via mitochondrial creatine kinase and the net reaction can be expressed by the equilibrium equation: creatine + Pi → phosphocreatine + H2O,
This implies that changes in creatine, Pi or phosphocreatine can regulate the rate of oxygen consumption (Jacobus & Diffley, 1986). During hibernation, ground squirrel hearts exhibited upregulation of creatine phosphate and creatine kinase compared with the SA group. The interconversion of phosphocreatine and creatine by creatine kinase supplies or stores this energy high-energy molecule for cells. The high levels of phosphocreatine during torpor might likely be intended to supply the ATP needs associated with rewarming the body during arousal and upregulating metabolic processes required after return to euthermia. For heart that could include using ATP-energy to repair myocardial damage and restore the heart to a normal euthermic rhythm. These changes might be an essential feature of the mitochondrial bioenergetics of the heart in hibernators, and mitochondrial respiration in the heart during hibernation must not tolerate any period of inefficiency.
4.6 Ferroptosis
Ferroptosis is an interesting but not well-known process (KEGG: rno04216) that was found to be enriched in both DEGs and DEMs of ground squirrels, suggesting an important role for ferroptosis in hibernation. Ferroptosis is a mode of cell death that is consistent with I/R injury dependent iron. It is based on the accumulation of lipid ROS substances, and is clearly distinguished from apoptosis, cell necrosis and cell autophagy by its cell morphology, and may be induced by cold stress (Dixon et al., 2012). Research suggests that ferroptosis can play an important role in hypothermia (Hattori et al., 2017) and, therefore, may be a target pathway of great interest for hibernation research.
A potentially important role for ferroptosis during hibernation is supported by the transcriptomic data in the present study that identified significant upregulation of multiple genes known to encode proteins involved in ferroptosis in the LT group as compared with SA. These included diamine acetyltransferases 1 (SAT1), microtubule-associated proteins 1A/1B light chain 3B (Map1lc3b), voltage-dependent anion-selective channel protein 2/3 (VDAC2/3), ferritin heavy chain (FTH1), phospholipid hydroperoxide glutathione peroxidase (GPX4), ubiquitin-like modifier-activating enzyme (ATG7), and diamine acetyltransferase 2 (SAT2) whereas serotransferrin (TF), and cellular tumor antigen p53 (P53) were significantly downregulated. In addition, the metabolomic data showed a significant decrease in arachidonic acid and adrenic acid. These results suggested that antiferroptosis-related signaling pathways may be activated during hibernation, as shown in Figure 10.
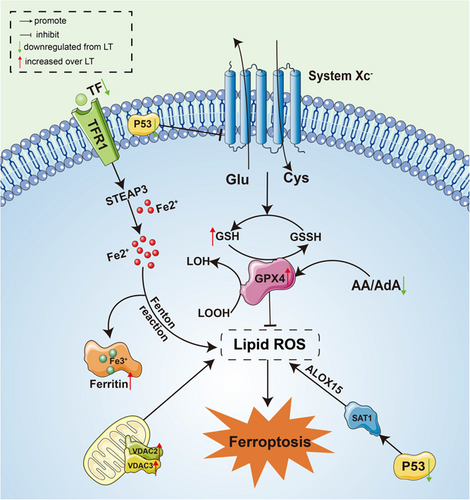
Information on ferroptosis in hibernators is scarce. Previous studies have shown that GPX4 is the only enzyme that can reduce lipid hydroperoxides within biological membranes, converting toxic lipid peroxides (LOOH) to nontoxic lipid-like alcohols (LOH). GPX4 requires GSH as a reducing agent, which is a cofactor necessary for GPX4 to exert its antioxidant effects (Eleftheriadis et al., 2019). In a study on liver of soft-shelled turtle (Pelodiscus sinensis juveniles), Gpx4 mRNA levels were found to be significantly elevated during hibernation and returned to pre-hibernation levels during arousal, (Tang et al., 2021) which was consistent with our findings. Another study reported that hibernating Syrian hamsters' renal tubular epithelial cells could escape lipid peroxidation-induced cell death via increased GPX4 activity, although it was not at low temperature (Eleftheriadis et al., 2018). Our results suggest that the increase in GPX4 and cofactor GSH during torpor may inhibit the onset of ferroptosis and protect against PUFAs.
Moreover, the upregulation of ferritin heavy chain (FTH1) protein during hibernation and the downregulation of transferrin (TF) may help ground squirrels reduce the reduction of Fe3+ to toxic Fe2+. The body stores the nontoxic form of Fe3+ to prevent excessive Fe2+ from Fenton reaction with H2O2 to increase ROS production, promote lipid peroxidation, and induce ferroptosis (Eleftheriadis et al., 2019; Faherty et al., 2018). Recent studies have shown that the p53 gene, an important oncogene, showed enhanced expression following the action of the ferroptosis inducer, erastin, by inhibiting the expression of the downstream cystine/glutamate reverse transport system (SystemXc), and leading to peroxidation of PUFAs in cell membranes, and ultimately causing ferroptosis (Jiang et al., 2015). And a specific siRNA silencing P53 was found in rat kidneys to alleviate cell apoptosis caused by I/R injury (Molitoris et al., 2009). Our findings also suggested that P53 was downregulated in the LT group as compared with the SA group and that this might also contribute in inhibiting ferroptosis and resisting I/R injury. The voltage-dependent anion, VDAC, located in the outer mitochondrial membrane regulates mitochondrial metabolism and ion transport and is involved in the regulation of cell survival and death signals (Mazure, 2017). Action by erastin, the ferroptosis inducer, on VDAC increases the permeability of the outer mitochondrial membrane, leading to dysfunctional mitochondrial metabolism, oxidation, increased ROS production, and enhanced lipid peroxidation, ultimately causing cellular iron necrosis (Chen et al., 2018). However, VDAC2/3 is not required for ferroptosis, indicating that changes in VDAC during hibernation were only involved in the process of ferroptosis regulation (Lemasters, 2017). In addition to the above pathways, PUFAs, particularly arachidonic acid and adrenic acid, were more susceptible to lipid peroxidation, making cells more vulnerable to ferroptosis, and so hibernating ground squirrels might reduce the production of AA and ADA to make them more resistant to ferroptosis (Kagan et al., 2017; Liang et al., 2019). Overall, hibernating ground squirrels regulated ferroptosis-related genes and metabolites to maintain intracellular oxidative homeostasis and inhibit the occurrence of ferroptosis.
5 CONCLUSION
In summary, this study found no significant changes in the morphology of the heart in ground squirrels during hibernation, but significant changes in cardiac function occurred, including decreased HR, increased SV and CO, and increased serum BNP (a marker of cardiac function), in LT group as compared with SA and IBA groups. The serum markers CK-MB and cTnI, that are indicators of cardiac injury, were reduced during hibernation, suggesting that ground squirrels do not experience cardiac injury despite significant physiological fluctuations during hibernation. Transcriptomic and metabolomic analyses revealed significant changes in gene expression and metabolites during hibernation. A rebuilding of the cellular actin backbone and a reduction of protein synthesis and degradation may be crucial for maintaining cardiac function. Ground squirrels may resist oxidative stress induced by the extreme environmental conditions by adjusting amino acid metabolism and the glutathione system that acts in antioxidant defense. During torpor ground squirrels switch from a preference for unsaturated fatty acids to saturated fatty acids. The present study also revealed characteristic changes in energy metabolism, such as enhanced pentose phosphate pathway, mitochondrial oxidative phosphorylation, and phosphocreatine systems, that maintain the energy status of ground squirrel myocardium during hibernation. Our findings also support a role for 5-AMP and adenosine during hibernation induction. Interestingly, antiferroptosis-related pathways were activated, suggesting that ground squirrels can resist I/R-induced lipid peroxidation damage during hibernation and inhibit the occurrence of ferroptosis, adding to other data related to antiferroptosis in hibernators.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: Yingyu Yang, Ziwei Hao, Ning An, Yuting Han, Weilan Miao, Kenneth B. Storey, Etienne Lefai, Xiaoxuan Liu, Junshu Wang, Shuo Liu, Manjiang Xie, Hui Chang. Performed the experiments: Yingyu Yang, Yuting Han. Analyzed the data: Yingyu Yang, Yuting Han. Wrote the paper: Yingyu Yang, Kenneth B. Storey, Hui Chang. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was supported by funds from the National Nature Science Foundation of China 31640072, the Shaanxi Province Natural Science Basic Research Program (2023-JC-YB-179), the Shaanxi Fundamental Science Research Project for Chemistry & Biology (22JHQ040), and Ministry of Science and Technology - High end Foreign Expert Introduction Program (G2022040019L).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




