Effect of the secretome of mesenchymal stem cells overexpressing BMP-9 on osteoblast differentiation and bone repair
Abstract
The secretome present in the conditioned medium (CM) of mesenchymal stem cells (MSCs) is a promising tool to be used in therapies to promote bone regeneration. Considering the high osteogenic potential of the bone morphogenetic protein 9 (BMP-9), we hypothesized that the secretome of MSCs overexpressing BMP-9 (MSCsBMP-9) enhances the osteoblast differentiation of MSCs and the bone formation in calvarial defects. CM of either MSCsBMP-9 (CM-MSCsBMP-9) or MSCs without BMP-9 overexpression (CM-MSCsVPR) were obtained at different periods. As the CM-MSCsBMP-9 generated after 1 h presented the highest BMP-9 concentration, CM-MSCsBMP-9 and CM-MSCsVPR were collected at this time point and used to culture MSCs and to be injected into mouse calvarial defects. The CM-MSCsBMP-9 enhanced the osteoblast differentiation of MSC by upregulating RUNX2, alkaline phosphatase (ALP) and osteopontin protein expression, and ALP activity, compared with CM-MSCsVPR. The CM-MSCsBMP-9 also enhanced the bone repair of mouse calvarial defects, increasing bone volume, bone volume/total volume, bone surface, and trabecular number compared with untreated defects and defects treated with CM-MSCsVPR or even with MSCsBMP-9 themselves. In conclusion, the potential of the MSCBMP-9-secretome to induce osteoblast differentiation and bone formation shed lights on novel cell-free-based therapies to promote bone regeneration of challenging defects.
1 INTRODUCTION
Diseases such as arthritis and cancer, and traumas can generate defects in the skeleton, requiring surgical procedures to repair and/or replace the injured tissue (Bianco et al., 2008; Brydone et al., 2010; Walmsley et al., 2016). It is known that bone tissue exhibits a great capacity of regeneration when damaged; however, this ability is impaired or lost depending on the dimensions of the defect and/or the presence of systemic diseases (Walmsley et al., 2016). The available treatments for these situations, such as autografts, allografts, or synthetic bone grafts have limited availability, elicit adverse effects, or present low efficiency and, therefore, new strategies to treat bone defects have been investigated by the scientific community. Considering this context, tissue engineering and cell therapy strategies use biomaterials and cells to induce bone repair and among the latter mesenchymal stem cells (MSCs) have been the most studied (Adolpho et al., 2022; Almeida et al., 2019; Beloti et al., 2012; Ferraz et al., 2017; Freitas et al., 2017; Freitas, Lopes, Souza, Oliveira, Almeida, Coelho et al., 2019; Freitas, Lopes, Souza, Oliveira, Almeida, Souza, et al., 2019, 2021; Santos et al., 2015; Souza, Freitas, et al., 2018; Campos Totoli et al., 2023).
MSCs can be isolated from bone marrow, fat, skin, dental pulp, peripheral blood, umbilical cord, muscle, and placenta (Lessa et al., 2012; Merimi et al., 2021). Several studies have shown that MSCs injected into tissue defects display paracrine effects through the release of their secretome, which contribute to the tissue healing, including bone regeneration (Blaber et al., 2012; Gugliandolo et al., 2021; Ionescu et al., 2012; Linero & Chaparro, 2014; Oryan et al., 2017; Yang et al., 2013). Considering that MSCs release several molecules such as fibroblast growth factor 2 (FGF-2), vascular endothelial growth factor A (VEGF-A), transforming growth factor beta (TGF-β), angiopoietin 2 and extracellular vesicles, therapies based on the MSC-secretome became attractive (Eleuteri & Fierabracci, 2019; Merimi et al., 2021; Műzes & Sipos, 2022; Trapani et al., 2016).
The cell secretome is defined as a specific set of cytokines, growth factors, chemokines, proteins, and extracellular vesicles, secreted into the extracellular space that allows cell communication and can be regulated by the local physiological conditions (Beer et al., 2017; Eleuteri & Fierabracci, 2019; Vizoso et al., 2017). The use of MSC-secretome has been investigated as a therapeutic approach in regenerative medicine as a cell-free technique that offers advantages such as safety, low cost, and considering future clinic applications, the avoid of exposing the patient to invasive procedures for cell collection (Bar et al., 2021; Famian et al., 2017; Gugliandolo et al., 2021; Maguire, 2013; Osugi et al., 2012). Several studies have shown positive effects of the secretome on the repair of damaged cardiac tissue, recovery of renal function in chronic kidney disease, soft tissue wound healing, and bone repair (Chang et al., 2015; Chen et al., 2008; Linero & Chaparro, 2014; Mirotsou et al., 2011; Van Koppen et al., 2012; Osugi et al., 2012; Timmers et al., 2011; Tsuchiya et al., 2015; Walter et al., 2010).
Regarding the signaling molecules that act on osteogenesis, the bone morphogenetic protein 9 (BMP-9) is considered one of the most osteogenic, with broad proliferative and antiapoptotic actions (Herrera et al., 2013; Wang et al., 2013). As part of the TGF-β family, BMP-9 is reported as a pivotal player in the derivation of osteoprogenitor cells through the differentiation of MSCs probably due to its resistance to the inhibitory effect of noggin on BMP-Smad signaling pathway (Bharadwaz & Jayasuriya, 2021; Cheng et al., 2003; Liao et al., 2017; Wang et al., 2013). It was demonstrated that osteoblasts grown on nanotopographic titanium surface are more responsible to the osteogenic effect of BMP-9 (Souza, Bezerra, et al., 2018). Additionally, MSCs overexpressing BMP-9 (MSCsBMP-9) induces approximately twofold more bone repair than the control MSCs (Freitas et al., 2021).
Considering that the secretome of cultured MSCs is accumulated in the conditioned medium (CM) and the positive effects of MSCsBMP-9 on bone repair, we hypothesized that the MSCBMP-9-secretome enhances the osteoblast differentiation of MSCs and the bone formation in mouse calvarial defects (Cheng et al., 2003; Freitas et al., 2021). To test this hypothesis, MSCs were cultured under nondifferentiation-inducing conditions in the presence of CM of MSCsBMP-9 (CM-MSCsBMP-9) and cell migration, viability, and osteoblast differentiation were evaluated. Additionally, CM-MSCsBMP-9 was directly injected into mouse calvarial defects, and the bone formation was evaluated. The results indicated that the MSCBMP-9-secretome favors cell migration, osteoblast differentiation, and bone formation compared with the control MSC-secretome. Finally, we demonstrated that CM-MSCsBMP-9 is more efficient than the MSCsBMP-9 themselves to induce the repair of mouse calvarial defects.
2 MATERIALS AND METHODS
All animal procedures were approved by the Committee of Ethics in Animal Research of the School of Dentistry of Ribeirão Preto, University of São Paulo (Protocol # 2020.1.219.58.6).
2.1 Overexpression of BMP-9 in MSCs
The MSCs derived from the femur of 4-week-old male C57BL/6 mice were genetically edited using the clustered regularly interspaced short palindromic repeats (CRISPR-Cas9) technique, as previously described (Freitas et al., 2021). The cells were immortalized and genetically edited to overexpress BMP-9 (MSCsBMP-9). MSCs expressing only the constitutive dCas9-VPR endonuclease, without single-guide RNAs (sgRNAs), were used as control (MSCsVPR). The MSCsVPR and MSCsBMP-9 were cultured in a nondifferentiation-inducing medium composed of minimum essential medium, modification alpha (α-MEM Gibco-Life Technologies), 20% fetal bovine serum (Gibco-Life Technologies), 100 U/mL penicillin (Gibco-Thermo Fisher Scientific) and 100 µg/mL streptomycin (Gibco-Thermo Fisher Scientific), in 75 cm2 culture flasks (Corning Inc.) until reaching 80% confluence. The cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2 and 95% atmospheric air, and the culture medium was changed every 48 h.
2.1.1 Analysis of the Bmp-9 gene expression by real-time polymerase chain reaction (RT-qPCR)
To confirm the overexpression of Bmp-9, MSCsVPR and MSCsBMP-9 at passage 16 were cultured at a density 105 cells/well in six-well culture plates (Corning Inc.). On Day 3, cells were lysed, the total RNA was extracted, and complementary DNA (cDNA) strand was prepared from 1 µg of total RNA. The RT-qPCR reactions to quantify the gene expression were performed using the Fast SYBR Green Master Mix (Applied Biosystems) and primer sequences (Applied Biosystems, Table 1) in a QuantStudio™ 7 Flex System device for RT-qPCR (Applied Biosystems). The reactions (n = 3) were done, and the expression of Bmp-9 was based on the average of delta cycle threshold (ΔCt) (Albesiano et al., 2003).
| Gene | Sense/antisense sequence | bp |
|---|---|---|
| Bmp-9 | CAG AAC TGG GAA CAA GCA TC | 64 |
| GCC GCT GAG GTT TAG GCT G | ||
| Runx2 | CTT CAC AAA TCC TCC CCA AGT G | 150 |
| GGA ATG CGC CCTA AAT CAC TG | ||
| Alp | GGG GTA CAA GGC TAG ATG GC | 150 |
| CGG GCT CAA AGA GAC CTA AGA | ||
| Opn | CGA CCA TGA GAT TGG CAG TGA | 108 |
| GGC TGT AAA GCT TCT TCT CCT CT | ||
| Eif2β | ACC TCC CTG GAA TAC TCT GAC T | 136 |
| TCG CCC CGT CTT TGA TGA AT |
- Abbreviations: Alp, alkaline phosphatase; Bmp-9, bone morphogenetic protein 9; Eif2β, eukaryotic translation initiation factor 2B subunit 1 alpha; Opn, osteopontin; Runx2, runt-related transcription factor 2.
2.2 Collection of MSCsVPR and MSCsBMP-9 CM
After MSCsVPR and MSCsBMP-9 reaching 80% confluence in 75 cm2 culture flasks (Corning Inc.) in nondifferentiation-inducing medium, the medium was changed to α-MEM (Gibco-Life Technologies) with 100 U/mL penicillin (Gibco-Thermo Fisher Scientific) and 100 µg/mL streptomycin (Gibco-Thermo Fisher Scientific), and without fetal bovine serum. At 1, 2, 4, 8, and 24 h the CM of MSCsVPR (CM-MSCsVPR) and MSCsBMP-9 (CM-MSCsBMP-9) were collected, centrifuged for 5 min at 2000 rpm and 4°C, and stored at −80°C.
2.2.1 Analysis of the BMP-9 protein expression by enzyme-linked immunosorbent assay (ELISA)
The CM-MSCsVPR and CM-MSCsBMP-9 were thawed at room temperature and concentrated using Vivaspin® MWCO Polyethersulfone tubes (Sigma-Aldrich) in a swing-type centrifuge at 3000 relative centrifugal force and 4°C. The amount of BMP-9 was measure using 100 µL of CM and the mouse BMP-9 ELISA kit (ab267576; Abcam) following the manufacturer's instructions (n = 3).
2.3 Effect of the CM-MSCsVPR and CM-MSCsBMP-9 on cell migration and viability, and osteoblast differentiation of MSCs
2.3.1 MSCs
Primary MSCs were obtained from femurs of 8-week-old male C57BL/6 mice and cultured in the nondifferentiation-inducing medium, in 75 cm2 culture flasks (Corning Inc.) until reaching 80% confluence. Then, they were plated at a density of 105 cells/well in six-well plates (Corning Inc.) for cell migration, RT-qPCR, and western blot assays, and 2 × 104 cells/well in 24-well plates (Corning Inc.) for cell viability and alkaline phosphatase (ALP) activity assays. The cultures grown in either CM-MSCsVPR or CM-MSCsBMP-9 both at the proportion of 1:1 with nondifferentiation-inducing medium, supplemented to a final concentration of 20% fetal bovine serum (Gibco-Life Technologies) during the whole experimental time of cell migration and viability assays and in the last 48 h of RT-qPCR, western blot and ALP activity assays.
2.3.2 Analysis of the cell migration by scratch method
Cell migration was evaluated using the scratch method (Liang et al., 2007). After MSCs reach confluence, a scratch was done with a 100 µL pipette tip in the center of each well. At 6, 24, 48, 72, and 96 h, images of the cultures were obtained using a digital camera Nikon DS-Fi1c (Nikon Instruments Inc.) coupled to an inverted microscope Nikon Eclipse Ti-S (Nikon Instruments Inc.). The areas without cells were delimitated and measured (n = 5) using the Nis Elements Br 5.02 software (Nikon Instruments Inc.).
2.4 Analysis of the cell viability by colorimetric assay
At 24, 48, and 72 h, cell viability was evaluated by a colorimetric assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich) as described elsewhere (Mosmann, 1983). The absorbance was measured (n = 5) at the wavelength of 570 nm using a µQuant spectrophotometer (BioTek Instruments Inc.).
2.4.1 Analysis of the gene expression of bone markers by RT-qPCR
On Days 5 and 7, the gene expression of runt-related transcription factor 2 (Runx2), Alp, and osteopontin (Opn) was evaluated by RT-qPCR using appropriate primer sequences (Applied Biosystems, Table 1) as already described. The reactions (n = 3) were done, and the expression of Runx2, Alp, and Opn was analyzed based on the Ct values and normalized to the endogenous control eukaryotic translation initiation factor 2B subunit 1 alpha (Eif2β) according to the comparative method of (Livak & Schmittgen, 2001).
2.4.2 Analysis of the protein expression of bone markers by western blot
On Day 10, the protein expression of RUNX2, ALP, and OPN was evaluated by western blot. Samples with 30 μg of protein of each group were added to 8% acrylamide gels and transferred to polyvinylidene fluoride membranes (Bio-Rad Laboratories). After blocking the nonspecific sites, the membranes were incubated overnight at 4°C with primary antibody anti-RUNX-2 (#8486S, rabbit monoclonal antibody, 1:1000; Cell Signaling Technology), anti-ALP (#ab108337, rabbit monoclonal antibody, 1:1500; Abcam), anti-OPN (#MPIIIB10(1), mouse monoclonal antibody, 1:1000, Developmental Studies Hybridoma Bank) and antiglyceraldehyde-3-phosphate dehydrogenase (GAPDH, #sc-25778, rabbit polyclonal antibody, 1:3000; Santa Cruz Biotechnology). Then, the membranes were incubated with secondary antibody either donkey anti-rabbit immunoglobulin-horseradish peroxidase (IgG-HRP) (#7074S, 1:3000; Cell Signaling Technology) or goat anti-mouse IgG-HRP (#sc-2060, 1:3000; Santa Cruz Biotechnology) for 1 h at room temperature. The protein bands were detected using Clarity™ Western ECL Substrate (Bio-Rad Laboratories) and the images were acquired with a G-Box device (Syngene). RUNX2, ALP, and OPN were quantified (n = 3) using ImageJ Software (NIH) and normalized to GAPDH protein expression.
2.5 Analysis of the ALP activity by fast red staining
On Day 10, the ALP activity was detected using by fast red staining. The cultures were incubated with Tris buffer solution (Sigma-Aldrich) 120 mM, pH 8.4 containing 1.8 mM Fast Red TR (Sigma-Aldrich), 0.9 mM Naphthol–ASMX–Phosphate (Sigma-Aldrich) and 1:9 dimethylformamide (Merck Millipore) for 30 min at 37°C in a humidified atmosphere containing 5% CO2 and 95% atmospheric air. Then, the cultures were dried at room temperature and macroscopic images were acquired with a high-resolution camera (6.3 megapixels, with EF100 f/2.8 macrolens; Canon EOS Digital Rebel). The data of stained area (n = 5) were analyzed using ImageJ software (NIH).
2.6 Effect of the CM-MSCsVPR and CM-MSCsBMP-9 on bone repair of mouse calvarial defects
2.6.1 Surgical procedure to create and treatment of calvarial defects
Eight-week-old male C57BL/6 mice underwent surgery to create 4-mm diameter unilateral bone defects in the parietal bone of the calvaria. One-week postsurgery, which is equivalent to 1 year in humans (Wang et al., 2020), the bone defects were treated with 25 µL of either CM-MSCsVPR or CM-MSCsBMP-9 (n = 11 for each treatment) directly injected into the bone defects. Considering the calvarial defect size of 4-mm diameter and an average 0.4-mm height, 25 µL of CM was injected, which is the maximum volume supported by each bone defect. Untreated defects (n = 14) were used as control.
2.6.2 Microtomographic (µCT) analysis
Four weeks postdefect treatments, bone formation was evaluated using a high-resolution SkyScan 1172 system (Bruker) by a single-blinded operator. After scanning, the images were three-dimensionally reconstructed using NRecon Cluster software (Micro Photonics Inc.) and the following morphometric parameters were measured in the 4 mm-diameter of the calvarial defects: bone volume (BV), bone volume/total volume (BV/TV), bone surface (BS), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.S), according to recommendation of the American Society of Bone and Mineral Research (Bouxsein et al., 2010).
2.6.3 Histological analysis
After µCT analysis, the calvarias were decalcified in 4% ethylenediaminetetraacetic acid (EDTA, Merck Millipore), dehydrated in a sequence of alcohols (Merck Millipore), embedded in paraffin (Sigma-Aldrich), cut with 5 μm-thickness and stained with hematoxylin and eosin (Neon). The descriptive histological analysis was based on images acquired using a light microscope Axioskop 40 (Carl Zeiss) coupled to a digital camera Axiocam ICc3 (Carl Zeiss).
2.7 Effect of the MSCsBMP-9 and CM-MSCsBMP-9 on bone repair of mouse calvarial defects
As we previously demonstrated that MSCsBMP-9 induced more bone formation than MSCsVPR in rat calvarial defects (Freitas et al., 2021), here MSCsBMP-9 and CM-MSCsBMP-9 were compared in terms of induction of bone formation in mouse calvarial defects. Thus, 8-week-old male C57BL/6 mice underwent surgery to create 4-mm diameter unilateral bone defects in the parietal bone of the calvaria, which were treated with 2.5 × 106 cells in 25 µL of phosphate buffered saline (n = 7) directly injected into the bone defects. This number of cells was based on the defect volume and the size of the MSCs on the culture plate (Docheva et al., 2008). Four weeks postdefect treatment, bone formation was evaluated by μCT and histological analyses and compared with the defects treated with CM-MSCsBMP-9 of the previous experiment.
2.8 Statistical analysis
The data of BMP-9 protein expression (n = 3) and bone morphometric parameters (n = 14 for untreated and n = 11 for each, CM-MSCsVPR and CM-MSCsBMP-9) were compared by one-way analysis of variance (ANOVA) followed by the Tukey's posttest. The data cell migration (n = 5), cell viability (n = 5), RUNX2, ALP and OPN protein expression (n = 3), ALP activity (n = 5), and bone morphometric parameters (n = 7 for MSCsBMP-9 and n = 11 for CM-MSCsBMP-9) were compared by Student's t test. The data of Runx2, Alp, and Opn gene expression (n = 3) were compared by two-way ANOVA followed by Tukey's posttest. The statistical analyses of the cell migration and viability were done based on the area under the curve (Table 2). All data were expressed as mean ± standard deviation (p ≤ 0.05).
| Parameter | CM-MSCsVPR | CM-MSCsBMP-9 | p Value |
|---|---|---|---|
| Cell migration | 530 ± 88 | 601 ± 135 | 0.304 |
| Cell viability | 0.378 ± 0.015 | 0.385 ± 0.015 | 0.463 |
- Note: The data are presented as mean ± standard deviation.
- Abbreviations: BMP-9, bone morphogenetic protein 9; CM, conditioned medium; MSC, mesenchymal stem cell.
3 RESULTS
3.1 Overexpression of Bmp-9 in MSCs and detection of BMP-9 in MSCsBMP-9 CM
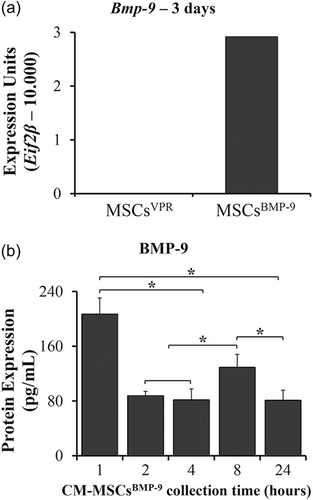
MSCs overexpressed the gene of Bmp-9 and secreted the protein to the CM (Figure 1). The overexpression of Bmp-9 was successful as determined by the Bmp-9 gene expression of MSCsBMP-9 compared with MSCsVPR, in which the expression was not detected (Figure 1a). The amount of BMP-9 protein the of CM-MSCsBMP-9 was higher at 1 h compared with 2, 4, 8, and 24 h (Figure 1b, p < 0.001 for all comparisons) and in 8 h compared with 2, 4, and 24 h (p < 0.001 for all comparisons). As expected, BMP-9 was not detected in the CM-MSCsVPR.
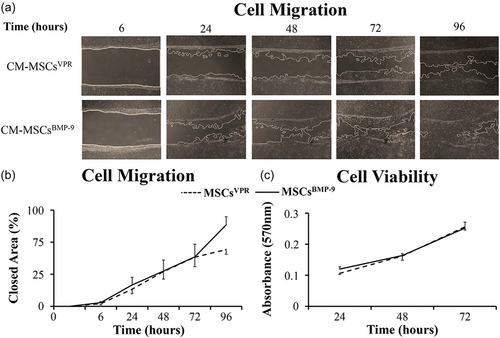
3.2 Effect of the CM-MSCsVPR and CM-MSCsBMP-9 on cell migration and viability, and osteoblast differentiation of MSCs
CM-MSCsBMP-9 did not increase the migration and viability of MSCs compared with CM-MSCsVPR (Figure 2). The micrographs showed similar progression of migration of MSCs cultured in both CM-MSCsVPR and CM-MSCsBMP-9 (Figure 2a), which was confirmed by the lack of statistically significant difference (p = 0.304) between them in terms of closed area (Figure 2b). Additionally, MSC viability was not affected (p = 0.463) by CM-MSCsBMP-9 compared with CM-MSCsVPR (Figure 2c).
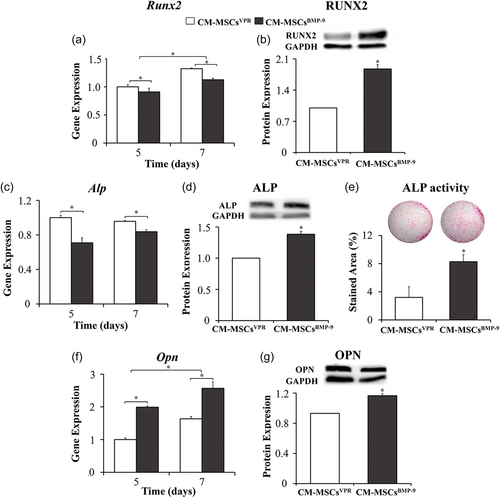
CM-MSCsBMP-9 increased osteoblast differentiation of MSCs compared with CM-MSCsVPR (Figure 3). The Runx2 gene expression (Figure 3a) was lower in MSCs cultured in CM-MSCsBMP-9 compared with CM-MSCsVPR on Days 5 and 7 (p < 0.001, for both) and was higher on Day 7 than Day 5 (p < 0.001). The RUNX2 protein expression (Figure 3b) was higher in MSCs cultured in CM-MSCsBMP-9 compared with CM-MSCsVPR (p < 0.001). The Alp gene expression (Figure 3c) was lower in MSCs cultured in CM-MSCsBMP-9 compared with CM-MSCsVPR on Days 5 and 7 (p < 0.001, for both), without statistically significant difference between Days 7 and 5 (p = 0.031). The ALP protein expression (Figure 3d) was higher in MSCs cultured in CM-MSCsBMP-9 compared with CM-MSCsVPR (p = 0.005). The ALP activity (Figure 3e) was higher in MSCs cultured in CM-MSCsBMP-9 compared with CM-MSCsVPR (p < 0.001). The Opn gene expression (Figure 3f) was higher in MSCs cultured in CM-MSCsBMP-9 compared with CM-MSCsVPR on Days 5 and 7 (p < 0.001, for both) and was higher on Day 7 than Day 5 (p < 0.001). The OPN protein expression (Figure 3g) was higher in MSCs cultured in CM-MSCsBMP-9 compared with CM-MSCsVPR (p = 0.003).
3.3 Effect of the CM-MSCsVPR and CM-MSCsBMP-9 on bone repair of mouse calvarial defects
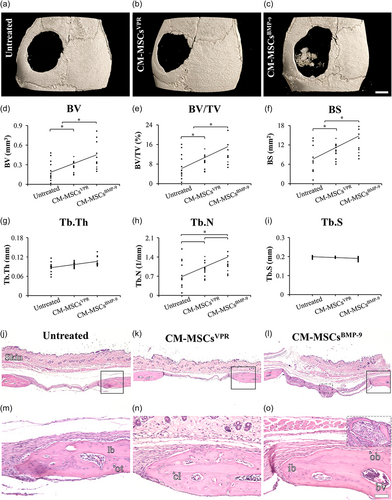
The µCT demonstrated that the defects treated with CM-MSCBMP-9 exhibited higher bone formation compared with both CM-MSCVPR and untreated ones (Figure 4a–i). The three-dimensional reconstructed images showed bone tissue in the center of defects treated with CM-MSCBMP-9, which was not observed in either defects treated with CM-MSCVPR or the untreated ones (Figure 4a–c). The morphometric parameters revealed that BV (Figure 4d), BV/TV (Figure 4e), and BS (Figure 4f) were higher in defects treated with CM-MSCsBMP-9 compared with CM-MSCVPR (p = 0.014, p = 0.014, and p = 0.024) and untreated ones (p < 0.001, p < 0.001, and p = 0.001), and in CM-MSCVPR compared with untreated ones (p = 0.644, p = 0.644, and p = 0.601). The Tb.Th (Figure 4g) was not affected by the treatments (p = 0.078). The Tb.N (Figure 4h) was higher in defects treated with CM-MSCsBMP-9 compared with untreated ones (p = 0.029), without statistically significant differences between CM-MSCsBMP-9 and CM-MSCVPR (p < 0.050), and between CM-MSCVPR and untreated ones (p < 0.050). The Tb.S (Figure 4i) was not affected by the treatments (p = 0.067). The histological sections corroborated the μCT data (Figure 4j–o). It was observed the presence of connective tissue, with no signs of adverse reactions in all defects regardless of treatment (Figure 4j–l). At the edges of all defects, it was noticed new bone tissue and the presence of immature bone, lamellar bone, and cement lines (Figure 4m–o). Additionally, as observed in the three-dimensional reconstructed images, bone tissue was observed in the center of defects treated with CM-MSCBMP-9, but not in defects treated with CM-MSCVPR or the untreated ones (Figure 4o, inset).
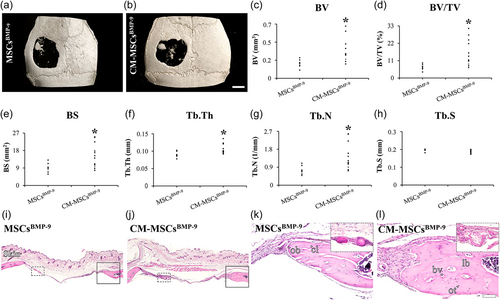
3.4 Effect of the MSCsBMP-9 and CM-MSCsBMP-9 on bone repair of mouse calvarial defects
Because MSCsBMP-9 induced more bone formation than MSCsVPR (Freitas et al., 2021) and CM-MSCsBMP-9 did the same compared with CM-MSCVPR, here MSCsBMP-9 and CM-MSCsBMP-9 were compared in terms of induction of bone formation in mouse calvarial defects. The µCT demonstrated that the defects treated with CM-MSCBMP-9 exhibited higher bone formation compared with MSCsBMP-9 (Figure 5a–h). The three-dimensional reconstructed images showed bone tissue in the center of defects treated with either MSCsBMP-9 or CM-MSCBMP-9 (Figure 5a,b). The morphometric parameters showed that BV (Figure 5c), bv/TV (Figure 5d), bs (Figure 5e), Tb.Th (Figure 5f), and Tb.N (Figure 5g) were higher in defects treated with CM-MSCsBMP-9 compared with MSCBMP-9 (p = 0.025, p = 0.025, p = 0.044, p = 0.026, and p = 0.034). The Tb.S (Figure 5h) was not affected by the treatments (p = 0.251). The histological sections corroborated the μCT data and followed the same pattern described before (Figure 5i–l). It is important to notice that the presence of bone tissue was observed in the center of defects treated with either MSCsBMP-9 or CM-MSCBMP-9 (Figure 5k–l, inset).
4 DISCUSSION
The osteoinduction potential of BMP-9 makes it an appealing molecule to be explored in the field of regenerative medicine focused on tissue regeneration of challenging bone defects. In this context, the present study evaluated the effect of the MSCBMP-9-secretome on osteoblast differentiation of MSCs and bone repair of mouse calvarial defects. Together, the results showed the osteogenic activity of the MSCBMP-9-secretome present in CM-MSCsBMP-9 evidenced by the enhancement of both osteoblast differentiation of MSCs and bone formation in mouse calvarial defects compared with MSCVPR-secretome. Based on these findings and because we previously demonstrated that MSCsBMP-9 elicited more bone repair than MSCsVPR (Freitas et al., 2021), here, the capacity of inducing bone repair of CM-MSCsBMP-9 was compared with MSCsBMP-9 and surprisingly, the secretome promoted more bone formation than the cells themselves.
Before investigate the osteogenic potential of MSCBMP-9-secretome, the expression of Bmp-9 was evaluated in both MSCsVPR and MSCsBMP-9. As expected, a significant amount of Bmp-9 gene expression was detected only in MSCsBMP-9, confirming the efficiency of the CRISPR-Cas9 technology as a tool for gene editing and corroborating our previous findings (Freitas et al., 2021). Furthermore, the presence of BMP-9 in the CM-MSCsBMP-9 indicated that this protein is secreted to the extracellular environment, a pivotal finding to the progress of this study (Wan et al., 2014). The BMP-9 was detected in the CM-MSCsBMP-9 for up to 24 h, the latest evaluated time point; however, the maximum amount was measured after the first hour, making this period the choice for collecting the CM to carry out the further experiments. The higher BMP-9 concentration detected after 1 h could be due to, at least in part, the stability of the protein in the extracellular medium as well as the proteolysis process that may occur at later time points (Wei et al., 2014). It is worth noting that circulating BMP-9 is poorly expressed in adult organisms varying from 2 to 10 pg/mL, making the overexpression an interesting approach to investigate its therapeutical potential. Additionally, even at low concentrations, starting from 50 pg/mL, BMP-9 can exert biological effects, which corroborates the effects of MSCBMP-9-secretome in terms of inducing osteoblast differentiation and bone formation (Van Baardewijk et al., 2013; Bidart et al., 2012; Wei et al., 2014).
The migration and viability of MSCs cultured in CM-MSCsVPR and CM-MSCsBMP-9 increased over time. Although without statistically significant difference, more MSCs migrate when cultured in CM-MSCsBMP-9 compared with CM-MSCsVPR at 96 h, which may be associated with the ability of these cells of proliferating and migrating in response to specific stimuli (Fu et al., 2019). These events are stimulated by secreted or exogenously added factors such as stromal cell-derived factor-1, basic FGF, VEGF-A, insulin-like growth factor 1 and 2, platelet-derived growth factor, TGF-β, and BMP-9, which can be found in CM of MSCs (Ball et al., 2007; Cheng et al., 2003; Dubon et al., 2018; Kowalski et al., 2016; Schmidt et al., 2006; Zou et al., 2011). Taking together, although not quantified, it is possible that some of these factors are increased in the CM-MSCsBMP-9 as the expression of genes related to the TGF-β/BMP signaling pathway was upregulated by BMP-9 overexpression (Freitas et al., 2021).
The positive effects of CM-MSCsBMP-9 on the osteoblast differentiation of MSCs was observed through the evaluation of classical osteoblastic markers such as RUNX2, ALP, and OPN (Lian et al., 2006; Millán, 2013). The gene expression of the initial and intermediate markers of osteoblast differentiation, Runx2 and Alp, was downregulated by CM-MSCsBMP-9, while the later marker Opn was upregulated. This finding could be explained, at least in part, by variations in the messenger RNA expression of bone markers during the progression of osteoblast differentiation (Owen et al., 1990). Thus, it is possible to suggest that the effect of MSC-secretome on these genes depends on the stage of osteoblast differentiation. The phenotypic bone markers RUNX2, ALP, OPN protein expression and ALP activity clearly showed that the MSCBMP-9-secretome enhanced the osteoblast differentiation of MSCs, which agrees with the increased osteoblast differentiation of MSCs induced by the secretome of either HEK-293 or HCT116 cells overexpressing BMP-9 (Li et al., 2016; Tang et al., 2009).
To address a future possible clinical application, the osteogenic potential of the MSCBMP-9-secretome was evaluated in terms of its ability for inducing bone repair. This evaluation was performed in a model of mouse calvarial defect treated with CM 1 week postdefect creation. This approach may allow the new connective tissue formed in the defect to act as a natural scaffold to retain the CM in the bone defect, avoiding the need for an additional biomaterial (Freitas, Lopes, Souza, Oliveira, Almeida, Souza, et al., 2019). In general, the morphometric parameters demonstrated more bone formation in defects treated with CM compared with untreated ones, with a clear advantage for defects treated with CM-MSCsBMP-9, which was corroborated by the histological analysis that additionally showed the lack of any adverse reaction signs irrespective of treatments. Furthermore, it was noticed bone formation in the center of defects treated with CM-MSCsBMP-9 close to blood vessels (bvs) that can be related to the osteogenic and angiogenic potential of BMP-9 (Herrera et al., 2013; Suzuki et al., 2010; Wang et al., 2013). These findings agree with the increased bone formation observed in experimental designs that used different approaches to deliver BMP-9 to the defect site (Freitas et al., 2021; Lee et al., 2019; Zhang et al., 2019). Because the MSCBMP-9-secretome enhanced bone formation, we compared the osteogenic potential of CM-MSCsBMP-9 with MSCsBMP-9 both directly injected into calvarial defects and observed that the secretome was more effective than the cells to induce bone repair. This is an interesting finding that may be related to the direct delivery of BMP-9 combined with MSCBMP-9-secretome to the bone defect site rather than the need for BMP-9 synthesis and secretion by the injected MSCsBMP-9.
In conclusion, the secretome of CRISPR-edited MSCs to overexpress BMP-9 favored the osteoblast differentiation of MSCs. Additionally, to the best of our knowledge, this is the first study to show that the MSCBMP-9-secretome effectively enhanced bone formation in mouse calvarial defects compared with MSCVPR-secretome or even with MSCsBMP-9 themselves. These promising results pave the way for further investigations on cell-free-based approaches such as the application of MSCsBMP-9 exosomes to promote bone regeneration of challenging defects.
AUTHOR CONTRIBUTIONS
Robson Diego Calixto: Data curation; formal analysis; investigation; methodology; writing-review and editing. Gileade Pereira Freitas: Conceptualization; data curation; formal analysis; investigation; methodology; writing—review and editing. Paola Gomes Souza: Methodology; investigation; writing—review and editing. Jaqueline Isadora Reis Ramos: Methodology; investigation; writing—review and editing. Isabela Cristine Santos: Methodology; investigation. Fabiola Singaretti de Oliveira: Data curation; formal analysis; investigation; methodology; writing-review and editing. Adriana Luisa Gonçalves Almeida: Data curation; formal analysis; investigation; methodology; writing-review and editing. Adalberto Luiz Rosa: Conceptualization; funding acquisition; data curation; formal analysis; supervision; writing-review and editing. Marcio Mateus Beloti: Conceptualization; funding acquisition; data curation; formal analysis; project administration; supervision; writing—original draft.
ACKNOWLEDGMENTS
The authors would like to thank Roger Rodrigo Fernandes for their technical assistance during the execution of the study. This research was supported by São Paulo Research Foundation (FAPESP, grants # 2017/12622-7, 2019/01346-4, and 2020/06599-5) and the National Council for Scientific and Technological Development (CNPq, grant # 303115/2019-8).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




