AMPK/mTOR downregulated autophagy enhances aberrant endometrial decidualization in folate-deficient pregnant mice
Yan Zhang and Rufei Gao contributed equally to this study and should be cofirst authors.
Abstract
Existing evidence suggests that adverse pregnancy outcomes are closely related to dietary factors. Folate plays an important role in neural tube formation and fetal growth, folate deficiency is a major risk factor of birth defects. Our early studies showed that folate deficiency could impair enddecidualization, however, the mechanism is still unclear. Dysfunctional autophagy is associated with many diseases. Here, we aimed to evaluate the adverse effect of folate deficiency on endometrial decidualization, with a particular focus on endometrial cell autophagy. Mice were fed with no folate diet in vivo and the mouse endometrial stromal cell was cultured in a folate-free medium in vitro. The decrease of the number of endometrial autophagosomes and the protein expressions of autophagy in the folate-deficient group indicated that autophagosome formation, autophagosome-lysosome fusion, and lysosomal degradation were inhibited. Autophagic flux examination using mCherry-GFP-LC3 transfection showed that the fusion of autophagosomes with lysosomes was inhibited by folate deficiency. Autophagy inducer rapamycin could reverse the impairment of folate deficiency on endometrial decidualization. Moreover, folate deficiency could reduce autophagy by disrupting AMPK/mTOR signaling, resulting in aberrant endometrial decidualization and adverse pregnancy outcomes. Further co-immunoprecipitation examination showed that decidual marker protein Hoxa10 could interact with autophagic marker protein Cathepsin L, and the interaction was notably reduced by folate deficiency. In conclusion, AMPK/mTOR downregulated autophagy was essential for aberrant endometrial decidualization in early pregnant mice, which could result in adverse pregnancy outcomes. This provided some new clues for understanding the causal mechanisms of birth defects induced by folate deficiency.
Abbreviations
-
- 3-MA
-
- 3-methyladenine
-
- mCherry-GFP-LC3
-
- monmer Cherry-green fluorescent protein-microtubule-associated protein 1 light chain 3
-
- AMPK
-
- adenosine 5‘-monophosphate (AMP)-activated protein kinas
-
- BSA
-
- bovine serum albumin
-
- 4E-BP1
-
- eIF4E binding protein 1
-
- Bmp2
-
- bone morphogenetic protein 2 Co-IP: co-immunoprecipitation
-
- CARM1
-
- co-activator-associated arginine methyltransferase 1
-
- D1
-
- the first day of pregnancy
-
- Hoxa10
-
- homeobox
-
- Lamp2
-
- lysosomal-associated membrane protein 2
-
- LC3
-
- microtubule-associated protein 1 light chain 3
-
- mES
-
- primary mouse endometrial stromal cell
-
- Mmp2
-
- matrix metalloproteinase 2
-
- Mmp9
-
- matrix metalloproteinase 9
-
- mTOR
-
- mammalian target of rapamycin
-
- OsO4
-
- osmium tetroxide
-
- PDCD4
-
- programmed cell death 4
-
- PtdIns3K
-
- class III phosphatidylinositol 3-kinase
-
- p-S6K1
-
- phosphorylation of p70 S6 kinase 1
-
- qRT-PCR
-
- quantitative real-time PCR
-
- Rapa
-
- rapamycin
-
- RPMI
-
- roswell park memorial institute
-
- TEM
-
- transmission electron micrographs
-
- TFEB
-
- the transcription factor EB
1 BACKGROUND
Adverse pregnancy outcomes, which place a heavy burden on families and society as a whole, have been increasingly reported. Current research suggests that adverse pregnancy outcomes are closely related to exposure to extrinsic environmental factors, including diet and chemical exposure. The nutritional status of the mother is undoubtedly one of the main factors that influence pregnancy. Folate (vitamin B9) plays a crucial role in fundamental cellular processes, including nucleic acid biosynthesis, methyl group biogenesis, and amino acid metabolism. Numerous studies have shown that maternal folate deficiency is a major risk factor of birth defects and fetal growth restriction, such as neural tube defects and anencephaly (Rosario, Powell, et al., 2017; Silva et al., 2017). The current study on maternal folate deficiency resulting in birth defects mainly focussed on fetal development, it has very little study from the point of view of the mother's uterus. Including fetal development, a successful pregnancy is also closely related to the receptivity and decidualization of the endometrium. During early pregnancy in mice, the onset of embryo implantation occurs in the receptive uterus, followed by a transformation of stromal cells to decidual cells in the morning on Day 5 of pregnancy (D5), this process is termed endometrial decidualization. These changes depend on the appropriate interaction between nutrients and cellular, hormonal, and molecular signals (Gellersen & Brosens, 2014). Our previous studies found that uterine receptivity and embryo implantation were not significantly compromised while endometrial decidualization was impaired by folate deficiency in early pregnant mice (Gao et al., 2012; Geng et al., 2015; Liao et al., 2015). However, the molecular mechanism has not been well elucidated. In this study, we aimed to explore how folate deficiency impairs endometrial decidualization during early pregnancy.
Autophagy (macroautophagy) is a highly regulated catabolic process involved in the maintenance of cellular homeostasis. Cellular autophagy could be activated by starvation, low oxygen, and external stimulation (H. Choi et al., 2016; Y. J. Kim et al., 2013). It begins with the engulfment of targeted components and damaged organelles in double-membrane-surrounded autophagosomes. The autophagosomes then fuse with lysosomes to degrade the contents with lysosomal enzymes (Klionsky & Emr, 2000). The impairment of autophagy may contribute to lots of pathological processes, including neurodegenerative diseases, heart failure, different types of cancer (Levine & Kroemer, 2008; Rubinsztein et al., 2011). For female mammals, autophagy participates in primordial folliculogenesis, endometrial function, and embryonic development (Q. Chen et al., 2018; Nakashima et al., 2017; Y. Zhang et al., 2018; Zhihan et al., 2019). In the endometrium, the autophagy marker LC3-II increases along with the progression of the menstrual cycle and reaches a maximum at the late stage of secretion (J. Choi et al., 2012). Autophagosomes were observed in decidual stromal cells during early human gestation (Avagliano et al., 2015). These research studies strongly confirm the positive role of autophagy in normal endometrial decidualization. Furthermore, autophagy can enhance the invasion of endometrial carcinoma is crucially implicated in the progression of endometriosis (J. Choi, Jo, et al., 2014; Sivridis et al., 2011). However, whether autophagy plays an important role in the aberrant endometrial decidualization induced by folate deficiency remains elusive.
The aim of this study was to evaluate the adverse effect of folate deficiency on endometrial decidualization during early pregnancy both in animal and cellular experiments, with a particular focus on endometrial cell autophagy. This information not only enriches the knowledge of autophagic function but also improves the understanding of mechanisms on birth defects from the perspective of maternal folate deficiency in early pregnancy.
2 MATERIALS AND METHODS
2.1 Animals and ethical approval
The Animal Facility of Chongqing Medical University provided the Kunming mice. All animal experiments were approved by the Laboratory Animal Centre of Chongqing Medical University, China (Certificate No.: SYXK (YU) 20120001) and the Ethics Committee of Chongqing Medical University. Mice were housed in a pathogen-free animal room under a controlled environment (12 h light/12 h dark).
The folate-deficient mouse model was established as previously reported (Gao et al., 2012; Liao et al., 2015). Mature female mice (25 ± 2.3 g) were randomly divided into two groups: the folate deficiency group (FD) fed a diet with no folate (Research Diets) and the normal diet group (control) fed normal fodder. After 5 weeks, mice were mated with mature males. The day a vaginal plug was found was considered the first day of pregnancy (D1). In mice, decidualization occurs with blastocyst attachment to the endometrium on Day 5 (D5) and is vigorous on Day 6–Day 8 of pregnancy (D6–8). Uterine tissues were collected from D6 to D8 during the critical period of mice endometrial decidualization and stored at −80°C for subsequent experiments. Serum folate levels were measured by electro-chemiluminescence (Zhao et al., 2006).
Uterine decidualization autophagy was blocked by the specific autophagy inhibitor 3-methyladenine (3-MA, Sigma) and intensified by the specific autophagy inducer rapamycin (Rapa, Sigma) (L. Wu, Feng, et al., 2013). Female pregnant mice were given a daily intraperitoneal injection from D1 to D6/7/8 with 2 mg/kg body-weight/day (mg/kg-bw/d) rapamycin or 15 mg/kg-bw/d 3-MA (Carmignac et al., 2011; X. Wu et al., 2014). The control group was given an equal volume of PBS using the same method.
2.2 Mouse endometrial stromal cell (mESCs) isolation and culture
The mESCs were isolated as previous study (Kover et al., 1995; Tan et al., 2004). The mice uterus tissues were minced and digested in enzyme I solution containing 6 mg/ml dispase II (Roche, MHG, GER) and 1% typsin (Sigma) at 4°C for 2 h, followed by 37°C in a 5% CO2 incubator for 30 min. Subsequently, a 70 μm cell strainer was utilized to filter the supernatant after further digestion using 0.05% collagenase, and the stromal cells were collected by centrifugation. The stromal cells were resuspended with complete medium and plated on culture plates in the 5% CO2 incubator at 37°C. After incubating about 1 h, the culture medium was changed to eliminate the nonadherent cells. Based on mice diet with or without folate, the stromal cells were cultured in Dulbecco's modified Eagle medium (DMEM)/F12 medium (Sigma) or folate-free roswell park memorial institute (RPMI) 1640 medium (Gibco), both containing 20% charcoal-stripped fetal bovine serum (Gibco) and 0.1% penicillin-streptomycin (Beyotime Biotechnology). Meanwhile, the mESCs were induced to decidualization in vitro by 10 nM β-estradiol (E2, Sigma) and 1 mM P4 (Sigma) for 72 h. The mESCs autophagy was blocked by 5 mM 3-MA and intensified by 100 nM rapamycin in a culture medium. The mESCs were isolated on more than three pregnant mice from each group.
2.3 Plasmid transfection
Microtubule-associated protein 1 light chain 3 (LC3) plays an important role in autophagy. To clearly realize LC3 expression level in the endometrium, plasmids containing monmer Cherry-green fluorescent protein-microtubule-associated protein 1 light chain 3 (mCherry-GFP-LC3, Gene Pharma) were transfected into the mESCs. According to the manufacturer's protocol, the mESCs were cultured in 24-well plates containing DMEM (Sigma) with 10% fetal serum (Gibco) (not containing antibiotics) for 6 h, then the prepared plasmids were added to the medium along with the Lipofectamine® 3000 Transfection kit (Invitrogen) and cultured for an additional 6 h. After successful transfection, the medium was changed to a complete medium containing 20% charcoal-stripped fetal bovine serum (Gibco) and 0.1% penicillin-streptomycin (Beyotime) for subsequent experiments. Cell images were taken by fluorescence microscopy (BX43, Olympus). A minimum of 10 microscopic views of cells were detected in each group, and each sample was assayed three times.
2.4 Transmission electron microscopy (TEM)
To explore whether autophagy occurs in endometrial cells, we collected uterine tissue and primary cells to observe autophagosomes under TEM (H7500, Hitachi). Samples were fixed in 2.5% glutaraldehyde in 0.1 M PBS buffer (pH 7.2) and 1% osmium tetroxide (OsO4) at 4°C, then postfixed in 1% OsO4 and dehydrated in an ascending series of alcohols. Subsequently, the samples were infiltrated in Araldite-Epon. Autophagosomes were photographed with a transmission electron microscope. TEM was performed on three pregnant mice in vivo and mESCs in vitro from each group, and each experiment was analyzed at least three times.
2.5 Immunofluorescence
Collected tissues were fixed in 4% paraformaldehyde (PFA) in PBS, transferred to 70% ethyl alcohol, and embedded in paraffin. After antigen retrieval, slides (5 µm) were incubated with the primary anti-LC3 antibody (1:200) overnight at 4°C. In addition, primary mESCs were plated at about 50%–70% confluence on slides (10 mm x 10 mm) and treated with hormone or no hormone. The slides were fixed by methanol and blocked by 5% bovine serum albumin (BSA), then we incubated the primary anti-LC3 antibody (1:200) overnight at 4°C. The slides were then incubated with secondary antibodies. Cell nuclei were stained using the 4, 6-diamidino-2-phenylindole stain (DAPI, Beyotime). Green fluorescence was defined as LC3 positive. All slides were viewed directly using a fluorescence microscope at a wavelength of 488 nm. Immunofluorescence was performed on four to five pregnant mice in vivo and mESCs in vitro from each group, and each experiment was analyzed at least three times.
2.6 Western blot analysis
According to previous reports (Li et al., 2015; Liao et al., 2015), protein samples were extracted from decidual tissues collected at various time points (D6, D7, D8) using cell lysis buffer (Beyotime). Then, samples were boiled in 5× sodium dodecylsulfate (SDS) sample loading buffer for 15 min. Cellular protein samples were collected using 1× SDS sample loading buffer, then boiled for 15 min. Subsequently, protein samples were analyzed using a 10% SDS-polyacrylamide gel (Beyotime). After electrophoresis, proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad). The membranes were blocked for 75 min at 37°C in 10% nonfat milk or 10% BSA and then incubated with the appropriate primary antibodies (Table S1) overnight at 4°C. After three washes with PBST (15 min each), the membranes were incubated with specific secondary antibodies for 1 h at 37°C. The bands were washed with PBST and visualized by chemiluminescence reagents (WBKLS0500, Millipore) and ChemiDocTM XRS + (Bio-Rad). Finally, target band densities were quantified with Quantity One version 4.4.0 software and normalized for loading using β-actin. Western blot was performed on more than three pregnant mice in vivo and mESCs in vitro from each group, and each experiment was analyzed at least three times.
2.7 Quantitative real-time PCR (qRT-PCR)
Total cellular RNA was isolated from the different groups using the RNAiso Plus Reagent (Takara). Reverse transcription was measured with a Prime ScriptTM RT reagent Kit (Takara) according to the manufacturer's protocols. The qRT-PCR was performed using a Bio-Rad CFX Manager 3.1 Detection System (Bio-Rad) with gene-specific primers (Table S2) and an SYBR Green PCR Kit (Takara). Experiments were conducted in triplicate for each sample, and the 2-ΔΔCt method was used to calculate the relative gene expression levels in different samples, with β-actin as the internal control. The specific primers for qRT-PCR were designed and synthesized by Sangon Biotech Co. Ltd. qRT-PCR was performed on more than three pregnant mice in vivo and mESCs in vitro from each group, and each experiment was analyzed at least three times.
2.8 Co-immunoprecipitation (Co-IP)
The tissue lysates were extracted from endometrial decidual tissues were collected at various time points (D6 and D7) and were precleared with Protein A/G-magnetic beads (Bimake Biotechnology) for 20 min. The solution was collected after magnetic separation. The Cathepsin L primary antibody was added to the solution and incubated overnight at 4°C. The tissue lysates further incubated for 1 h with Protein A/G-magnetic beads. After magnetic separation, the immune precipitates were collected and washed with cold lysis buffer three times. Finally, the mixture of precipitates and loading buffer was boiled and then used to detect Cathepsin L and Hoxa10 protein levels by Western blot. Co-IP was performed on six pregnant mice from each group, and each experiment was analyzed at least three times.
2.9 Statistical analysis
All experiments were replicated at least three times. Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software). Quantitative data were displayed as the mean ± SEM for each experiment. Statistical significance of the differences was determined using Student's t tests and one-way analysis of variance tests. Values of p < 0.05 were considered statistically significant.
3 RESULTS
3.1 Endometrial decidualization was impaired by folate deficiency in early pregnant mice and in primary mESCs
Folate is crucial for endometrial decidualization (Li et al., 2015; Liao et al., 2015). Serum folate concentration of folate-deficient pregnant mice was significantly lower than that in the control group (Figure 1a, p < 0.001), which was consistent with our previous study (Q. Chen et al., 2018). It indicated the successful establishment of a folate-deficient mouse model.
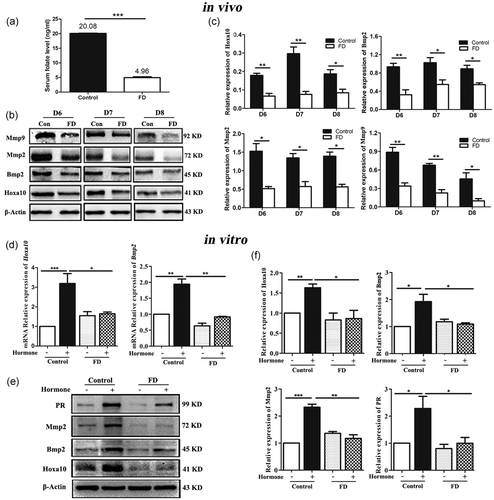
To validate whether folate deficiency could impair endometrial decidualization, the key proteins expressions of decidualization were detected, such as homeobox (Hoxa10), bone morphogenetic protein 2 (Bmp2), matrix metalloproteinase 2 (Mmp2), matrix metalloproteinase 9 (Mmp9), and progesterone receptor (PR) (Das et al., 2009; Sroga et al., 2012). The proteins expressions of Hoxa10, Bmp2, Mmp2, and Mmp9 were significantly decreased in the folate-deficient group compared with the normal diet group on D6, D7, and D8 which is the decidualization period in mice in vivo (Figure 1b,c, p < 0.05). It suggested that endometrial decidualization was impaired by folate deficiency, which was corroborated by our previous report (Liao et al., 2015).
The uterus is a complex organ comprising multiple types of cells, such as natural killer cells, endometrial epithelial cells, and stromal cells. During decidualization, stromal cells started transforming into decidual cells (C. Zhang et al., 2019). To further validate the effects of folate deficiency on the differentiation of endometrial stromal cells into decidual cells, the mESCs were isolated from folate deficient and normal mice, respectively (Figure S1), and were decidualized artificially by hormone in vitro. After being decidualized, the qRT-PCR results showed that the mRNA expression levels of Hoxa10 and Bmp2 were significantly decreased in the folate-deficient group when compared with that in the normal group (Figure 1d, p < 0.05). Western blot results showed that the protein expressions of Hoxa10, Bmp2, Mmp2, and PR were also significantly decreased in the folate-deficient group compared with the control group after mESCs were decidualized (Figure 1e,f, p < 0.05).
3.2 Autophagy was inhibited by folate deficiency during endometrial decidualization in early pregnant mice
There is some increasing evidence that connects autophagy with the normal development and health maintenance of the endometrium (Avagliano et al., 2015; J. Choi et al., 2012; Nakashima et al., 2017). Therefore, the role of autophagy in folate deficiency-impaired endometrial decidualization is described here. TEM was initially applied to observe the numbers of single- or double-membrane autophagosomes in the endometrium of pregnant mice on D6, D7, and D8. Compared with the normal diet group, the number of endometrial autophagosomes was drastically decreased in the folate-deficient group (Figure 2a). Immunofluorescence was performed to analyze the distribution of the autophagic marker LC3 protein in decidual cells on D8 during early pregnancy. The LC3 signaling accumulated in the normal diet group, while only weak signals were observed in the folate deficiency group (Figure 2b). The mRNA expression levels of LC3 were also significantly decreased in the folate-deficient group on D6, D7, and D8 by qRT-PCR (Figure 2c, p < 0.05). In addition, as a marker of autophagosomal membranes, LC3 including LC-I and LC3-II. After autophagosome formation, LC3-I can be transformed into LC3-II by conjugation with phosphatidylethanolamine located in the autophagosome membrane, where it is fused and degraded. Thus, LC3-II accumulation could reflect the number of autophagosomes within a cell (S. Choi, Shin, et al., 2014; Ko et al., 2017). Western blot results showed that folate deficiency dramatically reduced the conversion of LC3-I to LC3-II on D6, D7, and D8 (Figure 2d,e, p < 0.01). These data indicated that autophagy was significantly downregulated in folate deficiency mice during early pregnancy.
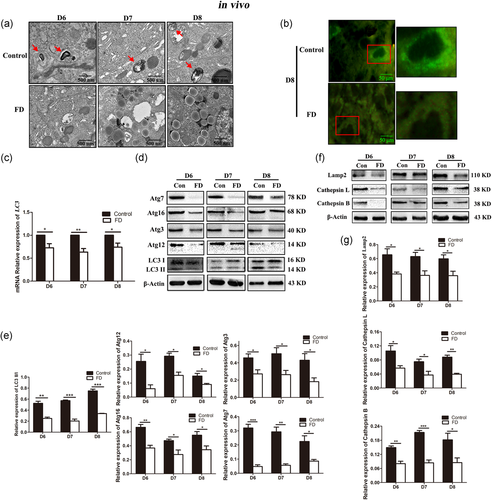
Additionally, we examined protein expressions of autophagy at different stages using Western blot. Compared with the normal diet group, protein expression levels of Atg12, Atg3, Atg16, and Atg7 involved in autophagosome formation were obviously decreased in the folate-deficient group (Figure 2d,e, p < 0.05). This result suggested that autophagosome formation was suppressed by folate deficiency. Furthermore, lysosomal-associated membrane protein 2 (Lamp2), Cathepsin B, and Cathepsin L were reported to play an essential role in lysosome-autophagosome fusion and the degradation process (Klionsky et al., 2012). The decreased protein expression levels of Lamp2, Cathepsin B, and Cathepsin L in the folate-deficient group (Figure 2f,g, p < 0.05) indicate that folate deficiency not only impaired autophagosome-lysosome fusion but also disturbed lysosomal degradation. These results also provided the evidence that autophagy was inhibited by folate deficiency in early pregnancy mice during endometrial decidualization.
3.3 Autophagy was inhibited by folate deficiency during decidualization in primary mESCs
We further wondered whether autophagy was reduced by folate deficiency in the primary mESCs model in vitro. A notably decreased number of autophagosomes was observed by TEM in the decidualized mESCs isolated from folate-deficient mice (Figure 3a). By immunostaining, numerous puncta of LC3 were detected in the control group, but only weak expression was exhibited in the decidualized mESCs isolated from folate-deficient mice (Figure 3b). After mESCs were decidualized by hormone, the mRNA expression levels of LC3 were also significantly decreased in the folate-deficient group when compared with that in the control group (Figure 3c, p < 0.05). Western blot analysis also showed that, after mESCs decidualized by hormone, the protein level of LC3-II/I was significantly decreased in folate-deficient group (Figure 3d,e, p < 0.05).
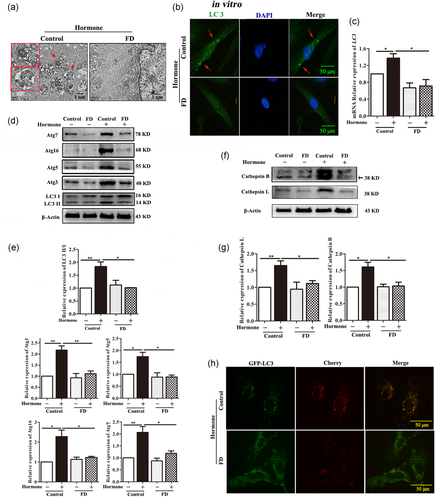
Meanwhile, obviously downregulated expression levels of autophagosome formation-related markers of Atg3, Atg5, Atg16, and Atg7 were found in the decidualized mESCs isolated from folate-deficient mice (Figure 3d,e, p < 0.05). Additionally, after mESCs were decidualized by hormone, the protein levels of Cathepsin L and Cathepsin B, which are the lysosomal degradation-related markers, were also significantly decreased in the folate-deficient group (Figure 3f,g, p < 0.05). Furthermore, autophagic flux was examined using a mCherry-GFP-LC3 transfection assay for assessing the autophagosomal-lysosomal fusion step (Kimura et al., 2007). By this approach, autophagosomes could exhibit green (GFP) and red (mCherry) fluorescence, resulting in yellow signals in merged images. After fusion with lysosomes, acidic autolysosomes quench GFP fluorescence and preferentially exhibit red fluorescence. Our results showed that the fusion step of autophagosomes with lysosomes was notably inhibited by folate deficiency in the decidualized mESCs (Figure 3h).
These results showed that autophagy could be inhibited by folate deficiency in the decidualized mESCs in vitro.
3.4 Aberrant decidualization induced by folate deficiency was reversed by activating autophagy in early pregnant mice and in primary mESCs
To further address whether folate deficiency-impaired endometrial decidualization was mediated by inhibited autophagy, autophagy was activated by the specific autophagy inducer rapamycin, which inhibits the mammalian target of rapamycin (mTOR) pathway by binding mTORC1 (Y. J. Kim et al., 2013). Besides this, autophagy could be blocked by the specific inhibitor 3-MA (Y. T. Wu et al., 2010). According to the results shown in Figure 4a,b, the number of embryo implantation sites on D6 was significantly reduced under the role of folate deficiency (control: 14.00 ± 0.84, FD: 7.60 ± 0.51, p < 0.001). After the mice received combined treatment with a folate-deficient diet and rapamycin, rapamycin could significantly reverse the inhibitory effect of folate deficiency on the number of embryo implantation sites (FD: 7.60 ± 0.51, FD + Rapa: 12.00 ± 0.84, p < 0.01). After combined treatment with a folate-deficient diet and 3-MA, the specific inhibitor of autophagy, 3-MA could enhance the inhibitory effect of folate deficiency on the number of embryo implantation sites (FD: 7.60 ± 0.51, FD + 3-MA: 5.80 ± 0.58, p < 0.05). Western blot results subsequently showed that the protein expression levels of Atg5 and Beclin1 were induced by rapamycin and reduced by 3-MA, indicating that autophagy was successfully activated by rapamycin and inhibited by 3-MA (Figure 4c). Further Western blot results showed that the decreased expression level of the endometrial decidualization related protein Bmp2 in the condition of folate deficiency could also be reversed by autophagy inducer rapamycin on D6, D7, and D8 during early pregnancy. However, it was not reversed by autophagy inhibitor 3-MA (Figure 4d). These results indicated that rapamycin may be able to reverse abnormal endometrial decidualization induced by folate deficiency while 3-MA could not. It suggested that autophagy played a critical role in the impaired endometrial decidualization induced by folate deficiency.
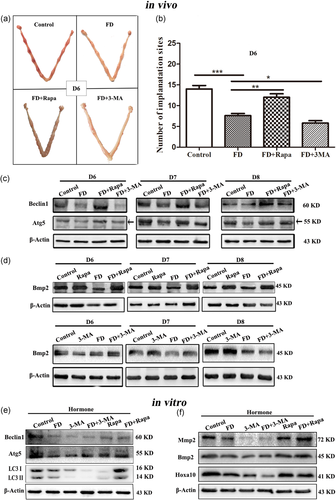
In addition, autophagy also was successfully induced by rapamycin and blocked by 3-MA in the decidualized mESCs (Figure 4e). Western blot analysis showed that the folate deficient-decreased protein expression levels of Hoxa10, Bmp2, and Mmp2 were significantly increased by autophagy inducer rapamycin and were further decreased by autophagy inhibitor 3-MA (Figure 4f).
Treatment with autophagy inducer rapamycin reversed the adverse impact of folate deficiency on endometrial decidualization both in vivo and in vitro, suggesting that a significant role of autophagy in folate deficiency-impaired endometrial decidualization.
3.5 Endometrial AMPK/mTOR signaling was disrupted in the impaired decidualization by folate deficiency in early pregnant mice and in primary mESCs
It has been reported that inhibition of adenosine 5‘-monophosphate (AMP)-activated protein kinase (AMPK) can activate the mTOR signaling pathway (Liu et al., 2017; Y. Zhou et al., 2017), which acted as one of the AMPK downstream molecules accompanied by its phosphorylated form (p-mTOR). To determine whether the AMPK/mTOR signaling pathway is involved in the folate deficiency-mediated regulation of autophagy, the AMPK/mTOR-related protein expression levels were evaluated by Western blot in vitro/vivo study. The results showed that folate deficiency significantly decreased AMPK phosphorylation and increased mTOR phosphorylation without inducing the expression of total AMPK or mTOR in the pregnant mice on D6, D7, and D8 (Figure 5a). Additionally, the protein expression level of mTOR downstream molecules p70 S6 kinase 1 (S6K1) phosphorylation was notably increased while eIF4E binding protein 1 (4E-BP1) phosphorylation was also obviously decreased in the folate-deficient group of pregnant mice (Figure 5a). The same results were obtained in the decidualized mESCs in vitro (Figure 5c). When the mTOR pathway was inhibited by rapamycin, the decreased protein expression of LC3-II/I, Atg5, and Beclin1 due to folate deficiency were significantly increased both in vitro/vivo studies (Figure 4c,e), suggesting that AMPK/mTOR was disrupted by folate deficiency to down-regulate autophagy during endometrial decidualization. Furthermore, rapamycin significantly reversed the inhibitory effect of folate deficiency on the number of embryo implantation sites (Figure 4a,b) and the endometrial decidualization-related protein expression levels of Hoxa10, Bmp2, and Mmp2 (Figure 4d,f). All data, both in vivo and in vitro studies, suggested that folate deficiency could reduce autophagy by disrupting AMPK/mTOR signaling, resulting in aberrant endometrial decidualization and adverse pregnancy outcomes.
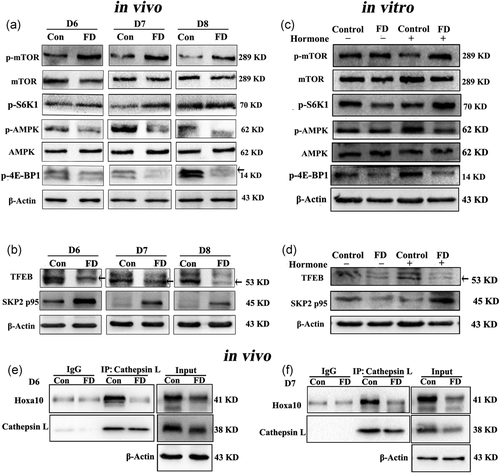
In addition, recent research has demonstrated a new axis of AMPK-SKP2-CARM1 signaling to initiate autophagy (Shin et al., 2016), and our research team reported that AMPK-SKP2-CARM1 signaling activated autophagy to disrupt primordial folliculogenesis in mice ovary exposure of Di (2-ethylhexyl) phthalate (Y. Zhang et al., 2018). We further explored whether the AMPK-SKP2-CARM1 signaling pathway was involved in folate deficiency-impaired aberrant endometrial decidualization in vitro and in vivo. The results revealed that the protein expression levels of p-AMPK and its downstream effector the transcription factor EB (TFEB) were markedly decreased, and the protein expression levels of SKP2 p95 were obviously increased by folate deficiency in vitro and in vivo (Figures 5b and d). These data suggested that the AMPK-SKP2-CARM1 signaling pathway could participate in the process of aberrant endometrial decidualization in folate-deficient pregnant mice.
Lastly, we further explored how the impairment of folate deficiency-induced decidualization was mediated by inhibited autophagy using Co-IP. The lysates were pulled down using the Cathepsin L antibody, and the precipitates were used to detect the endometrial decidualization-related protein levels by Western blot. The result on immunoprecipitation showed that Hoxa10 interacts with Cathepsin L. Furthermore, the interaction between Cathepsin L and Hoxa10 was notably reduced in the folate deficiency group of pregnant mice on D6 and D7 (Figure 5e,f).
4 DISCUSSION
It is well known that maternal folate deficiency is a major risk factor for birth defects and fetal growth restriction. Several studies about the effect of folate deficiency on birth defects have mainly focused on fetal development. Successful pregnancy requires not only the normal development of the embryo itself but also a suitable maternal endometrium, such as the establishment of uterine receptivity and decidualization. Our previous studies revealed that folate deficiency could impair endometrial decidualization during early pregnancy (Geng et al., 2015; Liao et al., 2015). However, the molecular mechanism is little known. Autophagy is a highly regulated catabolic process involved in the maintenance of cellular homeostasis. This study showed that AMPK/mTOR downregulated autophagy was essential for the aberrant endometrial decidualization in folate-deficient pregnant mice.
Several studies have addressed the influence of autophagy on uterine function (J. Choi, Jo, et al., 2014; S. Choi, Shin, et al., 2014; Li et al., 2018; Sivridis et al., 2011). Autophagy is upregulated both in mouse and human ESCs during decidualization but is diminished in obesity models with impaired decidualization (Rhee et al., 2016). And then, small interfering RNA (siRNA) knockdown of ATG7 and ATG5 (Mestre Citrinovitz et al., 2019), and loss-of-function for ATG16L1 (Oestreich et al., 2020) results in impaired endometrial decidualization both in mice and women. S. Choi, Shin, et al. (2014) found that glycogen content in ovariectomized uteri was increased by 3-MA treatment, indicating that autophagy may be related to glycogen metabolism in endometrial cells. Li et al. (2018) group reported that programmed cell death 4 (PDCD4) inhibited proliferation, migration, and invasion of endometrial cells by inhibiting autophagy in endometriosis. These studies suggested that autophagy plays an important role in maintaining endometrial function. In this study, we found that the number of autophagosomes was notably decreased in the decidual endometrium in folate-deficient pregnant mice. At the same time, the inhibited autophagosome formation, lysosome-autophagosome fusion, and the degradation process were all observed in the condition of folate deficiency. To further address whether folate deficiency-impaired endometrial decidualization was mediated by inhibited autophagy, autophagy inducer rapamycin and inhibitor 3-MA were applied in our study. Rapamycin can inhibit the mTOR signaling pathway to promote autophagy (Nalbandian et al., 2015; L. Wu, Feng, et al., 2013; X. Wu et al., 2014). 3-MA can inhibit class III phosphatidylinositol 3-kinase (PtdIns3K) to specifically block the formation of autophagosomes (Y. T. Wu et al., 2010; Y. Wu, Wang, et al., 2013). After treatment with rapamycin and 3-MA, the aberrant decidualization induced by folate deficiency was reversed by rapamycin, while aggravated by 3-MA. The same results were got both in vivo and in vitro. All these results gave the conclusion that suppressed autophagy was essential for the compromised decidualization induced by folate deficiency.
It was reported that AMPK inhibition activates the mTORC1 signaling pathway (Liu et al., 2017; Y. Zhou et al., 2017), which acts as one of the AMPK downstream molecules accompanied by its phosphorylated form (p-mTOR). As a nutritional condition sensor, AMPK/mTOR can control autophagy (Alers et al., 2012; J. Kim et al., 2011). Autophagy is promoted by AMPK, which is a key energy sensor and can regulate cellular metabolism to maintain energy homeostasis (Alers et al., 2012). Conversely, mTOR accepts biological signals which are passed to downstream target genes such as p-S6K to inhibit the formation of the ULK1 complex, thus inhibiting autophagy (Shaw et al., 2004; J. Zhou et al., 2013). In addition, AMPK directly inhibits the mTORC1 complex via phosphorylation, indicating that a proper balance of the AMPK/mTOR pathway is essential to maintain physiological conditions (Alers et al., 2012; J. Kim et al., 2011). Meanwhile, recent studies found that mTOR is very important for early pregnancy (X. Chen et al., 2009; Huang et al., 2018; J. Kim et al., 2010; Rosario, Nathanielsz, et al., 2017; Rosario, Powell, et al., 2017; Zeng et al., 2013). For example, the combination between SPP1 and integrin αβ3 and α5β1 regulate adhesion and migration of trophoblast cells through the activation of mTOR to ensure successful embryo implantation (J. Kim et al., 2010). Our previous study also confirmed that mTOR expression gradually increased in pregnant mice endometrium compared with nonpregnant mice from the time before implantation to the period of implantation. When mTOR was inhibited, the number of embryo implantation sites was obviously decreased (X. Chen et al., 2009). Moreover, mTOR, an important factor for embryo loss induced by carbon disulfide, could be downregulated by p-AKT and p-AMPK (Huang et al., 2018). In this study, we explored the AMPK/mTOR signal pathway and found that AMPK was inhibited and mTOR was activated in the aberrant decidualization induced by folate deficiency. When AMPK/mTOR inhibitor rapamycin was used to block signaling, the previously decreased autophagy-related protein expressions due to folate deficiency were significantly increased. In addition, rapamycin significantly reversed the inhibitory effect of folate deficiency on endometrial decidualization. It demonstrated that the AMPK/mTOR downregulated autophagy was essential for aberrant endometrial decidualization in early pregnant mice. However, this study did not conduct a drug intervention analysis for the AMPK signal pathway. Further studies are needed to confirm this hypothesis.
Recent research has demonstrated a new axis of AMPK-SKP2-CARM1 signaling for autophagy initiation (Shin et al., 2016), and our group reported that AMPK-SKP2-CARM1 signaling activated autophagy to disrupt primordial folliculogenesis in mice ovary exposure to di (2-ethylhexyl) phthalate (Y. Zhang et al., 2018). Here, this study also explored the role of the AMPK-SKP2-CARM1 signal pathway in the aberrant endometrial decidualization induced by folate deficiency. It found that the TFEB was significantly inhibited while SKP2 was notably increased by folate deficiency. AMPK-dependent phosphorylated FOXO3a downregulates SKP2 and stabilizes co-activator-associated arginine methyltransferase 1 (CARM1). CARM1 upregulates autophagy-related and lysosomal genes as a transcriptional co-activator with TFEB, and CARM1-dependent histone arginine methylation is required for proper autophagy (Shin et al., 2016). Whether the AMPK-SKP2-CARM1 signal pathway takes part in downregulated autophagy and results in aberrant endometrial decidualization induced by folate deficiency should be further explored.
5 CONCLUSION
In conclusion, here we explored the role of autophagy in the aberrant endometrial decidualization induced by folate deficiency. It was found that AMPK/mTOR downregulated autophagy was essential for aberrant endometrial decidualization in early pregnant mice, which could result in adverse pregnancy outcomes. This study provided some new clues for understanding the causal mechanisms of birth defects induced by folate deficiency during early pregnancy.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the helpful comments and technical assistance on this paper received from our colleagues. This study was supported by the National Natural Science Foundation of China [No. 31571554], and the Outstanding Graduate Student Cultivation Program of Chongqing Medical University [No. BJRC201904], Construction Project of Postgraduates Tutor Team of Chongqing (No. dstd201809).
AUTHOR CONTRIBUTIONS
Conceptualization: Yan Zhang, Rufei Gao, and Junlin He. Data curation: Yan Zhang, Rufei Gao, and Qiutong Chen. Formal analysis: Yan Zhang, Rufei Gao, Qiutong Chen, and Junlin He. Funding acquisition: Junlin He. Methodology: Yan Zhang, Lei Zhang, and Qiutong Chen. Resources: Junlin He. Supervision: Yanqing Geng, Xuemei Chen, Xueqing Liu, Xinyi Mu, Yubin Ding, and Yingxiong Wang. Validation: Yanqing Geng, Xuemei Chen, Xueqing Liu, Xinyi Mu, Yubin Ding, and Yingxiong Wang. Writing – original draft: Yan Zhang, and Lei Zhang. Writing – review and editing: Yan Zhang, Rufei Gao, and Junlin He.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.




