Moderate mechanical stress suppresses the IL-1β-induced chondrocyte apoptosis by regulating mitochondrial dynamics
Jiaming Zhang and Xiaoxia Hao contributed equally to this study.
Abstract
Mitochondrial dysfunction contributes to osteoarthritis (OA) onset and progress. Mitochondrial dynamics, coupled with mitophagy, is critical for the maintenance of mitochondrial fitness, involving many cellular processes, such as proliferation and apoptosis. Excessive mechanical stress induces chondrocyte apoptosis; however, the effects of mechanical stress on mitochondrial dynamics remain elusive. In this study, we performed fluorescence staining, flow cytometry, transmission electron microscope, Western blot analysis, and RNA-sequencing to assess the effects of different strength of mechanical stimulation on mitochondrial functions of chondrocyte treated with interleukin-1β (IL-1β). We found that moderate mechanical stress reduced the IL-1β-induced apoptosis by maintaining mitochondrial function and scavenging the reactive oxygen species, while excessive mechanical stress induced strong mitochondrial dysfunction and apoptosis. Moreover, RNAsequencing revealed that mitophagy and mitochondrial dynamics were involved in the regulation of mechanical stress on chondrocyte biology. In addition to the elevated mitophagy, moderate mechanical stress also promoted mitochondrial dynamics by enhancing the expression of MFN1/2 and OPA1 and the translocation of dynamin-related protein 1 from the cytoplasm to the mitochondria. However, an uncoupling of mitochondrial dynamics, characterized by strongly elevated fission, resulted in the unfavorable apoptosis of excessive mechanical stress-stimulated chondrocytes. This study revealed the effects of mechanical stress upon mitochondrial dynamics in chondrocyte.
Abbreviations
-
- GO
-
- gene ontology
-
- KEGG
-
- kyoto encyclopedia of genes and genomes
-
- MMP
-
- mitochondrial membrane potential
-
- OA
-
- osteoarthritis
-
- ROS
-
- reactive oxygen species
1 INTRODUCTION
Osteoarthritis (OA) is a common degenerative joint disease characterized by degradation of articular cartilage and subchondral bone (Glyn-Jones et al., 2015), which causes a significant socioeconomic burden (Felson, 2010; Zhang et al., 2019). Although accumulating evidence reveals the therapeutic targets and promising treatments, the pathological mechanisms of OA are still elusive.
Among multiple risk factors of OA, excessive mechanical loading is critical for the damaged integrity of the cartilage-subchondral bone unit, characterized by chondrocyte death, cartilage inflammation, and degeneration, and also subchondral bone remodeling (Jorgensen et al., 2017; Poulet et al., 2015; X. Yang, Guan, et al., 2016). However, physical exercise gives promise to OA management by alleviating the unfavorable symptoms and also the structural progress (Chang et al., 2017; Iijima et al., 2016). Some in vitro studies showed that moderate mechanical stress protects chondrocyte from apoptosis and promotes chondrocyte proliferation (Xiang et al., 2019; Xu et al., 2013; K. Yang, Wu, et al., 2016). In this context, it is necessary to explore the effects of mechanical stimulations of diverse strength on chondrocyte biology.
Mitochondrial dysfunction is widely accepted as a contributor to OA onset and progress. Mitochondria, which harbor the pathways involved in ATP synthesis through the tricarboxylic acid cycle and oxidative phosphorylation, regulate cellular energy and metabolism and participate in various biological and pathological processes. Mitochondrial fitness, including three layers (molecular, morphological, and functional), is indispensable for chondrocyte survival (Blanco & Rego-Perez, 2018). Mitochondrial dysfunction causes a series of metabolic alterations that increase the production of reactive oxygen species (ROS), decrease ATP synthesis, and trigger an inflammatory response, inducing the synthesis of cytokines and matrix metalloproteinases (Blanco & Rego-Perez, 2018). Mitochondria have a unique system, namely mitochondrial dynamics, including fission and fusion, to change the morphology and maintain their function in response to cellular demands and environmental imperatives. Fission segregates damaged parts of mitochondria from intact ones, whereas fusion connects neighboring depolarized mitochondria and mixes their contents to maintain membrane potential (Ikeda et al., 2015). When the coupling of fission and fusion is disturbed, abnormal dynamics disrupts the quality control, causing mitochondrial dysfunctions and cell death, which eventually culminate in tissue damage and diseases, such as cartilage and intervertebral disc degeneration (Kim et al., 2016; Lin et al., 2017).
In this study, we investigated the effects of different strength of mechanical stimulations on mitochondrial function of chondrocyte with IL-1β treatment to address several questions: (1) how mechanical stress on chondrocyte affects mitochondrial functions; (2) what are the roles of mitophagy and mitochondrial dynamics in this process; (3) what can we do in OA management. Our study demonstrated the roles of mechanical stress in chondrocyte biology, in particular, mitochondrial biology, and the relationship between mechanical stress and mitochondrial dynamics, providing the understanding of OA pathogenesis and also the basic evidence of OA management.
2 MATERIALS AND METHODS
2.1 Reagents
Dynamin-related protein 1 (DRP1) inhibitor Mdivid-1 (Catalog no. S7162) was purchased from Selleckchem (China). Recombinant Rat IL-1β (Catalog No.400-01B) was purchased from Peprotech (USA). The primary antibodies were listed as follows: Cleaved Caspase 3 (1:1000, #9664; CST), BAX (1:1000; 50599-2-Ig; Proteintech), ULK1 (1:1000; #8054; CST), Parkin (1:1000; 14060-1-AP; Proteintech), PINK1 (1:1000; 23274-1-AP; Proteintech), LC3-I/II (1:1000; #4108; CST), ERK (1:1000; #4695; CST), p-ERK (1:1000; #4370; CST), p38 (1:1000; #8690; CST), p-p38 (1:1000; #4511; CST), mTOR (1:1000; #2972; CST), p-mTOR (1:1000; #5536; CST), Akt (1:1000; #4691; CST), p-Akt (1:1000; #4060; CST), MFN1 (1:500; PB0263; Boster), MFN2 (1:1000; #11925; CST), DRP1 (1:1000; #8570; CST), OPA1 (1:1000; 27733-1-AP; Proteintech), COX IV (1:1000; #4850; CST), GAPDH (1:400; BM3876; Boster), and beta-actin (1:400; BM3873; Boster).
2.2 Isolation and culture of chondrocytes
Chondrocytes were isolated from the knee articular cartilage of 3-day-old Sprague-Dawley rats. The animal protocol was approved by the Experimental Animal Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). Briefly, cartilage tissue was minced and digested with 0.25% trypsin (Gibco) for 30 min and 0.25% collagenase II (Invitrogen) at 37°C for 6 h sequentially. Chondrocytes were collected and cultured in an incubator containing 5% CO2 at 37°C with Dulbecco's DMEM/F12 (HyClone) supplemented with 10% fetal bovine serum (FBS, Gibco) and antibiotics (100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate) (Gibco).
2.3 Mechanical stress stimulation
Mechanical stress was applied to chondrocytes by using the four-point bending system as previously described (Li et al., 2010; T. Xu et al., 2013). Briefly, cells were cultured on special vinyl plates until cells reached 80% confluence. Then the plate was transferred to the bending dishes filled with 20 ml of DMEM/F12 medium in an incubator containing 5% CO2 at 37°C. Cells were exposed to mechanical stress with different intensities (2000 and 5000 μstrain) at a frequency of 1 Hz for 4 h. For the control group, cells were cultured on the same plate and kept in the same incubator without mechanical stress stimulation for 4 h.
2.4 Protein extraction and Western blot analysis
Protein extraction and Western blot analysis were performed as the previous studies described (Yao et al., 2019). GAPDH and β-actin served as an internal standard for semiquantification. Protein blots were visualized using Western ECL Substrate Kit (Thermo Pierce) and exposed using Bio-Rad scanner (Bio-Rad). The intensity of bands was quantified by digital image analysis software (Bio-Rad).
2.5 Determination of cell proliferation
Cell proliferation was measured by 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay (RiboBio) according to the manufacturer's instructions. In brief, cells were incubated with 50 μM EdU for 2 h. Then we performed Apollo and 4′,6-diamidino-2-phenylindole (DAPI) staining keeping with the protocols and used a fluorescence microscope to detect the EdU-positive cells (red dots). The proportion of the EdU-positive cells was calculated as the rate of EdU-positive to total DAPI-positive cells (blue dots).
2.6 Estimation of apoptosis by annexin V-FITC/PI staining
Apoptosis of the cells was evaluated with Annexin V-FITC Apoptosis assay Kit (Absin) according to the manufacturer's instructions. After treatment, all the cells were harvested and washed three times with PBS, then resuspended in binding buffer followed by Annexin-V/PI staining at 37°C for 30 min in the dark. FACSCalibur flow cytometer (BD) was used to analyze the apoptotic cells.
2.7 Evaluation of intracellular reactive oxygen species
Levels of ROS were measured using fluorescent probe reactive oxygen species assay kit (Beyotime) as per the manufacturer's instructions. Briefly, the cells were stained with 10 μM DCFH-DA in the dark at 37°C. After 30 min of incubation, the cells were washed twice with serum-free DMEM media and immediately visualized with a fluorescence microscope (Evos fl auto; Life Technologies). To quantify the ROS level, cells were prepared as mentioned above and resuspended with serum-free DMEM media, and finally analyzed using a FACSCalibur flow cytometer.
2.8 Mitochondrial membrane potential (MMP, ΔΨm) measurement
Mitochondrial membrane potential was measured by the fluorescent probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1) detection kit (Beyotime) according to the manufacturer's instructions. JC-1 accumulates in mitochondria matrix with high membrane potential, resulting in the formation of JC-1 aggregates and the emission of red fluorescence. When the ΔΨm is low, the JC-1 monomer is formed and yields green fluorescence. In brief, the cells were stained with JC-1 staining solution in the dark at 37°C. After incubation for 30 min, the cells were washed twice with JC-1 staining buffer and immediately observed with a fluorescence microscope. To quantify the MMP, cells were prepared as mentioned above and resuspended with JC-1 staining buffer, and finally analyzed using a FACSCalibur flow cytometer.
2.9 Mitochondrial-specific fluorescence staining
Mito-Tracker Green (Beyotime) is a mitochondrial membrane potential independent of mitochondrial staining reagent. The experiment was performed according to the manufacturer's protocols (Yao et al., 2019). The mitochondrial morphology was observed and captured by a fluorescence microscope. The mitochondrial length was determined using ImageJ software v1.51k.
2.10 Transmission electron microscope (TEM)
TEM is an important method for the characterization of autophagosomes as it can directly visualize their inner architecture. The procedure was performed as previously described (Fu et al., 2019). In brief, the cells were washed with PBS and resuspended, and then post-fixed by 2% OsO4 (Electron Microscopy Sciences), dehydrated with different concentrations of alcohol and acetone, and then embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate (E. Merck) and lead citrate (Sigma-Aldrich). A TEM (JEM-100CXII) was used for observation.
2.11 Next-generation RNAsequencing (RNA-seq)
Total RNA was isolated from the IL-1β-induced chondrocytes subjected with the mechanical stress of 0 and 2000 μstrain, 1 Hz, for 4 h using TRIzol (Invitrogen) according to the manufacturer's instructions. Each group contains three biological replicates. Library construction and sequencing followed the standard protocols (Djebali et al., 2012). For differential expression analysis, gene abundances were quantified by R package edgeR (Nikolayeva & Robinson, 2014). Differentially expressed genes (DEGs) were defined using the criteria of FDR < 0.05 and |log2 (fold change)| > 1 and visualized by heatmap by the R package pheatmap. Gene ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed by the R package clusterProfiler and visualized by bubble plot (G. Yu et al., 2012).
2.12 Statistical analysis
The statistical methods used in this study include unpaired two-tailed Student's t test and one-way analysis of variance with Dunnett's multiple comparisons test; p < .05 was considered statistically significant. The application of each statistics method was specified in figure legends. GraphPad Prism 7.0 was used for the statistical analysis and data are presented as means ± SD.
3 RESULT
3.1 Moderate stress promotes chondrocyte survival by inhibiting IL-1β-induced apoptosis
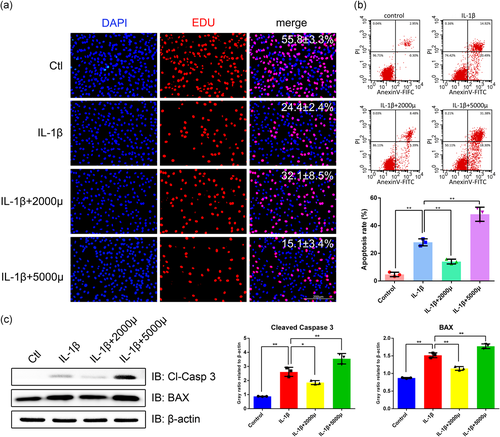
The chondrocytes treated with IL-1β (5 ng/ml) for 24 h were subjected to the mechanical stress of 2000 and 5000 μstrain, 1 Hz, for 4 h, respectively (T. Xu et al., 2013). As shown in Figures 1b and S1, there was no significant difference between the proliferation ratio of the 2000 μstrain group and the IL-1β group (p > .05), while the 2000 μstrain group had an elevated tendency. The proliferation ratio of the 5000 μstrain group was obviously reduced compared with both the 2000 μstrain and IL-1β group (p < .05). On the other hand, the apoptotic rate of the 5000 μstrain group was obviously elevated to 48.21%, which was higher than both the IL-1β (27.95%) and the 2000 μstrain group (12.76%). Interestingly, the apoptotic rate of the 2000 μstrain was reduced by 54.3% compared with the IL-1β group (p < .05, Figure 1c). Moreover, to investigate the regulation of mechanical stress on the apoptosis-related proteins, we detected cleaved-caspase 3 and Bax expression by western blot analysis. Accordingly, cleaved caspase 3 and Bax were increased in the 5000 μstrain group but reduced in the 2000 μstrain group (Figure 1d). Taken together, these results indicated that mechanical stress of 5000 μstrain is excessive for the IL-1β-treated chondrocytes because of its strong apoptosis-induced effect and 2000 μstrain rescues chondrocytes from IL-1β-induced apoptosis, which is considered as a moderate mechanical stimulation for the IL-1β-treated chondrocytes.
3.2 Moderate mechanical stress exerts protective effects by scavenging the IL-1β-induced ROS
IL-1β induces the ROS generation of chondrocytes, which causes structural and functional damage to the mitochondria and induces cell death signaling that eventually culminates in tissue damage (Ansari et al., 2018; T. Yu et al., 2018). As shown in Figure 2a, an obvious reduction of ROS generation was observed in the 2000 μstrain group compared with the IL-1β group. The mechanical stress of 5000 μstrain significantly increased the ROS production in the IL-1β-induced chondrocytes. To quantitatively investigate the elimination effect of moderate stress on ROS generation, a flow cytometry method was performed. The results showed that moderate stress of 2000 μstrain reduced the ROS generation by 38.5% compared with the IL-1β group (Figure 2b). Moreover, the ROS generation of the 5000 μstrain group was 1.98-fold to the IL-1β group, while 3.21-fold to the 2000 μstrain. These results suggested that moderate mechanical stress exerts protective effects by scavenging IL-1β-induced ROS.

3.3 Moderate mechanical stress protects chondrocytes against the IL-1β-induced collapse of the mitochondrial membrane potential (ΔΨm)
ΔΨm controls ROS production. The maintenance of physiologically optimal ΔΨm prevents the generation of ROS. Inversely, ROS induces the collapse of ΔΨm, which suggests mitochondrial dysfunction(Sanderson et al., 2013). As shown in Figure 2c, an increase in the fluorescence intensity of green JC-1 monomers was observed in the IL-1β group, indicating a significant collapse of ΔΨm induced by IL-1β. However, the fluorescence intensity of green JC-1 monomers was weakened in the 2000 μstrain group compared to the 5000 μstrain, which suggested that moderate mechanical stress protected chondrocytes against the loss of ΔΨm induced by IL-1β. Moreover, the mechanical stress of 5000 μstrain aggravated the collapse of ΔΨm. These results of JC-1 flow cytometry showed a similar tendency (Figure 2d). The fluorescence ratio of JC-1 monomers/aggregates in the 2000 μstrain group was significantly decreased by 59.1% compared with the IL-1β group (p < .05). Collectively, moderate mechanical stress protects chondrocyte against the IL-1β-induced mitochondrial dysfunction, while excessive mechanical stress aggravates mitochondrial dysfunction resulting in apoptosis.
3.4 Mechanical stress promotes chondrocyte mitophagy
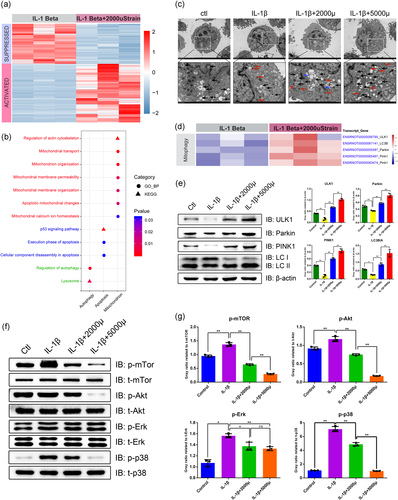
Next-generation RNAsequencing was performed to explore the underlying mechanisms of moderate mechanical stress in benefiting chondrocyte survival. As shown in Figure 3a, 5022 DEGs were identified by the threshold of FDR < 0.05 and log 2 (fold change) > 1 or < −1, including 2981 upregulated and 2041 downregulated genes. The functional analysis of these DEGs by GO and KEGG revealed the involvement of mitochondrial function, autophagy, and apoptosis, such as the regulation of mitochondrial membrane permeability, mitochondrial calcium ion homeostasis, and p53 signaling pathway (Figure 3b). Mitophagy is a unique form of autophagy for degrading damaged mitochondria. To investigate whether mechanical stress regulates autophagy and mitophagy, we observed the autophagosomes in chondrocytes by TEM (Figure 3c). The number of autophagosomes was decreased in the IL-1β group compared with the control group, whereas increased in the stress groups in a strength-dependent manner, which suggested elevated autophagy. Moreover, we plotted the abundance of mitophagy-related genes in the RNA-sequencing results (Figure 3d). Interestingly, the transcripts of Ulk1, Lc3b, Parkin, and Pink1 were consistently elevated in the 2000 μstrain group, while the Ulk1 expression in one replicant was not increased. These results were further confirmed at the protein level (Figure 3e). As a response to mechanical stimulation, the abundance of ULK1, Parkin, and PINK1 was increased consistently in a strength-dependent manner, suggesting that mechanical stress-induced mitophagy. Given that MAPK signaling and the Akt/mTOR signaling are two critical pathways regulating mitophagy, we also checked these pathways and found a strong suppression of these pathways by mechanical stress (Figures 3f,g). Taken together, these results revealed that mechanical stress promotes chondrocyte mitophagy by regulating MAPL/Akt/mTOR signaling in a strength-dependent manner.
3.5 Moderate mechanical stress promotes mitochondrial dynamics
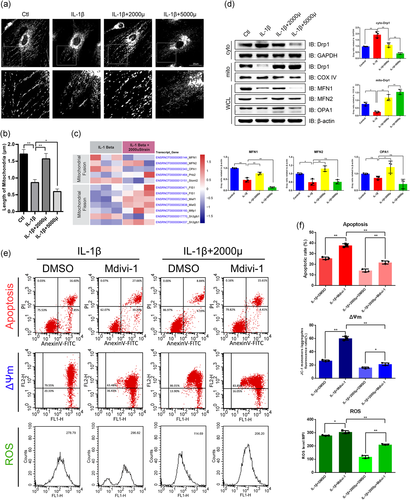
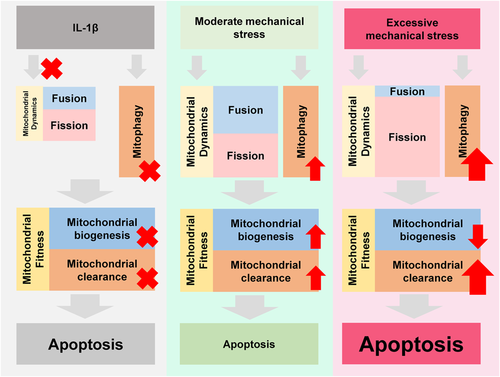
Mitochondrial dynamics is the upstream event of mitophagy. As shown in Figure 4a, IL-1β induced the morphological and distributional changes of mitochondria. The shortened mitochondria or mitochondrial fragments in the IL-1β group were in a scattered distribution. While the mitochondria were prolonged and surrounded the nucleus in the 2000 μstrain group, those in the 5000 μstrain group lost the normal morphology and the small fragments were heaped in the cytoplasm randomly. The quantitative results showed that the mitochondrial length was significantly reduced in the IL-1β group and 5000 μstrain aggravated this effect while 2000 μstrain rescued the IL-1β-induced mitochondrial shortening (Figure 4b). Then, we plotted the abundance of the genes involved in mitochondrial dynamics in RNA-seq data (Figure 4c). While the changes of the transcripts involved in fusion were conflicted, those in fission were upregulated in the 2000 μstrain group. As shown in Figure 4d, IL-1β suppressed both fusion and fission proteins. The expression of DRP1, a key participator in fission, was progressively increased in mitochondrial lysate while decreased in cytoplastic lysate in a strength-dependent manner. However, fusion proteins changed in a different pattern compared with fission proteins. The abundance of MFN1/2 and OPA1 was increased in the 2000 μstrain group while dropped in the 5000 μstrain group, which suggests the uncoupling of fusion and fission responding to excessive stress. To investigate whether mechanical stress regulates the mitochondrial functions and apoptosis by mitochondrial dynamics, we employed a DRP1 inhibitor, Mdivi-1 (10 μM), to treat the chondrocytes for 30 min before moderate mechanical stress stimulation (Park et al., 2015). Moderate mechanical stress rescued chondrocytes from Mdivid-1-induced apoptosis, while Mdivid-1 impaired the benefits from moderate mechanical stress on mitochondrial functions and survival (Figures 4e,f). Taken together, these results indicated that moderate mechanical stress promotes mitochondrial dynamics while an uncoupling of fission and fusion is induced by excessive stress, which breaks the balance and causes mitochondrial dysfunction and apoptosis (Figure 5).
4 DISCUSSION
Mechanical stress is a double-edged sword to chondrocyte biology and OA. Overloading promotes the OA-like damage of cartilage and chondrocyte death, resulting in OA development (Liu et al., 2016); however, physical exercise is efficient to attenuate symptoms and structural progress of OA (Bartels et al., 2016; Iijima et al., 2016) and the moderate mechanical stress also has been proven to benefit chondrocytes (Song et al., 2016; Xiang et al., 2019). However, the benefit window of mechanical stimulation seems to be narrow and various between different in-vitro mechanical stimulation models and the benefits of physical exercise is not robust and disturbed by the other factors. As a result, it is worthy to figure out this benefit window and the underlying mechanisms, which may provide evidence of the physical therapy and the prescription management in OA patients.
In our study, we employed a mechanical system to explore the role of mechanical stress in chondrocyte biology. Our results indicate that moderate mechanical stress promotes chondrocyte survival by inhibiting IL-1β-induced apoptosis. In particular, moderate mechanical stress scavenges the IL-1β-induced ROS, which is an indispensable element in chondrocyte death and OA. Moreover, moderate mechanical stress protected chondrocyte against the IL-1β-induced collapse of mitochondrial membrane potential, which is the reciprocal causation with ROS. These findings suggest the relationship between mechanical stress and mitochondrial biology. Indeed, we found that mitophagy was enhanced consistently in a strength-dependent manner as a response to mechanical stress, suggesting the involvement of mitophagy in IL-1β-induced chondrocyte apoptosis and OA progress. However, the mechanical stress of different strength showed distinct impacts on mitochondrial dynamics. The moderate mechanical stress promoted both mitochondrial fission and fusion to eliminate the damaged cellular elements, while an uncoupling of fission and fusion was induced by excessive mechanical stress, which broke the balance of mitochondrial dynamics and mitochondrial homeostasis and caused apoptosis. These results maybe give an explanation of underlying mechanisms to the distinct outcomes of the different magnitude of mechanical stimulation.
The disruption of mitochondrial homeostasis and oxidative stress are involved in the pathogenesis of OA (Sun et al., 2020). In addition to the significant decrease of mitochondrial respiration and energy production, the depolarization of mitochondria was reported in the chondrocytes from OA cartilage, which leads to the release of apoptotic factors, such as cytochrome c, from the mitochondria to the cytoplasm (Maneiro et al., 2003). It is widely accepted that mitochondria produce more ROS at high membrane potential. However, the opposite correlation between ΔΨ and ROS production was observed in OA, which means lower ΔΨ, decreased respiratory activity, and elevated ROS production. Indeed, overloading suppresses chondrocyte respiratory activity, decreases ΔΨ, and increases ROS formation(Coleman et al., 2016).
In some situations, such as energy shortage, ROS accumulation, and collapsed MMP, mitochondria undergo fission and fusion, for population renewal and adaptation. The dynamic balance of mitochondrial fusion and fission is indispensable for maintaining mitochondrial morphology, membrane potential, and function (Sebastian et al., 2017). Abnormal dynamics disrupt the mitochondrial quality control, which eventually culminates in tissue damage and diseases, such as cartilage and intervertebral disc degeneration (Kim et al., 2016; Lin et al., 2017). Mitochondrial fission is regulated by mitochondrial fission proteins, among which DRP1 is recruited to the outer mitochondrial membrane by FIS1, MFF, MID49, and MID51/MIEF1, forming dotted structures located on future mitochondrial scission sites (Smirnova et al., 2001), constricting mitochondrial tubules to mediate membrane fission. Moderate stress promotes DRP1 translocating from the cytoplasm to mitochondria rather than increasing total DRP1 mRNA expression. Besides, increased fusion proteins, such as MNF1/2 and OPA1, respond to moderate stress. Given that both fission and fusion are enhanced, mechanical stress seems to give more “energy” to this cycling machine of mitochondria, resulting in maintained mitochondrial fitness and survival. In our previous study, Yao et al. (2019) found that the number of shortened or granulated mitochondria dramatically increased in chondrocytes when treated by IL-1β, while FGF18 could restore the normal mitochondrial morphology of chondrocytes. Also, the expression of MFN2, FIS1, and parkin was upregulated when treated with IL-1β within 24 h and downregulated when treated for 48 h. In general, we speculate that a short time exposure of chondrocytes to IL-1β enhances mitochondrial fusion and fission and prolonged exposure to IL-1β suppresses both fusion and fission, whereas Mfn2 expression was decreased in IL-1β-treated chondrocytes (5 ng/ml, 24 h), which is inconsistent with the study performed by Yao et al. This difference may be attributed to the batch effects of the cell, IL-1β reagent, or the operation manner. However, the study performed by L. Xu et al. (2020) strongly indicates a strong positive correlation between MFN2 and OA. Their findings indicate that MFN2 has no response to IL-1β treatment (10 ng/ml, 24 h) in rat chondrocytes, while they show increased MFN2 contribution to mitochondrial dynamics impairment in chondrocytes during aging. These results have disagreed with the previous finding of MFN2 in other aging-relevant diseases that age-related MFN2 depletion links to obesity and type I diabetes, two diseases associated with OA (Blanco & Fernandez-Moreno, 2020). Moreover, Chen et al. recently reported that Mfn2 expression was decreased in human nucleus pulposus tissues during intervertebral disc degeneration. Removing the damaged mitochondria depends on the downstream events, mitophagy (Zhao et al., 2018). In this event, Parkin and PINK1 play an important role in mitochondrial clearance (Ansari et al., 2018). We found that the expression of Parkin and PINK1 was obviously upregulated in chondrocytes with the stimulation of mechanical stress, suggesting that their involvement in the overloading induced chondrocyte death.
Moreover, we noticed that mechanical stress regulates the cytoskeleton remodeling (RNA-sequencing results, Figure 3b), which is consistent with our previous studies (T. Xu et al., 2013; K. Yang, Wu, et al., 2016). As a mechanotransduction element, actin microfilament remodeling may be involved in the mitochondrial biology responding to mechanical stress. In many cases, actin is required in the location, movement, and redistribution of the mitochondrial network, suggesting the special role of the cytoskeleton in the regulation of mitochondrial dynamics (Bartolak-Suki et al., 2017). In our previous study, we found that the effect of mechanical stress on the regulation of PTHrP expression is dependent on, partially, the remodeling of F-actin microfilaments in growth plate chondrocytes (T. Xu et al., 2013). Moreover, we found that YAP- and ERK-mediated mechanical strain-induced cell cycle progression through RhoA and cytoskeletal dynamics (K. Yang, Wu, et al., 2016). In the present study, we found the possible involvement of cytoskeleton upon the regulation of mechanical stimulation in the RNA-seq results, while we did not export its roles by experimental validations. Whether mechanical stress regulates the mitochondrial dynamics by the actin cytoskeleton in IL-1β-treated chondrocytes is still unclear in our study, which would be addressed in future.
5 CONCLUSION
Moderate mechanical stress rescues chondrocyte from IL-1β-induced apoptosis by enhanced mitochondrial dynamics, including fission and fusion, while excessive stress induces an uncoupling of fission and fusion that breaks the balance and causes mitochondrial dysfunction and apoptosis.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant no. 81772440 and no. 82072556).
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and design of the studies, obtaining funding, data analyses and interpretations, drafting, and final approval of the article.
CONFLICT OF INTERESTS
The authors declared no conflict of interests about the publication of this paper.
Open Research
DATA AVAILABILITY STATEMENT
Raw data of this study are available from the corresponding author upon request.




