GDF-15: Diagnostic, prognostic, and therapeutic significance in glioblastoma multiforme
Abstract
Glioblastoma multiforme (GBM) is the commonest primary malignant brain tumor and has a remarkably weak prognosis. According to the aggressive form of GBM, understanding the accurate molecular mechanism associated with GBM pathogenesis is essential. Growth differentiation factor 15 (GDF-15) belongs to transforming growth factor-β superfamily with important roles to control biological processes. It affects cancer growth and progression, drug resistance, and metastasis. It also can promote stemness in many cancers, and also can stress reactions control, bone generation, hematopoietic growth, adipose tissue performance, and body growth, and contributes to cardiovascular disorders. The role GDF-15 to develop and progress cancer is complicated and remains unclear. GDF-15 possesses tumor suppressor properties, as well as an oncogenic effect. GDF-15 antitumorigenic and protumorigenic impacts on tumor development are linked to the cancer type and stage. However, the GDF-15 signaling and mechanism have not yet been completely identified because of no recognized cognate receptor.
1 INTRODUCTION
Glioblastoma multiforme (GBM) has been known as the commonest primary malignant brain tumor and has a remarkably weak prognosis and survival of 15 months (Ampie et al., 2015; Carlsson et al., 2014; Razavi et al., 2016; Van Meir et al., 2010; Weathers & Gilbert, 2015). GBM is capable of cell invasion to the neighboring brain parenchyma (Basirjafari et al., 2020; Buckner et al., 2007). On the basis of the World Health Organization (WHO) 2016 classification, GBM is classified into isocitrate dehydrogenase (IDH) wild-type (WT) GBM (90%) and IDH mutants 1 and 2 (10%). IDH 1/2 mutation show a better prognosis than the WT form (Carlsson et al., 2014; Razavi et al., 2016; Van Meir et al., 2010; Weathers & Gilbert, 2015). The primary GBM of the WT form can be observed in cases older than 55 years (Ohgaki & Kleihues, 2013). Younger ages are more likely to have secondary GBM which is the mutant type (Ohgaki & Kleihues, 2013). Both primary and secondary GBMs have poor prognosis along with vital genetic mutations affecting pathways, such as cellular proliferation, invasion, survival, and angiogenesis. Each one has a specific transcriptional pattern and recurrence of DNA copy number aberrations (Furnari et al., 2007). Several markers have been mentioned for the primary GBMs tumors, such as epidermal growth factor receptor (EGFR) amplification, mutations affecting phosphatase, tensin homolog (PTEN), and telomerase reverse transcriptase promoter (Bush et al., 2017). Secondary GBMs exhibit mutations in IDH 1/2, tumor protein 53 (p53), and alpha-thalassemia/mental retardation syndrome X-linked (Bush et al., 2017). Methylation enhances the tumor cells’ susceptibility to alkylating drugs (Bush et al., 2017). The signaling mechanism explaining the role of growth differentiation factor 15 (GDF-15) in hallmarks of cancer like angiogenesis, proliferation, stemness, drug resistance, and metastasis has been studied (Vocka et al., 2018). According to the aggressive form of GBM, identifying the accurate molecular mechanism associated with GBM pathogenesis is essential. In this paper, we review the effects of GDF-15 protein on GBM that might provide a basis to develop therapeutic approaches to prevent and treat GBM.
2 GDF-15: THE BASICS
GDF-15 is a member of the transforming growth factor (TGF)-β superfamily in the branch of bone morphogenetic protein-like group.
GDF-15 can be found in the blood, but its levels are different based on age and sex (Wesseling et al., 2020). Overexpression of the GDF-15 in illnesses, like advanced cancer, causes the anorexia/cachexia syndrome. Neurons of area postrema (AP) and/or nucleus tractus solitarii (NTS) are important to regulate food consumption and body weight through GDF-15 (Borner et al., 2017; Tsai et al., 2014). GDF-15 causes food avoidance and malaise as a peptide (Patel et al., 2019). GDF-15-related vomiting and diseases in line with the GDF-15 function concept, as a pathophysiological signal, are linked to the stimuli inducing sickness (Borner et al., 2020).
There is a link between C-reactive protein and GDF-15 with a crucial role in endothelial inflammation pathogenesis (Y. Kim et al., 2018). Moreover, upregulation of its expression after treatment with different anticancer drugs, such as doxorubicin, nonsteroidal anti-inflammatory drugs (NSAIDs), carboplatin, genistein, and peroxisome proliferator-activated receptor-γ (PPARγ) ligands has been reported (Meier et al., 2015). p53, SP1, phosphatidylinositol 3‑kinase/protein kinase B (PI3K/AKT) signaling, and early growth response-1 (EGR-1) induce GDF-15 transcription in different cancers (Eling et al., 2006). The signaling strategy to explain the GDF-15 effect in cancer symptoms, such as stemness, angiogenesis, resistance to medication, proliferation, and metastasis has been evaluated in different cancers involving insulin-like growth factor receptor 1, extracellular-signal-regulated kinase (ERK), ERB signaling. GDF-15 probably can be regarded as a point of amalgamation for cancer symptoms (Modi et al., 2019).
Intracellular GDF-15 exists predominantly in its proform. The GDF-15 active form is the dimer that is released mature. Nonetheless, considering various biosynthesized types, as well as the possibility of their interactions (binding partners), the mature dimer, proforms, and propeptides of GDF-15 are probably vital to modulate the GDF-15 biological effect (Wang et al., 2013). Sp1 transcription factors modulate the GDF-15 transcription by the GC box found within −133 bp of the GDF-15 promoter, while p53 sites are involved in dietary compound-related GDF-15 expression (Baek et al., 2002; P. X. Li et al., 2000).
Glial-derived neurotrophic factor family receptor α-like (GFRAL) is a special receptor for GDF-15 with expression in AP and NTS neurons in both mice and humans, in this context, lack of the GFRAL reduced food consumption and body weight (Mullican et al., 2017). GFRAL knockdown in the AP and NTS caused an increase in body weight and obesity in mice subjected to a diet high in fat (Tsai et al., 2019). Also, activating other intracellular signaling pathways, such as the mitogen-activated protein kinase (MAPK) and EGFR/ErbB routes can be triggered through GDF-15 (K. K. Kim et al., 2008; Y. J. Park et al., 2010).
GFRAL expression is carried out in hindbrain neurons (not peripheral tissues), suggesting a central mechanism for regulation of food consumption modulated by GDF-15-GFRAL. Obese mice were not found with an elevation in energy expenditure caused by GDF-15, the antiobesity effects of the cytokine may be due to a decrease in food intake (Yang et al., 2017). The GDF-15 and GFRAL interaction blockage using a monoclonal antibody inhibited the GDF-15 metabolic activities. Activation of GFRAL-expressing by GDF-15 in the AP and NTS of the mouse brainstem activates neurons in the parabrachial nucleus and central amygdala, as the “emergency circuit” shaping feeding reaction to stressful situations. Tissue stress and damage cause an elevation in GDF-15 levels. A mechanistic base has been reported for the non-homeostatic control of neural circuitry using a peripheral signal related to tissue injury and stress (Hsu et al., 2017). GDF-15 has various functions in its target tissues, such as heart, kidney, brain, and tumors, with findings being inconsistent or even contradictory (Emmerson et al., 2018).
3 BIOLOGICAL FUNCTION OF GDF-15
The GDF-15 gene has a high expression level in the placenta, as well as adult prostate tissue where GDF-15 exerts immunomodulatory functions, and at lower concentrations, in other normal tissues, including the liver, kidney, pancreas, and fetal brain (Fairlie et al., 1999; Hromas et al., 1997; Lawton et al., 1997; Paralkar et al., 1998; Segerer et al., 2012). GDF-15 has different roles to control biological processes. It is vital in cancer development rate and progression, controlling stress reactions, bone generation, hematopoietic development, and adipose tissue activity, and is contributed to cardiovascular disorders (Mimeault & Batra, 2010). GDF-15 plays a role in the pathogenesis and progression of several cancers (Bauskin et al., 2006; Karan et al., 2003), and also cachexia (Brown et al., 2007; Johnen et al., 2007; Tong et al., 2004; Wollert et al., 2007). GDF-15 induction is done by many cellular stresses, like anoxia, compounds damaging DNA and NSAIDs, regardless of p53 or hypoxia-inducible factor (HIF)-1α (Albertoni et al., 2002; Baek et al., 2001; Kannan et al., 2000; P. X. Li et al., 2000). It has been recently published that GDF-15 conveys somatic distress to the brain (Lockhart et al., 2020). The in vitro data on human macrophages showed that GDF-15 expression (at RNA and protein levels) was induced following treatment with phorbol 12-myristate 13-acetate/12-O-tetradecanoylphorbol-13-acetate and retinoic acid, which indicates the important role of oxidative stress through the GDF-15 induction (Fairlie et al., 1999; Schober et al., 2001). Insofar is a phorbol ester stimulating superoxide anion generation in cultured microglial cells and is possibly linked to the GDF-15 induction (Colton et al., 1998; Schober et al., 2001). Collectively, various mechanisms regulate GDF-15; thus, GDF-15 may be regarded as a molecular target for cancer chemoprevention (Wang et al., 2013).
4 ROLE OF GDF-15 IN BRAIN METABOLISM
GDF-15 is synthesized in the choroid plexus and its release to cerebrospinal fluid (CSF), where several signaling proteins can arrive at targeted cells in the brain and spinal cord (Dixon et al., 1997). Considering the expression sites and GDF-15 as a neurotrophic factor, it is secreted through plexus epithelial cells to the CSF; therefore, the peptide is able to access putative targeted cells over a long distance. In the brainstem, aminergic neurons have been shown with in vitro and in vivo functions for GDF-15 in the nervous system (Strelau et al., 2000).
Expression and temporal dynamics of GDF-15 in a cortical lesion model undoubtedly indicate its crucial role in the brain tissue's early response to damage. It should be assessed if an increase in GDF-15 near the wound edges happens in microglia, macrophages, astrocytes, and endothelial cells. In lesioned brains, two sites are available, where GDF-15 is located in neuronal cells. First, dorsal thalamus neurons, which are distant from the lesioned area, expressing GDF-15 messenger RNA (mRNA) 4 days following the cryogenic cortical lesion, which can be regarded as a response of a neuron population projecting to the lesioned site retrogradely. In the lesioned central nervous system (CNS), GDF-15 can possess anti-inflammatory activities that supplement the other superfamily members’ functions. Finally, GDF-15 localization of neurons in the lesion areas raises questions concerning its pro- or antiapoptotic functions in neurons (Schober et al., 2001). GDF-15 is a trophic factor in different CNS neuron classes and promotes their survival rates in vitro and in vivo (Krieglstein et al., 1995). Concerning the response rate in RE2, the GDF-15 promoter shows a greater affinity for p53 in comparison with p63/p73 (Klein et al., 2001; Osada et al., 2007).
GDF-15 is protective against iron-mediated cytotoxicity (Strelau et al., 2000). GDF-15 promotes the dopaminergic neurons' survival rate, as well (Strelau et al., 2000). It is a strong neurotrophic factor to develop lesioned aminergic neurons in vitro and in vivo, similar to the neurotrophic factor derived from glial cells (Strelau et al., 2000). GDF-15 may be considered as an astrocyte-originated activator of astrocyte remodeling associated with the strengthening of tight junctions at the blood–brain barrier (BBB; Malik et al., 2020).
5 GDF-15 PRESENCE IN CSF OF PATIENTS WITH GLIOBLASTOMA
GDF-15 is connected to the evolution of cancer both positively and negatively depending upon the cellular state and environment. In physiological conditions, GDF-15 inhibits early tumor promotion. However, its abnormal expression in advanced cancers causes proliferation, invasion, metastasis, cancer stem cell formation, immune escape, and a reduction in response to therapy (Fang et al., 2019). Elevated GDF-15 level was shown in the serum or tissues of patients with cancer and its expression was correlated with poor prognosis. In vitro and in vivo studies also corroborated a metastasis-promoting role for GDF-15. However, in some studies, GDF-15 was shown to suppress the metastatic properties of cells (D. D. Liu & Mei, 2017; Spanopoulou & Gkretsi, 2020). There are some studies that support that GDF-15 acts as an immune checkpoint and can be an emerging target for cancer immunotherapy (Wischhusen et al., 2020). As a biomarker, GDF-15 can be used for the diagnosis and therapy of extensive scope of cancers. But some basic functions of GDF-15 are unclear and complicated at the molecular level (Fang et al., 2019).
There is an association between high CSF levels of GDF-15 and glioblastoma. Also, GBM patients with high CSF GDF-15 levels have a shorter survival (Shnaper et al., 2009). Conversely, GDF-15 plasma levels did not show a significant change in the existence of intracranial tumors. It is not clear that CSF levels of GDF-15 are affected by plasma levels, because of the BBB disruption in cases with glioblastoma (Shnaper et al., 2009). Shnaper et al. (2009) reported no increase in GDF-15 CSF levels in GBM cases who received alkylating agents than cases with measurements at the beginning of operation (chemo naive); however, the patient groups showed different results suggesting inflammatory cells as the main source of GDF-15 in GBM, in particular, macrophages as the main source. In accordance, an immune gene expression signature is part of a classifier to differentiate the glioma malignancy grade (Godard et al., 2003). CSF can be used for repeatedly assessing response to treatment earlier than imaging and is also useful to adapt to treatment accordingly (Shnaper et al., 2009).
6 EFFECT OF GDF-15 IN GLIOBLASTOMA
Several researchers have noted that almost all gliomas express GDF-15 in vivo (Roth et al., 2010; Scrideli et al., 2008; Strelau et al., 2008). However, some studies reported that glioblastoma cells are GDF-15 negative in vivo (Shnaper et al., 2009). The role of GDF-15 to develop and progress cancer is complicated and has not yet been identified. GDF-15 possesses tumor suppressor activity, however, its oncogenic activity has been found. The antitumorigenic and protumorigenic impacts of GDF-15 on tumor growth are linked to the cancer type and stage (Table 1). An increase in GDF-15 mRNA expression level has been recorded through malignant progression to glioblastomas (A. Li et al., 2009). The GDF-15 expression levels have shown to be upregulated in glioblastoma cells against cytotoxic stimuli, anti-inflammatory agents, PPARα agonists, and anticancer drugs, such as etoposide, doxorubicin, and bexarotene during chemotherapy treatment (Chiu et al., 2011; Heo et al., 2016; Jeansonne et al., 2013; J. M. Kim et al., 2013; Mimeault & Batra, 2010; Yoshioka et al., 2008). There are different genetic routes of primary and secondary GBMs resulting in glioma (usual phenotypic endpoint) with little overlap (Kleihues et al., 2002). GDF-15 protein expression makes primary and secondary glioblastomas different, with the levels of GDF-15 transcripts in secondary being higher than the primary glioblastoma cells (Strelau et al., 2008). In the primary type, GDF-15 immunoreactivity was particularly found in tumor cells, whereas it was not found in blood vessels and perivascular spaces. On the contrary, there is notable GDF-15 immunoreactivity in the extracellular matrix (ECM) of perivascular areas of two anaplastic astrocytomas (Grade III) and a secondary glioblastoma (Grade IV), while tumor cells and microglia were found negative for GDF-15. Therefore, perhaps GDF-15 of secondary GBMs is almost found in the ECM. Interestingly, there is a correlation between increased stromal storage of GDF-15 in prostate tumors and a higher risk of maintaining disease-free. It is not clear whether the stromal store of proGDF-15 in the ECM is effective in secondary glioblastomas (Strelau et al., 2008). Concerning secondary glioblastoma cell cultures, GDF-15 in all WHO grades has a high expression level; however, it cannot be related to the level of malignancy. Therefore, GDF-15 is not crucial to promote the glioblastoma progress from less to a highly malignant phenotype (Strelau et al., 2008).
| Properties | Findings | References |
|---|---|---|
| Antitumorigenic properties | Inducing apoptosis (induced by HDAC inhibitor) | Yoshioka et al. (2008); Grunstein (1997); Kouzarides (1999) |
| Activating p-53 target genes | Baek et al. (2001); Bootcov et al. (1997); Kannan et al. (2000); P. X. Li et al. (2000) | |
| Lower expression level in GBM cells compared with benign glioma cells and healthy human astrocytes | Kadowaki et al. (2012) | |
| Basal expression and promoter hypermethylation of GDF-15, reversely associating with tumor grading (in response to staurosorine or TSA) | Wang et al. (2013); Kadowaki et al. (2012) | |
| Upregulating in GBM cells, against cytotoxic stimuli; lower level in primary GBM | Chiu et al. (2011); Heo et al. (2016); Jeansonne et al. (2013); J. M. Kim et al. (2013); Mimeault and Batra (2010); Yoshioka et al. (2008). | |
| Tumor suppressor in early stages of tumor in (P53, GSK-3B, and EGR-1 pathways) | Wang et al. (2013) | |
| Protumorigenic properties | Increased GDF-15 in malignant progression to GBM related to shorter survival | Shnaper et al. (2009) |
| Increased in secondary GBM compared to primary GBM (especially in ECM) | Strelau et al. (2008) | |
| Increasing cell migration and invasion | Louca, Gkretsi, et al. (2019) | |
| Inducing tumor formation by autocrine and paracrine regulation of tumor cells | Codó et al. (2016). | |
| Immunosuppressive cytokine that can protect the tumor microenvironment from effector immune cells | Yoshimura and Muto (2011) |
- Abbreviations: ECM, extracellular matrix; EGR-1, early growth response-1; GBM, glioblastoma multiforme; GDF-15, growth differentiation factor 15; GSK-3B, glycogen synthase kinase-3B; HDAC, histone deacetylase; TSA, trichostatin A.
In Baroni et al. (2018) experiment, although there was a specific molecular profile, the altered pathways in the adult GBM (U343 cell line) pediatric cell lines were different from pediatric GBM (KNS42 cell line). According to the cells’ clonogenic activity following GDF-15 silencing and the obtained results, the knockdown reduced colony generation in the KNS42 cells because the genes related to cell viability and survival were downregulated; however, there were no changes in the U343 cells (Baroni et al., 2018). Thus, there are differences in high-grade gliomas between pediatrics and adults. On the basis of collaborative molecular assessments, various chromatin regulation, developmental signaling pathways, and tumorigenesis mechanisms have been noted (Jones & Baker, 2014). It should be avoided to use the data resulted from investigations on adult glioblastomas in therapeutic methods (Baroni et al., 2018). More studies should be done for clarifying the contradictory results on the GDF-15 expression in tumors, specifically in glioblastoma.
Methylating the distinct promoter sequences result in transcriptional silencing of the GDF-15 locus in gliomas, possibly leading to tumor progression. Nonetheless, several tumors and cells have been found with a high expression level of GDF-15. Other tumors have an unknown methylation state, which can because of no CpG island methylation in GDF-15-overexpressing tumors. Future investigations should be carried out for clarifying the potential link to CpG island methylation (Wang et al., 2013). The GDF-15 promoter area was found with poor methylation and hypermethylation in cells with high and low GDF-15 basal expression, respectively. In glioblastoma cells, the basal expression and promoter hypermethylation of GDF-15 is reversely associated with tumor grading, and methylation of some promoter sequences (−53 and +55 CpG sites) causes transcriptional silencing of the GDF-15 locus in glioma and may play an important role in tumor progression (Kadowaki et al., 2012). There is a very low expression level of basal GDF-15 in the tumors, whereas there is a high level of promoter methylation (Kadowaki et al., 2012). Regulation and silencing of the GDF-15 in glioblastomas are done with methylation (Kadowaki et al., 2012). The relationship between hypermethylation and basal GDF-15 expression has not yet been identified in the human tumor specimens. In cells with GDF-15 promoter methylation, clusters of DNA methylation are associated with identified transcription factor-binding sites involved in the basal expression regulation (Kadowaki et al., 2012). A decrease in methylation levels in tumors compared with cell lines indicates diversity in cellularity, the signaling condition in vivo versus in culture. Also, DNA methylation in cells represents an effective higher growth rate in a culture that is not observed in tumors (Kadowaki et al., 2012; Stone et al., 2004). Various transcriptional factors and posttranscriptional methods regulate GDF-15 expression, suggesting a diverse regulation through antitumorigenic compounds. GDF-15 is a prominent downstream target of the p53 (Baek et al., 2002), Egr-137, and AKT/glycogen synthase kinase-3b (GSK-3b) routes (Kadowaki et al., 2012; Yamaguchi et al., 2004).
The mentioned pathways have distinct areas in the −133 to fl55 region of the GDF-15 promoter. Many transcription factors are active on the GDF-15 promoter. The Egr-1 and Sp-1 binding areas are crucial regulation sites to increase GDF-15 expression via COX inhibitors, troglitazone, and trichostatin A (TSA). Besides, they are important to regulate GDF-15 basal expression (Baek & Eling, 2006; Eling et al., 2006; Wang et al., 2011). Egr-1/Sp-1 regions have a high methylation level in glioblastoma cells and primary oligodendroglioma specimens with low GDF-15 basal expression (Kadowaki et al., 2012). A high level of methylation was also reported in the F55 region near the p53 binding site (Kadowaki et al., 2012). Methylation of the Egr-1/SP-1 region can decrease the basal expression of GDF-15 and prevent the increased expression following treatment with staurosporine (SS) or TSA. SS caused an increase in GDF-15 expression in T98G cells characterized by the GDF-15 promoter with poor methylation while treating U-118 cells with a promoter characterized by a high level of methylation caused no increase in GDF-15 expression (Kadowaki et al., 2012). Following removing the methyl group after 5-AZA-dC therapy, SS elevated GDF-15 expression in the U-118 cells (Kadowaki et al., 2012). SS caused an increase in the Egr-1 expression in T98G and U-118 cells, but there is a need for a transcription factor to elevate transcriptional properties of the SS, and the attachment of Egr-1 into the promoter region was stopped on the methylated promoter in U-118 cells approved through the Chip test (Kadowaki et al., 2012). In glioma, silencing the basal expression and blockage of the drug-related expression was done by hypermethylation of the GDF-15 promoter, which indicates that gliomas are resistant against several drug therapies (Kadowaki et al., 2012). More investigations should be done for determining the resistance of GDF-15 transgenic mice against the development of glioblastoma and silencing GDF-15 expression in other cancer types by hypermethylation (Kadowaki et al., 2012).
7 GDF-15 SIGNALING AND ITS TARGETING ON GLIOMA CELLS
GDF-15 is a new candidate for histone deacetylase (HDAC) inhibitors, which is vital to mediate the apoptosis against HDAC inhibitors in these glioblastoma cells (Yoshioka et al., 2008).
HDAC inhibitors impose their anticancer activities through changing histone acetylation, leading to changes in the targeted genes’ transcription/the transcriptional machinery components (Yoshioka et al., 2008). They induce cell differentiation, arrest in the cell cycle, as well as apoptosis in cancer cells (Grunstein, 1997; Kouzarides, 1999). Also, HDAC inhibitors change apoptotic signaling pathways, including Rb (Luo et al., 1998), PTEN (Pan et al., 2007), tumor necrosis factor-α (Y. H. Kim et al., 2004), p21 (Han et al., 2001; Y. K. Kim et al., 2003; Omotehara et al., 2002), and p53 (Condorelli et al., 2008; Habold et al., 2008; Roy & Tenniswood, 2007). Transcriptional and posttranscriptional methods are involved in HDAC inhibitors-associated induction of GDF-15. The effect of HDAC inhibitors on GDF-15 expression may be imposed directly by alterations in histone acetylation (Yoshioka et al., 2008). Sp-1 and Egr-1 are effective to induce GDF-15 expression with HDAC inhibitors. The COX inhibitor, sulindac sulfite, the PPARγ ligand, and troglitazone cause GDF-15 expression in many human cells linked to the Sp-1/Egr-1 region in the GDF-15 promoter (Yoshioka et al., 2008). Nonetheless, such a mechanism needs the MEK1/ERK1/2 pathway (Baek et al., 2004). ERK1/2 pathway is not needed for HDAC inhibitors-associated GDF-15 expression; thus, HDAC inhibitors can indirectly up-regulate GDF-15 expression through changing histone acetylation (Yoshioka et al., 2008). HDAC inhibitors induce the Egr-1 and Sp-1 expression, which then attaches into the SP-1/Egr-1 region in the –133 to +41 bp of the GDF-15 promoter increasing the GDF-15 mRNA and protein expression levels. Besides, HDAC inhibitors cause an elevation in the GDF-15 mRNA stability, leading to increased protein expression. HDAC inhibitors increase histones acetylation and GDF-15 expression that causes alterations in chromatin structure, and consequently, changed gene expression. This is a novel mechanism to regulate GDF-15 (Yoshioka et al., 2008). In GBM (except A172 cells), GDF-15 is less expressed compared with the low-grade gliomas and normal human astrocytes (Yoshioka et al., 2008). Therefore, the GDF-15 gene is silenced by the malignant transformation in malignant brain tumors. Briefly, GDF-15 induction by HDAC inhibitors is linked to HDAC inhibitors-related apoptosis. Such induction includes the transcriptional (by Sp-1 and Egr-1) and posttranscriptional regulations. Effective data were provided to develop optional combinational treatment approaches to be used in malignant brain tumors using HDAC inhibitors (Yoshioka et al., 2008). Also, GDF-15 activates p53 (Baek et al., 2001; Bootcov et al., 1997; Kannan et al., 2000; P. X. Li et al., 2000). It has been shown that GDF-15 has tumor-suppressing activity based on its effectiveness as a p53-target gene and the limited promoter hypermethylation in healthy tissues and cancer (Albertoni et al., 2002; Bauskin et al., 2006; Shnaper et al., 2009). GDF-15 caused p53- or p53-independent growth arrest and apoptosis (Albertoni et al., 2002). P53 mutations have rarely been observed in primary glioblastoma (<10%), whereas they are higher in secondary GBM (>65%) (Strelau et al., 2008). However, GDF-15 expression and p53 activation are poorly correlated (Strelau et al., 2008). The p53 family proteins’ attachment into DNA was defined through the sequence of quarter sites that possibly correspond to binding to the molecules of a p53 tetramer (Cho et al., 1994). Two mismatches are available in the majority of 30 quarter sites of RE2 in the residual resistivity ratio (RRR) stretch and TBP-associated factors (TAFs) in the core sequence that caused a low affinity for p63 and p73, leading to p53-specific activation. Transactivation of the response element is associated with its sequence. Also, p53 produces a tetramer and attaches to two repeats of RRRCWWGYYY at the DNA-binding domain and also to many components of the RNA polymerase II (RNA pol II) complex, likes TAFII31, TAFII70, and TAF1, at the transactivation domain for activating targeted gene transcription (Buschmann et al., 2001; Inga et al., 2002; H. H. Li et al., 2004; Lu & Levine, 1995; Osada et al., 2005, 2007). The p53 tetramer is kinked the DNA by binding to distinct targeted regions causing an alteration in its three-dimensional structure that allows the correlation between p53 and the RNA pol II complex that is needed for the transcription start region (Balagurumoorthy et al., 2002, 1995; Nagaich, Appella, et al., 1997; Nagaich et al., 1999; Nagaich, Zhurkin, et al., 1997; H. Zhou et al., 2001). Accordingly, different factors, such as the binding affinity for response element sequences, are effective in target gene activation of p53 family members (Osada et al., 2007). Activating the p53 pathway dramatically increases the GDF-15 expression; but, p53 undergoes a mutation in nearly 35% of GBM patients (Yin et al., 2005; Zanotto-Filho et al., 2012), which can make glioblastoma cells with p53 mutations resistant against medications associated with p53 activity. MIC-1 expression is subjected to upregulation by anoxia regardless of the p53 state (as shown for p53-null glioblastoma cell LN-Z308) (Albertoni et al., 2002). Upregulation of the GDF-15 protein expression and release in anoxia was detected against oxygen deprivation HIF-1-independently (Albertoni et al., 2002). It has been shown that overexpression did not affect the GDF-15 proliferation level of 3M1, 3M2, and 3M11 cells in vitro, and it was the same as the proliferation level of the parental cell and control pool, however, it showed an increase in vivo (Albertoni et al., 2002). Codó et al. (2016) did not observe the effect of hypoxic conditions on different other glioma cell lines, which indicates that hypoxia cannot be considered as the main driver of GDF-15 expression. NSAIDs increase the expression of GDF-15. There is some evidence that shows the expression of GDF-15 causes apoptosis in many cancer cells in vitro (Baek et al., 2001; Eling et al., 2006; S. H. Lee et al., 2010; Shimizu et al., 2013). The biochemical pathway related to NSAID-caused apoptosis does not need p53 induction and NSAID and p53 regulation of GDF-15 expression are possibly happened by independent strategies (Piazza et al., 1997). The increased GDF-15 expression with NSAIDs demonstrates the apoptotic impacts of NSAIDs independent of COX in cultured cells. GDF-15 overexpression could induce apoptosis in some cell lines of glioblastoma (U87 MG, U118 MG, U251 MG, and T98G), however, it cannot be observed in some cells (like A172 and LN-229 cells) (Z. Zhang et al., 2014). Apoptosis has two pathways: the extrinsic and the intrinsic pathways, started by the attachment of ligand of specific death receptors and started at mitochondria, respectively (Ganguly et al., 2010). Caspase-3 can be activated via upstream effector proteins, such as caspase-8 (extrinsic pathway) and caspase-9 (intrinsic pathway) (Xuejiao et al., 2013). Cleaved caspase-3 and caspase-9 are increased following GDF-15 overexpression, and GDF-15-related apoptosis is stopped via Ac-LEHD-FMK (Z. Zhang et al., 2014). The cytochrome c release, as well as alterations in ΔΨ, are impressive in the intrinsic pathway. Bcl-2 protein family is crucial to regulate the mitochondrial apoptosis pathway (Fesik, 2005). GDF-15 overexpression reduced Bcl-2 expression, enhanced Bax expression, increased cytosolic cytochrome c level, and declined mitochondrial membrane potential (ΔΨ). The mitochondrial apoptosis pathway is associated with GDF-15-caused glioblastoma cell apoptosis (Z. Zhang et al., 2014). There is no clear information regarding distinct receptors activated by the released GDF-15. GDF-15 mediates some cellular reactions by stimulating TGF-β receptors type I and II as well as Smad proteins (Mimeault & Batra, 2010). GDF-15 overexpression activates PI3K/Akt and Smad2/3 signaling pathways in glioblastoma cells and the GDF-15-associated apoptosis is increased by PI3K inhibitors and reduced via small interfering RNAs (siRNAs) to Smad2 and Smad3 (Naghizadeh et al., 2019; Z. Zhang et al., 2014). Therefore, apoptosis resistance occurs by the loss of the Smad3 function (Tarasewicz & Jeruss, 2012). Akt and sequesters unphosphorylated Smad3 are directly interacted (in the cell membrane and cytoplasm), indicating an improvement in survival by Akt kinase-independently (Conery et al., 2004; J. Li et al., 2013). Such interaction increased GDF-15-associated Smad3 phosphorylation levels through PI3K inhibitors in glioblastoma cells. PI3K/Akt and Smad2/3 signaling pathways have opposite impacts in glioblastoma cell apoptosis caused by GDF-15 (Z. Zhang et al., 2014). Also, no GDF-15 inhibitory effect on LN-Z308 cell proliferation can result from the failure in the TGF-β//Smad signaling route (Albertoni et al., 2002). On the contrary, the glioblastoma cell line LN- Z308 ability for forming tumors in nude mice was fully stopped after GDF-15 complementary DNA ectopic expression, whereas maintained in an assay with the vector control. Smad4 as an important factor in the TGF-β/GDF-15 signaling pathway exerts a key antiangiogenic activity (Schwarte-Waldhoff et al., 2000). Therefore, GDF-15, similar to TGF-β indirectly affects the regulation of Smad4 pathway-regulated angiogenesis (Schwarte-Waldhoff et al., 2000). At higher levels, TGF-β prevents angiogenesis via a decrease in the vascular endothelial growth factor receptor-2 (FLK-1) expression in vascular endothelial cells (Mandriota et al., 1996; Pepper et al., 1993). The identification of GDF-15 (an antitumorigenic gene regulated by NSAIDs) can lead to developing novel drugs to treat human cancers (Baek et al., 2001). The p53 areas are crucial to regulate GDF-15 expression and induce GDF-15 expression by food products, such as DADS and resveratrol (Baek et al., 2002; Bottone et al., 2002). Hence, the −128 to −53 site as well as the fl55 area of the GDF-15 promoter possibly silence the tumor suppressor gene in tumorigenesis of glioma (Kadowaki et al., 2012). Furthermore, the proteasome inhibitor MG132 and bortezomib (as a new class of antitumorigenic drugs), can stimulate GDF-15 expression in glioblastoma cells, leading to cell death and prevented cell growth, through the early phase of glioma tumorigenesis like other TGF-β superfamily members (Jennings & Pietenpol, 1998; Shimizu et al., 2013; Zanotto-Filho et al., 2012). Transient induction of p38 MAPK offers a survival signal and its persistent activation can induce cell death (Weng et al., 2005). MG132-induced GDF-15 expression relies on the p38 MAPK pathway that involves transcriptional and posttranscriptional strategies (Shimizu et al., 2013). Posttranscriptional stabilization of GDF-15 mRNA is the main cause to enhance GDF-15 protein expression. It has been suggested that GDF-15 expression forms a new pathway for the anti-glioblastoma effect of proteasome inhibitors (Shimizu et al., 2013). Overall, the GDF-15/p38 MAPK signaling pathway is possibly vital for the MG132 antiglioblastoma effect. MG132-related GDF-15 is crucial caused by stabilizing GDF-15 mRNA concerning the role of proteasome inhibitors in glioblastoma cells. MG132 enhances GDF-15 expression by the posttranscriptional method; therefore, hypermethylated cell lines, like A172 still react to MG132. Glioblastoma with hypermethylation of the GDF-15 promoter is vulnerable to the MG132-regulated GDF-15 induction effects (Shimizu et al., 2013). Nonetheless, there is no study on the transgenic mouse to determine whether GDF-15 suppresses glioblastoma growth, but in some others, it has been shown that GDF-15 possesses a tumor suppressor effect (Baek et al., 2006). Further studies should be conducted for addressing the clear dichotomous role of GDF-15 in tumorigenesis. It can be concluded that being aware of the induction of GDF-15 through the p38 MAPK pathway can result in promising and advanced treatment methods to apply proteasome inhibitors in glioblastoma cells (Shimizu et al., 2013).
Downregulation of GDF-15 is done in aggressive glioma cells (SW1088 and A172) versus nonaggressive ones (H4), with the expression completely different from Ras suppressor-1 (RSU-1) expression in glioma cells. Therefore, GDF-15 increases invasion of the H4 cells and suppresses it in A172 by changing in particularly interesting new cysteine-histidine rich protein (PINCH1), RhoA, and MMP-13 expression, all regulating cell migration, and invasion (Louca, Gkretsi, et al., 2019). RSU-1 suppresses migration and invasion in H4 and enhances them in A172 by regulating the PINCH1, RhoA, and MMP-13 expression (Louca, Gkretsi, et al., 2019). PINCH1 directly interacts with RSU-1 and perhaps, any alteration in RSU-1 expression can affect PINCH1 (Dougherty et al., 2005). GDF-15 silencing in H4 cells with a low level of RSU-1 expression and invasiveness can downregulate RSU-1 with no effect on invasion and gene expression (Louca, Gkretsi, et al., 2019). However, GDF-15 silencing in highly invasive A172 cells reduces RSU-1 expression that is consistent with the cell invasion model, as well as the results obtained after direct RSU-1 silencing (Louca, Gkretsi, et al., 2019). Glioma cell invasion is managed by RSU-1 differently considering the aggressiveness of the cells (Louca, Stylianou, et al., 2019). These cells (H4, SW1088, and A172) increase invasion capacity, whereas simultaneously, have reverse models of RSU-1 and GDF-15 expression (Louca, Gkretsi, et al., 2019). Glioma cells have different behavior based on cell migration and invasion associated with the relative RSU-1 and GDF-15 expression (Louca, Gkretsi, et al., 2019). Besides, PINCH1, RhoA, and MMP13 are critical because of their regulation by the RSU-1/GDF-15 interaction to affect the ultimate invasive phenotype of glioma cells (Louca, Gkretsi, et al., 2019). RSU-1 as a focal adhesion (FA) and an ECM adhesion protein regulates the migratory and invasive effect of glioma cells and is interacted with the LIM5 domain of the PINCH-1 at FA regions (Bokel & Brown, 2002; Donthamsetty et al., 2013; Dougherty et al., 2005; Gkretsi & Stylianopoulos, 2018; Hoffmann & Schwarz, 2013; Izdebska et al., 2018; Louca, Gkretsi, et al., 2019). Cell migration and invasion of glioma cells are regulated by RSU-1 according to their aggressiveness, with RSU-1 to promote an invasive behavior in aggressive cells (A172 and U87-MG) and inhibit them in cells with lower aggressiveness (H4 and SW1088) (Louca, Stylianou, et al., 2019). This suggests the presence of a complex molecular mechanism governing glioma cell invasion in vitro (Louca, Gkretsi, et al., 2019). Also, a signal transducer and activator of transcription 3 (STAT3) inhibitor, BP-1-102, upregulated GDF-15, and fibroblast growth factor 21 (FGF21) mRNA levels in GS6-22 and GS7-2 cells (X. Zhang et al., 2012). Surprisingly, despite the induction of neural genes, like FGF21 and GDF-15 by STAT3 inhibition, Jmjd3 overexpression or STAT3 inhibition can cause differentiation of the glioblastoma stem cells (Sherry et al., 2009).
STAT3 is critical in the proliferation and multipotency of the glioblastoma stem cell (G. H. Li et al., 2010; Sherry et al., 2009). Inhibiting STAT3 can induce H3K27 demethylation, as well as a further expression of target genes linked to GBM-SC neural differentiation (Sherry-Lynes et al., 2017). It preserves healthy neural and glioblastoma stem cells in a proliferative and self-renewing condition by suppressing the histone demethylase Jmjd3 (Sherry-Lynes et al., 2017). STAT3 inhibition was found to be irreversible in GBM-SC (Sherry-Lynes et al., 2017). Isochaihulactone (K8) activates GDF-15 in glioblastoma (Tsai et al., 2017). K8-induced GDF-15 expression caused 8401 cell apoptosis (Tsai et al., 2017). 8401 and G2T cells have a high level of PERK, without DDIT3 expression (Tsai et al., 2017). K8 decreases the glioblastoma cell viability while being applied in tandem with GSK 2606414. Analysis with western blotting indicated that GSK 2606414 suppressed PERK expression and DDIT3 expression was induced by K8 with GSK 2606414 (Tsai et al., 2017). Increased DDIT3 gene expression was associated with a remarkable elevation in GDF-15 gene expression (Tsai et al., 2017). DDIT3 siRNA was applied for inhibiting DDIT3 gene expression that decreased GDF-15 gene expression significantly, by over 40%.
DDIT3 regulates GDF-15 gene expression (Tsai et al., 2017). Anti-GRP 78 drug for blocking the endoplasmic reticulum (ER) stress repair system has been produced that induce cancer apoptosis (Booth et al., 2012; Martin et al., 2013). DDIT3 and GDF-15 were upregulated, resulting in tumor cell apoptosis following K8 therapy (Tsai et al., 2017). DDIT3 regulates GDF-15 expression in the transcription stage; therefore, DDIT3 can change GDF-15 mRNA expression and its stability (Tsai et al., 2017). The ER stress caused the DDIT3-regulated cytosolic translocation of human antigen R (HuR), as well as forming stress granules. The HuR was effective in GDF-15 gene regulation through the stabilization of the GDF-15 mRNA in ER stress. The GDF-15 expression is regulated in different stages, such as transcriptional and posttranscriptional stages (S. H. Park et al., 2012). In the cytoskeleton, K8 suppressed tubulin polymerization and the related pattern; however, the molecular mechanism has not yet been clearly recognized (Chen et al., 2006). The DDIT3-related apoptosis by the autophagy pathway has been noted recently (B'Chir et al., 2014). Besides, DDIT3 expression could affect p21, cyclin-dependent kinase (CDK1), and CDK2 (Zu et al., 2006).
According to the cancer genome atlas (TCGA) data analysis, GDF-15 expression is linked to PD-L1 in TCGA GBMs. Also, GDF-15 significantly elevated PD-L1 expression in U87, U251, and SHG44 GBM cells, whereas GDF-15 knockdown decreased PD-L1 expression in A172 and GIC6 GBM cells. Accordingly, GDF-15 is a new regulator for PD-L1 in GBM. Malignant glioma is promoted by selecting tumor cells with a high PD-L1 level, facilitating immune evasion. Similarly, an increase in PD-L1 expression level is remarkably associated with a weak prognosis of GBM (Baral et al., 2014; Y. Liu et al., 2013). Thus, the PD-1/PD-L1 signaling pathway can be targeted and is useful in immunotherapy to enhance antitumor immunity and improve the prognosis for GBM cases (Payandeh et al., 2020).
Nonetheless, there is no data on PD-L1 expression regulation by GDF-15. GDF-15 may enhance PD-L1 expression by Smad2/3 signaling in GBM cells, as Smad2/3 inhibition was effective to reduce PD-L1 expression through GDF-15 in U87 and U251 cells. Smad2/3 expression knockdown reduced GDF-15-induced PD-L1 levels in U87 and U251 cell lines. Accordingly, PD-L1 expression is partly managed via the GDF-15/Smad2/3 pathway in GBMs. Interferon (IFN)-associated signaling pathway enhanced PD-L1 expression in glioma (Qian et al., 2018; Silginer et al., 2017). Nevertheless, there is no correlation between IFN-γ and GDF-15 expression; thus, GDF-15 affects PD-L1 regardless of IFN signaling in GBM. Therefore, GDF-15 and PD-L1-mediated immune evasion of GBM cells are possibly involved in the GBM tumor progression. In conclusion, GDF-15 regulates PD-L1 expression by activating the Smad2/3 signaling pathway in GBM, which indicates the potential effectiveness of the blockage of the GDF-15/PD-L1 pathway to treat malignant GBM (Peng et al., 2019; Sadreddini et al., 2019).
8 GDF-15 IN IMMUNE-EVASION OF GLIOBLASTOMA
GDF-15 is involved in the malignant phenotype of glioblastoma (Albertoni et al., 2002; Codó et al., 2016; Roth et al., 2010; Shnaper et al., 2009). GDF-15 is effective in tumor formation by autocrine regulation of tumor cell development in GDF-15 sensitive cells (HCT-116 cells), as well as the paracrine mechanism of action on the host cells using cells unresponsive to GDF-15 (Albertoni et al., 2002).
An increase in the cancer cells’ invasion because of GDF-15 is observed in gastric cancers (D. H. Lee et al., 2003).
There are some variations between glioblastomas and their healthy counterparts’ white matter non-neoplastic brain tissues. GDF-15 showed remarkable diversities between glioblastoma and non-neoplastic brain tissue, which is associated with the tumorigenic process (Scrideli et al., 2008). Upregulation of GDF-15 is linked to the abrogation of PTEN, induction of EGFR in primary glioblastoma, and mutation of TP53, which is seen in secondary glioblastoma (Behin et al., 2003; J. S. Kim et al., 2007; Kleihues et al., 2002; Kraemer et al., 2006; Osada et al., 2007). GDF-15 is involved in cell tumor mechanisms, and also GDF-15 is correlated with chemoresistance (Corre et al., 2012; Huang et al., 2007; Mimeault et al., 2013; Proutski et al., 2009; Whiteside et al., 2004). However, Codó et al. (2016) showed no alteration in glioma cells’ vulnerability against the alkylating agent or irradiation. In addition, GDF-15 overexpression was correlated with radioresistant subclones of nasopharyngeal cancer cells (Chang et al., 2007).
GDF-15 has a tumor inhibitory effect (Kadowaki et al., 2012). It has been shown that GDF-15 suppresses tumor development in nude mice in xenograft models (Albertoni et al., 2002). GDF-15 expression shows downregulation in over 90% of the tumors. Nonetheless, hypermethylation at the −118 to −53 sites has not been noted in all human glioblastoma specimens (Kadowaki et al., 2012; Stone et al., 2004). GDF-15 has a lower expression level in glioblastoma cells compared with benign glioma cells and healthy human astrocytes (Kadowaki et al., 2012). GDF-15 expression causes glioblastoma cell death and suppresses glioblastoma cell development on soft agar (Kadowaki et al., 2012; Yoshioka et al., 2008). GDF-15 overexpression in human glioblastoma LN-Z308 cells prevents tumor development in nude mice in xenograft models (Albertoni et al., 2002). On the contrary, GDF-15 enhances the proliferation of glioblastoma cells in murine SMA-560 glioblastoma cells, and GDF-15 depletion can delay these cells’ development in syngeneic mice (Roth et al., 2010). There is a relationship between increased levels of GDF-15 in CSF of patients and GBM and short-term survival (Shnaper et al., 2009). The increased GDF-15 levels more reflect the late-stage tumor growth rather than being a force for tumor growth (Wang et al., 2011). Therefore, GDF-15 possibly is a tumor suppressor in the early phases of tumor growth (Wang et al., 2013). GDF-15 expression is upregulated through different tumor suppressor pathways, such as p53, GSK-3b, and EGR-1 (Wang et al., 2013).
Cells with stemness properties derived from different solid tumors, melanoma, and GBM have been shown to release GDF-15 as an immunosuppressive cytokine that can protect the tumor microenvironment from effector immune cells (Yoshimura & Muto, 2011). According to Roth et al. (2010), GDF-15 inhibition in a mouse glioma cell promoted a decrease in cell proliferation and an elevation in the immune reaction. The proliferation of glioma cells resulted from the deletion of GDF-15 was reversed by adding recombinant GDF-15 and by FCS, which indicated that in a complete medium, the absence of GDF-15 can be counterbalanced via a multitude of growth factors (Roth et al., 2010). However, immunologic investigations clearly noted a new and still unidentified effect of GDF-15 to act as a glioma-derived immunosuppressive molecule (Roth et al., 2010). The depletion of GDF-15 in glioma cells showed a higher susceptibility to the natural killer cells’ lytic activity.
The improved immune cell activation without GDF-15 has been approved by an elevation in lymphocyte-derived interleukin (IL)-2 and a reduction in IL-10 levels in coculture research. Therefore, anti-GDF-15 methods may in part relieve glioma-related immunosuppression and cause effective antitumor reactions in vitro (Roth et al., 2010). In syngeneic mice, subcutaneous-injected SMA-560 control cells showed a faster growth compared with SMA-560 shGDF-15 transfectants. In this regard, mice with intracranial tumors were found with longer survival derived from shGDF-15 cells. GDF-15 ectopic overexpression results in unphysiologically high cytokine concentrations affecting angiogenesis (Ferrari et al., 2005). The hypothesized immunomodulatory effect of GDF-15 can be confirmed when GDF-15-depleted tumors have higher infiltration with T cells and macrophages. Infiltration with regulatory T cells or the existence of tumor-associated macrophages has a relationship with no favorable outcome in other tumors (Curiel, 2008; Lewis & Pollard, 2006; Roth et al., 2010). Nonetheless, regarding brain tumors, regulatory T cells are prognostically neutral within the glioblastomas (Heimberger et al., 2008). Also, there is no association between the total tumor-infiltrating macrophages numbers and prognosis (Okada et al., 2009), while a (rare) severe lymphocytic infiltration is associated with remarkably longer survival (Palma et al., 1978). The T-cell infiltration magnitude has an inverse correlation with intratumoral TGF-β2 levels, as well as a positive correlation with clinical outcome (Liau et al., 2005) that is consistent with our results regarding the divergent TGF-β superfamily member GDF-15. The death of mice with GDF-15-depleted tumors has been reported suggesting the lack of GDF-15 compensated by other immune-inhibitory strategies, like TGF-β. Besides, there may be a selection process for glioma cell clones with a lower rate of significant GDF-15 expression knockdown; however, this hypothesis has not yet been tested. Overall GDF-15 provides the immune advantage to malignant gliomas, being responsible for the malignant phenotype of these cells, and consequently representing a new and potential target for future therapeutic methods (Roth et al., 2010).
Glioma cancer stem cells (gCSCs) are responsible to convert monocytes to an immunosuppressive macrophages/microglia (M2) phenotype using several factors (Wu et al., 2010). They release such factors and the gCSC-conditioned medium can elevate monocyte migration, which indicates that the gCSCs use monocytes into the tumor microenvironment (Wu et al., 2010). In Wei et al. (2010) study, they generated a high level of GDF-15 that was lost after differentiation. Also, GDF-15 induces STAT3 in neurons and the GDF-15 imposes immunosuppressive activities on macrophages (Bootcov et al., 1997; Johnen et al., 2007). GDF-15 inhibits phagocytosis and upregulates IL-10 and TGF-β1. Nonetheless, GDF-15 cannot significantly enhance phospho-STAT3 (p-STAT3) in macrophages and down-modulate the costimulatory molecules or cause the macrophage's capability for inhibiting T-cell proliferation (Wu et al., 2010). Physiological doses of GDF-15 induce IL-10 and TGF-β1, but induction of these molecules in lower doses of GDF-15 indicates that GDF-15 is not the only factor for M2 polarization. Accordingly, a complex interaction is available between the gCSCs and macrophages involving STAT3-dependent and STAT3-independent processes. Overall, there is not a certain association between GDF-15 and other gCSC-secreted compounds that control the M2 polarization, but p-STAT3 alone is not the only contributor in gCSC-mediated immune suppression, whereas p-STAT3-independent methods of immune suppression are also involved. For instance, GDF-15-mediated suppression of macrophages is not fully involved in p-STAT3 activation. Thus, other main transcriptional hubs, such as p-STAT3 playing a role to mediate different downstream immune-modulatory pathways should be identified for properly formulating therapeutic immune methods (Wu et al., 2010). GDF-15 is a crucial immunosuppressive cytokine in gliomas and other pathological situations (Kempf et al., 2011; Roth et al., 2010; Wu et al., 2010; Z. Zhou et al., 2013). GDF-15 pathways in GBM cells are shown in Figure 1.
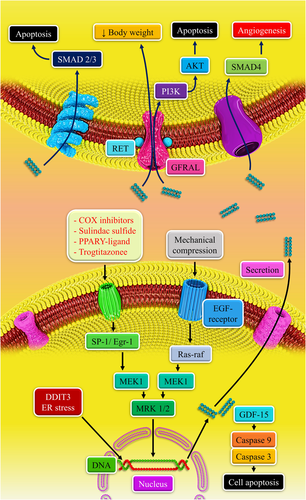
9 GDF-15 AND MIGRATION PROMOTION AND INVASION OF GLIOMA CELLS
Codó et al. (2016) have demonstrated that GDF-15 is involved in the control of cell migration and invasiveness. Promoting glioma cells’ migration and invasion using the autocrine GDF-15 signaling pathway is involved in the malignant phenotype of these tumors (Codó et al., 2016). GDF-15 expression is done differently in the proneural, neural, classical, and mesenchymal subtypes (Codó et al., 2016; Verhaak et al., 2010). The highest GDF-15 concentrations have been identified in the mesenchymal subgroup that possibly reacts well against different immunotherapeutic methods under development (Codó et al., 2016; Doucette et al., 2013; Weiss et al., 2015). In Codó et al. (2016) study, patients with tumors with the lowest GDF-15 level, regardless of their subtype classification and expression levels, had prolonged survival. Assessing different immunotherapeutic strategies that are currently under investigation in GBM cases indicated that the autocrine and paracrine activities of GDF-15 are a promising target for novel therapeutic approaches (Codó et al., 2016).
Mechanical forces with development in the tumor microenvironment and matrix hardness have been found as biomechanical dysfunctions that occurred in the tumor microenvironment and can influence tumor progression (Gilkes & Wirtz, 2017; Kalli & Stylianopoulos, 2018; Nia et al., 2016). Mechanical compression using for brain cancer cells is capable of activating the MEK1/Erk1 pathway by activating EGFR/Ras/Raf, as upstream of MEK1. MEK1/Erk1 can then regulate many migration-associated genes’ expression, such as GDF-15 for reorganizing the actin cytoskeleton with disrupted compression that finally can facilitate cell migration. GDF-15 binds to its receptor via a negative feedback loop, suppressing MEK1/Erk1 activation for regulating its levels in compressed cells. Mechanical compression that is less effective in the highly aggressive cells results in a potential tumor-suppressing pathway for preventing the compression-related MEK1/ERK1-mediated migratory effect. There are several unanswered questions on the whole mechanism associated with compression-related brain tumor progression; for example, if Erk1 is capable of directly regulating GDF-15 expression, how precisely the small GTPases can be regulated using compressive stress, and the exact GDF-15 function in brain cancer cell migration and reorganization cytoskeleton of undergoing compression, because first research that connected compression-related migratory profile of brain cancer cells to MEK1/Erk1 activation, small GTPases, and GDF-15 expression regulation, described these factors as possible targets to be used in future antimetastatic therapeutic strategies for treating brain tumors (Kalli et al., 2019).
10 CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Preclinical data indicate that GDF-15 is a therapeutic target for glioblastoma. Although the efficacy and safety of GDF-15 in humans remain unclear, understanding the biological function and physiological processes of GDF-15 to develop and progress the cancer is a key subject to elucidate its beneficial effects. In addition, receptor binding alteration and assessing downstream signaling pathways might be determined in the early and late stages of cancer. However, additional investigations are necessary to find complications of GDF-15 on GBM and confirm its therapeutic efficacy and safety. Considering the controversial evidence of GDF-15 in tumorigenesis is needed, as well.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Masoud Nouri-Vaskeh, Behzad Baradaran, and Mohammad Zarei devised the main conceptual ideas. Zahra H. Segherlou, Masoud Nouri-Vaskeh, and Sama N. Guilandehi wrote the initial draft of the manuscript. Amir Baghbanzadeh prepared the figure. Behzad Baradaran, Mohammad Zarei, Ramin Zand, and Amir Baghbanzadeh reviewed the manuscript and edited it critically for important intellectual content. Behzad Baradaran supervised the study. All authors read and approved the final version of the manuscript.




