The direct and indirect regulation of follicular T helper cell differentiation in inflammation and cancer
Yejin Cao, Lin Dong, Ying He, and Xuelian Hu contributed equally to this work as co-first author.
Abstract
Follicular T helper (Tfh) cells play important roles in facilitating B-cell differentiation and inducing the antibody response in humoral immunity and immune-associated inflammatory diseases, including infections, autoimmune diseases, and cancers. However, Tfh cell differentiation is mainly achieved through self-directed differentiation regulation and the indirect regulation mechanism of antigen-presenting cells (APCs). During the direct intrinsic differentiation of naïve CD4+ T cells into Tfh cells, Bcl-6, as the characteristic transcription factor, plays the core role of transcriptional regulation. APCs indirectly drive Tfh cell differentiation mainly by changing cytokine secretion mechanisms. Altered metabolic signaling is also critically involved in Tfh cell differentiation. This review summarizes the recent progress in understanding the direct and indirect regulatory signals and metabolic mechanisms of Tfh cell differentiation and function in immune-associated diseases.
1 INTRODUCTION
Follicular T helper (Tfh) cells were initially described as a subset of CD4+ T cells expressing high levels of CXCR5 in human tonsils (Breitfeld et al., 2000; C. H. Kim et al., 2001; Schaerli et al., 2000). Tonsils have large and active germinal centers (GCs). GCs are histologically distinct structures within B-cell zones of secondary lymphoid tissues. B cells accomplish hypermutation and selection, class switch recombination (CSR), plasma cell differentiation, and memory B cell differentiation in GCs. Therefore, the formation of GCs is crucial to humoral immunity (Bannard & Cyster, 2017; Victora & Nussenzweig, 2012). Many studies have shown that the formation of GCs depends on CD4+ T cells and CD4+ T cells that highly express CXCR5 migrate to GCs (Crotty, 2011). In subsequent studies, the Tfh cell subset was considered to be a subset of CD4+ T cells, such as Th1, Th2, and Th17 cells. Bcl-6 is regarded as a lineage-defining transcription factor of Tfh (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009).
The differentiation of Tfh cells is a complex multistep process. Naïve CD4+ T cells undergo fate selection under the influence of various factors and finally differentiate into Tfh or non-Tfh cells. The differentiation of Tfh cells requires the action of antigen-presenting cells (APCs). Dendritic cells (DCs) play important roles in the early stage of Tfh cell differentiation. Naïve CD4+ T cells primed by DCs are first differentiated into pre-Tfh cells and migrate to the T-B boundary. At the T-B boundary, pre-Tfh cells interact with activated B cells and further mature into GC-Tfh cells and induce the maturation and differentiation of GC-B cells (Figure 1).
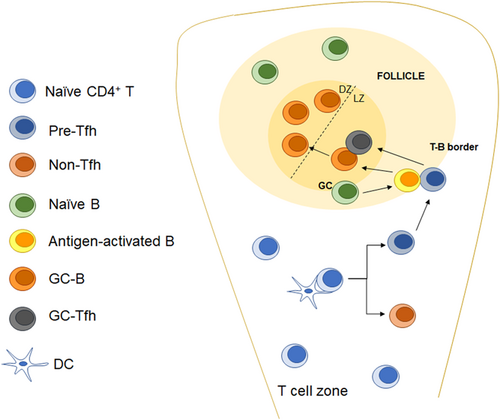
In recent years, the important roles of Tfh cells in humoral immune regulation have been extensively studied. Infectious diseases, autoimmune diseases, and other diseases closely related to humoral immunity have been shown to be related to Tfh cells. There have been studies showing that even Tfh is associated with cancer. Therefore, research on Tfh cells is also very important for the treatment of human diseases. In this review, we mainly summarize the recent progress in understanding the direct and indirect regulatory signals and metabolic mechanisms of Tfh cell differentiation and function in immune-associated inflammation.
2 TFH CELL DIFFERENTIATION AND FUNCTION IN HUMORAL IMMUNITY-MEDIATED DISEASES
2.1 Discovery of a helper T-cell subset that facilitates B cell differentiation and function
Miller et al. implied that thymocytes play irreplaceable roles in humoral immunity based on thymectomy performed 50 years ago. They found that the thymectomy of mice completely abrogated antibody responses to immunization with sheep red blood cells (Miller, 1965). They used cell transfer experiments to prove that the antibody response of B cells requires T-cell function (Miller & Mitchell, 1968; Mitchell & Miller, 1968; Nossal et al., 1968). With the discovery of the in vitro ability of interleukin-4 (IL-4) to increase the number of B cells (Howard et al., 1982) and the development of a Th1–Th2 helper T cell subset typing system (Mosmann et al., 1986), people simply inferred that Th2 is the CD4+ T cell subset that helps B-cell action. The role of CD40 and CD40 ligands in the process of T cells helping B cells suggests that the help of T cells to B cells is contact-dependent (Armitage et al., 1992; Y. J. Liu et al., 1989). Later, in vivo experiments also proved the importance of IL-4 and IL-21 in the B-cell response (Ozaki et al., 2002). However, experiments proved that the deletion of Th2-associated genes did not lead to defects in the GC in vivo (Kopf et al., 1995). Therefore, people were curious about the subset of helper T cells that help B cells.
Tfh cells were initially described as a subset of CD4+ T cells expressing high levels of CXCR5 in human tonsils (Breitfeld et al., 2000; C. H. Kim et al., 2001; Schaerli et al., 2000). Transcriptional repressor B-cell lymphoma 6 (Bcl-6) was identified as a lineage-defining transcription factor in Tfh cells. In contrast to Th2, which regulates IL-4 secretion through the characteristic transcription factor Gata3 (Liang et al., 2011), Tfh is regulated by SAP and protein kinase Cθ (PKCθ). In contrast to other helper T-cell subsets, Tfh cells reside in immune tissues such as lymph nodes (LNs) and interact with B cells to facilitate the development and function of B cells. The function of Tfh cells indicates that they have a transcription profile that differs from that of other helper T cell subsets. Therefore, during the development of naïve CD4+ T cells to Tfh cells, early cell fate determination for Tfh cells and non-Tfh cells is necessary and requires APC action.
2.2 The functions of Tfh cells in humoral immunity
Tfh cells play vital roles in humoral immunity as helper T cells that help B cells differentiate. The pre-Tfh cells differentiate from activated CD4+ T cells and migrate toward B-cell follicles and engage in GC reactions. The interactions between Tfh and B cells in the GC are the central aspects of humoral immunity regulation. The formation and maturation of GC-B cells, plasma cells, and memory B cells require the help of Tfh cells (Figure 2).
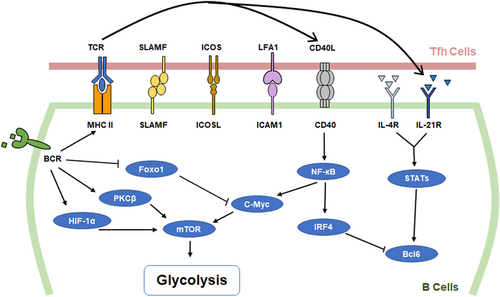
2.2.1 Tfh cells promote GC-B cell formation
Antigen-specific B cells expand and upregulate major histocompatibility complex class II proteins (MHC II) and costimulatory molecules, such as CD80, CD86, and ICOSL. The cognate interaction between activated B cells and Tfh cells migrating to the T-B boundary promotes the formation of GCs. Both B-cell mutations and antibody affinity maturation occur in GCs. However, the combination of an antigen and a BCR activates the metabolic activity of B cells, which is the first signal of GC formation. Without the second signals provided by Tfh cells, the activated B cells undergo rapid apoptosis, progressively losing mitochondrial function and glycolytic capacity (Akkaya et al., 2018). B cells compete for Tfh cells based on the amount of antigen-MHC II presented by the B cell. According to the research of Yeh et al. (2018) B cells with different capacities to express MHC II were used, and the results showed that MHC II + / + B cells are preferentially recruited to early GCs; however, under the condition of physiological antigen concentration, the competitive ability of B cells with MHC II haploinsufficiency in GCs is not affected. This result indicates that B-cell competition for Tfh help mainly occurs at the T-B boundary, not in GCs.
In GCs, B cells interact with follicular DCs (FDCs) and GC-Tfh cells in the light zone (LZ) to complete selection and undergo proliferation and mutation in the dark zone (DZ). FDCs play important roles in the maintenance of GCs. GC-B cells can also obtain complete antigens from FDCs through BCR-mediated endocytosis, and the antigens are processed and presented in the form of p:MHC II complexes (Heesters et al., 2013; X. Wang et al., 2011). With the combination of a transgenic strategy and a photoactivatable fluorescent reporter, Alexander et al. showed that cell division and the hypermutation of GC-B cells are proportional to the amount of antigen-presenting cells to follicular helper T cells in the LZ (Gitlin et al., 2014). GC-Tfh cells select high-affinity GC-B cells based on the expression of p:MHC II, and high-affinity GC-B cells migrate to the DZ with the help of Tfh cells and undergo proliferation and mutation in the DZ. Tfh cells facilitate B-cell proliferation and differentiation through CD40L signaling and IL-21 secretion (S. K. Lee et al., 2011). Luo et al. (2018) demonstrated that CD40 and BCR signals induced the transcription factor Myc through NF-κB and Foxo1, respectively. According to recent research, Myc mediates GC-B cell division. Conditional Myc haploinsufficiency or overexpression affects the division of B cells in the DZ. Myc protein content is proportional to the size and division capacity of GC-B cells (Finkin et al., 2019). In addition, when Tfh cells are completely differentiated into GC-Tfh cells, IL-4 is secreted, thereby maximally supporting GC-B cell differentiation (Weinstein et al., 2016). Thus, Tfh cells are required for the formation of GCs and the mutation of GC-B cells.
2.2.2 Tfh cells promote plasma cell and memory B-cell formation
In addition to their role in the selection of high-affinity B cells, of GC-Tfh cells also regulate the differentiation of GC-B cells into plasma cells (PCs) and memory B cells. IL-21 produced by GC-Tfh cells and IL-6 produced by stromal cells induce early GC-derived plasmablasts (Zhang et al., 2018). GC-B cells need to downregulate Bcl-6, upregulate IRF4, and express Blimp1 during differentiation into PCs (Ise et al., 2018). GC-Tfh cells transiently induce LZ GC-B cells to express SLAM (CD150), ICAM-1, and CD40, prolonging the interaction of Tfh cells and GC-B cells. In addition, this process promotes the expression of LFA-1 and CD40L on the surface of GC-Tfh cells (D. Liu et al., 2015; Meli et al., 2016). The extension of GC-Tfh cell:GC-B cell synaptic engagement ensures the multistep transcriptional reprogramming process.
Memory B cells (MBCs) are also important outputs of the GC response. Suan et al. (2017) reported that CCR6 uniquely marks MBC precursors. The transcriptional repressor Bach2 also plays an essential role in the differentiation of MBCs. Haploinsufficiency of Bach2 leads to the reduced generation of MBCs (Shinnakasu et al., 2016). When GC-B cells receive moderate help from GC-Tfh cells, GC-B cells tend to differentiate into MBCs. In addition, studies have proven that IL-9 derived from Tfh cells can also promote the development of MBCs (Y. Wang et al., 2017). Interestingly, IL-9 can also regulate humoral recall responses when memory Tfh cells and memory B cells rapidly interact upon re-exposure to the antigen (Takatsuka et al., 2018). These results show that Tfh cells facilitate long-term humoral immunity by inducing the outputs of PCs and MBCs.
2.3 Other follicular T cells
2.3.1 Follicular regulatory T (Tfr) cells
Tfr cells constitute a subset of follicular-resident T cells with functions similar to those of Tregs (Table 1). They can negatively regulate GC responses by directly suppressing Tfh and GC B-cell responses (Sage et al., 2016). Similar that of to Tfh cells, the differentiation of Tfr cells is composed of two phases: conventional Tregs primed by DCs migrate to follicles. TCR signals and costimulatory molecules, including CD28 and ICOS, play indispensable roles in the differentiation of Tfr cells. Coinhibitory molecules, including PD-1 and CTLA-4, negatively regulate the differentiation of Tfr cells (Sage & Sharpe, 2016). In addition to expressing Treg characteristic molecules, such as Foxp3, CD25, and CTLA4, differentiated Tfr cells also show the transcription characteristics of Tfh (Chung et al., 2011; Linterman et al., 2011; Sage & Sharpe, 2015).
| Cell subsets | Source | Flow cytometry detection | Characteristic molecule | Function |
|---|---|---|---|---|
| Tfh | Naïve CD4+ T Cells | CD4+CXCR5+Foxp3− | CXCR5 Bcl-6 PD-1 ICOS IL-21 IL-4 | Promotes GC formation and B-cell functions |
| Tfr | Tregs | CD4+CXCR5+Foxp3+ | CXCR5 Bcl-6 PD-1 ICOS Foxp3 CD25 CTLA4 | Negatively regulates the GC reaction |
| Tfc | Naïve CD8+ T Cells | CD8+CXCR5+ | CXCR5 Bcl-6 ICOS Tcf7 CD62L IL-7R | Resistant to chronic viral infection, related to CD8+ T-cell memory |
- Abbreviations: GC, germinal center; Tfh, follicular T helper.
Recently, to study the regulatory role of Tfr in the immune process, Rachel et al. constructed a Tfr cell-specific deletion mouse model. Tfr cells were induced to be deleted at different time points after immunization. Rachel et al. found that Tfr cells played only a regulatory role in the formation of early GCs. After the formation of GCs, the deletion of Tfr cells had little effect on the GC response of mice (Clement et al., 2019). The Tfr cell-specific deletion mouse model also demonstrated that Tfr cells control IgG and IgE responses to vaccines, allergens, and autoantigens. The Tfr cell:Tfh cell ratio represents the extent to which Tfr cells control GC reactions. Anatomical location, the nature of the antigen and the strength of the inflammation response can all affect the Tfr cell:Tfh cell ratio. The data showed that in MC38 tumors, the Tfr cell:Tfh cell ratio remained high. However, in influenza infection or lymphocytic choriomeningitis virus (LCMV) infection, the Tfr cell:Tfh cell ratio was substantially lower. The percentage of Tfr cells in Peyer's patches (PPs) that are continuously exposed to an antigen environment was also lower (Sage & Sharpe, 2016). These findings reflect the relationship between Tfr cells and the GC reactions.
2.3.2 Follicular cytotoxic CD8+ T cells
CXCR5+CD8+ T cells were first recorded in human tonsils (Quigley et al., 2007). This unique subset of CD8+ T cells was proven to have specialized functions during chronic viral infections and was termed follicular cytotoxic T (Tfc) cells (Table 1). In mice that were chronically infected with LCMV and patients with HIV, He et al. observed the virus-specific CXCR5+CD8+ T-cell subset. Moreover, in adoptive transfer experiments, the CXCR5+CD8+ T-cell subset showed greater therapeutic potential than the CXCR5− T-cell subset (R. He et al., 2016). Transcription profile analysis revealed that that the Tfc cell subsets showed a unique gene signature that was related to that of Tfh cells and CD8+ T-cell memory precursors. The expression of molecules typical of Tfh cells, such as ICOS, Bcl-6, transcription factor 7 (Tcf7), and pleiomorphic adenoma genelike 1 (Plagl1), is upregulated. In addition, Tfc cells express higher levels of genes associated with memory cells, including CD62L and IL-7R (Im et al., 2016). These data suggested that Tfc cells may function as memory stem cells during chronic infection. A recent study indicated that Tcf-1, Id2, and Bcl-6 could all affect the formation of Tfc cells (Y. Wang et al., 2019). However, the mechanism by which these transcription factors integrate and regulate Tfc cell differentiation needs further study.
2.3.3 Circulating Tfh subsets
In addition to GCs, CXCR5+CD4+ T-cell subsets have also been found in human blood. These CXCR5+CD4+ T cells contain long-lived memory cells with similar functional properties to Tfh and are considered as circulating Tfh cells (cTfh cells; Schmitt et al., 2014; Ueno, 2016). The cTfh subsets express environment-specific transcription factors in different kinds of immune responses, such as T-bet, Gata3, and RORγt (Huang et al., 2019). The combination of CXCR3 preferentially expressed by Th1 cells and CCR6 preferentially expressed by Th17 cells defines cTfh1, cTfh2, and cTfh17 within cTfh cells. The Th1-like cTfh1 cells express T-bet and secrete cytokines IFN-γ; similarly, cTfh2 cells express Gata3, and produce IL-4, IL-5, and IL-13; cTfh17 cells express the transcription factor RORγt, and produce IL-17A, IL-17F, and IL-22 (Ueno, 2016). Interestingly, a recent study reported that not limited to human blood, Tfh1 (CXCR3+ CCR6−), Tfh2 (CXCR3− CCR6−), and Tfh17 (CXCR3− CCR6+) were also found in the spleen of SLE in Est1 −/− mice (C. J. Kim et al., 2018). These results suggest that Tfh subsets play distinct roles in different immune-associated diseases.
3 DIRECT REGULATION OF TFH CELL DIFFERENTIATION AND FUNCTION
Compared with helper T cells in other subsets, Tfh cells have unique transcriptional regulation. Various cytokines and costimulatory molecules can promote or inhibit the differentiation of Tfh cells by regulating the expression of transcription factors. The differentiation and function of Tfh cells are directly regulated by these molecules. Many transcription factors play important roles in the process of Tfh cell differentiation and directly affect the fate decision and function of Tfh cells (Figure 3).
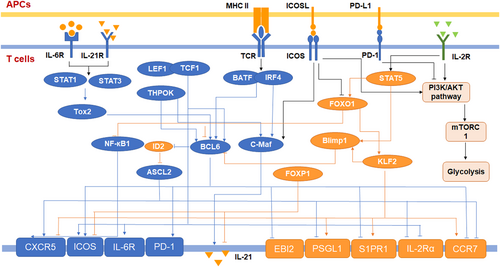
3.1 The repressor-of-repressor circuits of Tfh cell transcriptional regulation
There are multiple transcription factors that can positively regulate a series of key targets and play important roles in the development of Tfh cells. Bcl-6 is the defining transcription factor of Tfh cells and plays a vital role in the process of Tfh cell differentiation. Many studies have shown that Bcl-6 is an obligate repressor of gene expression (Béguelin et al., 2016; Hatzi et al., 2015). Through ChIP-seq, Katerina et al. found that Bcl-6 in Tfh cells can target the promoters and enhancers of many genes, including B-lymphocyte-induced maturation protein 1 (Blimp1), Krueppel-like factor 2 (KLF2), Epstein Barr virus inducible receptor 2 (EBI2), CC-chemokine receptor 7 (CCR7), CCR6, sphingosine-1-phosphate receptor 1 (S1PR1), and P-selectin glycoprotein ligand 1 (PSGL1). Moreover, the AP1 motif is highly enriched at the Bcl-6 binding site, which implies that Bcl-6, as a transcription repressor gene, can subvert the function of AP1 (Hatzi et al., 2015). Interestingly, Bcl-6 can also promote the expression of some Tfh cell effector molecules, such as PD-1, CXCR5, and CXCR4 (Y. S. Choi et al., 2011; Yu et al., 2009). Thus, J. Choi et al. proposed a repressor-of-repressor regulatory circuit for Bcl-6. They used an experimental method that integrated RNA-seq, CHIP-seq, and ATAC-seq to prove that Bcl-6 can negatively regulate the transcription factors of Tfh cell-related genes. According to the RNA-seq data, the researchers divided the differentially expressed genes of Tfh cell subsets into four clusters. Then, they performed CHIP-seq and ATAC-seq on the two clusters representing genes directly inhibited by Bcl-6 (Bcl6-r genes) and Tfh cell-specific genes directly inhibited by Bcl-6 (Bcl6-rr genes). These results showed Bcl-6 binding sites on the negatively regulated transcription factors of Tfh cell-related genes. In vivo experiments have also proven that in the absence of Bcl-6, inhibiting Bcl6-r genes, such as Gata3, positive regulatory domain 1 (Prdm1), inhibitor of DNA binding 2 (Id2), and KLF2 leads to the upregulated expression of Bcl6-rr genes, such as CXCR5, ICOS, and IL-21 (J. Choi et al., 2020). These data indicated that Bcl-6 is the nexus transcription factor of Tfh cells.
3.2 The molecular biology and immune metabolism regulation of Tfh cell differentiation
Many available data indicate that the expression of Bcl-6 is regulated by multiple pathways. Tfh-inducing cytokine IL-6 and IL-21-mediated Bcl-6 upregulation depend on signal transducer and activator of transcription 1 (STAT1) and STAT3 signaling (Y. S. Choi et al., 2013; Nurieva et al., 2008). In humans, STAT3 responds to IL-12 to enhance the development of Tfh cells (Ma et al., 2012). Wei Xu et al. demonstrated that STAT3 responds to IL-6 and that IL-12 induces the transcription factor TOX high mobility group box family member 2 (Tox2). Tox2 can regulate the expression of Bcl-6 by regulating chromatin accessibility, and Tox2 −/− mice exhibit defective Tfh cell differentiation (Xu et al., 2019). In addition, Lan He et al. proved that IL-6 and IL-21 induce secreted extracellular matrix protein 1 (ECM1), which upregulates Bcl-6 expression (L. He et al., 2018). Recent research has shown that deficiency of the H3K36me2 methyltransferase Nsd2 in T cells leads to decreased Bcl-6 expression and impaired Tfh cell generation. CD28 activation quickly induces the expression of Nsd2 (Long et al., 2020). In addition, the transcription factors BATF (basic leucine zipper ATF-like transcription factor), IRF4 (interferon regulatory factor 4), TCF-1, LEF-1 (lymphoid enhancer-binding factor 1), and Thpok (T-helper-inducing POZ/Krueppel-like factor) play essential roles in the regulation of Bcl-6 (Y. S. Choi et al., 2015; Vacchio et al., 2019; Vinuesa et al., 2016).
In addition to Bcl-6, other transcription factors also play an important role in the process of Tfh cell differentiation. c-Maf responds to ICOS signaling and induces IL-21 (Bauquet et al., 2009). Both BATF and Thpok promote the expression of c-Maf (Betz et al., 2010; Vacchio et al., 2019). The expression of the Tfh cell effector molecule CXCR5, which is related to cell migration, depends on E protein transcription factors, such as E2A and Ascl2 (achaete-scute family BHLH transcription factor 2; X. Liu et al., 2014). Interestingly, Shaw et al. demonstrated that E protein activity is inhibited by Id2, and depletion of Id2 via RNA-mediated interference increased the frequency of Tfh cells. They also found that Bcl-6 bound the Id2 locus and inhibited Id2 (Shaw et al., 2016).
Metabolic regulation also plays an important role in the differentiation process of Tfh cells. Activated naïve T cells increase glycolysis through metabolic reprogramming (Frauwirth et al., 2002). However, compared with Th1 cells, Tfh cell subsets exhibit less proliferation and glycolysis. IL-2 signaling activates the PI3K-AKT-mTORC1 axis to promote glycolysis and negatively regulate Tfh cell differentiation in the context of LCMV infection (Ray et al., 2015). The characteristic transcription factor Bcl-6 and the highly expressed molecule PD-1 in Tfh cell subsets have also been shown to inhibit cellular glycolysis (Oestreich et al., 2014; Patsoukis et al., 2015). Interestingly, many studies have proven that mTORC1 and mTORC2 signaling are required for Tfh cell generation and GC reactions (Yang et al., 2016; Zeng et al., 2016). Deficiency of mTORC1 and mTORC2 in T cells has a significant impact on the expression of signature genes in Tfh cells, and increased mTOR activity promotes the Tfh cell response (Zeng et al., 2016). Moreover, mTORC1 activation and the overexpression of glucose transporter type 1 (Glut1) have been linked to the expansion of autoreactive Tfh cells (Yang et al., 2016; Yi et al., 2017). These results may imply that proper mTOR activity and glycolysis can mediate the proper level of Tfh cell proliferation and adaptation to the GC environment. On the contrary, Choi et al. proved that the inhibition of glucose metabolism reduces the autoimmune Tfh subsets in lupus-prone mice but has no effects on the immunization-induced Tfh subsets. In contrast, glutaminolysis inhibition reduces both Tfh subsets. The altered expressions of solute transporters between autoimmune and immunization-induced Tfh subsets imply that these two Tfh subsets have different requirements for glucose metabolism (S. C. Choi et al., 2018). Deletion of cytochrome c oxidase, a critical regulator of oxidative phosphorylation (OXPHOS), also affect the GCs formation and antibody response in the immunized mice. However, no significant differences were observed in the number of Tfh subsets between COX dysfunction and wild-type (WT) mice. These results suggest the regulatory effects of metabolism on Tfh function (Tarasenko et al., 2017).
3.3 Negative regulation of Tfh cell differentiation
Tfh cell development depends on a balance between multiple transcription factors that either negatively or positively regulate a set of key targets. Therefore, a number of transcription factors oppose the actions promoting Tfh cell differentiation.
Foxo1 binds to a region close to the transcription start site of Bcl-6 and negatively regulates the expression of Bcl-6. During early Tfh cell differentiation, ICOS signaling inactivates Foxo1. Interestingly, Foxo1 −/− T cells have a reduced ability to differentiate into GC-Tfh cells, indicating that Foxo1 is essential for the final differentiation to GC Tfh cells (GC-Tfh; Stone et al., 2015). Blimp-1 and Bcl-6 are antagonistic regulators of Tfh cell differentiation (Johnston et al., 2009). IL-2 can induce Blimp-1 through STAT5 signaling, thereby negatively regulating Tfh cell differentiation (DiToro et al., 2018; Johnston et al., 2012). According to L. He et al., an ECM1 induced by IL-6 and IL-21 not only upregulates Bcl-6 but also inhibits Blimp-1 by downregulating the level of STAT5 phosphorylation (L. He et al., 2018). KLF2 prevents Tfh cell development by upregulating Blimp-1 and downregulating Bcl-6 (J. Y. Lee et al., 2015). The expression of molecules CXCR5 and CCR7, which are related to cell migration, is also regulated by KLF2 (Weber et al., 2015). Recent research proved that protein kinase D2 (Prkd2) binds to Bcl-6 and phosphorylates Bcl-6 to constrain it to the cytoplasm, thereby limiting Tfh cell development (Misawa et al., 2020). In addition, forkhead box P1 (Foxp1) is a critical negative regulator of Tfh cell differentiation that represses the expression of ICOS and IL-21 (B. Shi et al., 2018; H. Wang et al., 2014).
4 THE INDIRECT REGULATION OF TFH CELL DIFFERENTIATION AND FUNCTION
Tfh cells are the outcomes of a cell fate determination in which CD4+ T cells can differentiate into Tfh or non-Tfh cells. The differentiation of CD4+ T cells into Tfh cells requires the help of APCs, including DCs and B cells. In the early stages of Tfh cell differentiation, DCs play a major role due to the limited number and localization of cells. B cells are required for the late differentiation and GC-Tfh cell maturation stages (Crotty, 2019). APCs promote the differentiation of Tfh cells through the antigen-MHC II complex, costimulatory molecules, and cytokines (Zou et al., 2018).
4.1 DCs initiate the early differentiation of Tfh cells
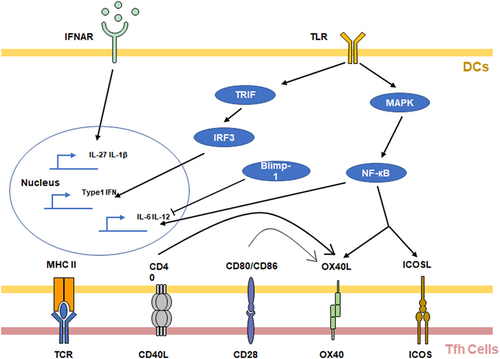
DCs are the most important APCs to promote the early differentiation of Tfh cells. Many studies have shown that TCR affinity and the interaction time between the TCR and antigen-MHC II complex signaling affects the differentiation of Tfh cells and IL-21 secretion (Benson et al., 2015; Tam et al., 2016; Tubo et al., 2013). These results suggested that different DC subsets have different abilities to induce Tfh cell differentiation, and DC subsets stably expressing the antigen-MHC II complex have more influence in this regard.
In addition to expressing the antigen-MHC II complex, DCs also promote Tfh cell differentiation through a variety of costimulatory molecules. ICOS signaling plays a major role in the priming of Tfh cell differentiation. Choi et al. showed that Icos −/− T cells could not undergo early Tfh cell development. However, the authors demonstrated that the loss of B cells has no effect on the early development of Tfh cells. These results indicate that the ICOSL signaling of DCs is very important for the early development of Tfh cells (Y. S. Choi et al., 2011). In addition, DCs induce Tfh cell differentiation through OX40L signaling. Blocking OX40L leads to a significant reduction in Tfh cell and GC-B cell frequencies. Tahiliani et al. (2017) also observed the colocalization of OX40L-expressing DCs and OX40+ T cells. Similarly, Pattarini et al. (2017) demonstrated that blocking OX40L in TSLP-activated human DCs reduced IL-21 and CXCL13 production in CXCR5+ PD-1+ Tfh cells in vitro. CD80/CD86-CD28 signaling and CD40-CD40L signaling are also required for the initiation of early differentiation of Tfh cells by DCs. These pathways can promote the differentiation of Tfh cells by upregulating the expression of OX40L on DCs and OX40 on T cells (Fillatreau & Gray, 2003; Linterman et al., 2014; Watanabe et al., 2017). Watanabe et al. (2017) used cre-mediated CD80/CD86 conditional knockout mice to prove that antigen-specific T-cell activation and Tfh cell development require B7 expression on DCs. Fillatreau et al. reported that Cd40 −/− mice have impaired T-cell accumulation in follicles. The upregulation of OX40L and CXCR5 are CD40-dependent events (Fillatreau & Gray, 2003).
DCs can also affect the microenvironment by secreting cytokines, thereby regulating the development of Tfh cells. In mice, IL-6 plays an important role in the early stage of Tfh differentiation. Previous studies have shown that Blimp-1 −/− DCs show upregulation of IL-6 expression in vitro and preferentially induce Tfh cell differentiation (S. J. Kim et al., 2011). In addition, Kim et al. also found that Prdm1 −/− DCs affect not only IL-6 secretion but also antigen processing, thereby inducing IL-21 secretion by cocultured T cells. The increased cathepsin S (Ctss) expression in DCs from DC-specific conditional Prdm1 −/− mice altered the presentation of antigen to CD4+ T cells (S. J. Kim et al., 2017). In contrast to murine DCs, IL-12 secreted by human DCs plays a critical role in human Tfh cell differentiation (Hercor et al., 2017; Schmitt et al., 2009). In contrast to that of murine DCs, IL-12 secreted by human DCs plays a critical role in human Tfh cell differentiation (Hercor et al., 2017; Schmitt et al., 2009). Type I IFN is also required by DCs to prime Tfh cell differentiation. Helena and colleagues demonstrated that interferon-alpha/beta receptor deficiency (Ifnar −/−) in DCs results in reduced IL-6 production and reduced frequencies of Tfh cells (Cucak et al., 2009). There is also evidence showing that IFNAR signaling in human DCs can drive Tfh cell differentiation by promoting IL-27 production (Gringhuis et al., 2014). Recently, Batten et al. indicated that IFNAR signals of DCs promoted Caspase 1- and Caspase 11-mediated production of IL-1β, in turn, driving the expression of BCL6, CXCR5, and ICOS in T cells (Barbet et al., 2018).
DCs recognize antigens through TLR signaling and activate downstream pathways to promote CD4+ T-cell differentiation. TLR agonists have been widely used to induce Tfh cell responses (Krishnaswamy et al., 2018; Ugolini et al., 2018). Many studies have shown that antigen-activated TLR signaling can induce DCs to secrete cytokines that promote Tfh cell differentiation through the NF-κB pathway, including IL-6 and IL-12 (Ballesteros-Tato & Randall, 2014; Fitzgerald & Kagan, 2020; Ugolini et al., 2018). In DCs, the NF-κB pathway can also upregulate the expression of ICOSL and OX40L (Shin et al., 2015). In addition, TLR signaling induces autocrine Type I IFN via a TRIF-dependent mechanism (Barbet et al., 2018; Cucak et al., 2009; Fitzgerald & Kagan, 2020). These studies indicate that DCs regulate the early differentiation of Tfh cells through TCR signaling, costimulatory molecules, and cytokines (Figure 4).
4.2 B cells as APCs facilitate Tfh cell differentiation
DC priming is not sufficient for Tfh cell differentiation. B cells play important roles as APCs in late Tfh differentiation and GC-Tfh cell maturation. However, B cells do not provide signals different from those of DCs for the development of Tfh cells. In the experiments performed by Deenick et al. (2010) although preventing T-B cell interaction in vivo impaired Tfh cell differentiation, augmenting the antigen presentation by non-B cells reversed this change. Additionally, Hong et al. (2018) used RNA phage Qβ-derived VLPs (Qβ-VLPs) as model antigens and proved that B cells are sufficient, as APCs, to prime Tfh cell differentiation in the absence of DCs. Thus, due to the location and number of B cells, DCs play a major role in the early differentiation of Tfh cells (Crotty, 2019). With the help of DCs, CD4+ T cells differentiate into pre-Tfh cells, express CXCR5 and other Tfh-related genes, and migrate to B-cell follicles. At the T-B cell border, activated B cells aggregate and interact with pre-Tfh cells, becoming the main APCs at this stage.
The interaction between T cells and B cells occurs in the GC. The reactions that take place in the GC are energetically demanding. However, the GC microenvironment is hypoxic (Cho et al., 2016). The metabolic reprogramming of Tfh cells has been mentioned above, and the metabolic regulation of GC-B cells cannot be ignored. GC-B cells show increased glycolysis (Figure 2). Evidence shows that the expression of glycolytic enzymes and glucose transporters Glut1 proportionally increased with increasing hypoxia-inducible factor 1 subunit alpha (HIF-1α) and c-Myc levels in activated B cells (Caro-Maldonado et al., 2014; Cho et al., 2016; Jellusova et al., 2017; Luo et al., 2018). mTORC1 is required for the metabolic reprogramming of GC-B cells. Blocking mTORC1 leads to the prevention of clonal expansion (Ersching et al., 2017). Protein kinase Cβ (PKCβ) plays an essential role in BCR-induced glycolysis in B cells. Using pharmacological inhibition and mice deficient in PKCβ, Blair et al. (2012) demonstrated that PKCβ could promote glycolytic rates with the engagement of B9CR. PKCβ-null (Prkcb −/−) B cells exhibited mTORC1 signaling and led to the impairment of metabolic reprogramming and mitochondrial remodeling (Tsui et al., 2018). BCR and CD40 signals can also regulate mTORC1 activity and cell metabolism by inducing the transcription factor c-Myc (Luo et al., 2018). In addition, Mendoza et al. (2018) showed that the Ras-related guanosine triphosphate hydrolase (GTPase) R-Ras2 can regulate the activation of the PI3K-Akt-mTORC1 pathway as an effector of both B-cell receptor (BCR) and CD40.
5 TFH CELL DIFFERENTIATION AND FUNCTION IN DISEASES
Tfh cells play important roles in the formation of GCs and the antibody response of B cells. Tfh cells are required for humoral immunity. Thus, abnormalities in Tfh cells are associated with many diseases (Table 2).
| Type of disease | Disease name | Tfh-related mechanisms |
|---|---|---|
| Infectious diseases | Recurrent tonsillitis (RT) | Streptococcus pyogenes infection results in an aberrant population of GC-Tfh cells expressing granzyme B and killing B cells |
| Acquired immune deficiency syndrome (AIDS) | HIV or SIV infection causes abnormal expression of CXCR3 in Tfh cells, which affects the location and function of Tfh cells | |
| Malaria | Malaria (caused by parasitic infection) can target DC-T-cell interactions, thereby affecting the normal differentiation of Tfh cells | |
| Autoimmune diseases | Systemic lupus erythematosus (SLE) | The abnormal Tfh cell subset, Tfh2 cells (Bcl6 + GATA3hi), mediates greatly exacerbated IgE synthesis |
| Rheumatoid arthritis (RA) | ELS formation is mediated by CXCL13+ CD4+ T cells | |
| Organ transplant diseases | Graft versus host disease (GVHD) | Alloantibodies secretion is driven by alloantigen-specific Tfh cells |
| Cancer | Breast cancer | Tfh cells induce the formation of ELSs at tumor sites and participate in the antitumor response. |
| Colorectal cancer |
5.1 Tfh cells in infectious diseases
The main function of Tfh cells is to prevent infectious diseases by regulating humoral immunity. Tfh cells facilitate Ab responses to infections. Many studies have proven that Tfh cells play important roles in the control of chronic infectious diseases and the clearance of viruses. (Greczmiel et al., 2017; Ryg-Cornejo et al., 2016). In the course of chronic infectious diseases, CD8+ T cells undergo exhaustion and CD4+ T cells tend to differentiate into Tfh cell subsets. By conditionally knocking out CXCR5 +/+ Tfh cells, Greczmiel et al. (2017) demonstrated that Tfh cells are essential for the production of late LCMV-specific neutralizing antibodies and the complete elimination of the virus.
Therefore, in many infectious diseases, pathogens cause diseases by targeting the normal differentiation of Tfh cells. Recurrent tonsillitis (RT) caused by Streptococcus pyogenes is a classic childhood disease. Comparing RT and non-RT tonsils from two independent cohorts, Jennifer et al. found that RT tonsils had smaller GCs, and antigen-specific GC-Tfh cells were underrepresented. The GC-Tfh cell population that expresses granzyme B and killing B cells were also aberrant in the GCs of the RT tonsils (Dan et al., 2019).
In studies focusing on HIV and SIV, abnormalities of Tfh cells have also been performed. A CXCR3+ GC-Tfh cell population was discovered in SIV-infected monkeys (Velu et al., 2016). In these SIV-infected monkeys, the proportion of CXCR3+ GC-Tfh cell subgroups in lymph nodes was increased significantly. The CXCR3+ GC-Tfh cell subsets not only expressed high levels of Tfh cell-related molecules such as ICOS and IL-21 but also expressed high levels of characteristic Th1 cell factors, such as IFNγ and T-bet. These results indicate that chronic SIV infection promotes the expansion of Th1 cell-biased GC Tfh cells (Velu et al., 2016). Ryg-Cornejo et al. also demonstrated that CXCR3+ Tfh cells are poor helpers to B cells in a mouse malaria model (Ryg-Cornejo et al., 2016). The expression of CXCR3 may impair the help that Tfh cells render to B cells by affecting the location of the GC-Tfh cells in follicles. PD-1 can inhibit the expression of CXCR3 in Tfh cells (J. Shi et al., 2018).
Studies on malaria have shown that parasitic infections can target DC-T-cell interactions, thereby affecting the normal differentiation of Tfh cells (Osii et al., 2020). Millington et al. found that DCs isolated from a mouse model at the initial stage of infection showed upregulation of CD40, CD80, and CD86. However, compared with the initial period (4 days after Plasmodium chabaudi infection), the level of antigen-specific IgG produced by mice during convalescence (days 12 and 21 postinfection) was significantly reduced. The DCs isolated from these mice did not upregulate costimulatory molecules (Millington et al., 2006). In addition, although hemozoin-treated DCs retained their capacity to process antigens, they were unable to form stable clusters with naïve T cells. This inability led to the generation of dysfunctional T cells that failed to migrate to B-cell follicles or produce effectors. The development and function of B cells are also affected by dysfunctional T cells (Millington et al., 2007).
However, in human infectious diseases, some mechanisms are different from those in animal models. This remains to be further investigated (Schmitt et al., 2016). In addition, Tfh cells and intestinal microbiota also affect each other. Tfh cells can generate high-affinity IgA responses in GC-rich PPs against pathobionts (Proietti et al., 2014). Gut microbiota also affects the response of Tfh cells by changing the secretion of cytokines. Segmented filamentous bacteria (SFB) can promote the effect of Tfh cells by reducing the IL-2 response (Teng et al., 2016).
5.2 Tfh cells in autoimmune diseases
Most autoimmune diseases are closely related to autoreactive antibodies. Therefore, the Tfh cell subset, which plays an important role in the humoral immune response, is considered to be associated with the occurrence of autoimmune diseases. Many studies have shown that Tfh cells play crucial roles in the disease progression of systemic lupus erythematosus (SLE; Gensous et al., 2018). Accumulation of Tfh cells was found in both a mouse SLE model and SLE patients (J. He et al., 2013; Linterman et al., 2009). Kim et al. proved that mice with Ets1-deficient Tfh cells develop spontaneous SLE. Ets1 inhibits signature Tfh and Th2 cell genes. Ets1-deficient Tfh cells coexpress a Th2 cell-associated gene and become Tfh2 cells (Bcl6+GATA3hi). Tfh2 cells mediate greatly exacerbated IgE synthesis, resulting in SLE (C. J. Kim et al., 2018). Low-dose IL-2, an inhibitor of Tfh cell, is a therapeutic treatment for SLE (J. He et al., 2016). In addition, IL-21 and OX40 molecules associated with Tfh cell differentiation are also proven to be related to SLE (J. Y. Choi et al., 2017; Jacquemin et al., 2015). These results further proved the roles of Tfh cells in SLE. Chang et al. (2011) found an ectopic lymphatic structure (ELS) in lupus nephritis patients.
Rheumatoid arthritis (RA) is another common autoimmune disease. A large number of studies on RA patients show that ELS is closely related to RA (Bombardieri et al., 2017). Evidence shows that ELS is associated with CD4+ T cells expressing CXCL13 (Manzo et al., 2008). In human lymphoid tissues, CXCL13 is mainly produced by GC-Tfh cells. CXCL13 can recruit B cells and promote the formation of GCs (Havenar-Daughton et al., 2016). However, CD4+ T cells expressing CXCL13 in RA express low levels of CXCR5 and Bcl-6 (Manzo et al., 2008). Nevertheless, these CD4+ T cells still express Tfh cell-related molecules, including IL-21, ICOS, and MAF, to help B cells, thereby promoting the formation of ELS (Rao et al., 2017). However, the relationship between CXCL13+ CD4+ T cells and Tfh cells needs further study.
5.3 Tfh cells in organ transplant rejection
Alloantibodies are associated with organ transplant rejection. GC alloantibody responses are mediated exclusively by Tfh cells (Conlon et al., 2012). Chenouard et al. (2017) indicated that Tfh cells in transplanted kidneys are associated with acute and chronic human kidney transplant rejection. IL-21 secreted by Tfh plays an important role in plasma cell differentiation and immunoglobulin production in renal transplant rejection patients (de Leur et al., 2017). In the allogeneic HSC transplant murine model and mouse skin GVHD disease model, disease development was associated with Tfh cells. Blocking CXCR5, CD40L, ICOS, or IL-21 can prevent the occurrence of diseases (Flynn et al., 2014; Taylor et al., 2018). The roles of Tfh in transplant rejection have also been demonstrated in the studies of liver transplant patients and heart transplant mouse models (Conlon et al., 2012; Crotty, 2019).
At present, immunosuppressive therapies targeting effector T lymphocytes cannot inhibit the differentiation of Tfh subgroups and even cause Tfh activation (Knechtle et al., 2009; Macedo et al., 2019). Therefore, new therapies that target Tfh to suppress humoral immunity after transplantation need to be studied. In vitro experiments and clinical results show that tacrolimus can partially inhibit Tfh function and keep the Tfh level of transplant patients stable (Cano-Romero et al., 2019; de Graav et al., 2017). In nonhuman primate kidney transplantation models, the CTLA4-Ig fusion protein has been shown to reduce the amount of Tfh in GC and the production of IL-21 (E. J. Kim et al., 2014). In the mouse model of heart transplantation, CTLA4-Ig also showed the effects of reversing the alloantibody responses (Young et al., 2016).
5.4 Tfh cells in cancer
Although the main function of Tfh cells is to regulate humoral immunity, some studies have reported a relationship between Tfh cells and cancer. Tfh cells may facilitate or support the development of ectopic lymphoid structures (ELSs), recruiting CD8+ T cells, macrophages, and natural killing (NK) cells that engage in antitumor immunity. Studying breast cancer, Gu-Trantien et al. found that CXCL13-expressing CD4+ T cells express multiple genes characteristic of GC-Tfh cells, including Bcl-6, CD200, PD1, and ICOS. CXCL13-expressing CD4+ T cells are correlated with ELSs (Gu-Trantien et al., 2017). Similar results were also found in colorectal cancer (Becht et al., 2016). On the contrary, Tfh cells may support antitumor Ab responses by B cells (Garaud et al., 2018). Considering the roles of follicular T cells in the antitumor process, the potential implications of PD1 and PDL1 immunotherapies on Tfh and Tfc cells have been noted. However, there are a few related studies, and further investigation is needed.
6 CONCLUDING REMARKS
In recent years, both broad and deep advances have been made in the research of Tfh cells. Tfh cells are demonstrated to contribute to both humoral immunity and immune-related diseases. Although the transcriptional regulation of Tfh cell differentiation has been intensively studied, the mechanism by which DCs and B cells regulate their own interactions with Tfh cells needs further study. Recent studies have also revealed the existence of different subsets of Tfh cells and found that they play unique roles in different disease processes. The function of other follicular T cells, such as Tfr and Tfc, during the immune process and the mutual regulatory effects with Tfh subgroups also need to be revealed in further studies. In addition, a large number of studies on Tfh cells and diseases are based on animal models. Many studies have shown that the regulatory mechanisms of Tfh cell differentiation and function are different in human and animal models. Some clinical research results related to Tfh cells and disease pathology are difficult to establish in animal models. In view of the close relationship between Tfh and humoral immunity, Tfh can be used as an important therapeutic target in autoimmune diseases, transplant rejection, and other humoral immunity-related diseases. Research on the relationship between the direct and indirect mechanisms of Tfh development and the various subgroups of follicular T cells will provide innovative and important ideas for the diagnosis and treatment of these diseases.
ACKNOWLEDGMENTS
The authors’ research is supported by grants from the National Natural Science Foundation for Key Programs of China (31730024, G.L.), National Natural Science Foundation for General Programs of China (31671524, G.L.), and Beijing Municipal Natural Science Foundation of China (5202013, GL).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Consulted the references: Yejin Cao, Lin Dong, Ying He, and Xuelian Hu. Participated in discussions: Yueru Hou, Yingjie Dong, and Qiuli Yang. Contributed to writing of the manuscript and participated in discussions: Yejin Cao, Yujing Bi, and Guangwei Liu.




