What makes leader cells arise: Intrinsic properties and support from neighboring cells
Abstract
Cancer cells collectively invading as a cohesive and polarized group is termed collective invasion, which is a fundamental property of many types of cancers. In this multicellular unit, cancer cells are heterogeneous, consisting of two morphologically and functionally distinct subpopulations, leader cells and follower cells. Leader cells at the invasive front are responsible for exploring the microenvironment, paving the way, and transmitting information to follower cells. Here, in this review, we will describe the important role of leader cells in collective invasion and the emerging underlying mechanisms of leader cell formation including intrinsic properties and the support from neighboring cells. It will help us to elucidate the essence of collective invasion and provide new anticancer therapeutic clues.
1 INTRODUCTION
It has long been observed that cancer cells can invade collectively by maintaining intercellular coordination and establishing multicellular structures, which is termed collective invasion. Collective invasion can be observed in many types of cancers, particularly epithelial cancers including pancreatic cancer, breast cancer, colon cancer, and lung cancer (Beerling, Oosterom, Voest, Lolkema, & van Rheenen, 2016; Sonoshita et al., 2015; X. Wang, Enomoto, Asai, Kato, & Takahashi, 2016). The collective invasion has been shown to require cancer cells to retain cell–cell adhesion and communication, accomplish group polarization and invade as one multicellular unit rather than spreading out as a single cell (Friedl, Locker, Sahai, & Segall, 2012). However, the specific principles controlling collective invasion are much more intricate and remain unclear.
Collectively, invading cancer cells consist of two subpopulations, leader cells at the invasive front and follower cells at the rear (Friedl et al., 2012). Leader cells are conferred with an elongated morphology with actin-based protrusive structures extending into the surrounding tissues after undergoing the supracellular cytoskeletal polarization along the direction of invasion (Caussinus, Colombelli, & Affolter, 2008). During the collective invasion, leader cells are responsible for exploring the microenvironment, determining the direction, generating traction force, paving the path of invasion, and transmitting information to follower cells. Follower cells are the majority of the multicellular unit and are essential for leader cells to obtain and consolidate leadership by steering leader cells polarization and influencing leader cells behavior (Mayor & Etienne-Manneville, 2016). When collectively invading along a two-dimensional (2D) substrate, cancer cells can move as cohesive sheets toward the same direction (Chapnick & Liu, 2014). Monolayered cancer cells display high junction stability established by E-cadherin- and β-catenin-based adherens junctions, apical desmosomal junctions, and tight junctions (Takeichi, 2014). These stable cell–cell junctions jointly mediate multicellular integrity and the apicobasal polarity of leader and follower cells. All cancer cells within the group are faced with the extracellular matrix (ECM) to exert traction force to initiate collective sheet movement (Chapnick & Liu, 2014). When moving in 3D tissues cancer cells tend to invade collectively as linear, branched, or network-like strands (Wolf et al., 2007). The cell–cell junctions are more flexible and complex and are mainly mediated by E-cadherin- and N-cadherin-based adherens junctions (Chapnick & Liu, 2014; Shamir & Ewald, 2015). Leader cells at the invasive front are faced with ECM and various types of extracellular stimulation to initiate protrusion formation and exert traction force with follower cells surrounded by other cells and not contacting ECM (Friedl et al., 2012). Compared with collective invasion along the 2D substrate, collective invasion through the 3D tissues means more complicated microenvironment and interaction between cancer cells and that leader cells need to acquire specific phenotype and functions to tackle with more obstacles from the microenvironment and cancer cell groups.
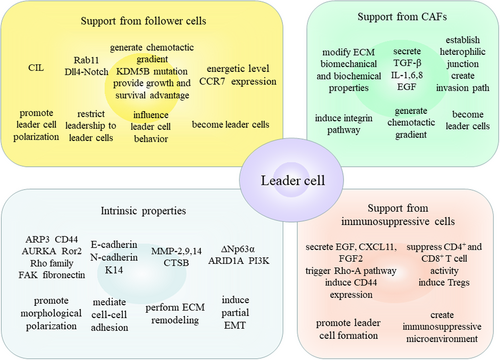
Here, we briefly review the emerging evidence investigating the underlying mechanisms of leader cell formation including intrinsic properties and the support from the neighboring cells and analyze how theses mechanisms coordinate to steer cancer cells to acquire morphological and functional phenotype of leader cells, exert and consolidate leadership to promote persistent collective invasion (Figure 1).
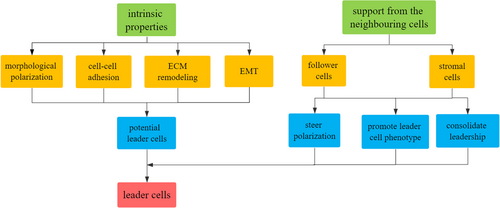
2 INTRINSIC PROPERTIES
Intratumor phenotypic heterogeneity is generally observed in cancer cell populations, which is generated by genomic heterogeneity and epigenetic modifications (Jacoby, Duncavage, & Walter, 2015; McGranahan & Swanton, 2015). The genetic heterogeneity between leader and follower cells includes the distinct gene expression profiles in leader cells that can provoke cytoskeletal rearrangement, structural reorganization, and morphological polarization (Carey, Starchenko, McGregor, & Reinhart-King, 2013; Mayor & Etienne-Manneville, 2016). Recently, substantial studies have revealed plenty of genes differentially expressed between leader and follower cells and the genes significantly expressed in leader cells are mainly involved in morphological polarization, cell–cell adhesion, extracellular matrix remodeling, and epithelial to mesenchymal transition (EMT), which altogether define the characteristics of leader cells.
2.1 Promoting morphological polarization
Morphologically, leader cells show an elongated morphology and display dynamic actin-based protrusive structures including filopodia and lamellipodia (S. Etienne-Manneville, 2004; Friedl et al., 2012). Actin-related protein 3 (ACTR3) mutation was one of the leader cell-enriched mutations leading to the overexpression of actin-related protein 3 (ARP3) that can facilitate invadopodia formation and lamellipodia protrusion (Goley & Welch, 2006). In non–small cell lung cancers, tumor spheroids consisting of unmodified cancer cells and cancer cells with ARP3 overexpression showed a prominent increase in invasive area and the number of invasive chains and cancer cells overexpressing ARP3 were found to be leader cells (Zoeller et al., 2019). During the invasion of the wounded epithelium, CD44 was expressed significantly higher in the epithelial cells at the invasive front than that in the bulk of epithelial cell clusters and was associated with the invasive properties of leader cells (Bleaken, Menko, & Walker, 2016). In breast cancer, CD44hi leader cells displayed a concomitant forward-moving lamellipodia at the leading edge with higher proliferation, invasiveness, and migration rate (Yang et al., 2019). AURKA, one of Aurora kinase family members, was related to leader cells’ actin rearrangement and the subsequent formation of filopodia and lamellipodia at the leading edge by mediating the phosphorylation of extracellular regulated kinase (ERK). The spatial expression of ERK at the leading edge is important to guide the collective migration of epithelial sheets (Chapnick & Liu, 2014; Choi & Helfman, 2014). Intraflagellar transport protein 20 (IFT20) expression induced by the orphan receptor tyrosine kinase-like receptor 2 (Ror2) signaling pathway is necessary for the polarized and continuous microtubules growth in cancer cells to induce the polarized leader cells morphology and collective invasion. Inhibiting either Ror2 or IFT20 expression drastically impaired cancer cells collective invasion in colorectal cancer (Aoki et al., 2019; Yadav, Puri, & Linstedt, 2009).
Rho family of small GTPases plays a predominant role in cell migration as the central regulator of the cytoskeleton and is crucial for the extension of protrusions in leader cells (Reffay et al., 2014; Ridley, 2015; Wakatsuki, Wysolmerski, & Elson, 2003). However, recent studies argued that in different tumor types, Rho signaling may exert contradictory effect on leader cells behavior via different downstream effectors. Dia1, one of Rho-GTPases effector proteins, was necessary for leader cells to form and stabilize protrusions and mediate the maturation process of focal adhesions. Knocking down Dia1 prevented the stabilization of protrusion followed by decreased collagen deformation and traction force, inhibiting leader cells formation and collective invasion in epithelial tissues (Fessenden et al., 2018). In contrast, targeting the rho pathway by inhibiting rho-associated coiled-coil containing protein kinase 1 (ROCK1) and ROCK2 activity in colorectal adenocarcinomas led to the formation of actin-rich protrusions at the leading edge. Inhibition of these two kinases activity triggered the supracellular polarization leading to the appearance of protrusive leader cells and was sufficient to initiate collective invasion. Further study revealed that in colorectal adenocarcinomas, ROCK2 inhibition triggered the protrusion formation through a dual mechanism including the activation of Rac family small GTPase 1 (RAC1) and the reduction of Myosin II to generate protrusive forces (Libanje et al., 2019).
The mature focal adhesions are the sites of biochemical signaling and mechanical links between cancer cells and ECM to promote filopodia and lamellipodia formation, matrix deposition, and traction force generation (Mayor & Etienne-Manneville, 2016). Focal adhesion kinase (FAK) and fibronectin were increased in leader cells to promote the maturation of focal adhesions while the inhibition of FAK or fibronectin could dampen collective invasion in colorectal cancer (Baker, Bird, Lang, Cox, & Erler, 2013). In lung adenocarcinoma, liver kinase B1 (LKB1) is a negative regulator of FAK and LKB1 deficiency in leader cells led to the accumulation and stabilization of FAK in a highly spatiotemporal behavior, leading to increased collagen alignment surrounding leader cells while the activation of LKB1 resulted in single-cell invasion (Gilbert-Ross et al., 2017).
2.2 Mediating cell–cell adhesion
At the invasive front, leader cells are linked to other cells through adhesive structures including adherens junctions by their tight association with the actin cytoskeleton (Harris & Tepass, 2010). Cadherin-mediated interactions orchestrated by both E-cadherin and N-cadherin can contribute to the polarization of leader cells along the appropriate direction and the persistent directed movement of the cell groups (Sandrine Etienne-Manneville, 2012). In the lack of adherens junctions, integrins along the entire cell periphery are consecutively engaged with the ECM, leading to focal adhesions and protrusions forming in random directions, significantly reducing the persistence of migration (Camand, Peglion, Osmani, Sanson, & Etienne-Manneville, 2012). Focal adhesions and protrusion can be restricted to the cell front to generate traction force along the direction of migration significantly increasing the efficiency of collective invasion because of adherens junctions located at the rear of leader cells and their antagonistic relationship with integrin-based adhesions (Borghi, Lowndes, Maruthamuthu, Gardel, & Nelson, 2010; Dupin, Camand, & Etienne-Manneville, 2009). Loss of E-cadherin in border cells inhibited the formation of protrusions and totally blocked collective migration of border cells in Drosophila oogenesis (Niewiadomska, Godt, & Tepass, 1999).
In breast cancers, leader cells were found to undergo a conserved basal epithelial program and strongly express keratin 14 (K14; Cheung, Gabrielson, Werb, & Ewald, 2013). The transcriptomes of K14+ leader cells were related to the increased expression of both cell–cell and cell–matrix adhesion genes including LAMA3, CD44, COL7A1, MPDZ, CXADR, PKP1, DSG3, TNC, HSPG2, COL12A1, TNN, ANTXR1, DST, ITGA2, POSTN, CTGF, FAT2, DSC3, and ABL2 (Cheung et al., 2016). Cancer cells with K14 overexpression rapidly formed compact tumor spheroids with significant outgrowth from original spheroids, while K14 knock-out cancer cells remained largely isolated and dispersed resulting in tumor organoids with borders displaying rounded morphology and the absence of leader cells, indicating the important role of K14 in cancer cells adhesion and spheroids assembly in breast cancer (Cheung et al., 2013). In addition, leader cells rely on cadherin-mediated adherens junctions to interact with their neighboring cells to interpret the polarized chemotactic gradient and promote chemotaxis of the cell group (Dupin et al., 2009).
2.3 Performing extracellular matrix remodeling
As large sheets or clusters of cancer cells move through the microenvironment, leader cells are responsible for remodeling extracellular matrix to pave the way for collective invasion. Leader cells can generate invasion tracks and create a chemotactic gradient to steer collective invasion by secreting multiple proteolytic enzymes to cut and remodel ECM fibers (Mayor & Etienne-Manneville, 2016). In luminal breast cancer, many genes were differentially expressed between leader and follower cells. Among the most differentially expressed genes are those related to cell motility and modification of the extracellular matrix, including CLDN-1, -3, -7, and -11, matrix metalloproteinase-2 (MMP-2), MMP-9, and Plau (Yang et al., 2019). In neuroblastoma, inhibiting MMP-2 significantly prevented leader cells from transmitting information to follower cells (Hotary, Allen, Punturieri, Yana, & Weiss, 2000). Mesenchymal cells at the front of the wounded epithelium could behave as leader cells to direct collective invasion of epithelial cells and the expression of MMP-9 at the invasive fronts was indispensable for leader cells to clear the path and decrease the resistance (Bleaken et al., 2016). In breast cancer, knocking down LIM kinases prevented invadopodia maturation and led to the loss of focalized MMP9 and decreased matrix degradation, dramatically impairing the formation of leader cells and collective invasion (Scott et al., 2010). In fibrosarcoma and breast cancer, MMP-14 was overexpressed in leader cells to promote focalized cleavage of collagen fibers and generate invasion tracks that were subsequently used by follower cells to form invasive strands. After knocking down MMP-14, collagen degradation, lytic tracks formation, and collective invasion was totally abolished, leading to the transition from collective invasion to single-cell invasion (Sabeh et al., 2004). In addition, cathepsin B was demonstrated to be important for leader cells to generate invasion tracks and play a more complex role as the regulator of the proteolytic network by mediating the activities of MMP-2,14 and signaling pathways in breast cancer cells and salivary adenoid cystic carcinoma (Bengsch et al., 2014; Liaudet-Coopman et al., 2006; Skrzydlewska, Sulkowska, Koda, & Sulkowski, 2005; Wu et al., 2019).
2.4 Inducing partial EMT
Vimentin-rich cells were found to be leader cells to promote collective invasion and vimentin overexpression could collaborate with the upregulation of CD44, MMP-2, and MMP-9 to impart leader cells with invasive manners, indicating the involvement of EMT in collective invasion of epithelial tissues (Bleaken et al., 2016). During collective invasion, leader cells still maintain E-cadherin-based adherens junctions with follower cells and have elongated morphology polarized along the direction of invasion with elevated motility at the same time (Cheung & Ewald, 2014; Friedl & Mayor, 2017). This hybrid phenotype ranging from purely mesenchymal phenotype to purely epithelial phenotype is indicative of partial EMT induced in leader cells (Grigore, Jolly, Jia, Farach-Carson, & Levine, 2016). Further studies have revealed colocalization of markers of both epithelial and mesenchymal phenotypes in collectively invading cancer cells and the unconventional expression of EMT-related markers in collective invasion (Piotrowski-Daspit, Tien, & Nelson, 2016; C. Wang et al., 2011). In luminal breast cancer, leader cells showed a slight increase in vimentin and Zeb1 expression and a lack of Snail expression. E-cadherin, ZO-1, β-catenin, and F-actin were still localized at the contact area with follower cells, indicating leader cells did not undergo a complete EMT program (Yang et al., 2019). In basal-like breast cancer, ΔNp63α could selectively increase Axl and Slug expression to confer leader cells with elevated motility. And the simultaneous activation of miR-205 by ΔNp63α could silence ZEB1 and ZEB2 expression to prevent a complete EMT program to retain cell–cell adhesions between cancer cells (Dang, Esparza, Maine, Westcott, & Pearson, 2015). Loss of AT-rich interaction domain 1A (ARID1A) induced the upregulation of vimentin, SNAI1, SNAI2, and TWIST1 leading to the conversion of endometrial epithelium toward mesenchymal phenotype, which was meanwhile antagonized by the upregulation of phosphoinositide 3-kinase (PI3K) pathway. The coexistence of ARID1A loss and PI3K pathway upregulation led to the partial EMT in epithelial cells, conferring them with strand-like morphology to lead the collective invasion of the endometrial epithelium into myometrium (Wilson et al., 2019).
3 SUPPORT FROM THE NEIGHBORING CELLS
The intrinsic properties of cancer cells can confer them with the potential to become leader cells but the conversion of latent leadership to leader cell behavior relies on the support from the neighboring cells (Clark & Vignjevic, 2015; Zoeller et al., 2019). The interaction between leader cells and the neighboring cells through cell–cell junctions, stimulation of chemokines and growth factors, and modification of the ECM can steer potential leader cells to initiate appropriate polarization, acquire leader cell behavior, consolidate leadership, and make sure there are always enough leader cells to lead collective invasion (Mayor & Etienne-Manneville, 2016; Wolf et al., 2007; Zhang et al., 2019). The support can come from follower cells and stromal cells, specifically cancer-associated fibroblasts (CAFs).
3.1 Support from follower cells
As cancer cell groups collectively invade through the microenvironment, the status of leader cells can be challenged. Follower cells are indispensable for leader cells to obtain and consolidate leadership by promoting leader cell polarization and influencing leader cell behavior (Stramer, Dunn, Davis, & Mayor, 2013) and follower cells can exchange positions and roles with leader cells to ensure replenishment of enough competent leader cells at the invasive front (Malet-Engra et al., 2015; Zhang et al., 2019).
3.1.1 Promoting leader cell polarization
It has been recently revealed that follower cells can promote the polarization of leader cells toward the appropriate direction and play an important role in the coordinated movement of the whole cluster by contact inhibition of locomotion (CIL). CIL is a process that two migrating cells colliding with each other stop their forward movement, collapse their protrusions at the site of contact and repolarize protrusions away from each other (Mayor & Carmona-Fontaine, 2010). CIL can make sure the absence of protrusions at the site of contact and promote the formation of protrusions at the leading edge to promote the directed movement of cell clusters (Stramer et al., 2013; Figure 2). The molecular mechanisms of CIL between leader and follower cells can be separated into three core mechanisms, including sensing the contact with each other, transmitting the signal from the cell surface to the inside of the cell, and inducing protrusion collapse at the contact and protrusion formation at the leading edge (Figure 3). Cell surface molecules involved in CIL include N-cadherins, ephrins and EPH receptors, and the planar cell polarity pathway members (Astin et al., 2010; Carmona-Fontaine, Matthews, & Mayor, 2008; Theveneau et al., 2010). The activation of cell surface molecules leads to the subsequent activation of the signaling pathway inside the cell to regulate the activity of small GTPases including Ras homolog family member A (RHOA), RAC1, and cell division control protein 42 homolog (CDC42; Batson, Maccarthy-Morrogh, Archer, Tanton, & Nobes, 2014; Theveneau & Mayor, 2010). The repolarized activity of the GTPases leads to the repolarization of the cytoskeleton, resulting in protrusion collapse at the contact and protrusion formation at the leading edge, promoting the polarization of leader cells toward the appropriate direction (Charrasse, Meriane, Comunale, Blangy, & Gauthier-Rouvière, 2002; Krause & Gautreau, 2014).
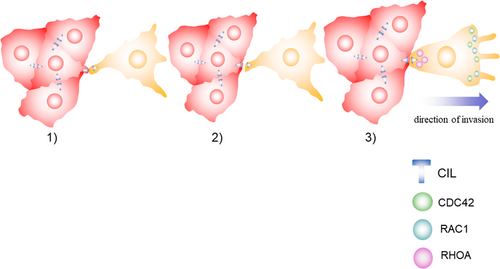
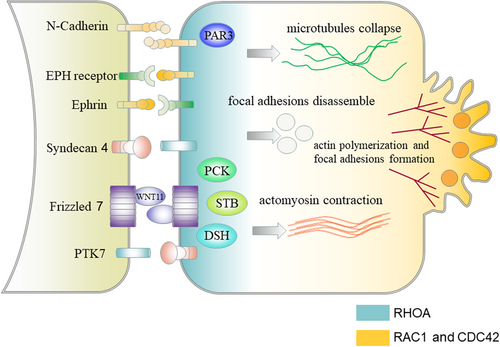
3.1.2 Restricting leadership to leader cells
The domination of leadership by leader cells is the result of the consecutive crosstalk between leader and follower cells. During Drosophila oogenesis, RAC signaling pathway can promote leader cell phenotype and the interaction between leader and follower cells can restrict the activation of RAC signaling in leader cells (Poukkula, Cliffe, Changede, & Rorth, 2011; Prasad & Montell, 2007). Through cell–cell communication, Rab11 can influence the behavior of the entire cell group by restricting RAC activity in leader cells (Ramel, Wang, Laflamme, Montell, & Emery, 2013). In tumor angiogenesis, vascular endothelial growth factor (VEGF) in the microenvironment can induce VEGF signaling pathway in leader cells to induce leader cell behavior and upregulation of Dll4 expression in leader cells that subsequently activates Notch signaling pathway in adjacent follower cells to downregulate the VEGF pathway activity to prevent follower cells from becoming leader cells (Hellstroem et al., 2007; Noguera-Troise et al., 2006). Leader cells consolidating and restricting leadership to themselves by halting other cells becoming leader cells is important for the efficient communication among cancer cells when discussing the direction of invasion (Riahi et al., 2015). In breast cancers, exogenous JAG1 could increase Notch signaling pathway activity and decrease the number of leader cells, restricting the sprouts formation toward random directions (Dean, Elias, Jamilpour, Utzinger, & Wong, 2016). In non–small cell lung cancers, inhibiting the Notch-1 activity could block persistent chain invasion in cancer cell spheroids (Konen et al., 2017).
3.1.3 Influencing leader cell behavior
During the collective invasion, follower cells are indispensable for leader cells to exert leadership and survive to make sure there are enough competent leader cells to lead collective invasion (Muinonen-Martin et al., 2014; Zoeller et al., 2019).
Recent studies have revealed that follower cells played a significant part in generating chemotactic gradient, demonstrating that follower cells are necessary for leader cells to respond to external signals. In melanoma, lysophosphatidic acid (LPA) is an important attractant for melanoma cells and is distributed homogenously in the microenvironment. Follower cells can degrade LPA to generate a gradient with low LPA level inside the cell cluster and high LPA level in the microenvironment. This follower cell-generated gradient is necessary for leader cells to determine the direction of invasion and induce the collective invasion of melanoma cells into the surrounding tissues (Muinonen-Martin et al., 2014). In non–small cell lung cancers, lysine demethylase 5B (KDM5B) mutation promoted the follower cell phenotype and the phenotypic heterogeneity in cancer cell clusters. Follower cells with decreased expression of KDM5B sustained chain-like cooperation between leader and follower cells to support leader cells to promote collective invasion (Zoeller et al., 2019). In non–small cell lung cancers, leader cells were found to have a variety of mitotic defects including cytokinetic instability and prophase-to-anaphase mitotic delay that inhibited the colony growth of leader cells. In contrast, follower cells were more proliferative and adding follower cells conditioned media to leader cells or coculture with follower cells could rescue the mitotic defects of leader cell leading to the strikingly increased colony number and area in leader cells population. Furthermore, the addition of follower cells conditioned media to leader cells also reduced leader cells death, indicating follower cells provided growth and survival advantage to leader cells to make sure there are enough leader cells at the invasive front (Konen et al., 2017).
3.1.4 Exchanging positions and roles with leader cells
Although leader cells can restrict leadership to themselves by multiple mechanisms, the lifetime of leader cells is limited especially when invading through a complex microenvironment and leader–follower turnover is important for cancer cell clusters to sustain persistent collective invasion (Malet-Engra et al., 2015). As leader cells quit from the invasive fronts, competent follower cells will gradually acquire leader cell phenotype and move to the invasive fronts to lead collective invasion. Leader cell metabolism status is closely correlated to collective invasion and metastasis as it demands energy to perform morphological polarization, generate traction force, and remodel the ECM (Han et al., 2013). Cancer cells prefer to utilize glycolytic pathway to increase lactate generation and secretion to promote protease-mediated ECM remodeling and enhance cancer invasion, even in the microenvironment with abundant oxygen, which is known as the Warburg effect (Hsu & Sabatini, 2008). Whereas oxidative phosphorylation at the invasive front can provide ATP in a more efficient way to meet the energetic demands of collective invasion, which requires leader cells to have enough high cellular energy level (Cunniff, McKenzie, Heintz, & Howe, 2016). In breast cancers, leader cells exhibited higher glucose uptake and higher cellular energy level, which was the driving force of collective invasion and was gradually consumed by forward invasion. When the energy level of leader cells dropped below a threshold, a follower cell with higher cellular energy level would take over the leader position to maintain persistent invasion (Zhang et al., 2019).
In addition, during the collective invasion of malignant lymphocytes, the exposure to CCL19 gradient induced the removal of surface CCR7 in leader cells, leading to the protrusion retraction and the loss of polarity. As leader cells lost their position at the invasive front and moved sideways, the gradually increased expression of CCR7 was observed in follower cells moving to the invasive front with a simultaneous extension of polarized protrusions. The internalization and recycle of CCR7 is the result of continuous crosstalk between leader and follower cells to make sure the replenishment of enough leader cells with high expression of CCR7 to promote persistent collective invasion (Malet-Engra et al., 2015).
3.2 Support from CAFs
To promote the leader cell, phenotype and collective invasion require not only the intrinsic properties of cancer cells but also the extracellular mechanisms by the reciprocal interplay between cancer and stromal cells. During the progression of collective invasion, cancer cells can recruit and reprogram a repertoire of stromal cells among which CAFs are the ideal stromal partners. CAFs have been demonstrated to play a significant role in cancer progression by increasing cancer cell motility and metastatic spread, regulating cancer cell metabolism, remodeling ECM, and preparing metastatic niche (Cirri & Chiarugi, 2011). Furthermore, recent studies have revealed the correlation between CAFs and collective invasion suggesting CAFs can participate in several stages of a collective invasion by modifying ECM and generating chemotactic gradient to make the microenvironment favorable to leader cells formation.
3.2.1 CAFs modifying extracellular matrix
CAFs can promote leader cell phenotype by modifying ECM biomechanical and biochemical properties, which results in ECM heterogeneity and complexity to promote leader cell morphological change, enhance cancer cell motility, and influence invasion direction and mode (Friedl & Wolf, 2010; Zhu, Liang, Jiao, Liu, & Allianc, 2015).
CAFs-induced modification of ECM biomechanical properties includes matrix stiffness, density, thickness, and porosity. Increased mechanical compression stimulated morphological change and cytoskeletal reorganization at the invasive front to accelerate the extension of protrusions (Tse et al., 2012). Increased matrix stiffness and density can facilitate fiber formation, strengthen focal adhesion, and ultimately generate traction force to accelerate cancer cell invasion (Baker et al., 2013). Increased ECM stiffness and spatial confinement by CAFs can influence invasion mode by determining the energetic costs for cancer cells to invade through the microenvironment. In breast cancer, high ECM stiffness is correlated with high energy level of cancer cells and increased ECM stiffness by CAFs could promote the establishment of cell–cell contacts and induce collective invasion to minimize energetic costs as more energy is demanded for a single cell to invade under high levels of mechanical compression (Estrada et al., 2016; Zanotelli et al., 2018). Conversely, cancer cells invaded individually with the deficiency of leader–follower behavior in low-density ECM (Malik, Lelkes, & Cukierman, 2015). The deposition of fibrillar collagens and the increased ECM stiffness by CAFs can trigger integrin signaling pathway in cancer cells to transduce downstream mechanical and chemical signals, which leads to morphological polarization, traction force generation, and MMPs activation (Boettiger, 2012; Dabiri, Lee, & Parker, 2012; Figure 4). Although increased ECM stiffness induced by CAFs is important for cancer cells to acquire leader cell phenotype and promote collective invasion, excessive mechanical stresses significantly impeded collective invasion. In fibrosarcoma, cancer cell spheroids embedded in a 6 mg/ml collagen matrix showed a shorter invasion distance than that embedded in 1 mg/ml collagen matrix (Jimenez Valencia et al., 2015).
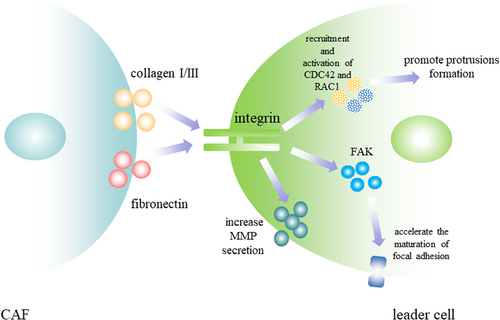
CAFs-induced modification of ECM biochemical properties includes the structure and constitution of the collagen (Malik et al., 2015). CAFs-induced modification of the constitution of collagens includes the accumulation of collagen I and collagen III and the degradation of collagen IV (Huijbers et al., 2010). In breast cancer, leader cells in invasive regions with protrusive morphology were related to an eightfold increase in collagen I fiber in ECM relative to noninvasive regions with no leader cells formation. After being re-embedded in collagen I, tumor organoids previously cultured in collagen IV rapidly became protrusive and initiated collective invasion, which indicates the correlation between leader cell behavior and collagen-I-rich microenvironment (Cheung et al., 2013). Fibronectin, another important component modified by CAFs, is the major ligand of the integrin-FAK pathway and is important for leader cells to generate traction force, stabilize cell–cell connections, and maintain persistent collective invasion (Biname, Lassus, & Hibner, 2008). In the collective migration of hepatocyte, cohorts lack of fibronectin abolished leader cells’ ability to lead follower cells (Kalluri & Zeisberg, 2006). Discoidin domain receptor 2 (DDR2) was increased in CAFs and the depletion of DDR2 in CAFs led to the deficiency of highly aligned collagen I and fibronectin in ECM and the dramatic decrease in the number of invasive tumor organoids. Whereas adding CAFs with DDR2 expression to the breast cancer organoids with no DDR2 expression rescued collective invasion. The absence of DDR2 in CAFs could impact ECM production and reorganization and paracrine functions of CAFs, which inhibited leader cell formation (Corsa et al., 2016).
3.2.2 CAFs generating chemotactic gradient
Recent studies suggested that apart from promoting the emergence of leader cells by remodeling ECM, CAFs can secret chemokines and growth factors to transmit information to adjacent cancer cells to confer them with leader cell phenotype and induce collective invasion. In breast cancer, CAF can trigger ΔNp63α expression in cancer cells by paracrine, leading to a partial EMT program in cancer cells to confer them with leader cell phenotype (Dang et al., 2015). In lung cancer, transforming growth factor-β secreted by CAFs increased the expression of cell–matrix adhesion proteins integrin β1 and integrin β3 in leader cells to enhance cell–matrix interaction to generate traction force (An et al., 2013). In ameloblastoma, CAFs could secrete interleukin (IL)-1, IL-6, and IL-8 to promote cancer invasion and mediate the interaction between CAFs and cancer cells. When cocultured with CAFs, ameloblastoma cells initiated tuft-like invasive processes and collectively invaded into surrounding tissues, whereas without CAFs, ameloblastoma cells formed blunt invasive process with rounded cell borders (Labernadie et al., 2017). CAFs can secret epidermal growth factor (EGF) to induce morphogenic modification of leader cells by enhancing CDC42 activity to accelerate actin skeleton repolarization, promote the extension of protrusion, and increase traction force (Cirri & Chiarugi, 2011; Grasset et al., 2018). In squamous cell carcinoma, the increased matrix stiffness by CAFs could sensitize cancer cells to EGF and enhance EGF pathway activity, demonstrating CAFs could promote leader cell formation by the collaboration between matrix stiffness and EGF pathway (Grasset et al., 2018).
3.2.3 CAFs becoming leader cells
Intriguingly, studies have revealed that leader cells could not only derive from cancer cells but also from CAFs. Spheroids of CAFs mixed with squamous cell carcinoma cells invaded the ECM by forming multicellular invasive strands led by CAFs. And the heterophilic E-cadherin/N-cadherin junctions between CAFs and cancer cells were found to be mechanosensitive and withstand the similar forces as the homophilic E-cadherin/E-cadherin junctions to promote force transmission, mechanotransduction and CAFs polarization toward leader cells. The knockdown of either N-cadherin or E-cadherin drastically decreased the number of leader cells and inhibited multicellular invasive strands formation (Gaggioli et al., 2007). In addition to increasing ECM stiffness to promote leader cell formation and collective invasion, CAFs can behave as leader cells by cutting ECM fibers and invading into surrounding tissues to create a path for follower cells to “hitch a ride.” As CAFs paved the way for collective invasion, the activity of Rho is enhanced in CAFs and the inhibition of Rho activity prevented cancer cells from moving behind, indicating the indispensable role of Rho for CAFs to lead collective invasion (Gaggioli et al., 2007).
3.3 Support from immunosuppressive cells
During cancer progression, cancer cells derived soluble factors can recruit multiple inflammatory cells to infiltrate into tumors and orchestrate the local immune response, conferring stromal cells with more complex roles in the microenvironment. Recent studies have revealed the latent involvement of immunosuppressive cells in the collective invasion, suggesting they can create the immunosuppressive tumor microenvironment and simultaneously promote leader cells formation. Tumor-associated macrophages (TAMs), one of the most abundant immunosuppressive cells in the microenvironment that can suppress CD4+ and CD8+ T cell activity, were found to promote the cohesion of fibrosarcoma cells and persistent collective invasion (Qian & Pollard, 2010). In breast cancers, TAMs could facilitate coordinated multicellular streaming of cancer cells by secreting EGF to promote cancer cells repolarization and protrusion extension (Chang, Carmona-Fontaine, & Xavier, 2013). In addition, direct contact with TAMs could trigger RhoA-dependent invadopodia formation in breast cancer cells (Roh-Johnson et al., 2014). Coculture with TAMs induced elevated CD44 expression in luminal breast cancer cells and cancer cells with increased CD44 expression were found to be leader cells with higher proliferation, invasiveness, and migration rate (Yang et al., 2019).
Myeloid-derived suppressor cells (MDSCs) are a group of immunosuppressive cells that can suppress antitumor immune response and promote tumor progression by a wide spectrum of effects including producing immunosuppressive cytokines and inducing Tregs (Marvel & Gabrilovich, 2015; Vesely, Kershaw, Schreiber, & Smyth, 2011). In breast cancers, MDSCs secreted CXCL11 and fibroblast growth factor 2 (FGF2) to accelerate CAFs migration and promote CAFs becoming leader cells to lead the collective invasion. In addition, multiple genes involved in the regulation of immune response were significantly depleted in leader cells of breast cancer, holding back MHC class II antigen presentation and T-cell activation, further suggesting the reciprocal interaction between immunosuppressive tumor microenvironment and the leader cell behavior (Cheung et al., 2016).
4 CONCLUSION
Taken together, to become leader cells, cancer cells need intrinsic properties including distinct genetic expression, protein synthesis, and signaling pathway to confer them with the potential to acquire leader cell phenotype. And the support from the neighboring cells by cell–cell junctions, the stimulation of chemokines and growth factors and modification of the microenvironment can convert the potential into specific leader cell behavior. And these mechanisms can coordinate to steer cancer cells to acquire morphological and functional phenotype of leader cells, exert leader cell behavior, and consolidate leadership to promote persistent collective invasion (Figure 5).
TAMs are revealed to promote leader cells formation by inducing CD44 expression in cancer cells and facilitate the coordinated multicellular movement of cancer cells by secreting EGF (Yang et al., 2019). MDSCs are revealed to steer CAFs to become leader cells by secreting CXCL11 and FGF2. Multiple genes involved in the regulation of immune response were significantly depleted in leader cells (Cheung et al., 2016). These suggested the latent reciprocal interaction between immunosuppressive tumor microenvironment and the leader cell behavior, which means the immunosuppressive tumor microenvironment can promote leader cells formation and leader cells can in turn create the immunosuppressive tumor microenvironment. Many cancer cells and immunosuppressive cells can coordinate to accomplish tumor immune escape by expressing inhibitory molecules including PD-1, PD-L1, and CTLA-4 (Sharma & Allison, 2015). Whether leader cells can express these inhibitory molecules to create a “shield” against antitumor immune response for the whole multicellular unit and initiate tumor immune escape? We expect more research investigating the correlation between immunosuppressive cells and leader cells to enrich the roles of leader cells in collective invasion and seek new therapeutic methods to decrease collective invasion by targeting immune checkpoints.
ACKNOWLEDGMENTS
This work was supported by the National Key R&D Program of China (No. 2019YFA09005700) and the National Natural Science Foundation of China grants (Nos. 81672672, 81972542, and 21838002).




