The effects of macrophage-mediated inflammatory response to the donor site on long-term retention of a fat graft in the recipient site in a mice model
Zijue Wang and Yunzi Chen contributed equally and are co-first authors.
Abstract
Inflammatory responses mediated by macrophages play a role in tissue repair. However, it is unclear whether the repair in the donor site after liposuction would have any effects on fat graft retention in the recipient site. This study is designed to evaluate the effects of a macrophage-mediated inflammatory response in donor sites on long-term retention of fat grafting. In this study, mice were randomly divided into two groups. One underwent simulated liposuction, called the fat procurement plus grafting (Pro-Grafting) group, and the other underwent sham surgery, called the fat grafting only (Grafting Only) group. The prepared fat (0.3 ml each) was engrafted and cellular events over a 90-day period were assessed. We found macrophages were infiltrated into adipose tissue at the recipient site in the Grafting Only group within 7 days and the repair essentially completed within 30 days. By contrast, few macrophages infiltrated the recipient site in the Pro-Grafting group within 7 days and the entire remodeling process took 30 days longer in the Pro-Grafting than the Grafting Only group. Moreover, C-reactive protein levels were immediately upregulated after surgery, and the inflammatory factors' expression was higher at the donor rather than the recipient site. However, the repair processes and the long-term retention rate became normal when the adipose tissue was grafted after the donor site did not require macrophages for repair. Therefore, we suggest higher inflammatory factors promote macrophage infiltration and the adipose tissue regeneration process at the donor site. This process is delayed at the recipient site, which may affect long-term retention of fat grafts.
1 INTRODUCTION
Autologous fat grafting is increasingly performed worldwide due to its biocompatibility, versatility, natural-looking outcomes, cost-effectiveness, and ease of performance (Coleman, 2001; Leuchter, Schweizer, Hohlfeld, & Pasche, 2010). Although fat graft retention has become more predictable, it remains less than ideal (Khouri et al., 2014; Khouri et al., 2015). Thus, efforts have focused on methods resulting in improved and more consistent fat graft retention.
The retention of grafts is mainly owing to the repair of adipose tissue, especially the inflammatory response in the early stage after grafting. Also, clinical studies and animal experiments have evaluated adipose tissue repair at the donor site and surrounding areas after surgery such as liposuction (Broughton et al., 2006; Liszka, Dellon, Im, Angel, & Plotnick, 1998; Mauer & Bartness, 1997; Reyne, Nougues, & Vezinhet, 1983). Indeed, after fat grafting, the trauma at the donor site immediately induces strong inflammatory signaling, recruiting macrophages to initiate an inflammatory response. Thus in autologous fat grafting, both the donor and recipient sites require recruiting macrophages. However, within certain periods of time, the body can recruit only limited numbers of macrophages (Martin et al., 2003; Pitchford, Hahnel, Jones, & Rankin, 2010; Wengner, Pitchford, Furze, & Rankin, 2008). Therefore, there may be a competition between the repair at the donor and recipient sites.
In fact, the most commonly used models in research on the retention mode after grafting, including C57/BL6 and nude mouse adipose transplantation models, have assessed histologic changes at the recipient site without evaluating the repair of the donor site (Kato et al., 2014; Zhan et al., 2017; Zhao et al., 2012). However, the interaction between donor and recipient sites cannot be ignored during fat grafting. This study, therefore, hypothesized that there may be a competition between the donor and recipient sites.
This hypothesis was tested using a new mice model. In this model, subcutaneous inguinal fat pads in donor sites were damaged, thereby simulating liposuction, and free fat was grafted in the back. The effects of the donor site on the inflammatory response were assessed, as were the relationships between the donor and recipient sites.
2 MATERIALS AND METHODS
2.1 Animals
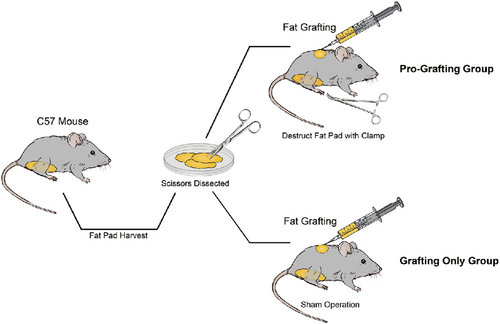
All experiments were approved by the Nanfang Hospital Animal Ethics Committee Laboratory and were conducted according to the guidelines of the National Health and Medical Research Council of China. A total of 252 8-week-old male C57/BL6 mice, obtained from Southern Medical University, were housed in individual cages with a 12 hr light/dark cycle and provided standard food and water ad libitum. One hundred and twenty-six of these mice were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg). To prepare fat grafts, bilateral subcutaneous inguinal fat pads were separated and removed from each mouse and placed in culture dishes on ice. Using corneal scissors, these fat pads were gently cut into very small pieces, similar to the size of aspirated fat tissue used clinically. A volume of 0.3 ml of prepared adipose tissue served as the fat graft baseline for each mouse.
2.2 Animal model
2.2.1 Pro-Grafting group and Grafting Only group
Eighty-four C57/BL6 mice were randomly divided into two groups of forty-two mice each using IBM SPSS, Version 21.0 (IBM Corp., Armonk, NY), with allocation determined using the random number table method. Bodyweight did not significantly differ between the two groups.
Mice in the Pro-Grafting group underwent simulated liposuction surgery, in that their inguinal skin was incised, their subcutaneous inguinal fat pads were dissected and crushed with a clamp, and their skin was closed with 7-0 nylon sutures. Mice in the Grafting Only group underwent sham operations, in that their inguinal skin was incised, followed by skin closure with 7-0 nylon sutures. Each mouse in both groups was injected subcutaneously on the back with 0.3 ml prepared fat using a 1 ml syringe and an 18-gauge cannula within 30 min of tissue harvesting.
Seven animals in the Pro-Grafting group and Grafting Only group were killed at 3, 7, 14, 30, 60, and 90 days after grafting. At sacrifice, the grafts (recipient site) were harvested and carefully separated from surrounding tissue, and their volumes were measured. Inguinal adipose tissue (donor site) was also harvested from the Pro-Grafting group at 0, 3, 7, 14, and 30 days after grafting. Each harvested sample was assessed by histology, immunofluorescence, western blot analysis, and real-time quantitative polymerase chain reaction (qPCR).
2.2.2 Days of the delayed group
Mice in the 3, 7, and 14 days of the delayed group underwent simulated liposuction surgery the same as the Pro-Grafting group, while the grafting time was delayed till 3, 7, and 14 days separately after surgery. Then, the three groups were compared to the Pro-Grafting group (0 day of the delayed group) and Grafting Only group (∞ days of the delayed group).
7 animals in each group were killed at 14, 30, and 90 days after grafting. At sacrifice, the grafts were harvested and carefully separated from surrounding tissue, and their volumes were measured. Each harvested sample was assessed by histology, immunofluorescence, western blot analysis, and real-time qPCR.
2.3 Enzyme-linked immunosorbent assay
Blood samples were collected in tubes without anticoagulant. Sera were separated and stored at −20°C until tested. The concentration of endotoxin in CRP was determined using the Limulus amebocyte lysate kit (QCL-1000) according to the manufacturer's instructions (Lonza).
2.4 Histological examination
Tissue samples were fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin for staining with hematoxylin–eosin. Tissue blocks were sectioned, examined under an Olympus BX51 microscope, and photographed using an Olympus DP71 digital camera.
2.5 Immunohistochemistry
Tissue samples were immunofluorescently stained with the primary rat anti-mouse Mac2 (1:200; Cedarlane Corp., Burlington, Ontario, Canada), rabbit anti-mouse CD206 (1:300; Abcam, Cambridge, MA), and guinea pig anti-mouse perilinpin(1:400; Progen, Heidelberg, Germany), and chicken anti-GFP (1:200; Abcam). After washing, the samples were incubated with donkey anti-rat Alexa Fluor 555 IgG (Abcam) and goat anti-rabbit Alexa Fluor 430 IgG (Invitrogen, North Ryde, NSW, Australia) antibodies. The nuclei were stained with DAPI (Sigma, St. Louis, MO).
2.6 Quantitative reverse transcription PCR (qRT-PCR)
Fat tissue was excised, snap-frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted from 50 mg tissue samples using an RNeasy Lipid Tissue Mini Kits (Qiagen, Hilden, Germany), according to the manufacturer's instructions. cDNA was synthesized using a QuantiTect Reverse Transcription Kit (Qiagen) and the genes of interest were amplified in 40 cycles using a Rotor-Gene 3000 Real-Time PCR Detection System (Corbett Research, Sydney, Australia) and primers specific to interleukin-1β (IL-1β: forward, 5′-CCTCGTGCTGTCGGACCCATA-3′; reverse, 5′-CAGGCTTGTGCTCTGCTTGTGA-3′); tissue necrosis factor-α (TNF-α: forward, 5′-CAGCAAGCACTCAACGGAAT-3′; reverse, 5′-CGTCCTCTGAACGACCAACA-3′); IL-6 (forward, 5′-GTTGCCTTCTTGGGACTGAT-3′; reverse, 5′-TTTCCACGATTTCCCAGAGA-3′); IL-10 (forward, 5′-AACATACTGCTAACCGACTC-3′ reverse, 5′-CACTGCCTTGCTCTTATT-3′); transforming growth factor-β (TGF-β: forward, 5′-GGACCCTGCCCCTATATTTG-3′; reverse, 5′-AGGAGCGCACAATCATGTTG-3′); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH: (forward, 5′-AACTTTGGCATTGTGGAAGG-3′; reverse, 5′-CCCTGTTGCTGTAGCCGTAT-3′). Levels of expression were calculated using the method, with levels of IL-1β, TNF-α, IL-6, IL-10, and TGF-β messenger RNAs (mRNAs) normalized to the level of GAPDH mRNA.
2.7 Western blot analysis
Total cell lysates of adipose samples were prepared using a M-PER Mammalian Protein Extraction Reagent (Thermo Fisher Scientific). A BCA protein assay (Thermo Fisher Scientific) was used to estimate the concentration of the protein. After separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a NuPAGE electrophoresis system, protein extracts were transferred to immobilon polyvinylidene difluoride membranes (Millipore, Billerica, MA). We blocked membranes in 5% milk and immunoblotted them with the following primary antibodies: anti-PPAR-γ (1:200; Abcam), anti-C/EBP (1:1,000; Abcam), and anti-AP2 (1:1,000; Abcam). Thereafter, the membranes were incubated with secondary antibodies. A WesternBreeze Chemiluminescent Detection Kit (Thermo Fisher Scientific) was used to detect signals. GAPDH and tubulin served as internal controls.
2.8 Statistical analysis
Data were expressed as mean ± SD and analyzed by repeated-measures analysis of variance. Independent Student's t tests were used to compare the two groups of mice at single time points, and one-way analysis of variance was used to compare the groups at all time points. A p value < .05 was considered statistically significant (Figure 1).
3 RESULTS
3.1 Comparison of grafts retention and CRP level in two groups
Grafts in the Grafting Only group were covered by a thin fibrous capsule from Day 3 to Day 7, with the surface of the fibrous capsule becoming thicker from Day 7 to Day 30. From Day 60 to Day 90, the appearance and volume of the grafts were stable, and their texture was soft and similar to that of normal fat tissue. Grafts in the Pro-Grafting group were dispersed from Day 3 to Day 7. On Day 30, fibrous capsules, similar to those observed in the Grafting Only group on Day 7, were observed in the Pro-Grafting group. These grafts showed a stable appearance and texture, similar to normal fat tissue, on Day 90. That is, it took an additional 30 days for the fat grafts in the Pro-Grafting group to attain a stable appearance and texture than the grafts in the Grafting Only group (Figure 2a).
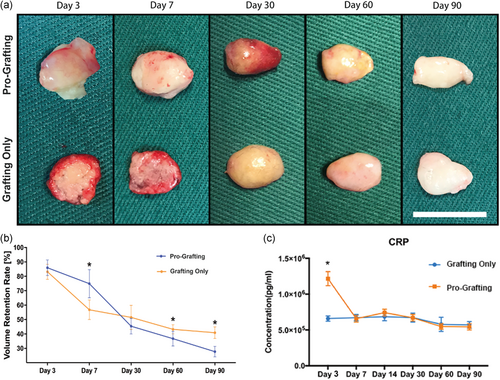
Quantification of graft volume indicated that the volume of adipose tissue in the Grafting Only group decreased sharply over 7 days. The rate of decline subsequently decreased, with the adipose tissue volume remaining stable from Day 60 to Day 90. By contrast, the volume of adipose tissue in the Pro-Grafting group decreased slowly from Day 3 to Day 7 and more sharply from Day 7 to Day 30, remaining constant thereafter. The 90-day retention rate was significantly higher in the Grafting Only than in the Pro-Grafting group (Figure 2b).
Serum concentrations of CRP increased rapidly after surgery in the Pro-Grafting group, decreasing to normal levels on Day 7. By contrast, serum CRP concentrations in the Grafting Only group remained at normal levels, with little fluctuation (Figure 2c).
3.2 The repair process and vascularization of adipose tissue in two groups
Histological analysis of samples from the Grafting Only group showed observable infiltration of inflammatory cells, as early as 3 days after transplantation (Initial Stage). Then, considerable infiltration of inflammatory cells (blue arrows) appeared on Day 7 (Peak Stage). However, the inflammatory cells gradually disappeared on Day 30 (Declining Stage). At the same time, small-sized adipocytes (yellow arrows), which first appeared on Day 30, gradually matured from Day 60 to Day 90, with the structure of adipose tissue being basically stable on Day 90. By contrast, the three stages of inflammation stage could also be observed in the Pro-Grafting group, though the points in time were later. Moreover, the adipose tissue structure in the Pro-Grafting group was not completely stable, and the degree of tissue fibrosis (green arrows) was higher than in the Grafting Only group. The most obvious difference between the two groups was the delay in the repair process observed in the Pro-Grafting group (Figure 3a).
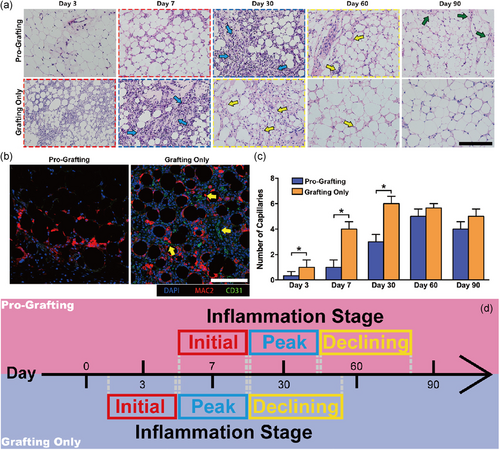
Immunofluorescence showed the appearance of newly formed blood CD31+ vessels on Day 7 in the Grafting Only, but not in the Pro-Grafting, group (Figure 3b). The numbers of newly formed blood vessels in the Grafting Only group increased from Day 3 to Day 30, stabilizing thereafter. In the Pro-Grafting group, the numbers of newly formed blood vessels increased slowly from Day 3 to Day 30, being significantly lower than in the Grafting Only group. The numbers of newly formed blood vessels in the Pro-Grafting group peaked at Day 60 and then stabilized. There was no significant difference between the two groups on Day 90 (Figure 3c).
Compare to the Grafting Only group, the three stages of inflammation stage were similar, but the whole inflammation stage was delayed and the Declining Stage was prolonged in the Pro-Grafting group (Figure 3d).
3.3 Macrophage infiltration in two groups
Immunofluorescence showed that MAC2+ macrophages had infiltrated fat tissue in the Grafting Only group on Day 3 (Figure 4a, white arrow), with additional MAC2+ and M2 (MAC2+/CD206+) macrophages appearing on Day 7 (yellow arrows). On Day 30, most of the inflammatory cells were M2 (MAC2+/CD206+) macrophages (yellow arrows), with the number of inflammatory cells being significantly lower on Day 30 than on Day 7. In the Pro-Grafting group, MAC2+ macrophage infiltration was observed on Day 7 (Figure 4a, upper panel, left, white arrows). On Day 30, large numbers of MAC2+ and M2 (MAC2+/CD206+) macrophages appeared in this group at a level similar to that in the Grafting Only group on Day 7 (yellow arrows). M1 macrophages mediate inflammatory responses, whereas M2 macrophages mediate tissue repair processes and resolve inflammation. A persistently high ratio of M2 macrophages causes a high degree of tissue fibrosis.
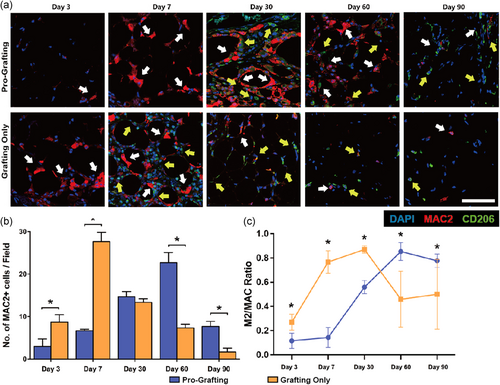
Quantitative analysis showed that the number of MAC2+ macrophages peaked on Day 7 in the Grafting Only group and then began to decline, becoming minimal on Day 90. By contrast, the number of MAC2+ macrophages in the Pro-Grafting group peaked on Day 60 and declined thereafter. On Day 90, the number of MAC2+ macrophages was significantly higher in the Pro-Grafting than in the Grafting Only group (Figure 4b). The ratio of M2 (MAC2+/CD206+) macrophages in the Grafting Only group increased rapidly from Day 3 to Day 7, and more slowly from Day 7 to Day 30. This ratio subsequently declined, stabilizing at a low level from Day 60 to Day 90. By contrast, the ratio in the Pro-Grafting group was low before Day 7, increased continuously from Day 7 to Day 60, and declined from Day 60 to Day 90. This ratio on Days 30–60 was higher in the Pro-Grafting than in the Grafting Only group. Moreover, although this ratio decreased from Day 60 to Day 90 in the Pro-Grafting group, it remained significantly higher than in the Grafting Only group (Figure 4c).
3.4 The three stages of the repair process is preferential in the donor site
To explore the difference between the Pro-Grafting group and Grafting Only group, we focused on the donor site in the Pro-Grafting group (Figure S1a).
Histological analysis of the donor sites in the Pro-Grafting group showed the Initial Infiltration Stage on Day 3 and the Peak Inflammation Stage on Day 7. Moreover, the Tissue Regeneration Stage was almost completed on Day 30 (Figure S1b). Immunofluorescence yielded similar results. Large numbers of MAC2+ inflammatory cells and M2 (MAC2+/CD206+) macrophages were observed at donor sites in the Pro-Grafting group from Day 3 to Day 7. On Day 30, the number of inflammatory cells was significantly lower, with most being M2 (MAC2+/CD206+) macrophages (Figure S1b). These changes were very similar to early changes in grafted fat observed in the Grafting Only group.
The levels of expression of mRNAs encoding three potent pro-inflammatory cytokines, IL-1β, TNF-α, and IL-6, were compared at the graft donor and recipient sites in the Pro-Grafting group by qRT-PCR. The IL-1β mRNA level was higher at donor than at recipient sites on Days 0 and 7, with the two becoming equal on Day 14. IL-6 and TNF-α mRNA levels were higher at donor than at recipient sites on Days 0–14, with the two becoming equal on Day 30 (Figure S1c).
3.5 Grafts in the groups with different grafting time after inguinal fat pads in donor sites were damaged
According to the delayed time of the inflammation stage in the Pro-Grafting group, we grafted after the inguinal fat was damaged for 3, 7, and 14 days (3, 7, and 14 days of delayed), which then was compared with the Pro-Grafting group (0 day of delayed) and Grafting Only group (∞ days of delayed; Figure 5a).
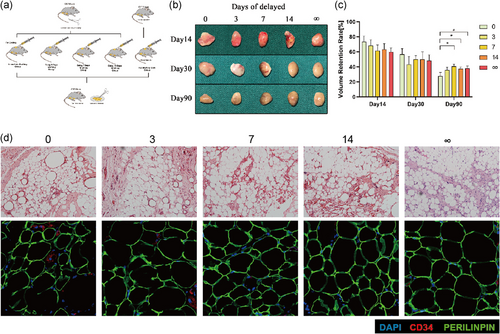
Grafts and quantification of graft volume showed the long-term volume retention rate in 3 days of the delayed group had no difference with the Pro-Grafting group, while that in 7 and 14 days of the delayed group was significantly higher than the Pro-Grafting group and had no difference with Grafting Only group (Figure 5b,c).
Histological analysis of long-term tissues showed more oil sacs in 0 and 3 days of the delayed group than 7, 14, and ∞ days of the delayed group. Also, the immunofluorescence showed tissues in 7, 14, and ∞ days of the delayed group had a more stable structure (Figure 5d). The number of CD34+ cells in 7, 14, and ∞ days of the delayed group also higher than 0 and 3 s of the delayed group on Day 14 (Figure S2).
Adipogenic associated protein, including peroxisome proliferator-activated receptor-γ (PPAR-γ), C/EBP, and AP2, were also explored on Day 30 (Figure S3a). The expression of PPAR-γ in 7, 14, and ∞ days of the delayed group was higher than 0 and 3 days of the delayed group (Figure 4d). The expression of C/EBP and AP2 in 3, 7, 14, and ∞ days of the delayed group were all higher than 0 day of the delayed group (Figure S3b).
3.6 Macrophages in the groups with different grafting time after inguinal fat pads in donor sites were damaged
Immunofluorescence showed that MAC2+ macrophages had infiltrated fat tissue in all groups on Day 14. However, most were M1 macrophages (MAC2+/CD206−) in 0 and 3 days of the delayed group, but M2 macrophages (MAC2+/CD206+) in 7, 14, and ∞ days of the delayed group on Day 14. On Day 30, many M2 macrophages were also observed in 0 and 3 days of the delayed group, however, the number of macrophages in 7, 14, and ∞ days of the delayed group were significantly decreased (Figure 6a).
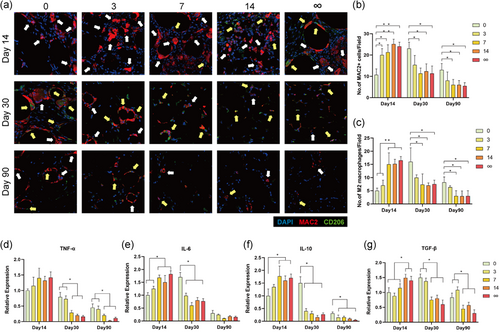
Quantitative analysis showed that the number of MAC2+ macrophages in 3, 7, 14, and ∞ days of delayed group were all higher than 0 day of the delayed group on Day 14, while on Days 30 and 90, the number of MAC2+ macrophages in 7, 14, and ∞ days of the delayed group were lower than that in 0 day of the delayed group (Figure 6b). The number of M2 macrophages in 7, 14, and ∞ days of the delayed group were significantly higher than 0 day of the delayed group on Day 14, and lower than 0 day of the delayed group on Days 30 and 90. However, the M2 macrophage number in 3 days of the delayed group was always similar to 0 day of the delayed group (Figure 6c).
The expression of TNF-α in 7, 14, and ∞ days of the delayed group was lower than 0 and 3 days of the delayed group on Days 30 and 90 (Figure 6d). The expression of IL-6 in 7, 14, and ∞ days of the delayed group were higher than 0 and 3 days of the delayed group on Day 14, however, 3, 7, 14, and ∞ days of the delayed group were all lower than 0 day of the delayed group on Day 30 (Figure 6e). The expression of IL-10 in 7, 14, and ∞ days of the delayed group were higher than 0 day of the delayed group on Day 14, however, 3, 7, 14, and ∞ days of the delayed group were all lower than 0 day of the delayed group on Days 30 and 90 (Figure 6f). The expression of TGF-β in 14 and ∞ days of the delayed group was higher than 0, 3, and 7 days of the delayed group on Day 14, however, 7, 14, and ∞ days of the delayed group were lower than 0 and 3 days of the delayed group on Days 30 and 90 (Figure 6g).
4 DISCUSSION
This study showed that the repair processes of fat grafts in the Pro-Grafting group were delayed and that the long-term volume retention rate was lower in the Pro-Grafting than the Grafting Only group. This study also showed that donor sites could increase serum CRP levels. And repair processes were faster at donor than at recipient sites, although the three stages of repair processes were similar at these two sites in the Pro-Grafting group. However, M2 macrophages persisted longer and the degree of tissue fibrosis was higher in the Pro-Grafting than in the Grafting Only group. However, the repair processes, and the long-term volume retention rate became normal when the adipose tissue was grafted after the donor site did not require macrophages for repair (Figure 7).
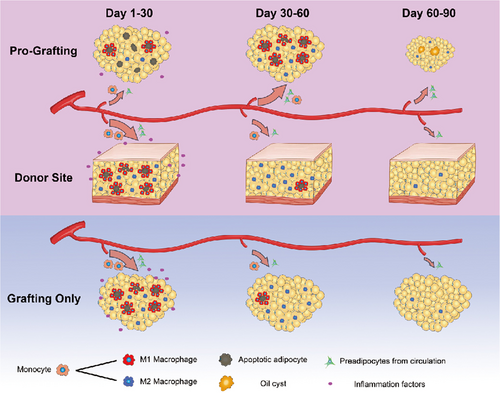
After grafting, the adipose tissue rapidly entered into the inflammation stage (Kato et al., 2014; Mashiko & Yoshimura, 2015), including the “Initial Stage,” “Peak Stage,” and “Declining Stage.” At the Initial Stage, a large number of M1 macrophages infiltrated into the tissue and then shifted into M2 macrophages at Peak Stage. At the Declining Stage, M2 macrophages gradually decreased to finish the inflammation stage. In this study, the whole inflammation stage was delayed, in which the Initial Stage was delayed to the 7th day after grafting, and also the Declining Stage was prolonged in the Pro-Grafting group. These mean that M1 macrophage infiltration was delayed and M2 macrophages persisted longer than the Grafting Only group. However, the inflammation stage was important to the long-term volume retention rate (Cai et al., 2017; Cai, Feng, Liu, Zhou, & Lu, 2018; Liu et al., 2018). According to the delayed time of Initial Stage, we grafted after the inguinal fat were damaged for 3, 7, and 14 days. Then, the inflammation stage was resorted normal when grafted after the inguinal fat was damaged for 7 days or longer. Also, the delay of reparation in the recipient site disappeared and the long-term volume retention rate had no difference with the Grafting Only group. That is to say, the delay of reparation in the recipient site was the main reason for the low long-term volume retention rate.
Indeed, macrophages have been reported to play a significant role in tissue inflammation and regeneration (Kato et al., 2014; Lech & Anders, 2013; Mashiko & Yoshimura, 2015; Mineda et al., 2014; Owen & Mohamadzadeh, 2013). M1, or classically activated, macrophages mediate inflammatory responses, which are associated with high levels of pro-inflammatory cytokines. By contrast, M2, or alternatively activated macrophages mediate tissue repair processes and resolve inflammation by producing anti-inflammatory cytokines (Honda & Inagawa, 2019; Lolmede et al., 2009; Ploeger, van Putten, Koerts, van Luyn, & Harmsen, 2012; Potente, Gerhardt, & Carmeliet, 2011). In this study, we observed that infiltration by M1 macrophages was delayed in the Pro-Grafting group, which may have slowed the release of angiogenic cytokines, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), and the recruitment signals for stem cells (Anghelina, Krishnan, Moldovan, & Moldovan, 2006; Modarai, Burnand, Sawyer, & Smith, 2005; Pfafflin & Schleicher, 2009). Delays in macrophages infiltration may have resulted in poorer vascularization at recipient sites, as well as higher degrees of necrosis and oil cysts, reducing long-term fat retention. As there was more necrosis and because oil cysts must be phagocytosed (Mashiko & Yoshimura, 2015; Mineda et al., 2014), M2 macrophage infiltration lasted for a long time. Thus, the whole inflammation stage was delayed and the Declining Stage was prolonged in the Pro-Grafting group (Kato et al., 2014; Lolmede et al., 2009).
To explore the difference infiltration of macrophages between the two groups, we contrast the inflammation levels of the body, which were indicated by serum CRP. CRP is both a marker and mediator of inflammation and a positive acute-phase protein produced by the liver (Li et al., 2016; Singh, Suresh, Voleti, & Agrawal, 2008). Trauma to local tissue can stimulate the release of IL-1β, IL-6, and TNF-α into the blood, from which they can enter the liver (Lelubre, Anselin, Zouaoui Boudjeltia, Biston, & Piagnerelli, 2013; Pfafflin & Schleicher, 2009; Vigushin, Pepys, & Hawkins, 1993). In the liver, these cytokines can stimulate the production of CRP, which circulates in the blood as a pentamer and promotes the repair of traumatized local tissue (Lelubre et al., 2013; Ridker, 2016). The present study found that the levels of IL-1β, IL-6, and TNF-α at donor sites were upregulated after surgery, thus explaining the reason that CRP levels were higher in the Pro-Grafting than in the Grafting Only group.
CRP can directly activate the complement system, inducing apoptosis, activating vascular cells, and stimulating monocyte recruitment (Calabro, Chang, Willerson, & Yeh, 2005; Juma et al., 2011; Kuta & Baum, 1986; Lelubre et al., 2013; Meuwissen et al., 2006). All of these activities can promote repair processes in adipose tissue (Gurtner, Werner, Barrandon, & Longaker, 2008; Spiller & Koh, 2017). However, the long-term volume retention rate of free fat grafting was lower in the Pro-Grafting than in the Grafting Only group. The blood supply is better at donor than at recipient sites in the Pro-Grafting group, as the groin fat pads have better blood supply due to the femoral artery than the back. After surgery, donor sites released IL-1β, IL-6, and TNF-α into the blood, upregulating CRP levels, which promote donor site repair (Lelubre et al., 2013; Ridker, 2016). Thus, vascular cell activation and monocyte recruitment may be greater at donor than at recipient sites. Indeed, the present study found that the repair process was faster at donor than at recipient sites.
However, the numbers of these cells and their ability to mobilize have been reported to be limited for a short period of time after fat transplantation (Cai et al., 2017; Pitchford et al., 2010; Wengner et al., 2008). As most cells from the circulation and bone marrow initially moved into donor sites for reconstruction, the numbers of cells infiltrating into recipient sites during early stages were insufficient for repair at these sites. Reconstruction and regeneration at recipient sites had to await repair at donor sites. Thus, monocyte infiltration and tissue reconstruction at recipient sites began after the end of these processes at donor sites. The numbers of macrophages were increased at later time points in the Pro-Grafting group, parallel to the sudden decrease in graft volume. Macrophages are involved in fat resorption (Kato et al., 2014; Mashiko & Yoshimura, 2015). The numbers of macrophages at recipient sites were limited at early time points due to the poor blood supply at these sites. This limitation was alleviated at later time points, allowing macrophages to attack the graft. Consequently, the level of macrophages increased and graft volume simultaneously decreased at later time points. Thus, the long-term volume retention rate of free fat grafting was lower in the Pro-Grafting than in the Grafting Only group.
Comparisons of repair processes at donor and recipient sites suggest that macrophage infiltration during the early stages of fat grafting is key to the repair process. Rapid infiltration and rapid evacuation of macrophages may lead to better tissue vascularization and regeneration after fat grafting (Anghelina et al., 2006; Modarai et al., 2005). Based on this study, we propose two clinical recommendations. First, injury at the donor site should be minimized by, for example, reducing the area of liposuction. Second, other surgical procedures should not be performed at the same time as large-volume fat grafting. Third, doctors could use macrophages assisting grafting after liposuction in the clinic. These may lead to better clinical outcomes and faster recovery.
ACKNOWLEDGMENTS
This study was supported by the National Nature Science Foundation of China (81601702, 81671931, 81772101, 81701920, 81801933, 81801932, 81871573, 81901976, 81901975, 81971852), the Medical Scientific Research Foundation of Guangdong Province of China (A2020542) and the Administrator Foundation of Nanfang Hospital (2019B021).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Z. D., J. G., and F. L. designed the research. Z. W., Y. C., S. Z., X. C., and Y. L. performed the research. J. G. and Y. Y. performed the histology. F. L. and Z. D. analyzed data. Y. L. and Y. C. wrote the paper. X. W. and Z. W. collected and assembled the data. J. G. has statistical expertise. Z. D. and Y. L. revised the manuscript critically. Z. D., F. L., and J. G. finally approved the article. All authors read and approved the submitted manuscript version.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The data used to support the findings of this study are included in the article.




