Mesenchymal stromal cells; a new horizon in regenerative medicine
Abstract
In recent decades, mesenchymal stromal cells (MSCs) biomedical utilizing has attracted worldwide growing attention. After the first report of the human MSCs obtaining from the bone marrow (BM) tissue, these cells were isolated from wide types of the other tissues, ranging from adipose tissue to dental pulp. Their specific characteristics, comprising self-renewality, multipotency, and availability accompanied by their immunomodulatory properties and little ethical concern denote their importance in the context of regenerative medicine. Considering preclinical studies, MSCs can modify immune reactions during tissue repair and restoration, providing suitable milieu for tissue recovery; on the other hand, they can be differentiated into comprehensive types of the body cells, such as osteoblast, chondrocyte, hepatocyte, cardiomyocyte, fibroblast, and neural cells. Though a large number of studies have investigated MSCs capacities in regenerative medicine in varied animal models, the oncogenic capability of unregulated MSCs differentiation must be more assessed to enable their application in the clinic. In the current review, we provide a brief overview of MSCs sources, isolation, and expansion as well as immunomodulatory activities. More important, we try to collect and discuss recent preclinical and clinical research and evaluate current challenges in the context of the MSC-based cell therapy for regenerative medicine.
1 INTRODUCTION
Today, researchers are exploring for steady, safe, and highly available stem cells source with a huge capacity for regenerative medicine (Frenette, Pinho, Lucas, & Scheiermann, 2013). The isolated cells from mouse bone marrow (BM) upon culture demonstrated plastic adherence possessions and shaped spindle-shaped colonies known as colony forming unit fibroblasts (Via, Frizziero, & Oliva, 2012). Because of their remarkable differentiation potentials into specialized cells emerging from mesoderm, they were referred to as mesenchymal stromal cells (MSCs; Vodyanik et al., 2010). Mesenchymal stromal cells are unique types of the multipotent cells found in varied types of the human tissues with great ability to differentiate into several adult cell lineages (Gazit, Pelled, Sheyn, Yakubovich, & Gazit, 2019; Figure 1). Their special properties, such as self-renewality, multipotency, and availability in association with little ethical concern highlight their significance in the context of regenerative medicine (Hasan et al., 2018; Marofi, Vahedi et al., 2019). After the first report of the hMSCs isolation from BM tissue (Pittenger et al., 1999), researchers have been able to isolate them from a wide range of adult tissues, such as perivascular area (Crisan et al., 2008; Kugo, Moriyama, & Zaima, 2019). While there is not a distinct description and quantitative assay, providing MSCs recognition in diverse cells population (Saalbach & Anderegg, 2019), the International Society for Cellular Therapy has offered minimum principles to describe MSCs. These criteria include presenting plastic adherence property, expressing CD73, D90, CD105 without expressing CD14, CD34, CD45 and human leukocyte antigen-DR (HLA-DR) and (c) being able to differentiate in vitro into adipocyte, chondrocyte, and osteoblast. The listed features are usable for all types of MSCs, however, some differences found in MSCs isolated from several cell sources (Ullah, Subbarao, & Rho, 2015). In the current review, we present a brief review of MSCs source, isolation, and expansion and immunomodulatory activities. More important, we try to collect and discuss recent preclinical and clinical research in the context of MSC-based regenerative medicine.
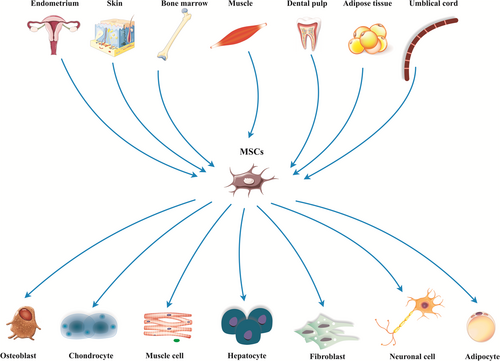
2 DIFFERENCES BETWEEN MSCs ISOLATED FROM VARIOUS TISSUES
MSCs are found in both fetal tissues and numerous adult tissues with some exclusion. Well-organized populations of MSCs have been isolated from BM (Marofi, Hassanzadeh et al., 2019; Park et al., 2012). Moreover, cells showing MSCs minimal properties were isolated from adipose tissue (AT; Kraskiewicz et al., 2020; Wilson, Chee, Butler, & Boyd, 2019; Zuk et al., 2002), dental pulps (DP; Tsutsui, 2020), endometrium (Zhu et al., 2019), peripheral blood (Jain et al., 2020), skin (Hartwig et al., 2019), placenta (Silini et al., 2017), umbilical cord (UC; Storms et al., 2017; Tan et al., 2019), synovial fluid (SF; Baboolal et al., 2016), muscles (Jackson et al., 2009), Wharton's jelly (WJ; Tantrawatpan et al., 2013), and so forth. Although MSCs isolated from various tissue commonly have comparable rates of the surface antigen expression, Jin et al. found that umbilical cord-derived MSCs (UC-MSCs) had the highest levels of cell progression, and dramatically lower expression of p53, p21, and p16, participating in cell cycle regulation and proliferation, compared with MSCs isolated from BM and AT in rat models (Jin et al., 2013). In another study, Wang et al. showed that cell development and clonality of the BM-MSCs and WJ-MSC were more prominent than AD-MSCs; on the other hand, they found that WJ-MSCs had remarkable immunosuppressory potential through suppressing of the activation of Th1 and Th17 but not Th2 and Treg (Wang et al., 2016). Moreover, a study on the MSCs isolated from BM, UC, AT, and tendons revealed that MSC numbers achieved from AT, tendon or UC were 222-fold higher than those achieved from BM. Furthermore, MSCs isolated from tendon and AT present most swift proliferation, as well as osteogenic differentiation was more obvious in BM-MSCs compared with the cells derived from other tissues (Burk et al., 2013). Conversely, Wehrle et al. reported that the osteogenic differentiation capability of the adipose tissue-derived MSCs (AT-MSCs) was more prominent than UC and BM-derived MSCs in three-dimensional (3D) cell culture using hydrogel (Wehrle et al., 2019). Other analyses between BM-MSCs, SF-MSCs, and AT-MSCs indicated that although BM-MSCs had the highest migration and wound healing abilities, cell proliferation rate in SF-MSCs was more prominent that BM and AT derived cells. These results signified that while BM, SF, and AT derived stromal cells are a dependable source cell-based therapy in regenerative medicine, they support various functional characteristics (Arevalo-Turrubiarte, Olmeo, Accornero, Baratta, & Martignani, 2019). In another research, neural differentiation capacity of AT, BM, skin, and UC isolated MSCs were evaluated to achieve perfect candidate for cell-based therapy in neurodegenerative diseases. Based on results, although MSCs from various sources can differentiate into neuron-like cells and differentially present progenitors and mature neural markers, AT- MSCs showed the highest rate of neural markers expression and also proliferation (Urrutia et al., 2019). In 2014, Stanko et al. found that the proliferation levels of DP-MSCs and UC-MSCs were more visible than BM-MSCs and AT-MSCs, but there were some differences in gene expression patterns of DP-MSCS compared with BM-MSCs, AT-MSCs, and UC-MSCs (Stanko, Kaiserova, Altanerova, & Altaner, 2014).
Interestingly, the oral cavity hosts varied cell populations' showing MSCs like-attributes. Because oral tissues are relatively easy to access and the number of isolated cells is higher, so they are a commendable and attractive source for therapeutic research (Xiao & Nasu, 2014). In this context, human periapical cyst MSCs (hPCy-MSCs) display features similar to other dental-derived MSCs, entailing their widespread proliferative capacity, cell surface marker profile and the potential to differentiate into several cell types, in particular, osteoblasts, chondrocyte and neural cells (Tatullo et al., 2017). Prominently, hPCy-MSCs are simply achieved from the surgically removed periapical cysts and exert anti-inflammatory and trophic impacts by the releases of the several immunosuppressive agents, providing self-regulated healing procedure in injured tissues and preparing a regenerative microenvironment (Yazid, Gnanasegaran, Kunasekaran, Govindasamy, & Musa, 2014). Regardless of the various barricades to their clinical use, MSCs, such as DP-MSCs have exposed satisfactory aptitude to further investigate in the field of regenerative medicine (Ballini, Cantore, Scacco, Coletti, & Tatullo, 2018). In sum, we believe that the special types of MSCs from particular sources according to the patient's conditions and disease properties must be administered to provide a promising therapeutic outcome.
3 MSCs EXPANSION IN CULTURE
MSCs can be isolated commonly easily from BM and AT of patients, aiming autologous transplantation, or from allogeneic donors (Mazini, Rochette, Amine, & Malka, 2019). For example, MSCs harvesting from BM and mononuclear cells (MNCs) is conducted using Ficoll density gradient centrifugation (Figure 2) (Bieback, Schallmoser, Klüter, & Strunk, 2008). Although other sources of the MSCs, in particular, DP and skin, are less invasive source than BM and AT (Ledesma-Martínez, Mendoza-Núñez, & Santiago-Osorio, 2016; Mastrolia et al., 2019), a much smaller amount of starting materials are harvested, and thereby cells need to expand in an expensive process before being transplanted. To date, it is not entirely responded whether the MSCs retain all of their biological properties and demonstrated their normal physiological activities after transplantation and whether they can generate a perfect lineage of required cells (Pittenger et al., 2019).
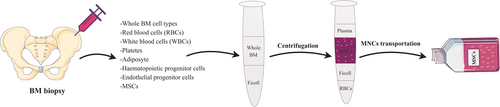
To in vitro MSCs expansion for clinical use, the existence of the appropriate culture system and cell expansion according to a good manufacturing practice grade protocol, supporting quality and long-term proliferation of MSCs is enormously important (Neri, 2019; Phinney & Galipeau, 2019). Moreover, the culture strategy must be cost-effective such that it could be utilized to many experimental and therapeutic workflows (Russell, Lefavor, & Zubair, 2018). The ex-vivo expansion of MSCs, which performed directly following cell isolation, supports the free ex vivo proliferation of MSCs to achieve adequate numbers for transplantation (Fekete et al., 2012). Based on investigations, MSCs can proliferate for about 19 doublings in culture system without showing cellular senescence associated with growth arrest and apoptosis (Pountos, Corscadden, Emery, & Giannoudis, 2007). But, expansion has exposed to steadily mitigated MSCs normal physiological functions and maximal differentiation capability (Fafián-Labora, Morente-López, & Arufe, 2019; Parekkadan & Milwid, 2010). Although it has already been revealed that MSCs lifespan could be improved for about 260 doublings, other results verified that prolonged culture leads to spontaneous transformation and obtaining tumorigenic ability (Pountos et al., 2007). Undoubtedly, the medium used is the most significant factor for the successful expansion of MSCs. Culture medium generally contains a basal medium containing glucose, amino acids and ions comprising calcium, magnesium, potassium, sodium, and phosphate in association with fetal animal sera in concentrations of 5–20% (Pountos et al., 2007). In 2017, Caroti et al. presented a reproducible expansion method to establish murine MSCs (mMSCs) exerting hypoxia and basic fibroblast growth factor (FGF) supplementation. Upon this condition, cells were expanded 2.8 times quicker than under ordinary methods and the mMSCs exhibited attenuated senescence and preserved their multipotency and differentiation capability (Caroti et al., 2017). Also, dynamic MSC culture expansion has been described as an advanced culture system that not only maintains cell unique features but also reduces contact-inhibition of cell progression, leading to an augmentation in cell numbers (Majd, Quinn, Wipff, & Hinz, 2011). In another study, Narcisi et al. found that WNT3A signaling proteins in association with FGF2 promote the prolonged expansion of the BM-MSCs. These results demonstrated that by supporting multipotency during expansion and averting hypertrophic maturation following differentiation, the modification of the WNT signaling pathway eliminates two main hindrances, impeding MSCs clinical application in cartilage repair (Narcisi et al., 2015). Another study showed that human platelet lysate (hPL) accelerates the fast expansion of MSCs without showing any adverse effect on cell immunophenotype. Interestingly, cellular rejuvenation indicated by diminished doubling time as well as smaller cell size was exhibited following aged and senescent MSCs culture using the hPL (Griffiths, Baraniak, Copland, Nerem, & McDevitt, 2013). While the use of fresh platelet units for platelet lysate (PL) manufacturing increases concerns as the restricted accessibility for platelet donors, scientists described expired units as an alternative candidate for PL production (Astori et al., 2016). In total, minimal manipulation settings have not been exactly described for the assemblage and isolation of several sources of the MSCs. On the other hand, in the context of cells ex vivo expansion, there exist three major problems, including using supplemented cell growth media, enzymatic treatment, and long-term cell expansion, which finally affect MSCs quality.
4 MSCs IMMUNOMODULATORY FUNCTIONS
Considering preclinical studies, scientists believe that MSCs can modify immune responses during tissue repair and preparer an appropriate soil for tissue repair as well as regeneration (Han et al., 2019). Although a large number of preclinical studies have been revealed the immunosuppressory effect of the MSCs, their acting mechanisms and involved signaling pathways have not been entirely clarified. However, it seems that various soluble factors and cell contact-related process in response to the immune cells participate in MSCs immunomodulatory activities (Figure 3) (Joel et al., 2019; Sobacchi, Palagano, Villa, & Menale, 2017). Based on reports, MSCs adjust the adaptive and innate immune responses upon inhibition of T cells and maturation of dendritic cells (DCs), decreasing B-cell activation and progression, as well as suppressing activation and cytotoxicity of NK cells in association with an elevation in T regulatory (Treg) cells proliferation and function by soluble factors or cell-cell contact mechanisms (Cheung, Galleu, von Bonin, Bornhäuser, & Dazzi, 2019; Gao et al., 2016). Varied types of the soluble factors have been suggested to participate in the immunosuppression process, comprising transforming growth factor-β1 (TGF-β1), prostaglandin E2 (PGE2), hepatocyte growth factor (HGF), indoleamine-pyrrole 2, 3-dioxygenase (IDO), nitric oxide (NO) and interleukin-10 (IL-10; Cheung et al., 2019; Gao et al., 2016; Liu et al., 2010). Considering other results, proinflammatory cytokine interferon-γ (IFN-γ), alone or in relation with tumor necrosis factor-α (TNF-α), IL-1α or IL-1β, triggers MSCs to release several enzymes and soluble factors, including cyclooxygenase 2, PGE2 and IDO, facilitating immunosuppressive functions (Gao et al., 2016). PGE2 suppresses T-cell proliferation (Coimbra, Junger, Liu, Loomis, & Hoyt, 1995) and IDO modify proliferation and activation of immune cells through catalyzing breakdown of tryptophan (Gualdoni et al., 2019). In 2007, Sato et al. reported suppression of T cell proliferation following inhibition of STAT5 phosphorylation by MSCs-secreted NO (Sato et al., 2007); On the other hand, NO and NO-derived reactive nitrogen species can interact with various enzymes, ion channels as well as receptors (Edwards & Rickard, 2007). Also, it has a prominent role in macrophage activities, TCR signaling, cytokine receptor expression, T cells phenotypes (Niedbala, Cai, & Liew, 2006). Other results verified by consequences from a murine sepsis model revealed that MSCs administration ameliorates bacterial clearance by the promotion of neutrophil migration and phagocytic activity following an increase in IL-6, IL-8, and GM-CSF levels, leading to infection removing and enabling tissue repair and regeneration (Joel et al., 2019). Although various soluble factors are identified to participate in the MSCs immunomodulation, the exact correlation between theses has not been understood. The impacts of the soluble factors on MSCs functions may differ depending on the MSCs sources (Eljarrah, Gergues, Pobiarzyn, Sandiford, & Rameshwar, 2019) and target microenvironment (Kusuma, Carthew, Lim, & Frith, 2017). For instance, Cheng et al. described that the immunomodulatory potential of AT-MSCs was more prominent than BM-MSCs on anti-CD3/CD28-stimulated peripheral PBMC proliferation; On the other hand, AT-MSCs were more effective than BM-MSCs on suppression of monocytes differentiation to DCs (Cheng, Ghetu, Wallace, Wei, & Liao, 2014). In another study, analysis exposed high levels of IL-2, IL-4, IL-13, and GM-CSF cytokines in chorionic plate-derived MSCs compared with AT-MSCs and BM-MSCs (Lee et al., 2012). Additionally, Fazekasova et al. reported that imunosuppressory potential of the BM-MSCs is stronger than placenta-derived MSCs, and described BM-MSCs as appropriate candidates to use in tissue repair and regenerative medicine due to their remarkable inhibitory effect on T cell activation as well as proliferation (Fazekasova, Lechler, Langford, & Lombardi, 2011). Overall, while there is not any disagreement about the MSC therapy immunosuppressory properties, further clarification of the detailed biological mechanisms participated in these characteristics is of paramount importance.

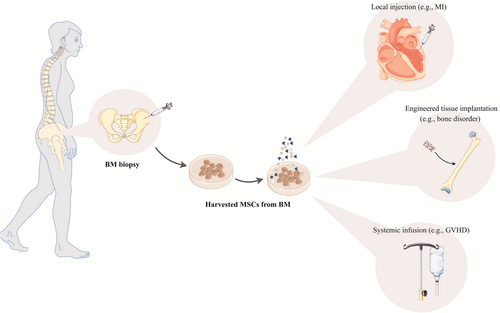
5 MSCs IN REGENERATIVE MEDICINE
Since MSCs facilitate tissue repair, progression, wound healing, and cell replacement, it could be employed widely in regenerative medicine. Though MSCs have been applied extensively depending on their special application through various routes in clinical trials to achieve promising therapeutic consequences, the appropriate route to use MSCs to acquire their maximum advantages has not yet been exclusively determined (Figure 4).
5.1 MSCs in neurodegenerative disorders
MSCs hold the desired ability for cell therapy due to their potential to expand ex vivo and safely grafted autonomously after isolation from a wide spectrum of the human adult tissues (Wei et al., 2013). Importantly, MSCs can be differentiated into neural fates and generate and release comprehensive types of soluble factors, enabling nervous tissue repair and regeneration. In the last decade, various beneficial effects were showed upon hMSCs transplantation into rodent models of neurodegenerative diseases, including neurotrophic factor-mediated protection, promoted neurogenesis, suppression of inflammation, and toxic collected proteins clearance (Sadan, Shemesh, Cohen, Melamed, & Offen, 2009). Also, researchers try to use ex vivo manipulation for boosted expression of possibly favorable factors, aiming to promote MSCs homing toward the injured area (Torrente & Polli, 2008). Though neurodegeneration is commonly identified through advanced neuronal loss, several neurodegenerative diseases demonstrate particular neuronal pathologies. Parkinson's diseases (PD) entail dopaminergic (DA) neurons degeneration in the substantia nigra (SN), amyotrophic lateral sclerosis (ALS) is characterized by motor neurons loss in the brainstem and spinal cord, global neuronal degeneration in the cerebral cortex and hippocampus is found in Alzheimer's diseases (AD), and Huntington's diseases involve projection neurons loss in the dorsal striatum (Volkman & Offen, 2017). Chierchia et al. examined rat AT-MSCs neuroprotective potentials against pro-oxidizing compounds, in particular, hydrogen peroxide or the DA neurons selective toxin 6-hydroxydopamine exerted to mimic PD neurodegeneration. They found that a reduction in reactive oxygen species (ROS) cellular levels in association with an increase in the expression of antioxidant enzyme sirtuin 3 accelerates possible AT-MSCs-mediated neuroprotection (Chierchia et al., 2017). In another study, Bodart-Santos et al. found that EVs secreted by human Wharton's jelly mesenchymal stromal cells (hMSC-EVs) preserved hippocampal neurons from ROS and synapse injury stimulated by amyloid β oligomers in AD. They found that catalase contained in EVs play a key role in neuroprotection mediated by hMSC-EVs (Bodart-Santos et al., 2019). On the other hand, beneficial effects of the intranasal administration of MSCs were reported through a significant enhancement in DA neurons numbers in the SN of a mouse model of PD (Salama et al., 2017). In 2014, Shin et al. showed that MSC administration meaningfully diminished the level of Aβ in the hippocampus of the mouse model of AD, leading to an elevation in the viability of hippocampal neurons. Moreover, upregulated Beclin-1 expression in AD models indicates that ameliorated autophagy and promoted Aβ clearance in AD models promote neuronal viability toward Aβ toxicity (Shin et al., 2014). In another study on a murine model of multiple sclerosis using placenta-derived MSCs (PMSCs) and PMSC-EVs, the motor function of treated animals was reinforced compared with the untreated animals (control group). Moreover, PMSC-EVs improved myelin regeneration upon induction of endogenous oligodendrocyte progenitor cells differentiation toward mature myelinating oligodendrocytes (Clark et al., 2019).
In Phase 2 randomized controlled trials, 64 participants with ALS were enrolled. In addition to the verifying of the safety of intrathecal (IT) injections of BM-MSCs in the treated group, this group presented attenuated proinflammatory and boosted anti-inflammatory cytokines. Moreover, TGF-β1 dramatically exhibited an inverse association with monocyte chemoattractant protein-1. However, results did not show any evidence indicating MSCs administration significant effect on the long-term survival of treated subjects. Overall, IT injections of BM-MSCs exhibited a probable clinical advantage lasting at least 6 months by switching from pro- to anti-inflammatory situations in patients with ALS (Oh et al., 2018). In another Phase 1/2 trial using induced MSCs to secrete neurotrophic factors (MSC-NTF), six participants with early-stage ALS were injected intramuscularly (IM) and six patients with more advanced disease were transplanted IT. In the second step, a Phase 2a dose-escalating study, 14 patients with early-stage ALS delivered a combined IM and IT transplantation of autologous MSC-NTF cells. Results verified the safety of MSC-NTF cells in all participants and exposed a reduction in the progression of ALS in 13 of the 14 patients participated in Phase 2a study (Petrou et al., 2016).
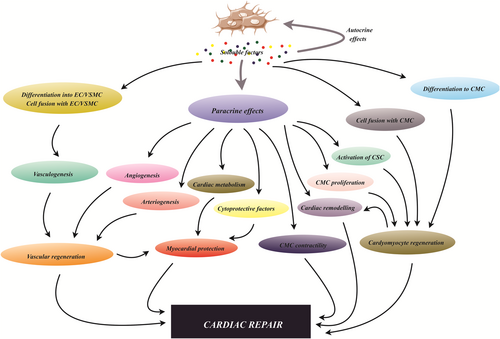
5.2 MSCs in cardiovascular disorders
MSCs can be differentiated into cardiomyocytes, endothelial cells as well as vascular smooth muscle cells through a wide spectrum of the paracrine effectors, enabling cardiac repair and regeneration (Figure 5; Taylor & Robertson, 2009). These stromal cells can stimulate the generation of reparative growth factors, substitute injured cells and establish an environment that supports endogenous cardiac repair. 5-Azacytidine, a well-known inhibitor of DNA methylation, is a chemical agent that is able to stimulate BM-MSCs differentiation into cardiomyocytes in murine models (Jia, Chang, Sun, Li, & Guo, 2020; Makino et al., 1999). Moreover, the stimulatory effects of the miR-1a overexpressing on BM-MSCs differentiation toward myocardial cells in the particular conditioned medium have been evidenced (Zhao, Yang, Ma, & Dong, 2016). Other reports have exposed that IL-1β participates in pathogenesis, progression, and function of cardiomyocytes in damaged heart and could mediate the neovascularization process following myocardial infarction (MI; Guo et al., 2018). A recent study conducted by Lu et al. demonstrated that nestin-positive BM-MSCs administration ameliorated cardiac function in an acute myocardial infarction mouse model through induction of cardiac endothelial cell migration to the infarcted border area using CXCL12/CXCR4 chemokine pathway (Lu et al., 2019). In another study, the theory indicating the central role of MSCs-secreted soluble factor in their cardioprotective effect was assessed by evaluation of the impacts of the encapsulated and free MSCs in the rat model of MI. Investigation indicated that MSC-derived growth factors and cytokines play a key role in cardioprotection provoked by MSC in rat models of MI. These consequences suggested that the use of life, but encapsulated, cells for MI treatment has the potential to substitute with a heart-targeted-persistent transfer of growth factors and cytokines (Karpov et al., 2019). Similarly, Han et al. found that human UC-MSC-derived exosomes encapsulated in hydrogels could improve maintenance and permanence of the exosomes and reinforce cardiac function in a rat model of MI (Han et al., 2019). Other reports described that cardiac MSCs-secreted exosomes not only retain acute ischemic myocardium from reperfusion damages, but also their intramyocardial injection after MI improves cardiac angiogenesis, boosts cardiomyocyte progression, and support heart function (Ju et al., 2018). On the other hand, Deng et al. showed that administration of exosomes from AT-MSCs diminished cardiac injury upon mitigation of cardiac dysfunction, cardiac apoptosis as well as fibrosis, and also inhibition of inflammatory responses in preclinical and clinical models of MI. Moreover, they suggested that M2 polarization in association with overexpression of NFκB and TGF-β1, in turn, led to the suppression of cardiac fibrosis and the inflammatory response elicited by MI (Deng et al., 2019). Another study on mice models of MI demonstrated that SDF1 overexpression in MSCs-derived exosomes suppressed apoptosis and autophagy of ischemic myocardial cells and augmented endothelial cells generation through activation of the phosphoinositide 3-kinase signaling pathway (Gong, Liu, Wang, Liang, & Wang, 2019).
A Phase 1/2 randomized controlled trial in 30 participants with compensated HF in dilated phase with the aim to evaluate differences between intravenous (IV) inaction of allogeneic UC-MSCs and BM-MSCs showed a remarkable upsurge in the expression of HGF, which induce myogenesis, cell migration as well as immunosuppression, in UC-MSC-treated group compared with BM-MSC-treated group. In addition to the verification of the IV administration of UC-MSCs for patients with HF, advancement in left ventricular function and life quality was detected in patients treated with UC-MSCs (Bartolucci et al., 2017). Another trial in six patients undergoing coronary artery bypass grafting revealed that the IM administration of autologous MSCs led to a concordant amelioration in regional activity, tissue perfusion, and eventually fibrotic burden (Karantalis et al., 2013). Another pilot study in 10 patients with chronic myocardial ischemia showed that not only autologous MSCs could be safely grafted to the hearts of these patients, but also may be concomitant with advances in cardiac function and Left ventricle remodeling (Guijarro et al., 2016).
5.3 MSCs in bone disorders
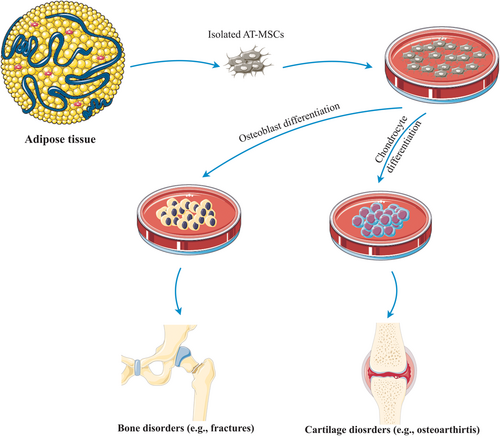
MSCs play a fundamental role in bone repair and regeneration upon differentiation into bone-osteoblasts and chondrocytes, shaped bone, and cartilage tissue, respectively (Figure 6). Cartilage provides a template for required bone generation by the endochondral ossification process, enabling the replacement of the developing cartilage by bone to shape the growing skeleton (Scotti et al., 2010). According to molecular research, special transcription factors support differentiation of MSCs into osteoblasts and augment the specific-cell type differentiation procedure via induction of enhancement in the expression of genes associated with the specific cell type. Impairment of the bone formation resulting from runt-related transcription factor 2 (runx2 or Cbfa1) and transcription factor Sp7 (SP7 or Osx) deficiencies signifies their essential role in triggering of the osteogenesis (Sepulveda et al., 2017); on the other hand, both the canonical BMP signaling and noncanonical BMP signaling associated in the process of fate determination of the MSCs (Hu et al., 2018). Though healing and regeneration of bone impairments, ranging from fractures to bone cancer, is an intricate process, using of osteoinductive and osteogenic growth factors and MSCs currently have attracted significant attention. Novel methods of tissue engineering and regenerative medicine employ tissue scaffolds, healing-inducer growth factors, such as FGF-2 and IGF in association with MSCs to stimulate bone repair and regeneration (Oryan, Kamali, Moshiri, & Baghaban Eslaminejad, 2017). In this regard, synthesis and fabrication of special scaffolds from biodegradable poly glycerol sebacate and osteoinductive nanosilicates is described. It has already been found that nanocomposite scaffolds promote cell adhesion, propagation, and proliferation and improve the osteogenesis process. It seems that the augmentation of alkaline phosphatase (ALP) function and generation of matrix mineralization may be responsible for the promotion of the MSCs differentiation into osteoblast (Kerativitayanan et al., 2017). Taken together, Barry et al. proposed that the merging of bioactive nanomaterials within a biomaterial scaffold demonstrates ability for the progression of localized and prolonged treatment for patients suffered from bone disorders, such as osteoporosis (Barry, Pearce, Cross, Tatullo, & Gaharwar, 2016).
A large number of research support the evidence of MSCs ability to reinforce osteoinduction and osteogenesis, but little evidence has been obtained from clinical trials (Oryan et al., 2017). To evaluate the potential of hMSCs to improve a clinically significant bone defect, Bruder et al. implanted hBM-MSCs into the femurs of adult athymic rats. They found an elevated bone formation during 12 weeks, providing the proof of hMSCs capability to regenerate bone in a clinically substantial imperfection (Bruder et al., 1998). In another study, Linero and Chaparro examined the potential of AT-MSCs and their conditioned medium (CM) to ameliorate surgical bone injuries utilizing rabbit mandibles. The analysis revealed that both AT-MSC and CM stimulate bone regeneration in the caused wounds in the rabbit's jaws, signifying that AT-MSC repair bone lesions commonly upon secreting paracrine soluble factors (Linero & Chaparro, 2014). Another study verified the therapeutic capacity of human dental pulp-MSCs in the surgically stimulated rabbit temporomandibular joint lesions about 9 weeks after transplantation (Mohammed et al., 2019). Another study conducted by Lee et al., aiming to compare the osteogenic ability and bone repair potential of BM-MSCs and DP-MSCs in rabbit calvarial bone defect models, evidenced that although mineral deposition and expression rates of osteogenic marker genes, such as ALP and RUNX2 were promoted in BM-MSCs compared with DP-MSCs, the bone volume fraction in both MSC groups was meaningfully superior to the control group (Lee, Chan, Hsieh, Lew, & Feng, 2019). Furthermore, it has been found that lipocalin-2 and prolactin, isolated from BM-MSCs, adjust their functions, moderate BM-MSCs cellular senescence, and consequently can promote calvarial defects healing in mice (Tsai & Li, 2017). Similarly, other reports showed that that the overexpression of miR-135 (Xie et al., 2016) and miR-375 (Chen et al., 2019) in AT-MSCs robustly improved the osteogenic marker genes presentation as well as extracellular matrix calcium (ECM) deposition through induction of the miR-135/Hoxa2/Runx2 pathway (Xie et al., 2016) and inhibition of insulin-like growth factor binding protein 3 (Chen et al., 2019), respectively. Between 1986 and 1995, BM transplantation was employed for osteogenesis in 100 participants with tibial fractures, however first, Horwitz and his experimental team observed allogeneic BM-MSCs clinical efficacy in children with osteogenesis imperfecta (Oryan et al., 2017). In 2010, a Phase 1 study in 12 patients with lower limb long bone nonunion verified the safety of autologous BM-MSCs for treating nonunion; on the other hand, 3/12 (25%) patients exhibited the evidence of the occurrence of the bony union (Emadedin et al., 2017). Other clinical trials demonstrated that MSCs administration in combination with PL product can induce regeneration of atrophied mandibular (Gjerde et al., 2018) and reconstruction of long bone nonunion (Labibzadeh et al., 2016) without showing any adverse effect.
5.4 MSCs in cartilage disorders
The MSCs administration to repair and regenerate cartilage tissue is due to their capability to perform as chondroprogenitor cells to substitute damaged cartilage or as regenerative cells to induce cartilage tissue regeneration using endogenous cells (Figure 6; O'Sullivan, D'Arcy, Barry, Murphy, & Coleman, 2011). MSC chondrogenic differentiation is exactly modified upon the coordination of various growth factors, cytokines, and signaling molecules. In addition to the varied signaling molecules comprising BMPs, SRY-related high-mobility group-box gene 9, parathyroid hormone-related peptide, Indian hedgehog, fibroblast growth factor receptor 3, and β-catenin, other factors including adenosine, O2 tension, and ROS levels are responsible for the cartilage generation (Li & Dong, 2016). In vitro research predominantly focus on BM-MSCs, followed by AT-MSCs and SF-MSCs due to their relatively easy separation and closeness to cartilage and related joints (Lo Monaco et al., 2018).
Studies to compare the chondrogenic potential of MSCs isolated from various sources revealed that BM-MSCs have superiority over other types of MSCs. These dissimilarities can be addressed by differences in the pattern of gene expression as well as signaling pathway activation (Lo Monaco et al., 2018). As inflammation resulting from synovial macrophages and infiltrated lymphocytes functions is the most pivotal hallmark of osteoarthritis (OA) described as a common form of arthritis, MSCs using because of their immunomodulatory properties is proposed as an authentic therapeutic option for OA treatment (Koh et al., 2013). In 2018, Zhang et al. examined cellular procedures affected by MSC exosomes and working mechanisms responsible for cartilage repair-mediated by theses exosomes (Zhang et al., 2018). They showed that exosome-induced repair of osteochondral abnormalities was identified by improvement in cellular proliferation and infiltration and promotion in matrix generation as well as regenerative immune phenotype. In total, they suggested that activation of AKT and extracellular signal-regulated kinase (ERK) signaling in association with the superiority of the infiltration of CD163-positive regenerative M2 macrophages over CD86-positive M1 macrophages, leading to decreasing in proinflammatory synovial cytokines IL-1β and TNF-α, play a central role in the process of the MSCs-exosomes-mediated cartilage repair (Zhang et al., 2018). Based on other investigations, Intra-articular (IA) injections of MSCs weaken cartilage degeneration and suppress inflammation reactions commonly exerting paracrine mechanisms in OA murine models (Mancuso et al., 2019). Similarly, Sato et al. showed that IA transplantation of MSCs suspended in hyaluronic acid in the knee joints of the pigs models of OA, enhanced MSCs differentiation into chondrocyte cells, boosted the expression of collagen type 2, and finally stimulate cartilage repair, partially (Sato et al., 2012). Another study in rat models of OA demonstrated that periodic, but not single injections of SF-MSCs sustained viable cells and suppressed OA development through induction of an enhancement in the expression of proteoglycan 4, BMP-2, and BMP-6 genes involved in chondroprotection, and TNF-stimulated gene 6, acting as inflammation suppressor gene (Ozeki et al., 2016). Likewise, other reports described that IV injection of hUC-MSCs expressing miR-140-5p (Geng et al., 2019) and AT-MSCs transplantation (Sasaki, Mizuno, Mochizuki, & Sekiya, 2019) stimulate cartilage repair in the rat (Geng et al., 2019) and dog models of OA (Sasaki et al., 2019), respectively.
A Phase 1/2 randomized clinical trial completed between 2012 and 2014 demonstrated that single intraarticular (IA) injection of autologous BM-MSCs was a safe and practical therapeutic method, providing long-term clinical and functional amelioration of knee OA (Lamo-Espinosa et al., 2018). Another study in 30 patients with OA exhibits that allogeneic BM-MSC therapy could be an effective alternative for OA treatment logistically more suitable than autologous MSC treatment (Vega et al., 2015). Similarly, other pilot and Phase 1/2 trial verified feasibility and safety of UC-MSCs application in knee OA following 12 months follow-up (Matas et al., 2019).
5.5 MSCs in wound healing
To sustain the human body's physiological homeostasis, the existence of healthy skin is of paramount importance. Chronic circumstances including diabetes mellitus or peripheral vascular disorders provide faulty wound healing (Sorg, Tilkorn, Hager, Hauser, & Mirastschijski, 2017). In this regard, several main phases, such as inflammation, proliferation, epithelialization, angiogenesis, remodeling, and scarring participate in wound repair and regeneration procedures. This is a complicated procedure that contains various cell types and mediators cooperating in a greatly intricate sequential pattern (Reinke & Sorg, 2012). Today, scientist describe MSCs as an appropriate candidate for cell-based therapy in wound healing because of their unique characteristic comprising self-renewal and multi-potency abilities, immunomodulatory activities, mitigation of inflammatory reactions, stimulating of the angiogenesis, adjusting of the ECM remodeling, and finally remarkable migration in association with generation and releasing of soluble growth factors and cytokines (Lee, Ayoub, & Agrawal, 2016). Considering observations, MSCs administration can trigger a sequence of organized cellular procedures, such as angiogenesis, inflammation, cell proliferation and migration, and epidermal terminal differentiation in the non-healing wounds (Sorg et al., 2017). While proinflammatory response supports the inducing of the wound repair process, promoted and persistent inflammation leads to unfavorable effects on skin wound closure. It has already been found that proangiogenic factors released by MSCs, in particular, vascular endothelial growth factor (VEGF) and FGF; uphold viability, proliferation, and branching of vascular cells both in vitro and in vivo (Aydemir, Öztürk, Sönmez, & Tuğlu, 2016). A study in severe combined immunodeficient mice administered by UC-MSCs showed the MSCs reinforced thickness of the regenerated epidermis, improved the dermal ridges and cell numbers in regenerated skin, and shaped healing tissue containing consistent alignment of fibers (Lee et al., 2016). Similarly, Martinello et al. evaluated the healing of the surgical-induced wound in sheep models upon the administration of allogeneic MSCs. They observed that lesions treated with MSCs had higher rates of wound closure and re-epithelialization, proliferation, neovascularization, and elevated contraction compared with the untreated lesions. In addition to the lack of the inflammation in lesions treated with MSCs, they had thicker cutaneous adnexa in comparison to the untreated lesions. Finally, hair-keratine and collagen1 gene expression levels were more prominent in treated-lesion than untreated lesions, signifying MSCs auspicious potential to generate ECM and its-related structural proteins (Martinello et al., 2018). Moreover, it was shown that MSCs genetically engineered to generate VEGF amplified blood flow in the ischemic limb of immune-deficient mice in comparison to the saline controls without presenting any undesired effects (Fierro et al., 2019). In another study, to evaluate the wound healing process using mouse BM-MSCs in C57BL/6 mice, a cutaneous wound was created by a scratch on mice. Based on consequences, treatment with autologous MSCs demonstrated the desired impact on wound healing at optimal dosage of 5 × 104 activated MSCs/8 cm2 of lesion site (Li et al., 2017). On the other hand, two randomized studies in 2009 (Dash, Dash, Routray, Mohapatra, & Mohapatra, 2009) and 2011 (Lu et al., 2011) demonstrated that autologous BM-MSCs injection into the injured limb meaningfully attenuated wound size as well as ulcer-associated wound pain and significantly augmented ulcer healing process compared with the control group. Interestingly, a study conducted by Falanga et al. at Roger Williams Medical Center proved that autologous BM-MSC could be safely and successfully delivered to skin lesions employing a fibrin spray system (Falanga et al., 2007). Results revealed that autologous BM-MSC topical administration induced closure of full-thickness lesions in diabetic mice (Falanga et al., 2007). In 2007, a double-blind, randomized, controlled trial, compared the effect of the BM-MSCs and BM-MSCs-derived MNCs (BMMNCs) in 41 patients with diabetic foot ulcers. In sum, various analyses, such as painless walking time and transcutaneous oxygen pressure support the superiority of the use of BM-MSCs on BMMNCs to stimulate wound healing in patients with the diabetic foot (Lu et al., 2011). Besides, A clinical study in 53 patients with severe symptoms diabetic foot showed a substantial upsurge in neovessels as well as demonstrated complete or partial wound healing 3 months after MSCs administration in the absence of serious adverse effect (Qin, Zhu, Zhang, Zhou, & Wang, 2016).
5.6 MSCs in liver disorders
MSCs transplantation introduces as an emerging therapeutic approach for the treatment of chronic liver disorders, ranging from diseases caused by viruses, such as hepatitis, to cirrhosis and liver cancers (Eom, Shim, & Baik, 2015; Fagone et al., 2016). Though the underlying mechanisms of MSCs transplantation have not yet been clarified, growing evidence has exposed that their special characteristic, such as immunomodulation, inhibition of inflammation, differentiation, and anti-fibrotic functions support liver restoration (Christ, Brückner, & Winkler, 2015). Undoubtedly, there exist tight associations between inflammation and liver disease. After the liver injury, immune cells-secreted pro-fibrotic factors, in particular, TGF-β, platelet-derived growth factor, IL-4, and IL-13, induce hepatic stellate cells (HSC) proliferation, growing ECM formation in the liver (Dewidar, Meyer, & Dooley, 2019). Hence, the anti-fibrotic effects of MSC on HSC are divided into direct or indirect effects. While the indirect anti-fibrotic influences on HSC are realized through MSC regulatory immune cells and consecutively suppressing the function of the HSC, the direct anti-fibrosis influences on HSC are facilitated by MSC suppressing the function of HSC. Upon MSC migration to the sites of inflammation containing high levels of inflammatory cytokine (e.g. IFN-γ and IL-1β), these cells release several soluble factors including NO, PGE2, IDO, IL-6, IL-10, and HLA-G, suppressing varied immune cells activation and proliferation and stimulating of Treg cells (Noronha et al., 2019).
Today, MSCs-differentiated hepatocyte-like cells are proposed as auxiliary sources for liver restoration. It has been verified that various cytokines and growth factors covering HGF, FGF2/4, epidermal growth factor (EGF), dexamethasone, oncostatin M, leukemia inhibitory factor as well as insulin-transferrin-selenium are responsible for hepatocyte-like cells differentiation of MSCs (Afshari, Shamdani, Uzan, Naserian, & Azarpira, 2020). Since ECM of the liver tissue stimulates MSC differentiation, damaged liver tissue enclosed with the ECM is commonly employed for MSC transplantation and differentiation (Kang, Kim, Eom, & Baik, 2019). In a study, Mansour at al. evaluated the hepatic regenerative potential of hUC-MSCs in a rat model of carbon-tetrachloride induced liver fibrosis. In addition to the remarkable immunosuppressory effects of the transplanted cell on the rat immune cells, they observed the cells proper homing in relation with the development of the liver architecture and functional anility in the transplanted rats compared with the control rats (Mansour, Greish, El-Serafi, Abdelall, & El-Wazir, 2019). In another study to examine the efficacy and underlying mechanisms of MSC transplantation in the treatment of liver failure (LF), rats MSCs were isolated and engrafted into the LF rat models. Analysis of the levels of alanine transaminase, aspartate aminotransferase, international normalized ratio, and serum ammonia, implied therapeutic efficacy of the MSCs on LF (Ding et al., 2019). Furthermore, other analyses exposed a positive correlation between the effect of the MSCs engraftment on hepatocyte proliferation with the upregulation of the AKT/GSK-3β/β-catenin signaling pathway in these models (Ding et al., 2019). Other reports presented that MSCs transplantation in a rat model of hepatic ischemia–reperfusion injury (IRI), meaningfully mitigated hepatic neutrophil infiltration and apoptosis-related proteins expression, and finally improved liver pathological damages in these models; on the other hand, MSCs promoted the intracellular activation of p38 mitogen-activated protein kinase (MAPK) phosphorylation, leading to reduction of CXCR2 expression on the neutrophils surface, and lessened neutrophil chemoattractant CXCL2 generation by suppression of nuclear factor-κB p65 phosphorylation in macrophages. In sum, consequences displayed that MSCs improve hepatic IRI by exerting inhibitory influences on hepatic neutrophil migration and infiltration (Li et al., 2018). Furthermore, it was found that BM-MSCs administration diminished IL17, IL2, and IL6 serum proteins level and reduced expression of STAT3, SMAD3, and TGFβR2 genes in liver fibrosis animal models. These results demonstrated that BM-MSCs could treat liver fibrosis upon suppression of the IL6/STAT3 signaling pathway (Farouk, Sabet, Abu Zahra, & El-Ghor, 2018). Other investigations introduced the overexpression of HGF and matrix metalloproteinase-2 (MMP-2) as the underlying mechanism of the MSCs anti-fibrotic characteristics in animal models (Mohamed et al., 2016). In the last years, various clinical trials evidenced the safety and efficacy of UC-MSC administration in acute-on-chronic liver failure patients associated with hepatitis B virus infection (Shi et al., 2012), autologous BM-MSC engraftment in patients with alcoholic cirrhosis (Suk et al., 2016) and allogeneic BM-MSCs transplantation in participants with liver cirrhosis (Liang et al., 2017).
5.7 MSCs in kidney disorders
Kidney diseases are categorized into acute kidney injury (AKI) and chronic kidney disease (CKD), leading to the functional organ failure (Chawla, Eggers, Star, & Kimmel, 2014; Levey & Coresh, 2012). As described, MSCs unique potential to suppress immune reactions facilitates restoration of injured tissue and the suppression of inflammation. Using the MSCs has been described as an attractive therapeutic method for AKI and CKD treatment (Cantaluppi et al., 2013). In the last years, a large number of studies have assessed the possibility, safety, and efficacy of MSC-based therapies for kidney disease; while their precise underlying mechanism remains unknown (Li & Wingert, 2013). The paracrine influences of MSCs on kidney restoration, concocting of the appropriate milieu for cell viability, and modification inflammatory reactions are supposed to be correlated to their associates with the injured kidney milieu (Yun & Lee, 2019). In the animal model of renal IRI, differentiation of the MSCs into the renal tubular epithelium, prompting renal tissue integrity and restoration, has been evidenced (Yun & Lee, 2019). In another study, Fang et al. showed that administration of the autologous AT-MSCs robustly reduced metabolic disorder symptoms in diabetic rats; on the other hand, their IV injection mitigated pathological modifications, attenuated ROS levels, diminished expression of proinflammatory cytokines in diabetic model kidney tissue. Also, a reduction in expression of p-p38, p-ERK, and p-JNK participated in MAPK signaling pathway was found upon administration (Fang et al., 2012). Another report revealed that The BM-MSCs administration suppressed p38 and ERK activation, inhibited expression of Bax and caspase-3, and eventually significantly promoted Bcl-2 expression in AKI rat model (Qi & Wu, 2013). Additionally, it was found that overexpression of CXCR4 by MSCs facilitated their homing process, and consequently ameliorated renal function and reinforced administered cells anti-inflammatory capability in kidney injury (Yun & Lee, 2019). In 2014, another study highlighted the importance of the enhancement of CD206-positive M2 macrophage infiltration in the protection of AKI mice from extreme tubular lesions upon MSCs injection (Yanqiu Geng et al., 2014). Bai et al. exhibited that MSCs administration decreased diabetic nephropathy (DN) profession by LXA4-ALX/FPR2 axis and suppressed kidney fibrosis through targeting TGF-β/Smad pathway and inhibition of TNF- α, IL-6, IL-8, and IFN-γ expression in DN rats (Bai et al., 2019). Recently, it has been suggested that downregulation of poly (ADP-ribose) polymerase 1, acting as caspase activator, accompanied with the suppressing of the expression of p57 cell cycle inhibitory protein ameliorate chronic kidney failure following MSCs infusion (Cetinkaya, Unek, Kipmen-Korgun, Koksoy, & Korgun, 2019). On the other hand, Zhang et al. showed that WJ-MSC-exosomes triggered the protection against AKI due to their antioxidative attribute mediated by the promotion of Nrf2/ARE activation (Zhang et al., 2016).
In 2018, a single-arm trial in seven patients with CKD evidenced the feasibility and safety of the autologous MSCs injection in these patients after 18 months of follow-up (Makhlough et al., 2018). Another trail in 18 participants with renovascular disease revealed that autologous AT-MSC intra-arterial administration not only was well tolerated but also improved kidney tissue oxygenation and cortical blood flow in patients with these patients (Saad et al., 2017).
Moreover, the safety and possible efficacy of UC-MSCs have been supported by randomized controlled trial in 42 renal allograft recipients that received 2 × 106/kg cells through the peripheral vein before renal engraftment, and 5 × 106 cells through the renal artery during the surgical process (Sun et al., 2018). However, another Phase 2 randomized trial in 156 patients did not evidence the efficacy of intra-aortic administration of allogeneic hMSCs in the diminishing of the recovery time from AKI after cardiac surgery (Swaminathan et al., 2018).
5.8 MSCs in lung disorders
During the progression of lung injuries, uncontrolled immune reactions accompanied by impaired restoration procedure induce irretrievable damage in lung tissue because of the fibrosis generation and progression, disrupting lung normal function (Bitterman, 1992; Kolb, Margetts, Anthony, Pitossi, & Gauldie, 2001). Though inhaled corticosteroids and other anti-inflammatory agents can alleviate inflammation ant its-mediated symptoms in lung disorders, these drugs' prolonged use presents severe adverse effects (Suda et al., 2011). Thus, finding of the innovative therapeutic strategies that trigger both of the inflammation suppression and damaged alveolar epithelial cells restoration is now determinative (Akram, Samad, Spiteri, & Forsyth, 2013). Considering biological and pathological reports, MSCs predominantly modify the proliferation, and activation of several immune cells that participated in chronic inflammatory lung disease pathogenesis (Ciencewicki, Trivedi, & Kleeberger, 2008). Regardless of the induction of inhibitory effect on lung-infiltrated immune cells, MSCs could be differentiated into alveolar epithelial cells in vitro and, thereby, propose unique belongings to use in cell-based therapies of the lung diseases (Harrell et al., 2019). For the first time, Ma et al. found that BM-MSCs can be differentiated into alveolar type II (ATII)-like cells upon coculturing with MRC-5 cells (derived from normal fetal lung mesenchymal tissue) in an altered small airway growth medium containing insulin, transferrin, bovine serum albumin, and pituitary extract and, epinephrine, FGF, cortisol, and EGF (Ma et al., 2011). In 2013, Akram et al. suggested that Wnt3a-stimulated activation of the canonical Wnt/β-catenin pathway augmented MSCs differentiation into ATII-like cells (Akram et al., 2013).
Preclinical studies showed that MSCs administration boosted ATII cells restoration, inhibited endothelial cells apoptosis, and promoted alveolar-epithelial barrier repairing in the acute respiratory distress syndrome (ARDS)-injured lungs by triggering of the production of keratinocyte growth factor, VEGF, and HGF (Harrell et al., 2019). On the other hand, the safety and efficacy of the IV, intratracheal, and intrabronchial administration of the MSCs were observed in chronic obstructive pulmonary disease animal models (Liu, Fang, & Kim, 2016). Another study in papain and Co60-stimulated pulmonary emphysema rats exhibited that BM-MSCs administration reduced apoptosis rates of the alveolar wall cells in treated rats compared with the control group (untreated rats; Liu et al., 2008). They found that the expression level of the Bcl-2 in treated rats was more prominent than the control group; on the other hand, the analysis revealed an attenuation in the expression level of the Bax in the treated rats compared with the control group (Liu et al., 2008). Other studies supported the safety and efficacy of AT-MSCs (Trzil et al., 2016) and UC-MSCs (Li et al., 2013) administrations in cat models of asthma and mice models of pulmonary fibrosis via fluctuating of the expression of MMP-2 and TIMP metallopeptidase inhibitor 1 (Li et al., 2013). Furthermore, it has been found that overexpression of the CXCR4 (Yang et al., 2015) and SDF1 receptor CXCR7 (Shao, Zhou, He, Zhang, & Shen, 2019) due to their fundamental roles in MSCs homing and migration procedure, upgraded lung restoration after acute lung injury in animal models. A Phase 1 clinical trial was carried out between 2013 and 2014 in nine patients with ARDS received a single IV infusion of allogeneic BM-MSCs. While two patients died during the weeks after the infusion and one patient was revealed to have embolic phenomena, none of these undesired effects were supposed to be MSC-associated (Wilson et al., 2015). Another trial although evidenced feasibility and safety of allogeneic AT-MSCs administration in patients with ARDS, did not support MSCs efficacy in treated patients (Chambers et al., 2017). Moreover, a Phase 1 trial explored the safety of the IV injection of allogeneic BM-MSCs to patients with progressive chronic lung allograft dysfunction. According to observations, MSC administration was tolerated without presenting process-associated ever adverse effects (Chambers et al., 2017). Finally, a Phase 1 trial piloted by Stolk et al. in patients with severe emphysema upon BM-MSCs injection supports the safety and feasibility of this therapeutic approach without showing reliable evidence of its clinical efficacy (Stolk et al., 2016).
6 THE CHALLENGES AND CONTROVERSIES
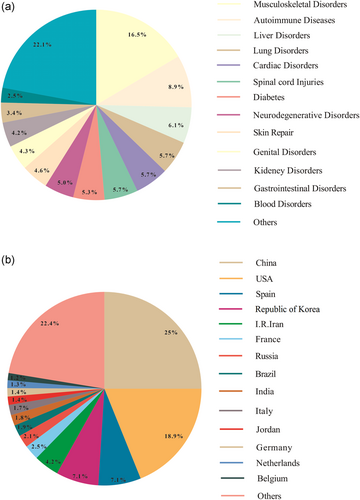
In last years, although many clinical trials have focused on assessing the safety and efficacy of MSCs-based therapy for regenerative medicine (Tables 1 and 2) (Figure 7), differences in cell sources, isolation, culture and expansion methods in correlation with dissimilarities in doses, dosing intervals, and administration route are current unanswered issues in this regard (Fitzsimmons, Mazurek, Soos, & Simmons, 2018). Moreover, poor cell survival, missing paracrine influences, and moderated homing capability have restricted the therapeutic advantages of MSC therapy (Musiał-Wysocka, Kot, & Majka, 2019). Regardless of the adaptability of autologous MSCs and the human immune system, their use is constrained by insufficient cell source as well as attenuation of the therapeutic potential or procurement unreliable MSCs isolated from elderly donors or unhealthy donors. On the other hand, autologous MSCs harvesting and expansion are a time-consuming process, restricting acute disorders therapy (Musiał-Wysocka et al., 2019). To defeat these restrictions, allogenic MSC administration has been suggested. On the other hand, during the ex vivo expansion, has been evidenced the role of oxidative stress in MSC biology, as their viability, proliferation, and activation are modified by oxidative stress. Overall, augmented ROS impede MSC proliferation, promote senescence, reinforce adipogenic but attenuate osteogenic differentiation, and significantly diminish MSC immunosuppression. Recently, modulation of sirtuin expression and activity has been signified as a possible therapeutic strategy to condense oxidative stress in MSCs (Denu & Hematti, 2016). These consequences have imperative suggestions in the clinical efficacy of MSCs for degenerative and immunological based malignancies (Denu & Hematti, 2016). On the other hand, Tatullo et al. showed that ROS generation in damaged area via various mechanism obstructs tissue repair and restoration (Tatullo et al., 2012). Furthermore, various reports have supported the immune-privileged or hypo-immunogenic nature of MSCs (Mulder, Lee, & Jeppesen, 2012), while some investigations exhibited that allogenic MSCs stimulate antibody generation and are susceptible to rejection (Consentius, Reinke, & Volk, 2015; Rasmusson, Le Blanc, Sundberg, & Ringden, 2007). However, because of the unique immunomodulatory properties, MSCs-based therapy is described as a promising therapeutic option in the treatment of inflammatory disease (Zhou, Kosaka, Xiao, & Ochiya, 2020). Interestingly, Hare et al. found that there were no significant differences between autologous and allogeneic MSCs in terms of the immunogenicity (Hare et al., 2012). Though Gu et al. indicated the nonimmunogenic nature of allogenic MSCs (Gu et al., 2015), their rejection was evidenced upon transplantation by several studies (Griffin, Ritter, & Mahon, 2010). Other reports revealed that severe inflammatory response in association with donor-specific antibody generation may responsible for allogenic MSC-based therapy failure (Zhang et al., 2015). Surprisingly, it has been suggested that there exist correlations between MSCs immunogenicity and their condition. For example, it was observed that the immunogenicity of the differentiated MSCs was robustly higher than the undifferentiated MSCs. Therefore, cell condition and location of the transplantation and its microenvironments play equally critical roles in determining the fate of transplants (Regmi, Pathak, Kim, Yong, & Jeong, 2019). Furthermore, whereas allogeneic MSCs safety and feasibility have been supported, according to various clinical trials, there exist reports presenting boosted infection risk after MSCs transplantation (Forslöw et al., 2012). Besides, though a large number of preclinical trials have shown MSCs therapeutic potential for regenerative medicine, the oncogenic ability of the uncontrolled MSCs differentiation must be comprehensively assessed before their application in the clinic (Lukomska et al., 2019).
| Condition | Study phase | Type of cell source | MSCs source | Study location | NCT number |
|---|---|---|---|---|---|
| Parkinson's disease | 1 | Allogeneic | UC | China | NCT03550183 |
| Alzheimer's disease | 2 | Allogeneic | N/A | United States | NCT02833792 |
| Alzheimer's disease | 1 | Allogeneic | UC | United States | NCT04040348 |
| Multiple sclerosis | 2 | Autologous | BM | United States | NCT03355365 |
| Multiple sclerosis | 1/2 | Autologous | BM | United States | NCT01745783 |
| Multiple sclerosis | 2 | Autologous | BM | United States | NCT03799718 |
| Ischemic heart disease | 1/2 | Autologous | BM | China | NCT03397095 |
| Ischemic heart disease | 2 | Allogeneic | BM | United States | NCT03925324 |
| Ischemic heart disease | 1/2 | Allogeneic | WJ | Colombia | NCT04011059 |
| Heart failure | 1 | Allogeneic | BM | United States | NCT02408432 |
| Heart failure | 1 | Allogeneic | BM | United States | NCT02962661 |
| Heart failure | 2/3 | Allogeneic | WJ | Poland | NCT03418233 |
| Ischemic cardiomyopathy | 2 | Allogeneic | WJ | China | NCT02368587 |
| Ischemic cardiomyopathy | 2 | Autologous | BM | France | NCT02462330 |
| Myocardial infarction | 1 | Allogeneic | WJ | Spain | NCT03798353 |
| Myocardial infarction | 1 | Allogeneic | UC | Taiwan | NCT04056819 |
| Osteoarthritis | 2 | Allogeneic | UC | China | NCT03383081 |
| Cartilage degeneration | 1/2 | Autologous | AT | China | NCT03955497 |
| Osteoarthritis | N/A | Autologous | AT | China | NCT03956719 |
| Osteoarthritis | 1/2 | Allogeneic | UC | China | NCT03166865 |
| Osteoarthritis | 1/2 | Allogeneic | WJ | Poland | NCT03866330 |
| Osteoarthritis | 2 | Allogeneic | AT | China | NCT04208646 |
| Osteoarthritis | 1 | Allogeneic | UC | Korea, Republic of | NCT04037345 |
| Cartilage injury | N/A | Autologous | SF | United Kingdom | NCT02696876 |
| Osteoarthritis | N/A | Autologous | AT | United States | NCT03379168 |
| Osteoarthritis | 1 | Allogeneic | UC | Indonesia | NCT03800810 |
| Osteoarthritis | 1/2 | Autologous | AT | Netherlands | NCT03869229 |
| Osteoarthritis | N/A | Autologous | BM | China | NCT03969680 |
| Osteoarthritis | N/A | Allogeneic | AT | China | NCT03357575 |
| Non-union fracture | 3 | Autologous | BM | Spain | NCT03325504 |
| Osteoarthritis | 1 | Allogeneic | BM | Mexico | NCT03602872 |
| Osteoarthritis | 1/2 | Allogeneic | BM | Taiwan | NCT03589287 |
| Pseudoarthrosis | 2 | Allogeneic | AT | Spain | NCT02483364 |
| Autologous | |||||
| Osteoarthritis | N/A | Autologous | AT | China | NCT04212728 |
| Osteoarthritis | 1 | Allogeneic | DP | China | NCT04130100 |
| Osteoarthritis | 2 | Allogeneic | BM | Iran | NCT03164083 |
| Osteoarthritis | 3 | Allogeneic | BM, UC, AT | United States | NCT03818737 |
| Autologous | |||||
| Osteoarthritis | 1/2 | Allogeneic | AT | Taiwan | NCT03943576 |
| Rotator cuff tear | 2 | Autologous | AT | China | NCT03279796 |
| Osteoarthritis | 3 | Autologous | AT | Korea, Republic of | NCT03990805 |
| Osteoarthritis | 1 | Allogeneic | WJ | Jordan | NCT02963727 |
| Osteoarthritis | 1 | Allogeneic | AT | Jordan | NCT02966951 |
| Osteoarthritis | 1/2 | Allogeneic | BM | Korea, Republic of | NCT04240873 |
| Osteoarthritis | N/A | Allogeneic | UC | China | NCT03358654 |
| Osteoarthritis | N/A | Allogeneic | UC | China | NCT03357770 |
| Cartilage injury | 1 | Autologous | BM | United States | NCT03477942 |
| Contour deformities | 2 | N/A | AT | Pakistan | NCT03564808 |
| Psoriasis | 1 | Allogeneic | UC | China | NCT03765957 |
| Psoriasis | 1/2 | Allogeneic | UC | China | NCT03745417 |
| Psoriasis | 1/2 | Allogeneic | AT | China | NCT03392311 |
| Diabetic foot ulcer | 1 | Allogeneic | UC | United States | NCT04104451 |
| Chronic venous ulceration | 1/2 | Allogeneic | Skin | Germany | NCT03257098 |
| Chronic venous ulceration | 1/2 | Autologous | Skin | Germany | NCT02742844 |
| Diabetic foot ulcer | 1 | Autologous | AT | Poland | NCT03865394 |
| Diabetic foot ulcer | 1 | Autologous | BM | China | NCT03248466 |
| Epidermolysis bullosa | 2 | Allogeneic | BM | United States | NCT02582775 |
| Systemic sclerosis | 1/2 | Allogeneic | BM | Netherlands | NCT03211793 |
| Liver cirrhosis | 2 | Allogeneic | UC | China | NCT03529136 |
| Liver cirrhosis | 2 | Allogeneic | UC | China | NCT03529136 |
| Liver cirrhosis | 1/2 | Autologous | BM | Singapore | NCT03626090 |
| Liver failure | 1 | Allogeneic | AT | Taiwan | NCT03629015 |
| Liver cirrhosis | 1 | Autologous | BM | United States | NCT03838250 |
| Liver cirrhosis | 4 | Autologous | BM | India | NCT04243681 |
| Liver cirrhosis | 1/2 | Allogeneic | UC | China | NCT02786017 |
| Liver cirrhosis | 1 | Allogeneic | UC | China | NCT03826433 |
| Acute kidney injury | 1/2 | Allogeneic | UC | N/A | NCT04194671 |
| Nephropathy | 2 | Allogeneic | UC | China | NCT04216849 |
| Chronic kidney diseases | 1/2 | Autologous | AT | Bangladesh | NCT03939741 |
| End stage renal disease | 1 | Autologous | AT | United States | NCT02808208 |
| Nephropathy | 1 | Allogeneic | UC | China | NCT04125329 |
| Acute kidney injury | 1/2 | Allogeneic | N/A | United States | NCT03015623 |
| Nephropathy | 1/2 | Allogeneic | WJ | Jordan | NCT03288571 |
| Diabetic kidney diseases | 1 | Autologous | AT | United States | NCT03840343 |
| Lupus nephritis | 2 | Allogeneic | UC | China | NCT03580291 |
| Pulmonary hemosiderosis | 1 | Allogeneic | BM | United States | NCT02985346 |
| COPD | 1 | Allogeneic | UC | Taiwan | NCT04206007 |
| ARDS | N/A | Allogeneic | UC | China | NCT03608592 |
| BPD | 1/2 | Allogeneic | UC | China | NCT03774537 |
| BPD | 1 | Allogeneic | UC | China | NCT03558334 |
| BPD | 1 | Allogeneic | UC | China | NCT03645525 |
| BPD | 2 | Allogeneic | UC | China | NCT03601416 |
| BPD | 1 | Allogeneic | UC | Vietnam | NCT04062136 |
| BPD | 1 | Allogeneic | UC | Taiwan | NCT03631420 |
| Coronavirus | 1 | Allogeneic | AT | China | NCT04276987 |
| Coronavirus | N/A | Allogeneic | UC | China | NCT04273646 |
| Cystic fibrosis | 1 | Allogeneic | N/A | United States | NCT03058068 |
| Ulcerative colitis | 1/2 | Allogeneic | AT | China | NCT03609905 |
| Crohn's disease | 1 | Allogeneic | BM | USA | NCT04073472 |
| Perianal fistula | N/A | Autologous | AT | Italy | NCT03913910 |
- Abbreviations: ARDS, acute respiratory distress syndrome; AT, adipose tissue; BM, bone marrow; BPD, bronchopulmonary dysplasia; COPD, chronic obstructive pulmonary disease; DP, dental pulp; MSC, mesenchymal stromal cell; SF, synovial fluid; UC, umbilical cord; WJ, Wharton's jelly.
| Condition | Study phase | Type of cell source | MSCs source | Study location | NCT number |
|---|---|---|---|---|---|
| Parkinson's disease | 1/2 | Allogeneic | BM | United States | NCT02611167 |
| ALS | 1 | Allogeneic | AT | Iran | NCT02492516 |
| ALS | 1 | Autologous | BM | Iran | NCT01759797 |
| ALS | 1 | Autologous | BM | Iran | NCT01771640 |
| ALS | 1 | Autologous | N/A | Brazil | NCT02987413 |
| Wilson syndrome | N/A | Allogeneic | BM | Turkey | NCT01378182 |
| ALS | 1 | Autologous | AT | United States | NCT01142856 |
| ALS | 1 | Autologous | AT | United States | NCT01609283 |
| Multiple sclerosis | 1 | Autologous | BM | Sweden | NCT03778333 |
| Multiple sclerosis | 1/2 | Autologous | BM | Iran | NCT01377870 |
| Multiple sclerosis | 1/2 | Autologous | BM | United Kingdom | NCT00395200 |
| Multiple sclerosis | 1/2 | Autologous | BM | Jordan | NCT01895439 |
| Multiple sclerosis | 1 | Autologous | BM | United States | NCT01933802 |
| Multiple sclerosis | 1 | Allogeneic | UC | Panama | NCT02034188 |
| Multiple sclerosis | 1/2 | Autologous | BM | Spain | NCT02035514 |
| Multiple sclerosis | 1 | Autologous | N/A | Canada | NCT02239393 |
| Multiple sclerosis | 1/2 | Autologous | AT | Spain | NCT01056471 |
| Multiple sclerosis | 1/2 | Allogeneic | UC | Jordan | NCT03326505 |
| Multiple sclerosis | 1/2 | Autologous | BM | Spain | NCT02495766 |
| Multiple sclerosis | 2 | Autologous | BM | Israel | NCT02166021 |
| Multiple system atrophy | 2 | Autologous | N/A | Korea, Republic of | NCT00911365 |
| Alzheimer's diseases | 1/2 | Autologous | AT | United States | NCT03117738 |
| ALS | 2 | Autologous | BM | United States | NCT02017912 |
| Alzheimer's diseases | 1 | Allogeneic | UC | Korea, Republic of | NCT01297218 |
| Coronary artery disease | 2 | Allogeneic | BM | Greece | NCT01753440 |
| Cardiomyopathy | 1/2 | Allogeneic | UC | Chile | NCT01739777 |
| Cardiomyopathy | 1/2 | Autologous | BM | Brazil | NCT01913886 |
| Heart failure | 1/2 | Autologous | BM | Denmark | NCT00644410 |
| Myocardial infarction | 1/2 | Autologous | BM | France | NCT01076920 |
| Cardiomyopathy | 1/2 | Autologous | |||
| Allogeneic | BM | United States | NCT01392625 | ||
| Myocardial infarction | 1 | Allogeneic | BM | United States | NCT00114452 |
| Myocardial infarction | 2/3 | Autologous | BM | Korea, Republic of | NCT01392105 |
| Myocardial infarction | 2 | Allogeneic | BM | United States Canada | NCT00877903 |
| Ischemic heart failure | 1/2 | Autologous | BM | United States | NCT00768066 |
| Myocardial infarction | 1/2 | Allogeneic | BM | India | NCT00883727 |
| Osteoarthritis | 2 | Autologous | BM | Iran | NCT01504464 |
| Osteoarthritis | 1 | Autologous | BM | Iran | NCT01499056 |
| Femoral fractures | 2 | Autologous | BM | Iran | NCT01788059 |
| Osteoarthritis | 1 | Autologous | BM | Iran | NCT01207661 |
| Osteoarthritis | 2 | Allogeneic | BM | India | NCT01453738 |
| Tibial fractures | 2 | Allogeneic | AT | Iran | NCT02140528 |
| Mandibular fractures | 3 | Autologous | AT | Mexico | NCT02755922 |
| Osteoarthritis | 1/2 | Autologous | BM | Spain | NCT02123368 |
| Osteoarthritis | 2 | Allogeneic | BM | Malaysia | NCT01448434 |
| Osteoarthritis | 2 | Autologous | BM | United States | NCT02958267 |
| Tibial fractures | 1/2 | Autologous | BM | Israel | NCT00250302 |
| Long bone fractures | 1 | Autologous | BM | Iran | NCT01206179 |
| Osteoarthritis | 1/2 | Allogeneic | BM | Spain | NCT01586312 |
| Bone fractures | N/A | Autologous | BM | United Kingdom | NCT02177565 |
| Osteoarthritis | 1/2 | Autologous | AT | China | NCT01809769 |
| Osteoarthritis | 1/2 | Autologous | BM | Brazil | NCT01895413 |
| Osteoarthritis | 1 | Autologous | BM | Iran | NCT01436058 |
| Osteoarthritis | 1/2 | Autologous | AT | Korea | NCT01300598 |
| Osteoarthritis | 1 | Allogeneic | UC | China | NCT02291926 |
| Osteoarthritis | 2/3 | Autologous | BM | Iran | NCT02291926 |
| Osteoarthritis | 1 | Autologous | BM | Iran | NCT00850187 |
| Bone fractures | 1/2 | Autologous | BM | France | NCT01842477 |
| Osteoarthritis | 2 | Autologous | BM | Jordan | NCT02118519 |
| Osteoarthritis | 1 | Allogeneic | AT | China | NCT02641860 |
| Osteoarthritis | 2 | Autologous | AT | China | NCT02162693 |
| Osteoarthritis | 1/2 | Autologous | BM | Spain | NCT01183728 |
| Leg length inequality | 1 | Autologous | BM | Iran | NCT01210950 |
| Osteogenesis imperfecta | 1 | Allogeneic | BM | Spain | NCT02172885 |
| Osteoarthritis | 1 | Autologous | SF | United States | NCT02468492 |
| Osteoarthritis | 2 | Autologous | AT | United States | NCT02674399 |
| Osteoarthritis | 3 | Allogeneic | UC | Korea, Republic of | NCT01626677 |
| Osteoarthritis | 3 | Allogeneic | UC | Korea, Republic of | NCT01041001 |
| Scars and Cutis laxa | 1/2 | Autologous | AT | Poland | NCT03887208 |
| Skin ulcers | 1 | Allogeneic | UC | China | NCT02685722 |
| Skin injuries | 1 | Allogeneic | UC | China | NCT02669199 |
| Diabetic foot | 1 | Autologous | BM | China | NCT00955669 |
| Diabetic foot | N/A | Autologous | AT | Italy | NCT03276312 |
| Liver cirrhosis | 1/2 | Allogeneic | UC | China | NCT01342250 |
| Liver failure | N/A | Autologous | BM | China | NCT00956891 |
| Liver failure | 1/2 | Autologous | N/A | Iran | NCT00420134 |
| Liver fibrosis | 1 | Autologous | BM | Iran | NCT01454336 |
| Liver cirrhosis | 2 | Allogeneic | BM | India | NCT01591200 |
| Renal failure | 1 | Autologous | BM | Iran | NCT02166489 |
| Kidney disease | N/A | Autologous | BM | China | NCT00658073 |
| Kidney disease | 1 | Autologous | BM | Iran | NCT02195323 |
| Kidney disease | 1 | Autologous | AT | United States | NCT01840540 |
| Kidney disease | 1/2 | Autologous | BM | Netherlands | NCT00734396 |
| IPF | 1 | Allogeneic | Placental | Australia | NCT01385644 |
| IPF | 1 | Autologous | BM | Spain | NCT01919827 |
| Pulmonary fibrosis | 1 | Allogeneic | UC | China | NCT02277145 |
| IPF | 1/2 | Allogeneic | BM | Russian | NCT02594839 |
| Pneumoconiosis | 1 | Allogeneic | UC | China | NCT02668068 |
| COPD | 2 | Allogeneic | BM | United States | NCT00683722 |
| ARDS | 1 | Allogeneic | BM | United States | NCT01775774 |
| Emphysema | 1 | Autologous | BM | Netherlands | NCT01306513 |
| ARDS | 2 | Allogeneic | BM | United States | NCT02804945 |
| Bronchiectasis | 1 | Allogeneic | BM | United States | NCT02625246 |
| Fecal incontinence | 1/2 | Autologous | AT | Spain | NCT02292628 |
| Fecal incontinence | 1 | Allogeneic | AT | Korea, Republic of | NCT02384499 |
| Crohn's disease | 2 | Allogeneic | BM | United States | NCT00294112 |
| Crohn's disease | 1/2 | Allogeneic | UC | China | NCT02445547 |
| Crohn's disease | 1/2 | Autologous | AT | Spain | NCT01157650 |
| Crohn's disease | 1/2 | Allogeneic | BM | Netherlands | NCT01144962 |
| Crohn's disease | 3 | Allogeneic | AT | International | NCT01541579 |
| Anal fistula | 3 | Autologous | AT | Spain | NCT01803347 |
| Rectovaginal fistula | 1 | Autologous | AT | Russia | NCT03643614 |
- Abbreviations: ALS, amyotrophic lateral sclerosis; ARDS, acute respiratory distress syndrome; AT, adipose tissue; BM, bone marrow; COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis; MSC, mesenchymal stromal cell; SF, synovial fluid; UC, umbilical cord.
7 CONCLUSION
In recent decades, optimizations of extraction, expansion as well as differentiation strategies have permitted MSCs to develop closer to clinical using for disorders therapy and tissue restoration. Some main MSC characteristics, such as immunoregulatory potential favorable to assuage immune reactions, paracrine or autocrine activities that support growth factors production and the unique capacity to differentiate into target cells, make them preferred candidates to use for regenerative medicine. Various clinical trials evidenced the feasibility and safety of the MSCs administration; however, its positive effects on human clinical outcomes have not yet been reliably achieved. Additionally, the oncogenic capability of uncontrolled MSC differentiation requires to be more evaluated. Further study is essential on cell physiology about how MSCs performances in vivo and how to attain favorable administration.
In sum, we believe that the promotion of the MSCs cultural environment and electing of the proper induction factors in association with the optimization of MSCs delivery dose and route in different disorders can lead to the promising therapeutic outcomes.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and the main idea of the work. S. T., H. R. G. J., M. A. J. K., M. Z., A. S., A. J., K. J., and F. J. drafted the main text, figures, and tables. A. N. supervised the work and provided the comments and additional scientific information. A. H. and S. T. reviewed and revised the text. All authors read and approved the final version of the work to be published.




