Advances and challenges in the treatment of primary central nervous system lymphoma
Abstract
Primary central nervous system lymphoma (PCNSL), a rare variant of non-Hodgkin's lymphoma, is characterized by distinct biological characteristics and clinical behaviors, and patient prognosis is not satisfactory. The advent of high-dose (HD) methotrexate (HD-MTX) therapy has significantly improved PCNSL prognosis. Currently, HD-MTX-based chemotherapy regimens are recognized as first-line treatment. PCNSL is sensitive to radiotherapy, and whole-brain radiotherapy (WBRT) can consolidate response to chemotherapy; however, WBRT-associated delayed neurotoxicity leads to neurocognitive impairment, especially in elderly patients. Other effective approaches include rituximab, temozolomide, and autologous stem-cell transplantation (ASCT). In addition, new drugs against PCNSL such as those targeting the B-cell receptor signaling pathway, are undergoing clinical trials. However, optimal therapeutic approaches in PCNSL remain undefined. This review provides an overview of advances in surgical approaches, induction chemotherapy, radiotherapy, ASCT, salvage treatments, and novel therapeutic approaches in immunocompetent patients with PCNSL in the past 5 years. Additionally, therapeutic progress in elderly patients and in those with relapsed/refractory PCNSL is also summarized based on the outcomes of recent clinical studies.
Abbreviations
-
- AB ± R
-
- HD-methotrexate, ifosfamide, vindesine, dexamethasone, carmustine, teniposide ± rituximab
-
- AKT
-
- protein kinase B
-
- ASCT
-
- autologous stem-cell transplantation
-
- BBB
-
- blood-brain barrier
-
- BCR
-
- B-cell receptor
-
- BKT
-
- bruton tyrosine kinase
-
- CHOP
-
- cyclophosphamide, doxorubicin, vincristine, prednisolone
-
- CR
-
- complete response
-
- D
-
- dexamethasone
-
- DeVIC
-
- dexamethasone, etoposide, ifosfamide and carboplatin
-
- FTP
-
- fotemustine, teniposide and dexamethasone
-
- GEMOX
-
- gemcitabine and oxaliplatine
-
- GFR
-
- growth factor receptor
-
- HD-MA
-
- HD-MTX and cytarabine
-
- HD-MTX
-
- high-dose methotrexate
-
- HD-MTX ± R
-
- HD-MTX ± rituximab
-
- M-CHOP
-
- HD-MTX, cyclophosphamide, doxorubicin, vincristine, prednisolone
-
- MBVP
-
- methotrexate, carmustine, teniposide, and prednisone
-
- MPV
-
- HD-MTX, rituximab, vincristine
-
- MRI
-
- magnetic resonance imaging
-
- mTOR
-
- mammalian target of rapamycin
-
- Mø
-
- macrophages
-
- OS
-
- overall survial
-
- PCNSL
-
- primary central nervous system lymphoma
-
- PD-1
-
- programmed cell death protein 1
-
- PD-L1
-
- programmed cell death-ligand 1
-
- PFS
-
- progression free survival
-
- PR
-
- partial response
-
- R/R PCNSL
-
- relapsed/refractory PCNSL
-
- RTX
-
- rituximab
-
- TAA
-
- tumor associated antigen
-
- TMZ
-
- temozolomide
-
- WBRT
-
- whole brain radiotherapy
1 INTRODUCTION
Primary central nervous system lymphoma (PCNSL) comprises 4% of all newly diagnosed brain tumors (Biccler et al., 2019) and 4–6% of all extranodal lymphomas (Grommes & DeAngelis, 2017). PCNSL is a rare and highly aggressive type of lymphoma confined to the brain, eyes, spinal cord, and leptomeninges, with no systemic involvement at the time of diagnosis. More than 90% of PCNSLs in immunocompetent patients are diffuse large B-cell lymphoma (DLBCL; Le et al., 2019), and the remaining 5% include T-cell, Burkitt, lymphoblastic, and marginal zone lymphomas (Biccler et al., 2019). According to one study, the majority of PCNSL cases (79/82) are classified as nongerminal center B-cell immunophenotypes (Camilleri-Broet et al., 2006).
In the past two decades, PCNSL has been diagnosed at an increasing frequency in immunocompetent patients, particularly in elderly individuals (Enblad et al., 2017; Shiels et al., 2016; Villano, Koshy, Shaikh, Dolecek, & Mccarthy, 2011). PCNSL occurs mostly among patients aged between 50 and 70 years (Agnieszka & Uwe, 2013), and the median age at diagnosis is 66 years. The annual incidence rate of PCNSL was 7 cases per 1,000,000 people in the United States (Shiels et al., 2016).
The outcome of PCNSL is poor (Haldorsen, Espeland, & Larsson, 2016). The reported median progression-free survival (PFS) and overall survival (OS) are 24.0 and 36.9 months, respectively (Camilleri-Broet et al., 2006), which are closely correlated with immune status of patients (Norden, Drappatz, Wen, & Claus, 2011) and age (Camilleri-Broet et al., 2006). Prompt diagnosis and treatment initiation are vital for improving clinical outcomes of PCNSL (Grommes & DeAngelis, 2017). The 5-year survival rate of immunocompetent patients with PCNSL in the United States has increased from 19.1% in the period 1992–1994 to 30.1% in the period 2004–2006 (Shiels et al., 2016). However, due to its rarity, best treatment modalities for PCNSL have not yet been identified (Liu et al., 2015). Current treatment approaches include chemotherapy, whole-brain radiotherapy (WBRT), targeted therapy, and autologous stem-cell transplantation (ASCT; Agnieszka & Uwe, 2013). Some newer drugs have also been reported to improve the prognosis of PCNSL. High-dose (HD)-methotrexate (MTX)-based regimens have shown promising early results, but the disease outcomes thus far have remained poor because of few long-term survivors (Kuitunen et al., 2017). In addition, approximately 36.3% (93 of 256) of patients with PCNSL have relapsed in one study (Langnerlemercier et al., 2016). Few appropriate second-line treatments are available after relapse.
In the present review, we will discuss recent progresses and challenges of surgery, induction chemotherapy, radiotherapy, ASCT, and novel therapeutic approaches in the treatment approaches for PCNSL over the past 5 years. Furthermore, we will focus on progress achieved in treatment strategies for elderly patients and those with relapsed/refractory PCNSL (Figure 1).

2 SURGERY
2.1 Biopsy
The pathological diagnosis of PCNSL primarily depends on biopsy, because the complications of biopsy are lower than those of surgical resection (Labak et al., 2019). To date, histopathological diagnosis based on stereotactic biopsy remains the gold standard for PCNSL diagnosis (Onder et al., 2015). Since classical neurosurgical teaching suggests that corticosteroid administration reduces the diagnostic yield of stereotactic brain biopsy for PCNSL, preoperative use of corticosteroids is not recommended (Porter et al., 2010). In one study, among patients who underwent stereotactic biopsy, the incidence of negative biopsy, which was 33.3% after a short steroid regimen (≤1 week), was increased to 57.1% with the increased duration of steroid treatment (>1 week; Manoj et al., 2014).
However, high corticosteroid doses are still recommended for patients with suspicious brain edema caused by PCNSL since it can rapidly relieve the neurological symptoms caused by intracranial masses in PCNSL (Bullis et al., 2019). In patients receiving preoperative corticosteroid therapy, biopsy is recommended within 1 week of corticosteroid initiation. Additionally, it is necessary to perform preoperative examination such as enhanced magnetic resonance imaging (MRI; including the brain, eyes, spinal cord, and leptomeninges), cerebrospinal fluid analysis, slit-lamp examination of both eyes, positron emission tomography/computed tomography (including chest, abdomen, and pelvis), bone marrow biopsy with aspiration and testicular ultrasound for males. Furthermore, the biopsy is guided by MRI to the greatest extent possible. In addition, experienced stereotactic neurosurgeons reduce technical errors during surgery. Finally, a second stereotactic scan to confirm the continued presence of the lesion before tissue sampling may also mitigate these concerns (Binnahil, Au, Lu, Wheatley, & Sankar, 2016).
2.2 Open surgery
Since PCNSL features an extensive range of lesions and deep brain involvement which could lead to poor efficacy of surgery, surgery is generally discouraged for patients with PCNSL (Shankar & Barker, 2014). Studies before 2010 suggested that complete resective surgery played no significant roles in improving either OS or PFS (Bellinzona, Roser, Ostertag, Gaab, & Saini, 2005; Farhi et al., 2018; Jellinger, Radaskiewicz, & Slowik, 1975; Schorb et al., 2019) and this approach might even be associated with higher mortality (Houillier et al., 2019; Lee et al., 2014b). In addition, even partial surgical resection could constitute an unfavorable prognostic factor (Bataille et al., 2000; Olivier et al., 2014; Pulczynski et al., 2015). Relapse and progression are the main causes for the failure of surgical approaches in the treatment of PCNSL.
A European study comprising 248 patients, including 116 and 132 patients who underwent surgical resection and biopsy, respectively, demonstrated that partial surgical resection was an unfavorable prognostic factor for PCNSL (Bataille et al., 2000). In another study, surgical resection did not improve the OS in 45 patients and only achieved palliative mass reduction (Kellogg, Straus, Karmali, Munoz, & Byrne, 2014). Surgery alone is infeasible, and chemotherapy is necessary after surgery. In support of this, a study comparing patients with newly diagnosed PCNSL reported median PFS and OS rates of 8.5 and 8.5 months in the surgery group, and median PFS and OS rates of 29 and 54 months in the biopsy or surgery plus chemotherapy group, respectively (Wang et al., 2017).
Over the last several years, some studies have proposed reconsidering the statement that efforts to resect PCNSL should be discouraged. The PFS and OS of 526 patients with PCNSL were significantly shorter in biopsied patients compared with those who received subtotal or gross total resection (Weller, Martus, Roth, Thiel, & Korfel, 2012). The median survival was 16.5 months longer in patients who underwent surgical tumor resection than in those who underwent biopsy alone (Villalonga et al., 2018). In addition, patients who underwent open surgery with total tumor reduction had significantly longer OS (median not reached) compared with that of patients who underwent partial tumor reduction or biopsy in whom the median OS was 23 months (Jelicic et al., 2016). In addition, surgical resection of PCNSL is relatively safe, with complication rates comparable to those of other intracranial neoplasms (Cloney et al., 2017).
Advances in surgical techniques are the main reason for outcome inconsistencies between recent (Jelicic et al., 2016; Villalonga et al., 2018; Weller et al., 2012) and previous (Bataille et al., 2000; Bellinzona et al., 2005; Farhi et al., 2018; Houillier et al., 2019; Jellinger et al., 1975; Lee et al., 2014b; Olivier et al., 2014; Pulczynski et al., 2015; Schorb et al., 2019) studies. Several shortcomings in the evaluation of surgical efficacy still remain in these studies. The vast majority of patients not only underwent surgery but also received other treatments. Therefore, evaluating the true impact of surgery alone on PCNSL remains a challenge. At present, surgical treatment is still not a class I recommendation because of variations in the technical level and discretion of operators. Surgery plays a limited role in most PCNSLs and is primarily used for diagnosis and intracranial decompression. Thus far, open surgery is important in rapidly reducing intracranial pressure and improving physical condition, thereby enabling on-time chemotherapy administration (Batchelor & Loeffler, 2006). However, the role of surgery should probably be reconsidered for single lesions and superficial PCNSL tumors.
Table 1 summarizes the clinical findings of studies on surgical excision and biopsy for PCNSL over the past 5 years.
| No. | Study | Design | Median (range) age, years | Number of treated patients/total patients | Type of surgery | Median PFS | Median OS |
|---|---|---|---|---|---|---|---|
| 1 | Kellogg et al. (2014) | Retrospective | 59.2 (37–86) | 7/45 | Tumor resection | / | HR of 0.86 (95% CI: 0.26–2.90, p = .81) |
| 1 | Kellogg et al. (2014) | Retrospective | 59.2 (37–86) | 37/45 | Biopsy | / | HR of 0.59 (95% CI: 0.26–1.36, p = .22) |
| 2 | Wang et al. (2017) | Retrospective | 63.5 (27.5–76) | 10/34 | Tumor resection | 8.5 months | 8.5 months |
| 3 | Villalonga et al. (2018) | Retrospective | 59 (25–84) | 18/47 | Tumor resection | / | 31 months (p = .016) |
| 3 | Villalonga et al. (2018) | Retrospective | 61 (27–81) | 29/47 | Biopsy | / | 14.5 months (p = 0.016) |
| 4 | Jelicic et al. (2016) | Retrospective | 50.5 (29–69) | 12/27 | Total tumour resection | / | NR (p = .014) |
| 4 | Jelicic et al. (2016) | Retrospective | 50.5 (29–69) | 15/27 | Partial resection or biopsy | / | 13 months (p = .014) |
- Note: NR: not reached; /: not mentioned.
- Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; PCNSL, primary central nervous system lymphoma; PFS, progression-free survival.
3 INDUCTION CHEMOTHERAPY
The first-line treatment of PCNSL is systemic chemotherapy. Many chemotherapeutic drugs for systemic lymphoma cannot access the brain due to the blood-brain barrier (BBB; Yang et al., 2019). For PCNSL, drugs which easily cross the BBB, such as HD-MTX, temozolomide, ifosfamide, thio-TEPA, and cytarabine, should be selected.
3.1 HD-MTX alone
The usual chemotherapy regimen for PCNSL is HD-MTX because regular doses of MTX cannot penetrate the BBB. An HD-MTX dose over 1 g/m2 is able to cross the BBB to penetrate the brain (Ferreri, Reni, & Villa, 2000). The optimal MTX dosage for PCNSL has not yet been determined; however, the general consensus is a minimum MTX dose of 3 g/m2 administered as a 4-hr intravenous infusion (Ferreri et al., 2004).
Nonetheless, the effect of HD-MTX alone is not satisfactory, and the average survival time after treatment is <2 years (Batchelor, Kolak, Ciordia, Foster, & Henson, 2003; Ulrich et al., 2005). The best evidence supporting the poor efficacy of MTX alone is the International Extranodal Lymphoma Study Group no. 20 randomized controlled trial, in which the OS in patients treated with MTX plus cytarabine was significantly better than that in patients treated with MTX alone (Joerger et al., 2010). Therefore, patients are more likely to be treated with HD-MTX-based systemic chemotherapy.
3.2 HD-MTX-based systemic chemotherapy
Treatment options for patients with newly diagnosed PCNSL are grouped into induction and consolidation chemotherapy. Induction chemotherapy based on HD-MTX is considered as the standard approach for newly diagnosed PCNSL (Han & Batchelor, 2017). Numerous studies have shown that HD-MTX-based systemic chemotherapy is effective and that the potential acute and delayed toxicities are manageable (Burton et al., 2017; Dalia et al., 2014; Han, Ji, Ouyang, Zhu, & Zhou, 2017; Hattori et al., 2017; Ichikawa et al., 2014; Sitthinamsuwan, Rujimethapass, Chinthammitr, & Treetipsatit, 2014). Therefore, HD-MTX-based systemic chemotherapy must be considered as the first-line chemotherapy regimen in PCNSL. However, the best chemotherapeutic regimen remains unclear. Many controlled studies (Burton et al., 2017; Dalia et al., 2014) focused on comparing the treatment efficacy between HD-MTX-based systemic chemotherapy and other chemotherapy regimens. In one study, 89 patients initially treated with HD-MTX-based therapy experienced improved PFS (Dalia et al., 2014), which was associated with an average OS improvement of 41.5 months, whereas the average OS improvement was only 4.5 months in patients who did not receive HD-MTX. The survival rate of patients who received HD-MTX-based therapy was superior to that of patients received radiation alone or other non-HD-MTX-based therapies (RCHOP or MTX; Burton et al., 2017). Of these, 52% (11/21 patients) received chemotherapy regimens that included systemic HD-MTX at the time of initial diagnosis, with a median OS of 22 months. In contrast, the patients who received other therapies, including WBRT or non-MTX-based chemotherapies, had a median OS of only 5 months. In addition, the median OS of patients receiving at least four cycles of HD-MTX was 40 months.
The complicated HD-MTX-based chemotherapies can easily lead to adverse effects, especially neurologic complications and hematological toxicities (Han et al., 2017). Bergner, Monsef, Illerhaus, Engert, and Skoetz (2012) compared the efficacy of HD-MTX plus additional chemotherapy with HD-MTX monochemotherapy as first-line PCNSL treatment in immunocompetent patients of all ages. Although the PFS improved significantly, adverse events were frequent in patients treated with HD-MTX plus cytarabine (Bergner et al., 2012). Other complex systemic chemotherapy schemes such as MBVP (MTX, teniposide, carmustine, and methylprednisolone plus two intrathecal injections of MTX, cytarabine, and hydrocortisone; Poortmans et al., 2003), CHOD/BVAM (cyclophosphamide, doxorubicin, vincristine, dexamethasone plus bis-chloronitrosourea; Laack et al., 2011), and HD-MTX plus cytarabine (Wu et al., 2018) have been reportedly associated with increased rates of toxicity. The rate of Grade 3 or higher neurotoxicity was 28% in patients on the CHOD/BVAM regimen (Laack et al., 2011). The cause of death was treatment-related toxicity in approximately 10% of patients on the MBVP regimen (Poortmans et al., 2003). FTD (fotemustine, teniposide, and dexamethasone) chemotherapy was associated with milder neutropenia than the combination use of HD-MTX with cytarabine in patients with PCNSL (Wu et al., 2018). The dose of MTX cannot be increased due to adverse effects. Han et al. (2017) found that the effects of MTX at doses of 5 and 3 g/m2 were similar in patients under 60 years of age whereas the effect of MTX at a dose of 3 g/m2 was better in those over 60 years of age, which may be related to reduced toxicity (Han et al., 2017).
Increasing brain uptake of MTX is another approach to reduce toxicity and limit side effects. Many new technologies can aid in the delivery of MTX across the BBB, facilitating reduction in MTX dosage. For example, tumor necrosis factor-α coupled with NGR (NGR-Htnf a peptide targeting CD13+ vessels; Ferreri et al., 2019), high-intensity focused ultrasound systems (Phenix, Togtema, Pichardo, Zehbe, & Curiel, 2014), multitargeted nanoparticles (Zhang et al., 2019), and nonviral nanovehicles (polymeric micelles, liposomes, polymersomes, dendrimers, solid lipids, porous metal and metal oxide particles, layered double hydroxides, and carbons with various nanostructures; Choi, Kim, Oh, & Choy, 2018) can all improve the brain uptake of MTX.
3.3 Temozolomide
In recent years, the role of temozolomide in lymphoma treatment has been confined to PCNSL because of its good BBB penetration and favorable toxicity profile (Makino et al., 2015). Wang et al. (2014) compared the efficacy and toxicity between MT (HD-MTX + temozolomide) and MC (HD-MTX + cytarabine) regimens in a study of 20 patients treated with the MT regimen and 20 patients treated with the MC regimens. The 5-year PFS and OS of the MT group were 36% and 62.2%, respectively, whereas the rate of Grades 3–4 hematological toxicities was 15%. Conversely, the 5-year PFS and OS of the MC group were 32.6% and 46.7%, respectively, and the rate of Grades 3–4 hematological toxicities was 85.7%. These results suggest that the MT combination regimen may be an effective and simplified regimen compared with the MC regimen for patients with newly diagnosed PCNSL. In a study by Wang et al. (2017), 24 patients with PCNSL who underwent surgery/biopsy were divided into the following four treatment cohorts: CHOP (cyclophosphamide, epidoxorubicin, vincristine, and prednisone), HD-MTX + dexamethasone + rituximab, HD-MTX + dexamethasone + temozolomide, and HD-MTX + dexamethasone + rituximab + temozolomide. The patients treated with the temozolomide-containing regimens had longer OS and PFS, suggesting that regimens including temozolomide may be a better choice.
Rituximab can improve the efficacy of temozolomide plus HD-MTX. Forty-six patients with PCNSL were treated with rituximab on day one, which was administered in combination with HD-MTX (Days 1 and 15) and temozolomide (Days 1–5) in 28-day cycles. The median OS and PFS were 26 and 8.6 months, respectively, and the toxicities were mild and manageable (Nagle et al., 2017). In another study, 53.2% of 32 patients receiving rituximab in combination with HD-MTX and temozolomide as induction therapy had complete response (CR) whereas both the 2- and 5-year OS rates were 82.3% (Chen et al., 2019). In contrast, 27.6% of the patients in the MT group had CR, with 2- and 5-year OS rates of 65.7% and 50.0%, respectively.
These studies have validated that temozolomide may be a promising new drug for treatment-naïve patients with PCNSL. In addition, the toxicity and side effects of temozolomide are acceptable. Liu et al. (2015) further suggested that high serum lactate dehydrogenase levels were associated with poor outcomes in patients treated with temozolomide-containing regimens.
3.4 Rituximab
A study confirmed that rituximab can cross the BBB under certain conditions (Lampson, 2011). However, due to the BBB, the cerebrospinal fluid levels of rituximab is approximately 0.1% of therapeutic serum rituximab levels in patients with PCNSL (Rubenstein et al., 2003).
Although some studies found that rituximab failed to improve OS (Kansara et al., 2015; Mocikova et al., 2016), most studies in recent years have suggested that rituximab may be an appropriate therapeutic option for improving both OS and PFS in PCNSL (Dalia et al., 2014; Karmali et al., 2017; Ly, Crew, Graham, & Mrugala, 2016; Matthias et al., 2014; Swinnen et al., 2018). One study reported that the combination use of rituximab and MT improved both OS and PFS compared to MT alone (Chen et al., 2019). Another study compared HD-MTX alone with HD-MTX plus rituximab for the treatment of patients with newly diagnosed PCNSL. The CR rate, which was 73% in 27 patients with PCNSL treated with HD-MTX plus rituximab, was only 36% in those treated with HD-MTX alone. The median PFS in the HD-MTX plus rituximab group was 26.7 months, compared with the median PFS of 4.5 months in the HD-MTX-alone group (Matthias et al., 2014). Ly et al. (2016) also reported a CR rate of 58% after treatment with HD-MTX plus rituximab. The median PFS was 22 months, but the median OS was not reached. Moreover, the 5-year PFS was superior in the rituximab-treated patients than in those who were not treated with rituximab among a cohort of 258 and 146 patients from Denmark and British Columbia, respectively (Biccler et al., 2019). In a study by Wang et al. (2017), the CR rate was 50.0% and the median PFS and OS were 8 and 15 months, respectively, in six patients in the rituximab group (HD-MTX + dexamethasone + rituximab).
These studies (Biccler et al., 2019; Chen et al., 2019; Ly et al., 2016; Matthias et al., 2014; Wang et al., 2017) suggest that the addition of rituximab improves outcomes in patients with PCNSL. However, another studies (Kansara et al., 2015) have reported contradictory findings. One study reported 5-year PFS rates of 17% and 38%, with no significant difference, in the HD-MTX and HD-MTX plus rituximab groups, respectively (Kansara et al., 2015). The addition of rituximab to HD-MTX did not appear to improve the prognosis of PCNSL. Since rituximab is correlated with poor CNS osmosis, it may be ineffective in patients with highly aggressive PCNSL and only those with less aggressive cancer might benefit (Kansara et al., 2015). Another study also found an ambiguous benefit of adding rituximab to MTX, teniposide, carmustine, and prednisone chemotherapies in patients with PCNSL (Bromberg et al., 2019). After a median follow-up of 33 months, the addition of rituximab did not improve the CR rate or the 1-year PFS or OS and the toxicity was similar. Importantly, subgroup analysis showed that application of rituximab may benefit patients under 60 years of age.
The role of rituximab in PCNSL requires further verification by additional large-scale clinical studies. The dosage, time at which rituximab is added, and suitable patient populations also require further clarification.
3.5 Summary
Currently, chemotherapy regimens for PCNSL are mainly based on empirical clinical experience. Both single- and multi-drug MTX regimens have been effective in the treatment of PCNSL. In addition, attention should be paid to the effects of rituximab and temozolomide. Chemotherapy regimens should consider not only treatment efficacy but also potential associated toxicities (Table 2).
| Study | Type of study | Median (range) age, years | Number of treated patients/total patients | Chemotherapy regimen | CR Rate (%) | Median PFS (months) | Median OS (months) | Toxic effects |
|---|---|---|---|---|---|---|---|---|
| Dalia et al. (2014) | Retrospective | 61 (17–70) | 67/89 | HD-MTX-based therapy | / | / | 41.5 | / |
| Dalia et al. (2014) | Retrospective | 61 (17–70) | 22/89 | Therapy without HD-MTX | / | / | 4.5 | / |
| Burton et al. (2017) | Retrospective | 65 (30–90) | 43,790 | HD-MTX-based therapy | / | / | 22 | / |
| Burton et al. (2017) | Retrospective | 65 (30–90) | 43,729 | Therapy without HD-MTX | / | / | 5 | / |
| Sitthinamsuwan et al. (2014) | Retrospective | 52.8 (23–78) | 18/69 | HD-MTX-based therapy | / | / | 18.3, 44.4% (2 years) and 16.7% (5 years) | Two had Grade 5 adverse effects |
| Han et al. (2017) | Retrospective | 56 (39–69) | 25/49 | HD-MTX ± R | 33.33 | 54.64% (2 years) | 74.62% (2 years) | 9 of 25 patients had Grades 3–4 toxicity |
| Han et al. (2017) | Retrospective | 57 (17–78) | 24/49 | AB ± R (methotrexate 3 g/m2) | 36.83 | 42.86% (2 years) | 71.43% (2 years) | 10 of 24 had Grades 3–4 toxicity |
| Ichikawa et al. (2014) | Prospective | 64.6 (50–78) | 24/24 | M-CHOP | 87.5 | / | 33 | No treatment-related mortality |
| Hattori et al. (2017) | Retrospective | 54.5 (47–55) | 43,748 | R-MPV | 70 | 56% (3 years) | 68.6% (3 years) | 40% Had Grade 4 toxicity |
| Wu et al. (2018) | Retrospective | 56 (20–69) | 24/49 | FTD | 33 | 17.4 | 48.8 | The HD-MA group showed more serious neutropenia (p = .009) and hepatic dysfunction (p = .010) than did the FTD group. |
| Wu et al. (2018) | Retrospective | 57 (16–67) | 25/49 | HD-MA | 40 | 16.7 | 44.9 | The HD-MA group showed more serious neutropenia (p = .009) and hepatic dysfunction (p = .010) than did the FTD group. |
| Wang et al. (2014) | Retrospective | 53.5 (18–72) | 21/41 | TMZ + HD-MTX | 45 | 36% (5 years) | 62.2% (5 years) | One treatment-related death |
| Wang et al. (2014) | Retrospective | 52 (34–76) | 20/41 | cytarabine +HD-MTX | 31.8 | 32.6% (5 years) | 46.7% (5 years) | One treatment-related death |
| Nagle et al. (2017) | Retrospective | 61 (21–85) | 27/31 | TMZ + HD-MTX | 70.4 | 22 | 55.3 | No serious toxic effects |
| Wang et al. (2017) | Retrospective | 55 (33–63) | 43,587 | CHOP | 20 | 15; 20% (2 years) | 25; 20% (5 years) | No treatment-related deaths |
| Wang et al. (2017) | Retrospective | 52.5 (51–67) | 43,638 | HDM + D + R | 50 | 8; 33.3% (2 years) | NR; 60.8% (5 years) | / |
| Wang et al. (2017) | Retrospective | 49 (25–59) | 43,699 | HDM + D + T | 50 | NR; 62.7% (2 years) | NR; 60.8% (5 years) | No treatment-related deaths |
| Wang et al. (2017) | Retrospective | 57 (57–67) | 43,546 | HDM + D + R + T | 66.7 | NR; 72.9% (2 years) | NR; 60.8% (5 years) | / |
| Chen et al. (2019) | Retrospective | 53.5 (29-77) | 30/62 | TMZ + HD-MTX | 27.6 | 29.1% (5 years) | 50.0% (5 years) | No treatment-related deaths |
| Chen et al. (2019) | Retrospective | 53.5 (29-77) | 32/62 | TMZ + HD-MTX + R | 53.2 | 53.3% (5 years) | 82.3% (5 years) | No treatment-related deaths |
| Swinnen et al. (2018) | Prospective | 57 (30–76) | 26/26 | HDM + vincristine+procarbazine+cytarabine+dexamethasone+rituximab | 28 | 34 | NR | 19 Subjects experienced Grade 4 toxicity |
| Ly et al. (2016) | Prospective | 62.5 (19–78) | 43811 | HDM + R | 58 | 22 | NR | 2 of 12 had Grade 3 toxicity |
| Kansara et al. (2015) | Retrospective | 62 (18–80) | 25/74 | HDM | 35 | 17% (5 years) | 38% (5 years) | 14% of patients required early cessation of chemotherapy due to treatment intolerance |
| Kansara et al. (2015) | Retrospective | 58 (29–74) | 49/74 | HDM + R | 37 | 17% (5 years) | 38% (5 years) | 15% of patients required early cessation of chemotherapy due to treatment intolerance |
| Bromberg et al. (2019) | Prospective | 61 (56–66) | 100/200 | MBVP | 30 | 49% (1 year) | 75% (1 year) | 3% Had treatment-related mortality |
| Bromberg et al. (2019) | Prospective | 61 (55–67) | 99/200 | MBVP + R | 33 | 52% (1 year) | 77% (1 year) | 3% Had treatment-related mortality |
- Note: NR: not reached; /: not mentioned.
- Abbreviations: AB ± R, HD-methotrexate, ifosfamide, vindesine, dexamethasone, carmustine, teniposide, rituximab; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisolone; CR, complete response; D, dexamethasone; FTP, fotemustine, teniposide and dexamethasone; HD-MA, HD-MTX and cytarabine; HD-MTX ± R, HD-MTX, rituximab; MBVP, methotrexate, carmustine, teniposide, and prednisone; M-CHOP, HD-MTX, cyclophosphamide, doxorubicin, vincristine, prednisolone; MPV, HD-MTX, rituximab, vincristine; OS, overall survival; PFS, progression-free survival; R, rituximab; TMZ, temozolomide.
4 RADIOTHERAPY
4.1 WBRT alone
PCNSL is sensitive to radiotherapy, which can quickly reduce intracerebral lesions (Han & Batchelor, 2017) and improve symptoms. WBRT is the most commonly used radiotherapy for PCNSL. Standard WBRT doses are typically 40–50 Gy with single fractions of no >2 Gy (Roth, Stupp, Eisele, & Weller, 2014). The outcomes with WBRT alone (doses ranging from 36 to 45 Gy as a single fraction) for PCNSL were poor in the 1970s and 1980s, resulting in a high proportion of radiation reactions and early recurrence, with most reported 5-year survival rates below 10%. (Leibel & Sheline, 1987; Littman & Wang, 2015; Nelson et al., 1992; Shibamoto et al., 1990; Watne et al., 1992).
Increasing WBRT dosage does not achieve good results (Nelson et al., 1992). In one study including 41 patients with PCNSL who received WBRT alone at a dose of 40Gy plus a 20-Gy boost dose to tumor bed, the median OS was only 11.6 months from the start of WBRT and 12.2 months from the time of diagnosis. Moreover, 61% (25/41) of the patients had recurrence and the majority of relapses occurred within the irradiated areas. However, the OS with WBRT alone was better in patients ≤60 years of age than in those >60 years of age. WBRT alone is no longer recommended as initial treatment due to the lack of sustainable response to radiation and associated neurotoxic risk (Han & Batchelor, 2017) and should be combined with chemotherapy (Burton et al., 2017; Hao, Ahluwalia, & Peereboom, 2015). Hao et al. (2015) analyzed 153 patients to demonstrate that HD-MTX-based chemotherapy with or without WBRT offered a better response rate and survival as initial treatment compared with WBRT alone.
4.2 WBRT and combination chemotherapies
In the 1990s, the development of HD-MTX-based chemotherapies improved the treatment outcomes of patients with PCNSL. The 5-year survival rate using this strategy is 22–40%, whereas that of WBRT alone is only 3–26% (Ferreri et al., 2000; Reni, Ferreri, Garancini, & Villa, 1997). Previous studies confirmed the marked survival benefit of WBRT in combination with HD-MTX-based chemotherapy (Abrey, Yahalom, & DeAngelis, 2000; Burton et al., 2017; Deangelis, Seiferheld, Schold, Fisher, & Schultz, 2002; Ferreri et al., 2000; Reni et al., 1997). Delayed neurotoxicity featuring incontinence, staggering gait, and memory deterioration have emerged as important complications, especially in elderly patients (Milgrom & Joachim, 2015). The PFS benefit afforded by WBRT plus chemotherapy must be weighed against the increased risk of neurotoxicity in long-term survivors (Chalise et al., 2017).
In the past 5 years, considerable effort has focused on whether WBRT in combination with chemotherapy can be used as first-line treatment in patients with PCNSL; however, the results remain controversial. Some studies have suggested that WBRT in combination with chemotherapy does not prolong survival compared with chemotherapy alone in patients with PCNSL (Korfel et al., 2016). While WBRT can extend PFS, no differences are observed in OS (Agnieszka et al., 2015; Chan et al., 2018; Dalia et al., 2014; Prica, Chan, & Cheung, 2014). Still, some studies (Ichikawa et al., 2014; Korfel et al., 2016) have even suggested that WBRT in combination with chemotherapy does not prolong PFS in patients with PCNSL. WBRT did not significantly prolong PFS or OS in a prospective study of 320 enrolled patients (Korfel et al., 2016). Ichikawa et al. (2014) analyzed 24 patients with PCNSL to show that WBRT did not prolong OS (Ichikawa et al., 2014). Conversely, many studies in the past 5 years (Adhikari et al., 2018; Burton et al., 2017; Chalise et al., 2017; Hattori et al., 2017; Karmali et al., 2017; Rudresha et al., 2017) have advocated HD-MTX-based chemotherapy in combination with WBRT as an effective therapeutic approach. HD-MTX-based chemotherapy in combination with WBRT can offer a better curative effect, and the neurotoxicity is acceptable (Burton et al., 2017). WBRT has also been associated with lower PCNSL relapse (Chanswangphuwana, Rojnuckarin, Cherdchoo, Raiyawa, & Uaprasert, 2018).
WBRT in combination with HD-MTX-based chemotherapy has not been considered as a routine approach for all patients with PCNSL due to the lack of evidence of its efficacy as an additional treatment and due to the high risk of neurotoxicity (Schlegel & Korfel, 2018). Although many clinicians tend to avoid WBRT in patients older than 60 years of age (Han et al., 2017; Houillier et al., 2019; Prica et al., 2014), employing a fixed age limit for all patients is too simplistic. Patients with PCNSL should be evaluated before considering WBRT in combination with HD-MTX-based chemotherapy. KPS score, leukoencephalopathy, and age are significantly associated with a decline in health-related quality-of-life score. Clinicians are strongly recommended to assess the KPS score of patients with PCNSL before administering WBRT in combination with HD-MTX-based chemotherapy (Okita et al., 2016).
4.3 Reduced-dose WBRT with or without lesional boost
Because the administration of WBRT in combination with HD-MTX-based chemotherapy has been questioned due to the high risk of delayed neurotoxicity, WBRT dose reduction or reduced-dose WBRT with a tumor bed boost may be a better choice for patients with PCNSL (Chanswangphuwana et al., 2018; Houillier et al., 2019; Kobayashi et al., 2019; Koh et al., 2017; Park et al., 2017; Sheu et al., 2018).
In a study on PCNSL, Park et al. (2017) found no significant difference in treatment outcomes between patients who received 36 Gy WBRT and those who received 45 Gy WBRT, especially among those who achieved partial response (PR) with induction chemotherapy. Chanswangphuwana et al. (2018) confirmed that both reduced-dose WBRT (30–36 Gy) with or without a lesional boost up to 45–50 Gy achieved satisfactory effects, with only mild neurocognitive toxicity. Low-dose WBRT with a tumor bed boost after MTX-based chemotherapy might be an effective approach for PCNSL management (Kim et al., 2014). Reduced-dose WBRT with a boost to residual PCNSL may be a viable treatment for patients who achieve PR with induction chemotherapy (Sheu et al., 2018).
However, reduced-dose WBRT after CR with HD-MTX-based chemotherapy may lead to a suboptimal clinical outcome due to a higher risk of recurrence, progression, and early death (Adhikari et al., 2018).
4.4 Partial-brain radiotherapy
Partial-brain radiotherapy (PBRT) has also been studied and evaluated in patients with PCNSL. A minimum safety margin of 4 cm for PBRT has been suggested (Shibamoto et al., 2003). The 5-year OS was 25% in patients treated with WBRT and 29% in those treated with PBRT (Shibamoto et al., 2014). In addition, among patients with a single tumor who were treated with HD-MTX chemotherapy, the OS and relapse-free survival did not differ between the WBRT and PBRT subgroups (Shibamoto et al., 2014). Iwabuchi et al. (2016) employed PBRT with wide margins in patients with single or few PCNSL lesions. The authors found that despite the higher 3–5-year PFS in the WBRT group (53% vs. 36%), the 3–5-year OS was comparable between the WBRT and PBRT groups (57% and 68%, respectively). The CNS recurrence rate was similar between the patients undergoing HD-MTX chemotherapy plus PBRT and those undergoing HD-MTX chemotherapy plus WBRT. Neurocognitive dysfunction developed in 3 of the 16 patients undergoing HD-MTX plus PBRT and in 4 of the 15 patients undergoing HD-MTX plus WBRT.
PBRT appears to be a feasible treatment option for PCNSL patients with a single tumor. The potential disadvantage of PBRT is risk of out-of-field recurrence, especially in multiple PCNSL lesions. Iwabuchi et al. (2016) reported that the out-of-field recurrence rate was 15% at 3 years in patients undergoing PBRT. The recurrence patterns following PBRT should be further investigated to determine whether PBRT is an acceptable treatment approach in patients with PCNSL.
4.5 Stereotactic radiosurgery (SRS)
SRS can be used as part of the multimodality management of patients with PCNSL, regardless of whether the disease is newly diagnosed or is relapsed/refractory (Shin et al., 2017). SRS following initial HD-MTX without WBRT provides excellent local control, acceptable OS, and a long period with preserved daily living activities. However, SRS should be validated in large patient populations, especially in patients with good KPS scores (Hirono et al., 2015).
SRS has been reported as an effective treatment approach in recurrent PCNSL (Kumar et al., 2015; Shin et al., 2017; Zheng, Wang, & Liu, 2018). The International Gamma Knife Research Foundation identified 23 patients with PCNSL who underwent SRS for relapsed/refractory disease. After 26 SRS procedures, 20 of the 23 patients showed treatment response. The 1-year PFS was 55%, and the SRS toxicity was low (Shin et al., 2017). Zheng et al. (2018) also reported a successful case of a patient with recurrent PCNSL who was treated with SRS and iodine-125 seeds plus temozolomide.
4.6 Summary
Over the past 5 years, many clinical studies have focused on different radiotherapy regimens for PCNSL. WBRT alone is not recommended as first-line treatment. Although WBRT in combination with chemotherapies is effective, serious toxicities are a concern. PBRT has no significant effect on improving OS in PCNSL. Reduced-dose radiotherapy and combination chemotherapies may be a better choice for patients with PCNSL under the age of 60 years (Table 3). Due to the small number of studies, further randomized studies are warranted to confirm the therapeutic impact of SRS (Table 4).
| Study | Design | No. of patients/total patients | Median (range) age, years | Induction chemotherapy | WBRT/booster dose or partial-brain radiotherapy (Gy) | Median PFS | Median OS | NT and other Grade 4 toxicity |
|---|---|---|---|---|---|---|---|---|
| Burton et al. (2017) | Retrospective | 9/21 | 65 (30–90) | / | / | / | 5 months | / |
| Sitthinamsuwan et al. (2014) | Retrospective | 14/69 | 52.8 (23–78) | HD-MTX-based regimen | 10–56 | / | 26.7 months, 50% (2 years) and 21.4% (5 years) | |
| Kim et al. (2014) | Retrospective | 64/64 | 56 (23–75) | HD-MTX-based regimen | 27/23.4 | 34 months, 47.9% (3 years) and 39.3% (5 years) | 63 months, 59.8% (3 years) and 52.6% (5 years) | 16 Of 55 patients who achieved CR experienced NT |
| Ichikawa et al. (2014) | Prospective | 9/24 | 58.3 (50–67) | M-CHOP | 36–60 | 26 months | 33 months (>65 year: 14 months) | 2 Of 9 exhibited no treatment-related mortality |
| Korfel et al. (2016) | Prospective | 273/551 | 63 (/) | HD-MTX-based regimen | / | 18.2 months | 35.6 months | / |
| Karmali et al. (2017) | Retrospective | 111/113 | / | With (29 of 111) or without (82 of 111) HD-MTX-based regimen | / | / | NR, a trend for improved OS with WBRT (p = .09) | / |
| Koh et al. (2017) | Retrospective | 179/179 | / | Most of the patients received MTX-based chemotherapy. | 30.6/15 | / | 64 months, 51.4% (5 years) | / |
| Hattori et al. (2017) | Retrospective | 10/10 | 54.5 (47–59) | R-MPV | 23.4 | 56% (3 years) | 69% (3 years) | 40% Had Grade 4 neutropenia |
| Chalise et al. (2017) | Retrospective | 21/35 | 60.5 (19–79) | DeVIC | 23.4/±20 | 37.4 months (p = .672) | 47.8 months (p = .435) | 31.6% Had NT |
| Chalise et al. (2017) | Retrospective | 4/35 | 67 (49–78) | HD-MTX monotherapy | 23.4/0 | 25.3 months (p = .672) | NR (p = .435) | 36.3% Had NT |
| Park et al. (2017) | Retrospective | 11/38 | 50 (NS) | HD-MTX-based regimen | 36 | 63.6% (5 years; p = .980) | 72.7% (5 years; p = .970) | None of the patients exhibited changes in memory |
| Park et al. (2017) | Retrospective | 27/38 | 45 (NS) | HD-MTX-based regimen | 45 | 62.6% (5 years; p = .980) | 77.8% (5 years; p = .970) | 37% Of patients exhibited changes in memory |
| Chanswangphuwana et al. (2018) | Retrospective | 12/12 | 56 (16–78) | HD-MTX or MTX-Ifos | 30–36/15–20 | 78.75% (3 years) | 87.5% (3 years) | Five patients were diagnosed with mild cognitive impairment |
| Rudresha et al. (2017) | Retrospective | 26/26 | 42.5 (NS) | HD-MTX-based regimen | 45 | / | 20.5 months | / |
| Adhikari et al. (2018) | Prospective | 10/19 | 51.5 (31–67) | HD-MTX-based regimen | 23.4 | 17.5 months (p = 0.77) | 19 months (p = .02) | No Grade 3/4 toxicity |
| Adhikari et al. (2018) | Prospective | 9/19 | 51.5 (31–67) | HD-MTX-based regimen | 45 | NR (p = .77) | NR (p = .02) | No Grade 3/4 toxicity |
| Chan et al. (2018) | Retrospective | 37/103 | 48.3 (30.0–72.5) | HD-MTX-based regimen | 45 | NR (p = .09, vs chemotherapy) | NR (p = .03, vs. chemotherapy) | / |
| Sheu et al. (2018) | Retrospective | 24/68 | 60 (31–77) | HD-MTX-based regimen | 26.7/±12.6 | 52.3 months | 52.3 months | Four patients had NT |
| Kobayashi et al. (2019) | Retrospective | 54/54 | 60.5 (31–78) | HD-MTX-based regimen | 30/10–20 | 42.5 months | 58.4 months | / |
| Houillier et al. (2019) | Prospective | 70/140 | 54.5 (22–60) | R-MBVP | 40 | 58% (2 years) and 40% (4 years) | 75% (2 years) and 64% (4 years) | One toxicity-related death |
| Shibamoto et al. (2014) | Retrospective | 969/1054 | / | HD-MTX-based regimen | 29.9–49.9/0 | / | 25% (5 years; p = .80) | |
| Shibamoto et al. (2014) | Retrospective | 85/1054 | / | HD-MTX-based regimen | (PBRT) over 30 | / | 29% (5 years; p = .80) | |
| Iwabuchi et al. (2016) | Retrospective | 15/55 | 60 (22–69) | HD-MTX-based regimen | 30–40 | 53% (3–5 years) | 57% (3–5 years) | 4 Of the 15 (26%) patients had NTp = .68) |
| Iwabuchi et al. (2016) | Retrospective | 16/55 | 68 (58–78) | HD-MTX-based regimen | (PBRT) 30–40 | 36% (3–5 years) | 68% (3–5 years) | 3 Of the 16 (19%) patients had NT (p = .68) |
- Note: NR: not reached; /: not mentioned.
- Abbreviations: DeVIC, dexamethasone, etoposide, ifosfamide and carboplatin; M-CHOP, HD-MTX, cyclophosphamide, doxorubicin, vincristine and prednisolone; NT, neurologic toxicity; OS, overall survival; PBRT, primary central nervous system lymphoma; PBRT, partial-brain radiotherapy; PCNSL PFS, progression-free survival; R-MPV, rituximab, methotrexate, procarbazine, and vincristine; WBRT, whole-brain radiotherapy.
| Study | Design | No. of patients/total patients | Median (range) age, years | Status | Median margin dose (Gy) | Median tumor volume (cm3) | Median PFS | Median OS |
|---|---|---|---|---|---|---|---|---|
| Hirono et al. (2015) | Retrospective | 20/51 | 67 (37–82) | Newly diagnosed | 15 | 0.45 | 17 months | 52 months |
| Kumar et al. (2015) | Retrospective | 14/14 | 71 (18–82) | Relapsed/refractory | 15.5 | 6.7 | 4 months | 9.5 months |
| Shin et al. (2017) | Retrospective | 23/23 | 62 (21–84) | Relapsed/refractory | 15 | 4 | 55% (1 year) | 55% (1 year) |
- Abbreviations: OS, overall survival; PFS, progression-free survival; SRS, stereotactic radiosurgery.
5 AUTOLOGOUS ASCT
High-dose chemotherapy in combination with ASCT may be an alternative approach to address chemoresistance and to overcome BBB. The first case of a patient with relapsed PCNSL who was successfully treated with ASCT was reported in 1996 (Khalfallah et al., 1996). Recent studies have revealed that ASCT is effective in both relapsed/refractory (Dholaria et al., 2019; Nelson et al., 1992; Elisabeth Schorb et al., 2013; Soussain et al., 2008; Soussain et al., 2001) and newly diagnosed PCNSL (Bojic et al., 2015; Ferreri et al., 2017; Illerhaus et al., 2016; Kuitunen et al., 2017; Nakasu et al., 2016; Omuro et al., 2015; Table 5).
| Study | Design | Number of patients who underwent ASCT/total patient | Median (range) age, years | Relapsed or refractory | HD-MTX-based chemotherapy as first-line treatment | Rituximab as first-line treatment | Number of patients exhibiting CR after ASCT | Median PSF | Median OS | Toxicity-related deaths |
|---|---|---|---|---|---|---|---|---|---|---|
| Omuro et al. (2015) | Prospective | 26/32 | 57 (23–67) | No | Yes | Yes | 21 | 81% (2 years) | 81% (2 years) | 0 |
| Illerhaus et al. (2016) | Prospective | 73/79 | 56 (51–62) | No | Yes | Yes | 61 | 65% (5 years) | 79% (5 years) | 0 |
| Ferreri et al. (2017) | Prospective | 59/59 | 58 (26–70) | No | Yes | Yes | 55 | 69% (2 years) | 71% (2 years) | 0 |
| Dholaria et al. (2019) | Retrospective | 13/18 | 56 (47–73) | No | 12 of 13 | 8 of 13 | 10 | 71% (2 years) | 71% (2 years) | 0 |
| Bojic et al. (2015) | Prospective | 5/5 | 42 (33–52) | No | Yes | 4 of 5 | 5 | / | / | 0 |
| Houillier et al. (2019) | Prospective | 70/140 | 55 (25–60) | No | Yes | Yes | 34 | 70% (2 years) and 64% (4 years) | 66% (2 years) and 66% (4 years) | 5 |
| Langnerlemercier et al. (2016) | Retrospective | 13/256 | 67 (24–92) | Yes | Yes | Yes | / | 13.5 months | NR | 0 |
| Kuitunen et al. (2017) | Retrospective | 25/25 | 57(40–71) | Yes | Yes | Yes | 19 | 61% (2 years) | 57% (2 years) and 47% (5 years) | 0 |
| Welch et al. (2015) | Retrospective | 8/8 | 58 (41–65) | Yes | Yes | Yes | 8 | 93% (3 years) (includes seven patients with SCNSL) | 93% (3 years) (includes seven patients with SCNSL) | 0 |
- Note: /: not mentioned.
- Abbreviatrions: ASCTCR, autologous stem-cell transplantation; CR, complete remission; HD-MTX, high-dose methotrexate; NR, not reached; OS, overall survival; PCNSL, primary central nervous system lymphoma; PFS, progression-free survival; SCNSL, secondary central nervous system lymphoma.
5.1 ASCT as postremission consolidation after induction therapy in first-line treatment
In a study of 33 immunocompetent adult patients (Omuro et al., 2015), those with CR or PR proceeded with consolidation HD-MTX-base chemotherapy plus rituximab, followed by ASCT without radiotherapy. Both the 2-year PFS and OS were 81% in the transplanted patients. HD-MTX-base chemotherapy in combination with HDC-ASCT as first-line treatment of PCNSL was associated with excellent disease control and survival, with an acceptable toxicity profile. In addition, no evidence of neurotoxicity was reported. In another study, 73 patients with newly diagnosed PCNSL started induction HD-MTX-base chemotherapy plus rituximab and ASCT (Illerhaus et al., 2016). CR was observed in 77.2% (61/73) of the patients, and the 5-year PFS and OS were 65% and 79%, respectively. Fifty-nine patients with newly diagnosed PCNSL receiving HD-MTX-base chemotherapy plus rituximab and ASCT also achieved satisfactory outcomes, with 2-year PFS, OS, and CR rates of 69%, 71%, and 93%, respectively (Ferreri et al., 2017).
5.2 ASCT for relapsed/refractory PCNSL
ASCT is a known effective treatment for relapsed/refractory non-Hodgkin lymphoma (Philip et al., 1995). Importantly, in the past 5 years, several studies have demonstrated that ASCT is a feasible salvage therapy for patients with relapsed and refractory PCNSL (Langnerlemercier et al., 2016; Soussain et al., 2008; Welch et al., 2015).
5.3 Biomarkers for the administration of ASCT in patients with PCNSL
Cho et al. (2017) examined the prognostic significance of CD68 and FoxP3 expression in tumor samples of 76 immunocompetent patients with newly diagnosed PCNSL and found that high CD68 expression was associated with inferior OS and PFS among those who did not receive upfront ASCT (n = 60) but not in patients who did. This finding suggests that the negative prognostic value of CD68 may be less important under consolidation upfront ASCT. In addition, the time at which ASCT should be performed requires further clinical studies.
6 SALVAGE TREATMENT OF RELAPSED/REFRACTORY PCNSL
Disease recurrence is commonly observed in patients with PCNSL. Up to 60% of patients experience relapse, and 10–15% experience primary refractory disease (Langnerlemercier et al., 2016). Patients with primary refractory or relapsed PCNSL have a poor prognosis and a median survival of only 2 months without additional treatment (Reni, Ferreri, & Villa, 1999). The median time to relapse is 10–18 months, and most relapses occur within the first 2 months following initial diagnosis (Jahnke et al., 2006). Treatment of relapsed or refractory PCNSL has not yet been defined (Rubenstein et al., 2018). The following relates to salvage treatment approaches for relapsed/refractory PCNSL investigated in the past 5 years (Table 6).
| Study | Type of study | Number of salvage treatment patients/total patients | Median (range) age, years | HD-MTX-based chemotherapy as first-line treatment | Salvage treatments | Number of patients with CR | Median PSF | Median OS |
|---|---|---|---|---|---|---|---|---|
| Rubenstein et al. (2018) | Prospective | 10/14 | 66 (47–79) | Yes | lenalidomide/rituximab | / | 6 months | 45 months |
| Ghesquieres et al. (2019) | Prospective | 34/50 | 69 (46–86) | Yes | lenalidomide plus rituximab | 13 | 7.8 months | 17.7 months |
| Miyakita et al. (2017) | Prospective | 15/30 | 64 (40–77) | Yes | Rituximab + HD-MTX | 10 | 7.8 months | NR |
| Miyakita et al. (2017) | Prospective | 15/30 | 64 (45–79) | Yes | HD-MTX | 10 | 9.1 months | NR |
| Zhao et al. (2015) | Prospective | 27/27 | 53.5 (35–63) | Yes | Rituximab + pemetrexed | 6 | 6.9 months | 11.2 months |
| Collignon et al. (2018) | Prospective | 13/13 | 71.4 (49.5–82.5) | Yes | rituximab + GEMOX | 2 | 3.2 months | 8.2 months |
| Nagao et al. (2018) | Retrospective | 18/18 | 58.5 (28–77) | Yes, 237 (92.6%) | ESHAP | 4 | / | / |
| Chamberlain (2014) | Retrospective | 29/256 | 67 (24–92) | IFO-based regimen plus ACST | / | 13.5 months | NR | |
| Langnerlemercier et al. (2016) | Retrospective | 12/12 | 59 (43–74) | Yes, 11 (91.7%) | bendamustine | 29 | 3.5 months | NR |
| Chamberlain (2014; second recurrence) | Retrospective | 39/39 | 66 (41–82) | Yes | MTX | 3 | 16 months | 41 months |
- Note: /: not mentioned.
- Abbreviations: ACST, autologous stem-cell transplantation; CR, complete remission; ESHAP, etoposide, solumedrol, high-dose cytarabine, and platinum; GEMOX, gemcitabine and oxaliplatin; IFO, ifosfamide; MTX, methotrexate; OS, overall survival; PFS, progression-free survival.
6.1 Lenalidomide
Lenalidomide is a second-generation immunomodulatory agent with pleiotropic antitumor effects. Lenalidomide is active in activated B-cell type DLBCL, whereas rituximab is effective in CNS lymphoma. Therefore, lenalidomide in combination with rituximab may be an option for relapsed/refractory PCNSL (Ghesquieres et al., 2019; Rubenstein et al., 2018). Treatment with lenalidomide in combination with rituximab led to a median PFS of 6 months in patients with relapsed/refractory PCNSL (Rubenstein et al., 2018). In another study to determine the efficacy of rituximab plus lenalidomide in 34 patients with relapsed/refractory PCNSL, the median PFS and OS were 7.8 and 17.7 months, respectively (Ghesquieres et al., 2019).
6.2 Rituximab
In addition to lenalidomide, rituximab in combination with other chemotherapy regimens may be effective in relapsed/refractory PCNSL. Thirteen patients benefited from the (R)-GEMOX regimen (rituximab, gemcitabine, and oxaliplatine), and the overall response rate was 38%, with two and three patients achieving CR and PR, respectively (Collignon et al., 2018). The median PFS and OS were 3.2 and 8.2 months, respectively. Moreover, RXT plus pemetrexed was marginally effective and well tolerated in 27 patients with PCNSL who failed HD-MTX-based first-line treatment regimens (Zhao, Chen, Shi, Tian, & Tao, 2015). The CR rate was 22.2% (6/27), and the median PFS and OS were 6.9 and 11.2 months, respectively.
Conversely, Miyakita et al. (2017) reported that the median time to tumor progression was 9.0 months with HD-MTX and 5.7 months with rituximab in combination with HD-MTX. There was no significant difference in mean time to progression, suggestion that the addition of rituximab to HD-MTX may not be a promising strategy for recurrent PCNSL.
6.3 High-dose cytarabine
In one study, four patients with relapsed/refractory PCNSL treated with ESHAP (etoposide, solumedrol, high-dose cytarabine, and platinum) chemotherapy achieved CR (Ungur et al., 2015). In another study, 18 patients with refractory/recurrent PCNSL were administered ESHAP chemotherapy, the response rate after the final ESHAP course was 61.1% (11/18 patients), and four patients (22.2%) achieved CR. No fatal adverse events occurred (Nagao et al., 2018). However, high-dose cytarabine alone has limited activity in recurrent PCNSL. One study of 14 patients with recurrent PCNSL treated with high-dose cytarabine as single-agent reported a median PFS of 3 months and a PFS of 0% at 6 months (Kasenda et al., 2017). The median survival after the initiation of high-dose cytarabine was 12 months. The observed poor outcomes may be associated with significant toxicity.
6.4 Ifosfamide
Ifosfamide is believed to improve response rates and PFS in patients with relapsed/refractory PCNSL. In a study comparing various regimens, the ifosfamide-based regimens were associated with a response rate of 42.4% (Langnerlemercier et al., 2016). Therefore, ifosfamide-based regimens plus ASCT may be considered as a treatment for patients with relapsed/refractory PCNSL.
6.5 Other treatment options
As mentioned earlier, SRS (Kumar et al., 2015; Shin et al., 2017; Zheng et al., 2018), bendamustine (Chamberlain, 2014), and HD-MTX (Elena, Deangelis, & Antonio, 2014) may also be considered as options for treating patients with relapsed/refractory PCNSL.
7 TREATMENT OF ELDERLY PATIENTS WITH PCNSL
According to the statistical analysis of the period between 1975 and 1999, patients over 60 years of age account for 50% of all PCNSL cases (Mendez et al., 2018). The significantly improved survival of elderly patients with PCNSL in the last 40 years (median OS, 14 months in 1987–1997 vs. 35 months in 2007; Kasenda et al., 2015) may be due to the emergence of more intensive and efficient treatments. However, many elderly patients are still likely to be undertreated solely because of advanced age. Consequently, treatment of PCNSL in elderly patients remains unsatisfactory (Table 7).
| Study | No. of patients/total patients | Median (range) age, years | Design | MTX | Other treatment options | CR rate (%) | Median PFS | Median OS |
|---|---|---|---|---|---|---|---|---|
| Lee et al. (2014b) | 38/38 | 69 (60–80) | Retrospective | Yes | Procarbazine, steroids | 42 | 18 months | 43 months |
| Pulczynski et al. (2015) | 27/66 | 70 (66–75) | Prospective | Yes | ARAC, TMZ | 69.2 | 44.4% (2 years) | 55.6% (2 years) |
| Olivier et al. (2014) | 35/35 | 65 (60–70) | Prospective | Yes | VIND, IDA, steroids | 17 | 13 months | 19 months |
| Makino et al. (2015) | 63/91 | /(All over 60) | Retrospective | Yes | Procarbazine, steroids | / | 7 months | 31 months |
| Schorb et al. (2019) | 52/52 | 68.5 (65–77) | Retrospective | No | Thiotepa-based HDT-ASCT | 0.692 | 51.1 months | 122.3 months |
| Han et al. (2017) | 7/24 | /(All over 60) | Retrospective | Yes | Rituximab | / | 33.33% (2 years) | 33.33% (2 years) |
| Han et al. (2017) | 11/23 | /(All over 60) | Retrospective | Yes | AB ± R | / | 54.55% (2 years) | 81.82% (2 years) |
- Note:/: not mentioned.
- Abbreviations: AB ± R, HD-methotrexate, ifosfamide, vindesine, dexamethasone, carmustine, teniposide, rituximab; ARAC, cytarabine; IDA, idarubicine; ASCT, autologous stem-cell transplantation; OS, overall survival; PFS, progression-free survival; TMZ: temozolomide; VIND, vindesin.
HD-MTX-based chemotherapy is the most widely used and studied regimen in both elderly and young patients with PCNSL. Currently, a considerable number of elderly patients with PCNSL benefit from HD-MTX-based chemotherapy (Gaelle et al., 2015; Kasenda et al., 2015; Lee et al., 2014a; Makino et al., 2015; Pulczynski et al., 2015). HD-MTX plus high-dose cytarabine (Farhi et al., 2018), HD-MTX plus high-dose cytarabine followed by ASCT (Schorb et al., 2019), and HD-MTX plus procarbazine (Makino et al., 2015) are among the reportedly effective treatment regimens for elderly patients with PCNSL.
A multicenter meta-analysis of 783 patients assessed the prognosis of immunocompetent patients over 60 years of age with newly diagnosed PCNSL who received different first-line regimens, including HD-MTX, WBRT, and multidrug chemotherapies. The prognosis of patients receiving the HD-MTX regimen was significantly better than that of those who did not. However, there is no evidence that adding other chemotherapeutic agents to HD-MTX improves prognosis (Kasenda et al., 2015). Studies investigating adverse reactions in elderly patients revealed that these patients could tolerate MTX at doses below 3.5 g/m2 (Fritsch et al., 2011; Ghesquieres et al., 2010; Han et al., 2017; Illerhaus et al., 2009; Zhu et al., 2009). Dose adjustment according to the glomerular filtration rate is necessary in elderly patients receiving MTX as a single dose of 4–8 g/m2 or as a dose of 1.5–3.5 g/m2 in combination with other cytotoxic drugs (Bessell, Dickinson, Dickinson, & Salmon, 2011).
Radiotherapy is not recommended for elderly patients, whether in combination with chemotherapy or alone. Although it may improve outcomes in elderly patients with PCNSL, WBRT is associated with an increased risk of neurological side effects and is related to increases in chemotherapy-related side effects (Han et al., 2017; Houillier et al., 2019; Prica et al., 2014). The median OS was significantly shorter in patients older than 65 years who received M-CHOP (HD-MTX, cyclophosphamide, doxorubicin, vincristine and prednisolone) plus WBRT compared to those who received M-CHOP alone (14 vs. 32 months). WBRT is also related to more frequent neurotoxicity (Ichikawa et al., 2014).
Finally, intensive treatment approaches, including high-dose chemotherapy followed by ASCT (HDT-ASCT), are offered only to younger patients (<65 years of age; Illerhaus et al., 2008; Soussain et al., 2008). As recently concluded by Schorb et al. (2017), HDT-ASCT plus thio-TEPA-based conditioning regimes are feasible and effective, as demonstrated in a cohort of 52 elderly patients with PCNSL (KPS score: >80), with only two HDT-ASCT-associated deaths (3.8%). Thus, further prospective and comparative studies are warranted to further evaluate the role of HDT-ASCT in elderly patients with PCNSL. A multicenter, prospective Phase II trial aimed to determine the feasibility and efficacy of HDT-ASCT in fit patients >65 years with newly diagnosed PCNSL is in progress; the results are anticipated in the near future (Schorb et al., 2019).
8 NOVEL PCNSL THERAPEUTIC APPROACHES
8.1 Ibrutinib
The B-cell receptor signaling pathway is mutated more frequently in PCNSL than in systemic DLBCL. Ibrutinib is a small molecule inhibitor of Bruton tyrosine kinase and has been shown as a promising candidate drug for the treatment of PCNSL (Lionakis et al., 2017). In a study of 18 patients with relapsed or refractory PCNSL, 94% (17/18) of the patients showed tumor reduction with ibrutinib monotherapy alone and 11% of the patients developed severe pulmonary infections (Lionakis et al., 2017). In one study comprising 14 patients with relapsed or refractory PCNSL, all of whom were previously treated with HD-MTX-based chemotherapy (Chamoun et al., 2017), three and four patients achieved CR and PR, respectively. The median PFS was 6 months. In another study, clinical response to ibrutinib was observed in 10 of 13 (77%) patients with relapsed or refractory PCNSL, including five patients who achieved CR (Grommes et al., 2017).
Ibrutinib is effective not only in patients with relapsed PCNSL but also in untreated patients. In one study of six patients with relapsed or refractory PCNSL and five untreated patients with PCNSL, ibrutinib was incorporated into the novel regimen DA-TEDDI-R (temozolomide, etoposide, doxil, dexamethasone, ibrutinib, and rituximab) (with intraventricular cytarabine) (Dunleavy et al., 2015). Three patients died during the study. With ibrutinib alone, seven of the eight evaluable patients achieved PR, and one patient had a mixed response. All five patients who completed the DA-TEDDI-R regimen achieved CR.
8.2 Nivolumab
One study has revealed the membranous expression of programmed death-ligand 1 (PD-L1) in 30% (13 of 43) of specimens by immunohistochemistry, suggesting that programmed cell death-1 (PD-1)/PD-L1 inhibitors may be useful in a subset of patients with PCNSL (Nayyar et al., 2019). PCNSL frequently harbors 9p24.1/PD-L1/PD-L2 copy number alterations and additional translocations of these loci (Chapuy et al., 2016). Nivolumab is a monoclonal antibody that blocks PD-1 to prevent T lymphocyte activation. One study determined the efficacy of nivolumab in four patients with relapsed/refractory PCNSL (Nayak et al., 2017). All patients had clinical and radiographic response to nivolumab. Additionally, three patients achieved PFS for 13–17 months and only one patient developed grade 4 nephrotoxicity. Furthermore, a 50-year-old patient with PCNSL was treated with MTX-based chemotherapy followed by HDCT/ASCT at initial diagnosis, as well as two later relapses. Nivolumab was used as maintenance therapy for 12 months after achieving a third CR (Terziev et al., 2018).
8.3 Temsirolimus
Temsirolimus is an inhibitor of mammalian target of rapamycin (mTOR) signaling. The mTOR signaling is often imbalanced in PCNSL, and inhibition of mTOR can effectively slow down PCNSL progression. In a Phase II trial (NCT00942747) of 37 patients with recurrent/refractory PCNSL, the radiographic response was 54% (Korfel et al., 2016). Unfortunately, the median PFS was only 2.1 months.
8.4 Chimeric antigen receptor (CAR)-T-cell therapy
CAR-T-cell therapy is expected to be used in B-cell-derived CNS lymphoma. At present, no report has evaluated CAR-T-cell therapy in PCNSL. A 68-year-old female with DLBCL and brain involvement who was insensitive to several chemotherapy regimens including ASCT was included in the clinical trial of JCAR017, and the efficacy from CAR-T-cell therapy was confirmed in the CNS. Subcutaneous lesions were resected at the 2-month follow-up without further treatment. CR was confirmed at the 3-month follow-up and sustained remission was confirmed at 12 months. No neurotoxicity, graft-versus-host disease, or cytokine release syndrome was observed (Abramson, 2017).
The genes, cellular interactions, or signaling pathways targeted by novel therapeutic approaches for PCNSL are illustrated in Figure 2.
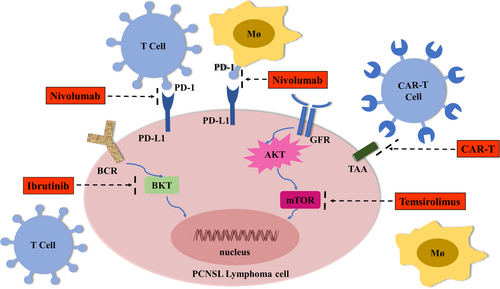
9 OUTLOOKS AND FUTURE PERSPECTIVES
The PCNSL survival rate has been improved despite the progress in treatment approaches for PCNSL over the past 5 years. HD-MTX remains the first-line treatment; however, the identification of patients who will benefit most from HD-MTX is necessary. The effects of rituximab and temozolomide should be emphasized, and more effective therapeutics should be developed. Regarding adjuvant radiotherapy for patients under 60 years of age, reduced-dose WBRT and boost radiotherapy for the tumor bed should be considered to decrease neurotoxic side effects. The role of SRS should not be neglected. ASCT should be promoted in PCNSL, in both newly diagnosed and relapsed/refractory cases; however, the exact timing of ASCT requires further clarification. Radiotherapy is still not recommended for patients with PCNSL who are over the age of 60 years, HD-MTX-based regimens are still preferred in elderly patients with PCNSL, and the MTX dosage depends on the glomerular filtration rate. Lenalidomide in combination with rituximab is expected to become popular for patients with relapsed or refractory PCNSL. Future studies require the cooperation of multicenter studies, and further evidence necessitates additional investigation on PCNSL.
ACKNOWLEDGEMENTS
This project in Fang Liu’ lab was funded by National key research and development program (No. 2018YFA0902702), the National Science and Technology Major Project (No. 2018ZX10731301-003), National Natural Science Foundation of China (No. 81570202) and Research Start-up Foundation for Lingnan Scholar of Foshan University. Hua You is supported by the National Natural Science Foundation of China (No. 81911530169, 81903088, 81850410547, 81670180, and 81711540047). Hua Yang is supported by the Medical Research Fund of Guangdong (No. A2020466).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Hua Yang and Yang Xun contributed equally to the work. Hua You and Fang Liu conceived the hypothesis and revised the manuscript. Hua Yang, Yang Xun, and Anping Yang did the literature search, Hua Yang and Yang Xun wrote the manuscript. Hua Yang and Hua You prepared the tables and figures.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




