Recent advances in the antilung cancer activity of biosynthesized gold nanoparticles
Abstract
Gold nanoparticles (Au-NPs) have been widely used in biomedical fields such as imaging, diagnosis, and treatment because of their special characteristics. Au-NPs can be synthesized using several methods, including the biological method, also called green or eco-friendly synthesis. Recent studies have reported the anticancer activity of biosynthesized Au-NPs, especially in lung cancer. This review focused on the advances in the antilung cancer activity of biosynthesized Au-NPs and its potential mechanisms.
1 INTRODUCTION
With the development of nanotechnology, nanoparticles (NPs) have been widely used in industry, agriculture, medicine, and daily life (Aghebati-Maleki et al., 2020; Ben Haddada et al., 2020; Falahati et al., 2020; Liu, Lu, Huang, Li, & Xu, 2018; Magro & Vianello, 2019; Milincic et al., 2019). NPs are particles with at least one dimension of <100 nm, which can be made of several metals, such as copper, titanium, silver, and gold. Gold nanoparticles (Au-NPs) are frequently studied and widely used in the field of medicine, such as imaging and disease diagnosis and treatment, due to their unique properties, including optical properties, chemical stability, biocompatibility, simple preparation, easy modification, and plasmonic properties (Aghaie et al., 2019; Bouche et al., 2020; Ning, Zhu, & Gao, 2017; Sharifi et al., 2019; Singh et al., 2018b). Several physical, chemical, and biological methods for the synthesis and assembly of Au-NPs have already been reported. Among these methods, the biological method, also called green or eco-friendly synthesis, is eco-benign, clean, less toxic, and more cost-effective compared with other synthesis methods (Khan, Ullah, Khan, Mashwani, & Nadhman, 2019; Lee et al., 2020; Lopes, Alves, Pereira, Granjeiro, & Leite, 2019; Siddiqi & Husen, 2017). Previous studies have reported on the biological synthesis of Au-NPs using microorganisms (bacteria, fungi, and yeasts; Narayanan & Sakthivel, 2010; Pei et al., 2017; Tripathi, Shrivastav, & Shrivastav, 2018) and natural plants (Salix alba leaves, Sambucus nigra fruit extract, Mentha piperita leaf extracts, flower petals of Moringa oleifera, root extracts of Glycyrrhiza uralensis, Garcinia mangostana fruit peel, Panax ginseng fresh leaves, Magnolia officinalis, and Rabdosia rubescens, etc.; Ahmad et al., 2017; Klekotko et al., 2015; Lee et al., 2020; Simu et al., 2019; Tiloke, Phulukdaree, Anand, Gengan, & Chuturgoon, 2016) and their biomedical applications. Recently, a growing number of studies have paid attention to the biological functions of the anticancer activity of biosynthesized Au-NPs, especially in lung cancer. Liu et al. (2019) reported that biosynthesized Au-NPs exerted anticancer activity by inducing cellular apoptosis in renal carcinoma A498 cells. A study by Patil et al. (2018) showed that biosynthesized Au-NPs exhibited anticancer activity by inhibiting cell proliferation and inducing cellular apoptosis in gastric adenocarcinoma AGS cells. Ji, Cao, and Song (2019) study indicated that Au-NPs fabricated by Cordyceps militaris extracts exerted anticancer effects on hepatocellular carcinoma cells by activating the gene expression of Bax, Bid, caspase 3, and caspase 9. The anticancer activity of biosynthesized Au-NPs was also observed in colon carcinoma HCT-116 cells, breast adenocarcinoma MCF-7 cells, bladder cancer T24 cells, cervical carcinoma Hela cells, and human chronic myelogenous leukemia K-562 cells (Ismail, Saqer, Assirey, Naqvi, & Okasha, 2018; Raghunandan et al., 2011; Wu et al., 2019). Moreover, several molecular events, such as reactive oxidative species (ROS)-induced apoptosis, cell cycle arrest, antiangiogenesis, oncogene downregulation, and tumor suppressor gene upregulation have been reported to be involved in the anticancer activity of Au-NPs (Ji et al., 2019; Liu et al., 2019; Nagalingam, Kalpana, Devi Rajeswari, & Panneerselvam, 2018; Mukherjee et al., 2005; Tiloke et al., 2016). This review focused on the advances in the antilung cancer activity of biosynthesized Au-NPs and its potential mechanisms.
1.1 Antilung cancer activity of Au-NPs synthesized via microorganisms
Microorganisms, including bacteria, fungi, and yeasts, are often utilized to biologically synthesize NPs. Four studies reported the antilung cancer activity of Au-NPs synthesized via bacteria (Table 1). The size and shape of synthesized Au-NPs vary among studies, which may be due to the different growth phases of particles among various microorganisms. Rajeshkumar (2016) fabricated Au-NPs using marine bacteria Enterococcus sp. The biosynthesized Au-NPs are mostly spherical in shape with size ranging from 6 to 13 nm. An MTT assay was performed to assess the anticancer activity of Au-NPs against A549 human lung adenocarcinoma cells. Data showed that 100 μg/ml of Au-NPs significantly inhibited the proliferation of A549 cells. Chandrakasan, Seetharaman, Gnanasekar, Kadarkarai, and Sivaperumal (2017) prepared Au-NPs using bacteria Xenorhabdus stockiae KT835471, which exhibits spherical, ovoid, and triangle NPs with size ranging from 10 to 30 nm (average: 14 ± 5 nm). The antilung cancer activity of the eco-friendly synthesized Au-NPs was evaluated by cell viability, acridine orange/ethidium bromide (AO/EtBr) staining, and DNA fragmentation assays. The results demonstrated that treatment with Au-NPs significantly inhibited cell proliferation in a dose-dependent manner and induced cellular apoptosis in A549 cells. However, the cytotoxicity of Au-NPs to normal cell lines was not observed in these two studies. The ideal antilung cancer agents are toxic to cancer cells, but not to normal cells. Thus, the cytotoxic effects of Au-NPs on normal cells should be investigated, while evaluating the antilung cancer activity of biosynthesized Au-NPs.
| Study | Biological agents | Size | Tested cells | Methods | Results |
|---|---|---|---|---|---|
| Rajeshkumar (2016) | Marine bacteria Enterococcus sp | 6–13 nm | A549 cells | MTT assay | Positive |
| Chandrakasan et al. (2017) | Bacteria Xenorhabdus stockiae KT835471 | 10–30 nm | A549 cells | MTT assay, acridine orange/ethidium bromide (AO/EtBr) staining and DNA fragmentation assay | Positive |
| Patil et al. (2019) | Marine bacteria Paracoccus haeundaensis BC74171 | 20.93 ± 3.46 nm | A549 cells, HaCat cells, and HEK293 cells | WST assay | Positive for A549 cells and negative for HaCat cells and HEK293 cells |
| Kumar, Poornachandra, and Chandrasekhar (2015) | Streptomyces clavuligerus bacteria | Average size of 8.2 nm | A549 cells and MRC-5 cells | MTT assay | Positive for A549 cells and negative for MRC-5 cells |
| Nagalingam et al. (2018) | Leaf extracts of Alternanthera bettzickiana | 80–120 nm | A549 cells | MTT assay, DNA fragmentation assay and nuclear staining | Positive |
| Sun et al. (2019) | Marsdenia tenacissima | Around 50 nm | A549 cells | MTT assay and fluorescent staining | Positive |
| Vijayan, Joseph, and Mathew (2019) | Leaf extracts of Bauhinia purpurea | Unknown | A549 cells | MTT assay | Positive |
| Zheng et al. (2019) | Magnolia officinalis | 10–70 nm | A549 cells | MTT assay | Positive |
| Vijayan et al. (2018) | Indigotera tinctoria leaf extracts | 6–29 nm | A549 cells | MTT assay | Positive |
| Francis, Koshy, and Mathew (2018) | Stereospermum suaveolens root bark extracts | 27.19 ± 5.96 nm | A549 cells | MTT assay | Positive |
| Vijayakumar et al. (2017) | Peel extracts of Musa paradisiacal | Within 50 nm | A549 cells | MTT assay | Positive |
| Zhang Zhang, Tan, Jia, Zhang, and Dang (2019) | Rabdosia rubescens | About 130 nm | A549 cells and MRC-5 cells | MTT assay, DAPI staining and TUNEL assay | Positive for A549 cells and negative for MRC-5 cells |
| Singh et al. (2017) | Panax ginseng fresh leaves | 10–20 nm | A549 cells, HaCat cells, and 3T3-L1 preadipocytes | MTT assay | Positive for A549 cells and negative for HaCat cells and 3T3-L1 cells |
| Ahmad et al. (2017) | Leaf extracts of Mentha piperita | Around 70 nm | A549 cells and 3T3-L1 preadipocytes | MTT assay | Positive for A549 cells and negative for 3T3-L1 cells |
| Tiloke et al. (2016) | Leaf extracts of Moringa oleifera | 10–20 nm | A549 cells and peripheral blood mononuclear cells (PBMCs) | MTT assay and Annexin-V-Fluos assay | Positive for A549 cells and negative for PBMCs |
| Wang et al. (2016) | Dendropanax morbifera leaf extracts | 10–20 nm | A549 cells and HaCat cells | MTT assay | Negative |
| Park Park, Ahn, and Park (2017) | Garcinia mangostana pericarp waste extracts | 15.37 ± 3.99–44.20 ± 16.99 nm | A549 cells and NIH3T3 mouse fibroblast cells | WST assay | Negative |
| Singh et al. (2018a) | Euphrasia officinalis leaf extracts | 49.72 ± 1.2 nm | A549 cells | MTT assay | Negative |
| Kumar et al. (2016) | Genipa americana fruit extracts | 15–40 nm | A549 cells | MTT assay | Negative |
- Abbreviations: Au-NPs, gold nanoparticles; DAPI, 4′,6-diamidino-2-phenylindole.
Patil et al. (2019) biologically synthesized Au-NPs, which are spherical in shape and have an average size of 20.93 ± 3.46 nm, using marine bacteria Paracoccus haeundaensis BC74171. They examined the proliferation of immortalized human keratinocyte HaCat cells, human embryonic kidney HEK293 cells, and A549 cells treated with various concentrations of synthesized Au-NPs (25, 50, 75, 100, 125, 150, 175, and 200 μg/ml) and observed that treatment with Au-NPs obviously inhibited the proliferation of A549 cells but did not have effects on the proliferation of normal cell lines (HaCat and HEK293). Kumar et al. (2015) study fabricated Au-NPs using a culture supernatant of Streptomyces clavuligerus bacteria, which are monodispersed and spherical in shape with an average size of 8.2 nm. The antilung cancer activity of the Au-NPs was measured by cell viability testing. The authors observed that synthesized Au-NPs by Streptomyces clavuligerus bacteria displayed anticancer activity in A549 cells but showed noncytotoxic effects on normal human embryonic lung MRC-5 fibroblasts (Kumar et al., 2015). These two studies indicate that Au-NPs prepared from P. haeundaensis BC74171 bacteria and Steptomyces clavuligerus bacteria specifically targeted lung cancer cells but did not kill normal cells, which can be used in lung cancer therapy.
1.2 Antilung cancer activity of Au-NPs synthesized via plant extracts
Plant extracts have been widely used to synthesize Au-NPs. Plant biomolecules, such as proteins, enzymes, vitamins, terpenoids, flavonoids, polyphenols, and polysaccharides, can act as reducing, stabilizing, and capping agents. Plant extracts consist of different kinds and doses of plant biomolecules, which might influence the biological activity of synthesized NPs. Fifteen studies reported the antilung cancer activity of Au-NPs synthesized using plant extracts (Table 1). Among them, 11 studies showed positive results, but four studies exhibited negative results. Nagalingam et al. (2018) found that Au-NPs (range, 80–120 nm) prepared using leaf extracts of Alternanthera bettzickiana exhibited anticancer activity in A549 cells. A study by Sun et al. (2019) demonstrated that biosynthesized Au-NPs (~50 nm) from Marsdenia tenacissima manifested anticancer activity in a dose-dependent manner by inhibiting cell proliferation and inducing cellular apoptosis in A549 cells. Vijayan, et al. (2019) reported that biosynthesized Au-NPs using leaf extracts of Bauhinia purpurea demonstrated anticancer activity against A549 cells in a dose-dependent manner measured using MTT assay. Zheng et al's. (2019) study found that eco-friendly synthesized Au-NPs (average size between 70 and 10 nm) from M. officinalis inhibited A549 cell proliferation and induced apoptosis, which suggests that Au-NPs produced from M. officinalis exhibited antilung cancer effects. Vijayan, Joseph, and Mathew, (2018) fabricated Au-NPs using Indigofera tinctoria leaf extracts and investigated their anticancer activity in A549 cells by MTT assay. The results showed that Au-NPs (range: 6–29 nm) treatment (6.25, 12.5, 25, 50, and 100 μg/ml) induced dose-dependent cell growth inhibition compared with negative control (Vijayan et al., 2018). Data from Francis et al. (2018) showed that Au-NPs (average size: 27.19 ± 5.96 nm) produced from Stereospermum suaveolens root bark extracts inhibited A549 cell growth in a dose-dependent manner (12.5, 25, 50, 100, and 200 μg/ml). Vijayakumar et al's. (2017) study found that Au-NPs (50 nm) prepared from the peel extracts of Musa paradisiaca displayed antiproliferative effects in A549 cells at higher concentration of 100 μg/ml. These seven studies indicated that these Au-NPs, which were synthesized from plants, affected the proliferation and apoptosis in A549 cells but did not test the cytotoxicity of Au-NPs to normal cells. The side effects of these biosynthesized Au-NPs on normal cells are still unknown. Thus, further studies should be performed to assess the cytotoxicity of the Au-NPs to normal cells.
Zhang et al. (2019) prepared Au-NPs from R. rubescens and measured their anticancer effects against A549 cells using MTT assay, 4′,6-diamidino-2-phenylindole staining, and TUNEL assay. The findings showed that 25 and 50 μg/ml of Au-NPs significantly inhibited A549 cell proliferation and induced cellular apoptosis but did not show obvious toxicity to MRC-5 cells (Zhang et al., 2019). Singh et al. (2017) reported that 100 μg/ml Au-NPs synthesized from P. ginseng fresh leaves exhibited anticancer activity in A549 cells but did not have effects on cell viability of either HaCat cells or 3T3-L1 preadipocytes. Ahmad et al. (2017) fabricated Au-NPs from the leaf extracts of M. piperita and analyzed their anticancer activity against A549 cells using MTT assay. The results indicated that biosynthesized Au-NPs (~70 nm) at various concentrations of 18.75, 37.5, 50, 75, 150, and 300 μg/ml exhibited significantly antilung cancer activity in A549 cells, but did not have obvious toxicity to 3T3-L1 preadipocytes. Tiloke et al. (2016) investigated the antilung cancer activity of Au-NPs prepared from leaf extracts of M. oleifera. Data showed that treatment with Au-NPs (range: 10–20 nm) caused a dose-dependent proliferation decline in A549 cells but showed no cytotoxicity in normal healthy peripheral blood mononuclear cells (Tiloke et al., 2016). These four studies suggest that Au-NPs prepared from natural plants, such as R. rubescens, P. ginseng fresh leaves, M. piperita leaf extracts, and M. oleifera leaf extracts specifically killed lung cancer cells but did not impair normal cells. These biosynthesized Au-NPs could be potentially applied for lung cancer therapy.
Wang et al. (2016) fabricated Au-NPs using Dendropanax morbifera leaf extracts. MTT assay was performed to examine the cytotoxicity of synthesized Au-NPs to HaCat and A549 cells. The results indicated that biosynthesized Au-NPs (10–20 nm) did not exhibit cytotoxicity in both cell lines at the concentrations of 1, 10, and 100 μg/ml after 48 hr treatment. Park et al. (2017) reported that the biosynthesized Au-NPs (average size: 15.37 ± 3.99 to 44.20 ± 16.99 nm) from G. mangostana pericarp waste extracts did not show any significant cytotoxicity to A549 cells and NIH3T3 mouse fibroblast cells in WST assay. Singh, Du, Singh, and Yi (2018a) observed that Au-NPs (49.72 ± 1.2 nm) produced by Euphrasia officinalis leaf extracts (1, 5, 10, and 100 μg/ml) for 48 hr treatment did not exert antiproliferative effects in A549 cells measured by MTT assay. Kumar et al's. (2016) study indicated that Au-NPs (15–40 nm) prepared using Genipa americana fruit extracts showed no cytotoxicity against A549 cells in MTT assay when cells were treated with 0.01, 0.1, 1, 10, and 20 μM Au-NPs for 48 hr. These four studies suggest that these Au-NPs prepared from D. morbifera leaf extracts, G. mangostana pericarp waste extracts, E. officinalis leaf extracts, and G. americana fruit extracts did not exert antilung cancer activity. Perhaps, these biosynthesized Au-NPs could be used in the field of drug delivery.
1.3 Antilung cancer activity of Au-NPs modulated by several factors
Two studies have reported the effects of surface charge of NPs and synthesis methods on antilung cancer activity. Muthukumarasamyvel et al. (2017) reported that the cytotoxicity of Au-NPs to A549 cells was positively correlated with surface hydrophobicity of dicationic amphiphiles. Dicationic cysteamine-conjugated lithocholic acid templated Au-NPs showed better inhibitory activity, followed by dicationic cysteamine-conjugated deoxycholic acid Au-NPs and dicationic cysteamine-conjugated cholic acid Au-NPs. Kumar, Ghosh, and Pandey (2019) explored the effects of Au-NP synthesis method on antilung cancer activity. Data showed that the optimized synthesis of Au-NPs from Millettia pinnata included 100 μg/ml enzyme concentration, pH 5.4, 0.45 mM substrate concentration, and 12-hr reaction time.
1.4 Possible mechanisms of antilung cancer activity of Au-NPs
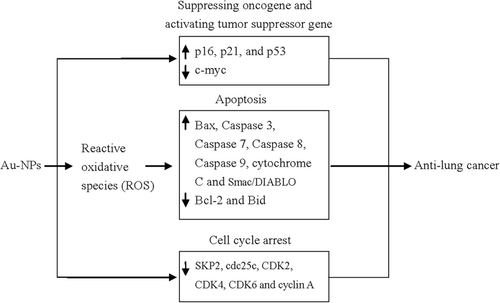
Although a number of published studies have investigated the antilung cancer activity of biosynthesized Au-NPs, the detailed mechanisms underlying this process are not well understood. Based on the current studies, several molecular events might be involved in the antilung cancer activity of Au-NPs (Figure 1). Zheng et al. (2019) reported that Au-NP treatment provoked ROS production in A549 cells, which may result in oxidative stress-induced apoptosis, accompanied with downregulation of BCL-2 and Bid and upregulation of Bax, caspase 3, and Beclin-1. Muthukumarasamyvel et al's. (2017) and Zhang et al's. (2019) studies also revealed that Au-NP treatment induced ROS generation in A549 cells. Nagalingam et al's. (2018) study showed that A. bettzickiana-mediated Au-NPs induced A549 cell apoptosis with downregulation of BCL-2 and upregulation of Bax, caspase 3, caspase 9, and cytochrome C. Treatment with Au-NPs downregulated the cell cycle-related proteins, such as cdc25c, CDK2, CDK4, CDK6, and cyclin A, and upregulated the tumor suppressor genes, such as p16, p21, and p53. The proinflammatory genes of IL1β, IL6, NF-kb, and TNF-α were downregulated and IL10 was upregulated in Au-NPs exposed A549 cells (Nagalingam et al., 2018). Treatment with Au-NPs produced from M. tenacissima upregulated caspase 3, caspase 8, caspase 9, and Bax protein expression and downregulated Bid and BCL-2 protein expression (Sun et al., 2019). M. oleifera-mediated Au-NPs treatment significantly increased caspase 3, caspase 7, and caspase 9 activity and decreased ATP levels in A549 cells. BCL-2, HSP70, SKP2, FBW7a, and c-myc expression levels were significantly decreased in Au-NPs-treated A549 cells and p53, SRP30a, Bax, and Smac/DIABLO, and cleaved PARP1 expression levels were significantly increased (Tiloke et al., 2016).
2 SUMMARY
Most studies reported positive results, but few reported negative results. The difference in results among published studies may be due to the following: different plant sources for preparing Au-NPs; different parts of plants (e.g., root, leaf, and fruit); different particle sizes and shapes; different surface charges; and different reaction conditions, such as enzyme concentration, reaction time, pH value, and substrate concentration. To the best of our knowledge, the A549 cell line was solely used to investigate the antilung cancer activity of biosynthesized Au-NPs. To obtain confirmed conclusions on the antilung cancer effects of certain biosynthesized Au-NPs, additional lung cancer cell lines should be applied to verify the anticancer activity of biosynthesized Au-NPs. In vitro experiments are easy to perform, time saving, and cost-effective, but the metabolic dynamic process and effects of biosynthesized Au-NPs on tumor microenvironment could not be evaluated by in vitro experiments. Published studies have investigated the antilung cancer activity of biosynthesized Au-NPs using in vitro experiments, but few studies reported the antilung cancer activity of biosynthesized Au-NPs in in vivo experiments. Thus, in vivo experiments should be performed to further assess the antilung cancer effects of biosynthesized Au-NPs. To determine the nonspecific effects of biosynthesized Au-NPs on normal tissues, the toxic effects of biosynthesized Au-NPs on normal cells especially derived from lung tissue (e.g., human bronchial epithelial cells and human fetal lung fibroblast cells) should be tested while assessing the antilung cancer activity. Moreover, the human immune system plays an important role in cancer treatment, but recent studies used monoculture of cells to test the antilung cancer activity of biosynthesized Au-NPs. The effects of biosynthesized Au-NPs on lung cancer cells in the presence of immune cells are unknown. Thus, the cocultured system composed of lung cancer cells, human monocyte-derived macrophage, and dendritic cells should be established to evaluate the antilung cancer effects of biosynthesized Au-NPs.
ACKNOWLEDGMENTS
We would like to thank Dr. Oppong Timothy Bonney for his kind help in editing the English language of this manuscript. This work was funded by the National Natural Science Foundation of China (81973105 and U1404815). The funder had no role in the preparation of the manuscript or decision to publish.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this review as no datasets were generated or analyzed during the current study.




