Rapid human-derived iPSC osteogenesis combined with three-dimensionally printed Ti6Al4V scaffolds for the repair of bone defects
Abstract
Human-induced pluripotent stem cells (iPSCs) are an alternative source of mesenchymal stem cells used for bone regeneration. However, the current osteogenically induced methods for iPSCs are slow and complex. We have used retinoic acid (RA) to induce osteogenic iPSCs within 10 days and assess whether a rapid differentiation could improve the osteogenic potential of the three-dimensionally printed Ti6Al4V (3DTi) scaffolds. First, the osteogenic differentiation of iPSCs was induced with RA, and the osteogenic potential of iPSCs was evaluated using standard assays. In addition, a 5-mm mandibular bone defect was generated in rats and was repaired with 3DTi scaffolds that were seeded with iPSC-induced osteoblasts. The capacity of seeded scaffolds for the enhancement of bone regeneration in vivo was assessed. Finally, we tested the potential mechanisms of RA-dependent iPSC bone induction and its effect on the Wnt/β-catenin pathway. The results showed that iPSCs could form osteocytes within 10 days. Animal experiments confirmed that rapid osteo-induced iPSCs could enhance the bone regeneration and osteointegration capacity of the 3DTi scaffolds. Mechanistically, RA could activate the AKT/GSK3β/β-catenin pathway during the process of iPSCs osteogenesis. The rapid osteoinduction of iPSCs combined with 3DTi scaffolds is a safe, effective, and reproducible method for repairing mandibular bone defects.
Abbreviations
-
- 3D
-
- three-dimensional
-
- 3DTi
-
- three-dimensionally printed Ti6Al4V
-
- ALP
-
- alkaline phosphatase
-
- ARS
-
- Alizarin red staining
-
- BMSCs
-
- bone marrow mesenchymal stem cells
-
- EDS
-
- energy dispersive spectroscopy
-
- iPSCs
-
- induced pluripotent stem cells
-
- MSCs
-
- mesenchymal stem cells
-
- NB
-
- new bone
-
- OI
-
- osteogenic induction
-
- PCR
-
- polymerase chain reaction
-
- RA
-
- retinoic acid
-
- RARs
-
- retinoic acid receptors
-
- SEM
-
- scanning electron microscopy
1 INTRODUCTION
The three-dimensionally printed porous Ti6Al4V (3DTi) scaffolds are a potential candidate in bone defects. The regeneration of bone defects remains a great challenge for surgeons, especially in the case of maxillofacial defects in view of their function and facial aesthetics. The traditional autogenous bone grafting cannot mimic the defect sections anatomically. In view of the considerable advantages of three-dimensional (3D) printing technology in precision medicine, the 3DTi scaffolds can reconstruct the defective areas anatomically through the unaffected mirror side. Despite the titanium's several advantages, such as its low corrosion and good biocompatibility, the applications of 3DTi scaffolds are limited owing to their poor bioactivity. Owing to the absence of stem cells in the bone defect area, several studies have used cytokine or stem cell incorporation to improve the bone ingrowth associated with 3DTi scaffolds (Levi et al., 2012; Lewallen et al., 2016; Nune, Misra, Bai, Li, & Yang, 2019). We tried to improve the bioactivity of the 3DTi scaffolds with stem cells.
Human pluripotent stem cells have been introduced as an attractive source for bone regeneration. Bone marrow mesenchymal stem cells (BMSCs) constitute the first source of mesenchymal stem cells (MSCs) and are considered as an important cell type for bone tissue regeneration. However, the proliferation rates and osteogenic differentiation abilities of BMSCs decrease during cell culture expansion in vitro and with age (H. T. Chen et al., 2012). Induced pluripotent stem cells (iPSCs) have better-unlimited growth capability and pluripotency than BMSCs (Bastami et al., 2017). Furthermore, the extraction of iPSCs from the patients' own somatic cells constitutes one of the major breakthroughs in tissue regeneration (Huangfu et al., 2008). Several studies have focused on the osteogenically induced bone-like nodules obtained from iPSCs (Ardeshirylajimi, Soleimani, Hosseinkhani, Parivar, & Yaghmaei, 2014; Loh et al., 2016). However, these methods are relatively slow, which usually takes several steps and more than 3 weeks to induced iPSCs into osteoblasts or osteocytes. Furthermore, the final results are inconsistent.
Retinoic acid (RA) was used to accelerate the osteogenic induction (OI) of iPSCs. RA is a metabolite of vitamin A1 that plays critical roles in growth and development, such as osteochondral development and osteoblastogenesis (Scholtes et al., 2018; S. Wang et al., 2020). Here, different concentrations of RA were used to osteo-induce the iPSCs. The addition of RA could accelerate the bone regeneration from iPSCs within 10 days. Through this method, we were able to perform rapid osteogenic differentiation of iPSCs within 10 days.
The aim of this study was to investigated the effect of rapidly osteo-induced iPSCs combined with 3DTi scaffolds for bone regeneration and further elucidated the molecular mechanism of the RA-induced osteogenic differentiation of iPSCs.
2 MATERIALS AND METHODS
2.1 3D-printed Ti6Al4V scaffolds
The 3DTi scaffolds were manufactured from Ti6Al4V powder as described previously (He et al., 2019; Yu et al., 2018). The structures of 3DTi scaffolds were designed in disc-like forms, with diameters equal to 5 mm, thickness values of 1 mm, with strut widths of 200 μm, and pore sizes of 600 μm. In addition, the porosity of scaffolds was more than 85%. Scanning electron microscopy (SEM; MIRA3 TESCAN, Czech) and energy dispersive spectroscopy (EDS; C-NANO, Oxford Instruments, UK) were, respectively, used to observe the surface morphology and the distribution of chemical elements on the surface of these 3DTi scaffolds.
2.2 iPSC cell culture
Feeder-free iPSCs (Y00275) (provided by Takara) were established from human peripheral blood mononuclear as reported previously (Nakagawa et al., 2014). Furthermore, these were maintained with mTeSR1 (STEMCELL Technology) on laminin-coated six-well plates. When the cells had grown to a confluency of 70–80%, 0.5% TrypLE Select (Life Technologies) was used (300 μl/well) to digest cells. A volume of mTeSR1 medium (STEMCELL Technology) of 1.5 ml was then added with 4.8-μl iMatrix–511 (Takara) and 1.5-μl 10 mM Y–27632 (Wako, Japan) in a new well of the six-well plate. Subsequently, 1.3–2.0 × 104 live cells were distributed over the plate surface. And the media (without Y–27632) were changed every other day. The feeder-free iPSCs were frozen in STEM-CELLBANKER (Nippon Zenyaku Kogyo) at a concentration of 2 × 105 cells per cryovial.
2.3 OI method
We induced the osteogenic differentiation of iPSCs into osteoblast and osteocyte cells within 10 days, as reported previously (Kawai et al., 2019). Briefly, at Day 0, 4 × 105 iPSCs were seeded in a well of 12-well plates in the presence of 1.5 ml 80% OI medium and 20% iPSCs standard medium. The iPSCs standard medium was composed of mTeSR1 medium with 2.4-μl iMatrix–511 and 1.5-μl 10 mM Y–27632. In addition, the OI medium was composed of KnockOut Dulbecco's modified Eagle medium (Gibco), 20% fetal bovine serum (Thermo Fisher Scientific), 2 mM l-glutamine (Thermo Fisher Scientific), 1% minimum essential medium, nonessential amino acids (Thermo Fisher Scientific), 0.1 mM β-mercaptoethanol (Thermo Fisher Scientific), 10 mM β-glycerophosphate (Sigma-Aldrich), 1 nM dexamethasone (Sigma-Aldrich), 50 μg/ml l-ascorbic acid, 1X penicillin–streptomycin, and different concentrations (10 nM–1 μM) of RA (Wako). On the second day, the medium was replaced with 100% OI medium and changed every 3 days.
2.4 Staining with Alizarin red
The calcium deposition of iPSCs was evaluated with Alizarin red staining (ARS). After osteogenic differentiation for 10 days, the cells in each group were fixed in 4% formaldehyde for 20 min and stained with ARS (Sigma-Aldrich). The visualisation was achieved with microscopy. Calcium was quantified with 10% acetic acid (Sigma-Aldrich) followed by neutralisation with 10% ammonium hydroxide (Sigma-Aldrich) as described previously (He et al., 2019).
2.5 Alkaline phosphatase activity
Alkaline phosphatase (ALP) activity was analysed through an ALP kit (Sigma-Aldrich) according to the manufacturer's instructions after osteo-induced for 10 days. The cells in each group were lysed with 0.1% Triton X-100. The p-nitrophenyl phosphate and lysate were mixed and incubated at 37°C for 15 min. Finally, the reaction is followed by monitoring the increase in absorbance at 405 nm.
2.6 Western blot analysis
Western blot was used to evaluate protein levels. Cells were harvested and protein was harvested with a protein extraction kit (Thermo Fisher Scientific) after osteogenic differentiation for 10 days. Equal amounts of proteins were resolved using sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. After blocking with a solution which contained 5% nonfat dry milk for 1 hr, probing was done at 4°C overnight with a primary antibody against β-catenin (1:1,000; Cell signal), pAKTser473 (1:2,000; Cell signal), protein kinase B (AKT; 1:1,000; Cell signal), pGSK3βser9 (1:1,000; Cell signal), GSK3β (1:1,000; Cell signal), glyceraldehyde 3-phosphate dehydrogenase (1:1,000; Cell signal), histone H2A.X (1:1,000; Cell signal), followed by a secondary antibody. The blots were analysed with an enhanced chemiluminescent kit (Thermo Fisher Scientific) and quantified with the ImageJ software (NIH).
2.7 Real-time quantitative polymerase chain reaction
Total messenger ribonucleic acid (mRNA) was extracted with the RNeasy Kit (Qiagen) at Day 10. It was then reversed-transcribed into complementary DNA (cDNA) using random primers (Table 1) and a cDNA synthesis kit (Thermo Fisher Scientific). The real-time polymerase chain reactions (PCRs) were performed using a Real-Time PCR system (ABI 7500; Thermo Fisher Scientific). The mRNA levels of osteoblastic markers (Col-1, OCN, BSP, PHEX, SOST) were calculated based on the method.
| Gene | Sequences (5′-3′) | |
|---|---|---|
| Forward | Reverse | |
| GAPDH | GACTTCAACAGCAACTCCCAC | TCCACCACCCTGTTGCTGTA |
| COL-1 | CCTCGCTTTCCTTCCTCTCC | GTCAAGGCTGAGAACGGGAA |
| OCN | CTCACACTCCTCGCCCTATTG | CGCCTGGGTCTCTTCACTAC |
| BSP | AAGGACAAGGCTACGATGGC | CGGATGCAAAGCCAGAATGG |
| PHEX | AAGAGGACCCTGGGAGAAAA | GGCAGCTTCTGGTCTGTAGG |
| SOST | CCCTTTGAGACCAAAGACGTG | GGCCCATCGGTCACGTAG |
- Abbreviation: qRT-PCR, real-time quantitative polymerase chain reaction.
2.8 Immunofluorescence staining
The expression of the specific protein of osteocytes was assessed and compared among different groups by immunofluorescence staining following 10 days culturing using the OI medium. Cells were fixed for approximately 20 min, followed by solution treatment with 0.2% Triton X-100. Cells were then incubated with the primary antibody sclerostin (SOST; 1:100; Sigma-Aldrich) overnight. The secondary antibody was used for incubation. Samples were visualised using fluorescence microscopy and quantified using ImageJ.
2.9 Mandibular bone defect models
Sprague Dawley (SD) rats (n = 18, weight = 260 g) were obtained from China Medical University (CMU). In vivo experiments were approved by the Institutional Animal Care and Use Committee of CMU (2018-21). Standardised mandibular ramus defects (full thicknesses = 5 mm) were drilled in each SD rat under anaesthesia (6 mg/100 g ketamine hydrochloride; Figure 1). After culturing for 7 days, cells were harvested and mixed with the HyStem hydrogel (Glycosan BioSystems). They were then used to fill/seed the inner parts of 5-mm 3DTi scaffolds before they were transplanted into the 5-mm mandibular defect. The rats were divided into three groups: (a) 3DTi group (only scaffold), (b) MSC group (scaffolds with bone marrow stromal cells), and (c) RA+ group (scaffolds with RA [1,000 nM] osteo-induced cells). The mandibles were harvested after 8 weeks.
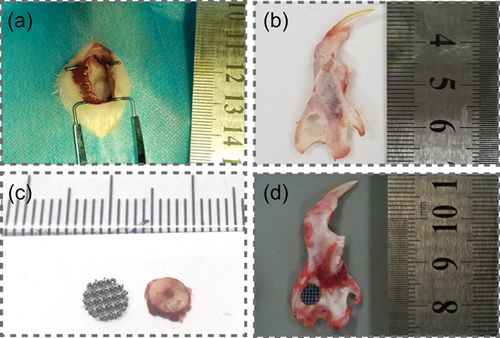
2.10 Micro-CT scanning
To track new bone (NB) formation, the mandibles were harvested at 8 weeks postoperatively. All mandible bone samples were scanned using a micro-CT (Bruker, MA). The bone volume/total volume (BV/TV) ratio was calculated using Mimics software (Materialise, Belgium) to determine the level of bone regeneration.
2.11 Histological analyses
Sections with a thickness of 200 μm were made and polished to 50 μm. The trinitrophenol (1.2%) and acid fuchsin solutions (1%; van Gieson staining) were used to stain these sections. The stained sections were imaged under an optical microscope. The ImageJ software was used to determine the proportion of bone tissue in each microscopic field. In addition, the newly formed bone was identified as red; fibrous tissues as blue; and scaffolds as black, respectively.
2.12 Transfection and luciferase reporter assay
iPSCs were transfected with TCF/β-catenin responsive luciferase reporter pTop-Luc and Renilla luciferase expression vector using Lipofectamine (Invitrogen; Gao et al., 2013). After transfection, iPSCs were treated without RA, with RA (concentration of 1,000 nM) and BMS493, or with RA (1,000 nM). After 48 hr, cells were lysed for luciferase assays using the luciferase assay kit (Promega). Recorded luciferase activity was normalised by the Renilla luciferase activity.
2.13 Statistical analyses
All the results were biologically replicated three times, and six images were used for analysis. All data were shown as the mean ± standard deviation. Statistical differences were analyzed by the Student's t-test and one-way analysis of variance. p < .05 were considered statistically significant.
3 RESULTS
3.1 3D-printed scaffold characterisation
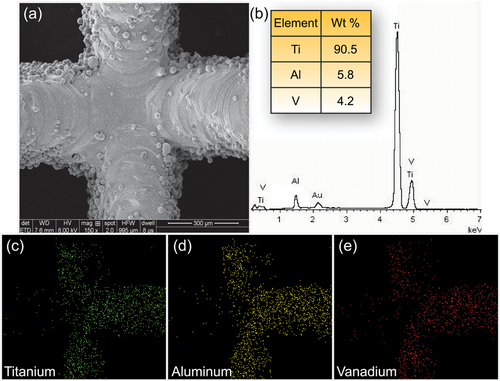
The surface morphology of the 3DTi scaffolds was detected with SEM. As shown in Figure 2a, the structural geometry of these scaffolds was in concordance with our design: the strut widths were 200.2 ± 3.4 μm, pore sizes were 602.4 ± 6.4 μm. The distributions of the chemical elements on the surface of scaffolds were observed by EDS data and mapping analyses (Figure 2b,c). The results indicated that 90.5 ± 2.2% titanium, 5.8 ± 0.4% aluminium, and 4.2 ± 0.2% vanadium composed the scaffolds. According to the previous reports, this type of scaffold is suitable for cell adhesion and bone ingrowth (Yin et al., 2016).
3.2 Fast osteogenic differentiation of iPSCs by RA
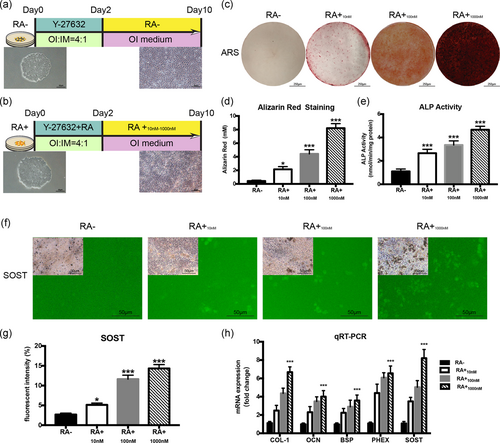
iPSCs were cultured in an OI medium as reported previously (Kawai et al., 2019). The schematic of the protocol is also shown for OI of iPSCs with/without RA (concentrations in the range of 0 nM [RA−], 10 nM [RA+10 nM], 100 nM [RA+100 nM], and 1,000 nM [RA+1,000 nM], totally four groups). Figure 3a,b shows that the iPSCs colonies are seeded in plates on Day 0, and osteogenic differentiation occurred during the subsequent 10-day OI culturing period. However, iPSCs without RA contained fewer calcified nodule formations compared with the results evoked with the use of RA. The results of ARS indicated that the mineralisation of iPSCs were enhanced under the RA condition on Day 7 (Figure 3c), and the quantitative analyses clearly showed that the calcium deposits in all RA+ subgroups were significantly higher than in RA− group (Figure 3d). The results associated with the ALP activity were coincident with those of ARS (Figure 3e). There was a significantly higher ALP activity in all three RA+ subgroups. The immunofluorescence results are shown in Figures 3f. The osteocyte marker SOST cannot be detected in the cells without RA. In addition, the quantitative analysis of the fluorescence intensity showed that the expression of SOST gradually rises with ascending RA concentration (Figure 3g). Real-time PCR was performed to assess the expression of osteogenesis-related mRNA, COL-1, OCN, BSP, PHEX, and SOST (Figure 3h). All these gene expressions were upregulated in the RA+ group. However, the RA1,000 nM group yielded the highest expression levels of osteogenic mRNA among the three RA+ subgroups. All these results showed that RA could promote the osteogenic differentiation of iPSCs, and the results in the RA1,000 nM group were much better than those in RA10 nM and RA100 nM group. Furthermore, there was no significant difference between groups with or without 3DTi scaffold in RA− or RA1,000 nM group (Figure S1).
3.3 Rapid osteogenesis of iPSCs combined with 3DTi scaffold-enhanced bone regeneration in vivo
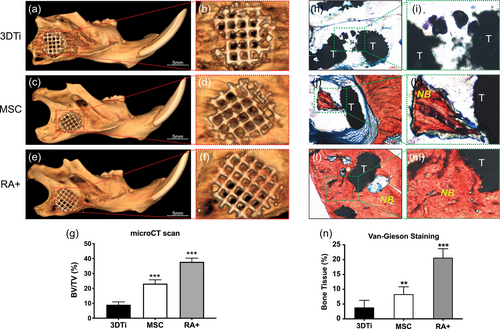
Eight weeks after surgery, all skin wounds were healed well and no weight loss or other complications were observed in host animals. Histological analyses were used to characterise NB formation in all samples. Mineralised tissue formation was assessed using micro-CT. The 3DTi group showed nearly no bone formation (Figure 4a,b), and the MSC group exhibited only minor bone formation (Figure 4c,d). However, micro-CT scanning showed that the newly formed bone and scaffold were better integrated in the RA+ group (Figure 4e,f). And quantitative analysis showed that the BV/TV value was the highest in the RA+ group (p < .001; Figure 6g). The van Gieson staining results indicated that minor quantities of mineralised tissue were detected within the 3DTi group without iPSCs (Figures 4h,i), but additional fibrous tissue was detected in the MSC group (Figure 4j,k). Furthermore, better bone regeneration and osteointegration were detected in the RA+ group (Figure 4l,m). The scaffold pores in the RA+ group were filled with newly formed bone, whereas fibrous tissue was found between the scaffold in both the 3DTi and MSC groups. Quantitative analysis confirmed that the NB ingrowth was higher in the RA+ group than that in the 3DTi group (Figure 4n).
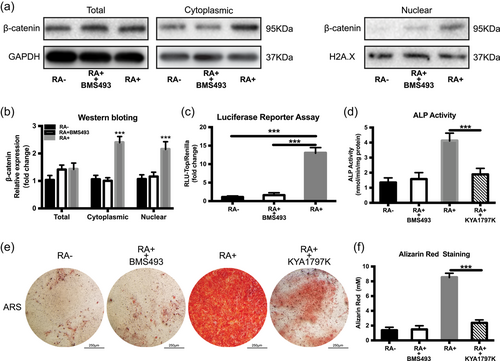
3.4 Retinoic acid crosstalk with the Wnt signal pathway in iPSCs osteogenic differentiation
The canonical Wnt signal pathway plays an important role in osteogenic differentiation. We aimed to investigate the relationship between RA and the Wnt signal pathway. Previous reports have shown that the RA transmits its signal via retinoic acid receptors (RARs; Janesick, Wu, & Blumberg, 2015). In addition, it has also been found that RARα and RARβ dramatically increased during the OI of iPSCs (Kawai et al., 2019). Thus, a pan-RAR antagonist (BMS493) was used to block the RA signals to evaluate the effect of RA on the osteogenesis of iPSCs. The western blotting results indicated that the β-catenin protein levels were increased in both the cytoplasm and nuclear under RA condition and decreased dramatically in response to BMS493 (Figure 5a,b). Accordingly, the RA could effectively stimulate the luciferase activities of the pTOP-Luc reporter, which was further inhibited by the combination of BMS493 (Figure 5c). Subsequently, the potential role of Wnt/β-catenin signalling on the osteogenic differentiation of iPSCs was investigated in the presence of RA. Pretreatment with KYA1797K (Selleck; a selective Wnt/β-catenin pathway inhibitor), partially blocked the RA-induced increase in ALP activity (Figure 5d) and also decreased the calcium deposits in the ARS assay (Figure 5e,f). These results indicated that RA can at least partly regulate osteogenesis of iPSCs through the Wnt/β-catenin signalling pathway.
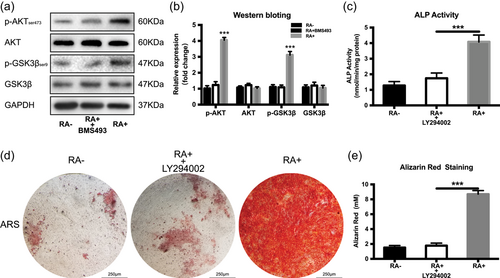
3.5 RA promotes osteogenesis through the AKT/GSK3β/β-catenin in iPSCs
It has been reported that RA can activate the PI3K/AKT pathway (Liu et al., 2014), and AKT can activate β-catenin by inhibiting GSK3β (Kolegraff, Nava, Helms, Parkos, & Nusrat, 2011). Therefore, we examined the mechanism based on which RA activates osteogenesis. As shown in Figure 6a,b, the pAKT (ser473) increases in the RA+ group but is inhibited by the RAR antagonist (BMS493). Furthermore, GSK3β, a negative regulator of Wnt/β-catenin signalling is phosphorylated at ser9 which causes its inactivation. The pGSK3β activity (ser9) is highest in GSK3β but is suppressed in combination with BMS493. The PI3K inhibitor LY294002 which could also inhibit AKT effectively, significantly reduces the calcium deposits in the ARS assay and also decreases the ALP activity (Figure 6c–e). These data suggest that the AKT/GSK3β pathway plays a critical role in the promotion of the activity of β-catenin in the osteogenesis process of iPSCs.
4 DISCUSSION
We demonstrated that with RA stimulation, the OI medium can induce the osteogenic differentiation of iPSCs rapidly within 10 days, which shortens considerably the time required for bone regeneration. In addition, it has been shown that the osteogenic capability of iPSCs was enhanced by the increased concentration of RA. And a concentration of 1,000 nM was ideal among three groups, which was consistent with Kawai et al. (2019). Mori et al. (2019) reported that the concentration of RA could affect the differentiation of iPSCs. RA is also an important modular for osteogenic differentiation (C. Chen et al., 2019). And the RA is an important morphogen known for its pleiotropic effects via RARs according to its concentration of RA, the cell type and other signals (Henning, Conaway, & Lerner, 2015). In our study, the RAR antagonist could significantly reduce the RA-mediated bone formation, suggesting that the RA is important for iPSCs osteoinduction.
P. Wang et al. (2015) reported that better osteogenic potential was observed in vivo for iPSCs than BMSCs. In our study, both the micro-CT and histopathological results confirmed that iPSCs could significantly enhance bone regeneration of 3DTi scaffolds in vivo, which is better than the BMSCs. No complications or teratomas were found in all the animal models. Therefore, RA osteo-induced iPSCs constitute a safe and effective method for the enhancement of osteogenesis in 3DTi scaffolds. Furthermore, the iPSCs could form osteocytes within 10 days in vitro. These data indicated that iPSCs are expected to replace BMSCs in bone regeneration endeavours.
Wnt/β-catenin signalling plays a critical role in osteogenic differentiation. Therefore, we explored whether the Wnt/β-catenin signalling was involved in the regulation of RA-induced iPSCs osteogenic differentiation. It has been reported that all-trans RA modulates Wnt3A induced osteogenic differentiation of MSCs (Zhang et al., 2016). However, the administration of Wnt alone cannot substitute the osteogenesis effect of RA (Kawai et al., 2019). The relationship between RA and Wnt should be investigated in iPSCs, which could provide better evidence for the clinical use of iPSCs. In this study, it was also shown that the Wnt/β-catenin pathway inhibitor KYA1797K could diminish the stimulatory effect of RA-induced ALP activity and calcium deposition. Furthermore, RA also enhanced the pTOP-Luc reporter activity and the translocation of β-catenin into the nucleus in the osteo-induced iPSCs. Thus, our data indicated that RA may modulate osteogenesis of iPSCs via the Wnt/β-catenin pathway. However, the crosstalk between RA and the Wnt/β-catenin pathway during osteogenesis has not been investigated adequately. Some studies have shown that RA could activate PI3K/AKT signalling (Lopez-Carballo, Moreno, Masia, Perez, & Barettino, 2002). As expected, AKT phosphorylation at ser473 and GSK3β phosphorylation at ser9 significantly increased in the RA+ group. Furthermore, pAKT and pGSK3β could prevent β-catenin from degradation and effectively induce its translocation into the nucleus. As the β-catenin accumulates in the nucleus, it displaces the inhibitor Groucho from the transcription factors TCF/LEF and induces osteogenic gene transcription (Lerner & Ohlsson, 2015). We also found that the PI3K/AKT inhibitor LY294002 could abolish the RA-induced osteogenesis of iPSCs. Given that the PI3K could mediate AKT activity, this further suggests and justifies the positive role of RA in PI3K/AKT signalling. Other studies have also shown that the PI3K/AKT signalling inhibited GSK3β through the phosphorylation of GSK3β (Liu et al., 2014; Wong, Xue, Baxter, Pavlakis, & Smith, 2016). These data emphasize the potential role of AKT/GSK3β signalling in the crosstalk between RA and the Wnt/β-catenin pathway (Figure 7).
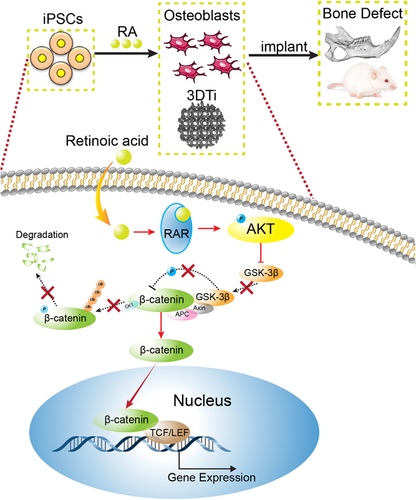
In conclusion, this study investigated the feasibility of rapidly induced iPSCs in combination with 3DTi scaffolds to repair bone defects and revealed the potential mechanisms of RA-induced osteogenic differentiation of iPSCs. Therefore, the combination of iPSCs and 3DTi scaffolds may be potentially used as a therapeutic strategy for mandibular or other bone defects in the clinical setting.
ACKNOWLEDGEMENTS
This study was funded by grants from the youth research programme of the School of Stomatology, China Medical University (D12110700420000). The authors are indebted to Christakis for manuscript English correction.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualisation, supervision, validation: L. Y. and Q. F. Funding acquisition, project administration: L. Z. Methodology, investigation: Y. Y. and Q.F. Formal analyses and data curation: B. Z., X. B. and L. Z.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




