Overexpression of Foxc1 regenerates crushed rat facial nerves by promoting Schwann cells migration via the Wnt/β-catenin signaling pathway
Wenzheng Xia, Jin Zhu, and Xueyi Wang contributed equally to this study.
Abstract
Facial paralysis can result in severe implications for patients. A good prognosis depends on the degree of nerve regeneration. Schwann cells (SCs) play an important role in facial nerve development and regeneration through migration. Forkhead box C1 (Foxc1), a member of the forkhead transcription factor family, is implicated in cell migration. However, the role of Foxc1 in the progression after facial nerve crush remains unknown. Our aim was to evaluate the effect of Foxc1 overexpression on SC migration and recovery of facial nerves after crush injury. The rat facial nerve crush injury model was established through the use of unilateral surgery. The results showed that the expression of Foxc1 was increased in the surgery group compared to that of the control group. SCs were isolated from the sciatic nerves and cultured. Foxc1, delivered by an adeno-associated virus in vivo, or adenovirus in vitro, both induced overexpression of Foxc1, and increased the expression of CXCL12 and β-catenin. After the transfection of Foxc1, the migration of SC was increased both in vitro and in vivo, was reduced by the inhibition of CXCL12 or β-catenin. The facial nerve function and the nerve axon remyelination of the rats transfected with Foxc1 were significantly improved after nerve crush injury. Overall, the results demonstrated that overexpression of Foxc1 promoted SC migration by regulating CXCL12 via the Wnt/β-catenin pathway, thus contributing to improved facial nerve function after crush injury.
1 INTRODUCTION
The facial nerve is important for physiological and social functions (Coulson, O'Dwyer N, Adams, & Croxson, 2004; Masterson, Vallis, Quinlivan, & Prinsley, 2015), and is affected by trauma due to its long extratemporal segment, which results in facial nerve paralysis (Foecking et al., 2012). Posttraumatic lesions often present sequela, even in those repaired by delicate surgery (Pereira et al., 2019), thus leading to serious functional deficits (Lal et al., 2008). Although facial motor neurons have the ability to regenerate their injured axons, the degree of functional recovery is determined by the rate of regeneration (Lal et al., 2008). The successful regeneration of facial nerve lesions depends on the support of Schwann cells (SCs; Saez et al., 2019). Following an injury, SCs migrate to the damaged site to assist fibroblasts and macrophages in phagocytosis and clearing of debris, thus promoting axon regeneration (Scheib & Hoke, 2013). However, the neurons regenerating axons may be impeded by insufficient SC migration (Motta, Endres, Wesdemiotis, Willits, & Becker, 2019). Axonal regeneration may, therefore, be facilitated by new strategies involving enhancement of the migration of SCs, to optimize the growing support of the injured nerve in combination with delicate nerve repair (Fu & Gordon, 1997).
Forkhead box C1 (Foxc1), a member of the forkhead transcription factor family, is characterized by a distinct DNA-binding forkhead domain (Kaestner, Knochel, & Martinez, 2000). The Foxc1 gene is located in chromosome 6p25 and encodes transcriptional factors, which regulate many biological processes including cell differentiation, cellular proliferation, and migration (Gong et al., 2019; Omatsu, Seike, Sugiyama, Kume, & Nagasawa, 2014). As an important inducer of cellular migration, previous studies revealed that an increase of Foxc1 was accompanied by increased cellular migration (L. Xia et al., 2013). With respect to tissue regeneration, Foxc1 showed its proregeneration ability by promoting tissue stem cell migration and preserving long-term tissue-regenerating potential (Lehmann et al., 2000). Mutations in Foxc1 hampered cellular migration (L. Huang et al., 2017). However, the biological functions of Foxc1 during SC migration have not been investigated.
Stromal cell-derived factor (CXCL12, also known as SDF-1α) is one of the most widely studied chemokines. CXCL12 induces the directional migration of cells by binding to its specific CXCR4/CXCR7 receptor. Recent studies have confirmed that CXCL12 promotes functional recovery in many diseases such as multiple sclerosis (Carbajal, Schaumburg, Strieter, Kane, & Lane, 2010), stroke (Ardelt, Bhattacharyya, Belmadani, Ren, & Miller, 2013), and spinal cord injury (Zendedel et al., 2016). For example, CXCL12 directs interneurons to migrate to the subventricular zone to induce the growth of thalamic axons (Abe et al., 2015). A recent study showed that Foxc1 also promoted tissue regeneration by upregulating CXCL12 (Omatsu et al., 2014). A previous study reported that the CXCL12 gene was a direct transcriptional target of Foxc1 during the neuron migration (Zarbalis, Choe, Siegenthaler, Orosco, & Pleasure, 2012). In the present study, we, therefore, determined whether Foxc1 overexpression induced CXCL12 release to promote SCs migration.
Wnt/β-catenin signaling is known to serve essential roles in cell growth, survival, and migration (Park et al., 2015). Modulating the Wnt/β-catenin signaling pathway affects SC migration (Y. Li et al., 2018). As a target gene of Foxc1, β-catenin is associated with cellular proliferation and migration (Wang et al., 2019; Yun, Han, & Park, 2020). Loss-of-function studies revealed that Foxc1 silencing inhibited β-catenin, leading to impaired migration in glioma cells (Cao et al., 2019). However, the role of Foxc1-associated activation of the Wnt/β-catenin pathway in SC migration remains unclear.
In this study, we determined the role of Foxc1 using the facial nerve injury model. We determined if Foxc1, which plays important roles in tissue regeneration and other biological processes by affecting cell migration, promoted SC cell migration by activating Wnt/β-catenin signaling pathway. Our study suggested that activation of Foxc1 might be helpful for clinical treatments of facial injury.
2 MATERIALS AND METHODS
2.1 Microarray analysis
The nerve tissues of the sham group and facial nerve injury (FNI) group were lysed immediately in 500 μl of TRIzol (Thermo Fisher Scientific, Waltham, MA) and were stored at −80°C before purification using a standard phenol-chloroform extraction protocol with an RNAqueous Micro Kit (Thermo Fisher Scientific). The transcriptome was subjected to microarray analysis using an Affymetrix human array (Thermo Fisher Scientific) and normalized based upon quantiles.
2.2 Quantitative reverse transcription-polymerase chain reaction
Real-time polymerase chain reaction (RT-PCR) was performed as described previously (Shan, Tang, Xia, & Qian, 2020). The primers are listed as follows: Rat Foxc1 forward: TGGACCGCTTTCCCTTCTATC, reverse: GCCCTTGCCTGGCTTCTT; rat CXCL12, forward: CAGAGCCAACGTCAAGCA, reverse: AGGTACTCTTGGATCCAC; rat β-catenin, forward: CGTTTCFCCTTCATTATGGACTACCT, reverse: GCCGCTGGGTGTCTGATGT; rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward: GGCTCTCTGCTCCTCCCTGTT, reverse: GGCTCTCTGCTCCTCCCTGTT. In brief, total RNA was extracted using TRIzol reagent, reverse-transcribed to complementary DNA, and then amplified using an SYBR-Green master mix kit. All procedures were performed in triplicate. The messenger RNA levels were calculated relative to the control GAPDH using the method.
2.3 Cell culture and treatment
As described in our previous report (Gao et al., 2019), SCs were isolated from the sciatic nerves of Sprague Dawley rats at postnatal Day 3. The collected nerves were dissociated with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA; Gibco, Grand Island, NY) for 30 min at 37°C, and single cells suspended in MEM/F12 (HyClone, South Logan, UT), containing 10% fetal bovine serum (Hyclone), were plated onto poly-l-lysine-coated dishes. After overnight incubation, the cultures were treated with cytosine arabinoside (10 mM, Sigma-Aldrich, St. Louis, MO) for 48 hr to eliminate fibroblasts. Then, the cells were routinely cultured with the SC medium to expand the cells. The maintenance medium was changed every 3 days.
The SCs were cultured with CXCL12 neutralizing antibodies to neutralize the function of CXCL12. The concentration of neutralizing antibody was set at 10 μg/ml and maintained in the medium as described previously (Chatterjee et al., 2015).
2.4 Plasmid transfection
Adenoviral vectors expressing Foxc1 (Ad-Foxc1; Gene ID: 364706) and control scrambled sequence (Ad-ctrl) were designed and synthesized. SCs were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) at a final concentration of 100 nM (W. Xia, Zhuang, & Hou, 2018).
2.5 Immunofluorescence analysis in vitro
The cell cultures were collected and permeabilized with 0.1% Triton X-100, blocked with 5% bovine serum albumin for 30 min, incubated overnight with primary rabbit anti-Foxc1 antibody (1:100; ab227977; Abcam, Burlingame, CA), and then incubated with Alexa Fluor® 488 (1:100; ab150077; Abcam) for 1 hr. Finally, nuclear staining was performed using 4′,6-diamidino-2-phenylindole (Thermo Fisher Scientific, Scotts Valley, CA) staining. Images were captured with a fluorescence microscope (Olympus, Tokyo, Japan).
2.6 Transwell migration assay
The effect of the transfection of Foxc1 on the migration of SCs was studied using 8 μm pore size Transwell chambers (Corning, NY). The SCs of different groups were transferred to the top chamber at a density of 3 × 105 cells/well and were cultured in a serum-free medium. At the same time, 600 μl complete medium was added to the lower chamber. The cells were allowed to migrate for 24 hr at 37°C in 5% CO2. The cells on the upper surface of each membrane were wiped away with a cotton swab, and the filters were fixed in 4% paraformaldehyde followed by Crystal Violet staining for 15 min. Five random fields were counted per chamber using an inverted microscope (Olympus, Tokyo, Japan).
2.7 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
We tested the potential influence of Foxc1 transfection on the SCs. A total of 300 µl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent (Sigma-Aldrich) was added to each well 2 hr before harvest. The supernatant was then removed and incubated with 400 µl dimethyl sulfoxide for 10 min. Absorbance at 540 nm was recorded using an enzyme-linked immunosorbent assay (ELISA) plate reader (W. Xia, Xie, Jiang, & Hou, 2015).
2.8 Cell proliferation assay
The rate of cell proliferation was estimated using a cell counting kit-8 (CCK-8 assay; ab228554; 100 µl; Abcam, Cambridge, UK) according to the manufacturer's protocol. Briefly, 1 × 105 cells grown in 96-well plates were incubated with CCK-8 solution for 1 hr at 37°C, after which the absorbance of each well at 450 nm was recorded (W. Xia & Hou, 2018).
2.9 Cell cycle assay
Cold anhydrous ethanol (70%) was used to fix the cells. The cells were then treated with propidium iodide (Sigma-Aldrich) and RNase A. A flow cytometer equipped with CellQuest software (BD Biosciences, San Jose, CA) was used to detect the cell cycle distribution.
2.10 Enzyme-linked immunosorbent assay
The concentration of secreted CXCL12 in the cell culture medium of SCs transfected with Ad-Foxc1 or Ad-Ctrl was measured using an ELISA kit. Assays were conducted in 96-well microplates according to the manufacturer's instructions.
FNI rats were transfected locally with AAV-Foxc1 or AAV-Ctrl. The rats were then killed at 1, 3, 7, and 28 days after surgery. A 0.8 cm length of facial nerve from the extracranial trunk was harvested and frozen for processing. Then, 100 μl radioimmunoprecipitation assay (RIPA) was added to 50 mg of the nerve tissue. Cytokine CXCL12 in the tissue lysates was detected using an ELISA kit (Meimian, Jiangsu, China), according to the manufacturer's instructions. Triplicates were included in each sample and experiments were repeated three times.
2.11 Western blot analysis
Nerve tissue block and the SCs were harvested and total protein was extracted using RIPA solution. Protein samples were denatured, separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5% nonfat milk for 2 hr at room temperature and then incubated with Foxc1 (1:750; ab227977; Abcam), β-catenin (ab32572, 1:1,000, Abcam), and β-actin (1:1,000; ab179467; Abcam) primary antibodies overnight at 4°C. The membranes were further incubated with IgG-horseradish peroxidase goat anti-rabbit secondary antibody (1:2,000; ab7090; Abcam, Cambridge, UK) for 2 hr at room temperature. Signals were developed by enhanced chemiluminescence (Sigma-Aldrich). The stained protein bands were visualized using a Bio-Rad ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA) and analyzed using Quantity One software (Bio-Rad).
2.12 Small interfering (si)RNA transfection
β-Catenin expression in SCs was knocked down using siRNAs, with a nontargeting siRNA as a negative control (Santa Cruz Biotechnology, Santa Cruz, CA). The procedures were conducted as described previously (W. Xia et al., 2018). Transfection efficiency was detected by qRT-PCR and western blot analysis.
2.13 Animals
Male Sprague Dawley rats (200–250 g) were maintained in accordance with guidelines published by the US National Institutes of Health. All study procedures were approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine. This study was conducted in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Academy Press (NIH, revised in 1996). Rats were randomly divided into four groups, respectively: Sham group, FNI group, FNI+AAV-Foxc1 group, and the FNI+AAV-Control group; n = 12 rats per group.
2.14 Construction and validation of the facial nerve injury model
The rat facial nerve injury model was established based on previously published studies (Mattsson, Janson, Aldskogius, & Svensson, 2001). Briefly, animals were anesthetized with an intraperitoneal injection of ketamine (60–90 mg/kg) and xylazine (100–150 mg/kg). The left extracranial portion of the facial nerve trunk was exposed, and a crush injury was made at the site of the facial nerve, exiting from the stylomastoid foramen, using mosquito forceps to clamp about 2 mm in length with one power grip for 60 s (Hadlock, Heaton, Cheney, & Mackinnon, 2005). The loss of facial nerve functions on the operated side was confirmed by examining the loss of the blink reflex and the loss of VMs (Chen, Li, Chi, Chen, & Li, 2012).
2.15 Local in vivo transfection
Anesthetized rats were injected intraperitoneally with ketamine (60–90 mg/kg) and xylazine (100–150 mg/kg). After the rats were deeply anesthetized, FNI tissue was processed. A syringe containing 10 μg of plasmid DNA (JiKai Gene, Jiangsu, China) was then injected near the injured facial nerve (Yeo, Kang, & Ji, 2019).
2.16 Analysis of VM
Using digital videos, VM recovery was measured and recorded 28 days after FNI. Rats were put in a box on a blackboard, then VMs were recorded using a video camera (A7RII; Sony, Tokyo, Japan). The frontal-occipital line (perpendicular to the line connecting the two orbital angles) was designated as the medial sagittal line. We measured the rostral-open angle between the frontal-occipital line and the most frontal hair shafts. The amplitude degree was the maximal difference between protraction and retraction. The neurological recovery of the crushed facial nerve was assessed by examination of VMs using the following scoring system: 0, no movement; 1, barely detectable movement; 2, slight movement; 3, significant but asymmetric movement; and 4, symmetric movement, as previously reported (Kano, Matsubara, Ueda, Hibi, & Yamamoto, 2017). The scores of the observed VMs were recorded 0, 3, 7, and 28 days after FNI, and then the scores of the different treatment groups were compared by statistical analyses.
2.17 Morphological evaluation of regenerated nerve segments
Four weeks after the induction of FNI, the tissue segments from the facial nerves were isolated from rats and fixed with 2.5% glutaraldehyde overnight at room temperature. Semi-thin sections (1 µm) were cut vertically, stained with 1% toluidine blue solution, and examined using a light microscope. The density of the myelinated fibers was analyzed from three nonoverlapping visual fields per specimen.
2.18 Immunohistochemical analysis in vivo
The tissue segments were isolated from rats and embedded, and 4 µm sagittal sections were generated with a cryostat. The sections were permeabilized with 0.1% Triton X-100 in phosphate buffered saline (PBS) for 20 min, blocked with 5% bovine serum albumin for 30 min, and incubated overnight with primary rabbit anti-S-100β antibody (1:50; ab41548; Abcam), followed by secondary donkey anti-rabbit IgG (heavy and light chains; Alexa Fluor® 647; 1:200; ab150063; Abcam). Tissue images were captured with a Universal fluorescence microscope.
2.19 Statistics
Data are expressed as the mean ± standard deviation. Statistical significance of differences among groups was tested by one-way analysis of variance. Comparisons between two groups were conducted using Student's t test. A value of p < .05 was considered statistically significant.
3 RESULTS
3.1 Functional overexpression of Foxc1 promotes SC migration
According to the results of biological examinations and thermograms, compared with normal rats, the expression of Foxc1 in rats with facial nerve injury increased (Figure 1a). qRT-PCR showed that the expression of Foxc1 in rats with facial nerve injury was significantly increased when compared with the normal group (Figure 1b). We then promoted Foxc1 expression using adenovirus transfection. The expression of Foxc1 in SCs was significantly higher than that in the control group after being transfected with Foxc1 by adenovirus transfection, which was confirmed by qRT-PCR (Figure 1c), western blot analysis (Figure 1d,e) and cellular immunofluorescence (Figure 1f).
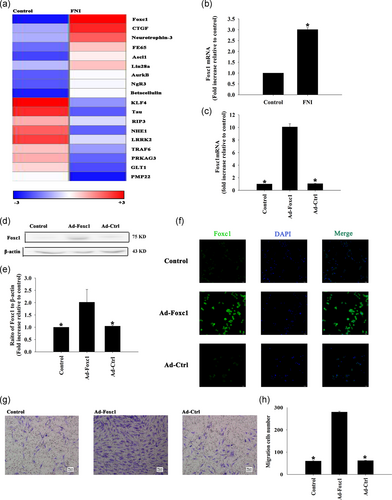
The migration of SCs was significantly increased after transfection with Foxc1 adenovirus compared with the control group, and there was no significant change after transfection by adenovirus with the blank control gene (Figure 1g,h).
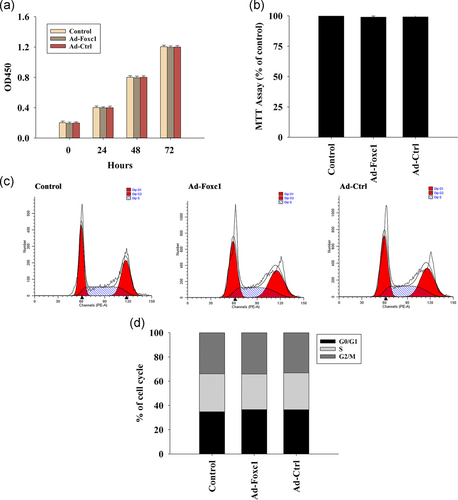
To determine whether transfection with Foxc1 influenced SCs, we examined cell viability using the MTT assay, which indicated that overexpression of Foxc1 did not influence cellular viability (Figure 2a). In addition, our results revealed that overexpression of Foxc1 had no effect on cellular proliferation (Figure 2b) and cell cycle (Figure 2c,d).
3.2 Foxc1 modulates SC migration by inducing CXCL12 release
After Foxc1 transfection, the expression of CXCL12 in SCs increased significantly when compared with the control group, not only at the mRNA level (Figure 3a), but also by promoting the release of CXCL12 (Figure 3b).
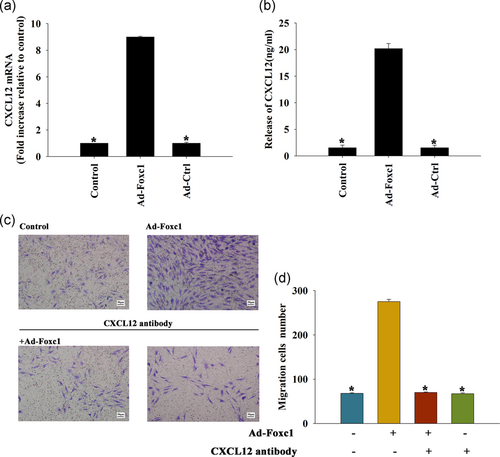
Compared with the control group, the migration of SCs after Foxc1 transfection was significantly increased, and there was no significant change after Foxc1 transfection with CXCL12 neutralizing antibody or CXCL12 neutralizing antibody alone. These results suggested that Foxc1 promoted Schwann cell migration by regulating CXCL12 release (Figure 3c,d).
3.3 The effect of CXCL12/β-catenin on Foxc1-induced SCs migration
After Foxc1 transfection, the expression of protein β-catenin in SCs was significantly higher than that in the control group. Compared with the control group, there was no significant change in groups treated by Foxc1 transfection accompanied with CXCL12 neutralizing antibody (Figure 4a,b). The results showed that Foxc1 increased β-catenin expression by regulating CXCL12.
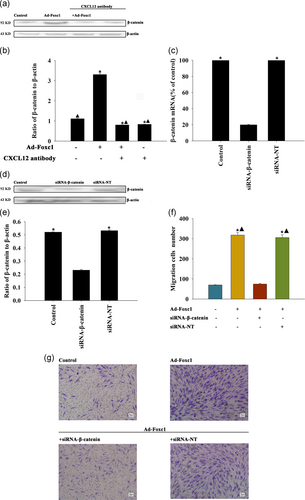
After gene silencing, the qRT-PCR results showed that the content of β-catenin RNA expression in the β-catenin-RNA silenced group was significantly lower than that in the blank control group, and there was no significant change in the unrelated sequence of the silenced group compared with the control group (Figure 4c). In addition, siRNA-β-catenin decreased the β-catenin protein in SCs (Figure 4d,e).
Compared with the control group, the number of SC migrations significantly increased after Foxc1 transfection, while stimulation of cell migration was abolished by silencing of β-catenin, but not by transfection with siRNA-NT (Figure 4f,g).
3.4 Inducing Foxc1 in vivo activates the CXCL12/β-catenin signaling pathway
We induced local Foxc1 overexpression in rat facial nerve using plasmid DNA, which attained high transfection efficiency. To quantitate the transfection efficiency, we used qRT-PCR. After transfection with Foxc1, the expression of Foxc1 in the facial nerve tissue was significantly higher than that in the control group and the unrelated gene transfected group, in a time-dependent manner (Figure 5a,b).
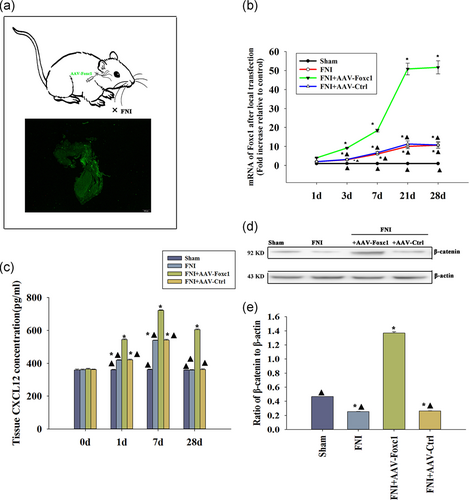
To investigate changes of CXCL12 levels after Foxc1 overexpression in facial nerve injury (FNI), we detected CXCL12 at different times using an ELISA assay. In sham-operated rats without nerve injury, the expression of endogenous CXCL12 protein was stable, while there was an increase in CXCL12 expression following facial nerve injury. Overexpression of Foxc1 induced CXCL12 release in a time-dependent manner (Figure 5c).
Next, we determined if there was any change in β-catenin with Foxc1 activation. Western blot analysis revealed that FNI impaired β-catenin in vivo, while a general inducement of β-catenin after Foxc1 overexpression was observed; there was no difference in the AAV-Ctrl infection, indicating Foxc1 overexpression induced β-catenin (Figure 5d,e).
3.5 Foxc1 overexpression restores facial nerve function after FNI
The evaluation of facial nerve function showed that the decrease of vibrissa activity reached a peak 3 days after FNI. After 4 weeks, the recovery of activity of the facial nerve vibrissa of the rats transfected with Foxc1 was significantly better than that of the rats in the FNI group. The function of facial nerves in the blank control group was normal and without change (Figure 6a).
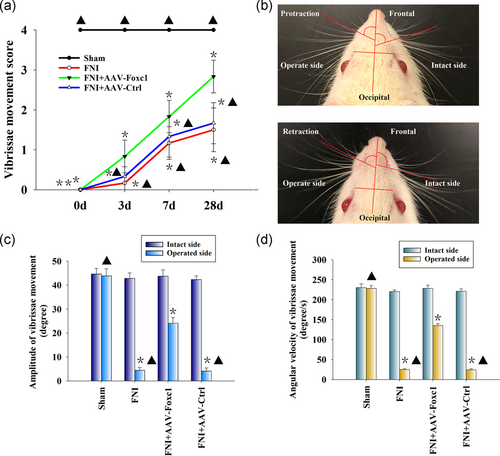
We also evaluated the recovery of the amplitude and angular velocity of the vibrissae movements (VMs) by digital photo recording. Four weeks after surgery, the PBS group showed that only the operated side was paralyzed with slight VMs. On the intact side, there was a significant change in the rostral open angle between the frontal-occipital line and the frontal hair shaft during protraction and retraction (Figure 6b). The mean amplitude and angular velocity of the operated side of the PBS and AAV-Ctrl groups were lower than that of the contralateral side, while local Foxc1 overexpression rescued the damage (Figure 6c,d). Together, these results showed that Foxc1 promoted the functional recovery of the FNI.
3.6 The effects of transfection of Foxc1 on myelination and SC migration in vivo
To evaluate facial nerve regeneration, we performed a histological analysis of semi-thin sections of nerve segments. Four weeks after FNI, the facial nerves of the AAV-Foxc1 group showed significant regeneration, with each exhibiting a large nerve fascicle containing many myelinated axons, while, the AAV-Ctrl group did not show the remyelinated effect (Figure 7a,b).

After 4 weeks of facial nerve injury, compared with the control group, the number of SCs in the Foxc1 transfection group decreased, and the unrelated sequence transfection in the simple facial nerve injury group was significantly reduced. After Foxc1 local transfection, the number of SCs in the facial nerves of rats was significantly higher than that in the simple facial nerve injury group and the unrelated sequence transfection group after FNI (Figure 7c).
4 DISCUSSION
The facial nerve is important for both communication and expression, and impairment of its function can severely affect the quality of life (Coulson, O'Dwyer, Adams, & Croxson, 2004). SCs, the main type of glial cells in the peripheral nervous system, undergo proliferation and help clearance of myelin debris after nerve injury, which plays an important role in mediating nerve regeneration (Richner et al., 2014). More important, SC migration is a necessary prerequisite for the injury site to guide and accelerate axonal outgrowth (Torigoe, Tanaka, Takahashi, Awaya, & Hashimoto, 1996). However, the ability of SCs to promote nerve regeneration is to some extent impaired by limited migration. Previous studies have found that limited SCs migration hampered nerve injury repair (Yi et al., 2019). Although some factors and pathways related to promotion of SC migration have been reported, the related mechanisms still need to be determined.
In the present study, microarrays showed that the expression of Foxc1 in the FNI group was higher than that of the control group. Foxc1, characterized by a common 100 amino acid winged-helix DNA-binding domain, has important roles in cell growth, survival, differentiation, and migration (Wang et al., 2012). For example, a recent study reported that Foxc1 promoted the migration of hepatocellular carcinoma cells (W. Huang et al., 2015). In breast cancer, overexpression of Foxc1 increased cell proliferation, migration, and invasion, whereas Foxc1 knockdown had the opposite effects (Ray et al., 2010). In the present study, our results showed that an induced increase in Foxc1 expression was accompanied by elevated SCs migration, both in vivo and in vitro. Consistent with the effects of Foxc1 on cellular migration, overexpression of Foxc1 in SCs promoted its migration. As the major glial cells of the peripheral nervous system, SCs form myelin sheaths, and provide support and nutrition to neurons (Castelnovo et al., 2017). Axonal regeneration is frequently accompanied by SC recruitment following peripheral nerve injury (Su et al., 2019). In our study, after facial nerve crush, we observed increased remyelination in rats with specific locally overexpressed Foxc1 compared to the control rats; this was accompanied with more SC accumulation.
The CXCR4-CXCL12 chemokine axis is required for normal development of the nervous system (Ma et al., 1998). A previous study showed that Foxc1 transcription factor played an important role in cellular migration by controlling CXCL12 expression (Hayashi & Kume, 2008). In the present study, after Foxc1 transfection, the expression of CXCL12 both in SCs and injured facial nerve tissue increased significantly when compared with the control group, consistent with a previous study, which suggested that exogenous CXCL12 induced recovery of facial nerve injury (Gao et al., 2019). Furthermore, use of CXCL12 neutralizing antibody impaired the migration of SCs after Foxc1 transfection, which suggested that Foxc1 promoted SC migration by regulating CXCL12 expression. The association of CXCL12 with cell migration has implicated activation of several signaling factors, including VEGFR2/VLA-4 (Jung, Beauvais, Adams, & Rapraeger, 2019), phospho-FoxO3a (J. Li et al., 2019), and Wnt/β-catenin (Chang et al., 2018), leading to regeneration, survival, and angiogenesis. In the present study, we found that CXCL12 inhibition deactivated β-catenin induced by Foxc1, thus showing that β-catenin activation depended on CXCL12 mediation.
Gain-of-function studies revealed that Foxc1 overexpression, leading to β-catenin stabilization and nuclear translocation, activated the Wnt/β-catenin signaling pathway (Wang et al., 2019). In the peripheral nervous system, activation of β-catenin treatment promotes remyelination of sciatic and facial nerves after crush injury (Makoukji et al., 2011). Our results also showed that overexpression of Foxc1 promoted β-catenin activation, accompanied by facial nerve remyelination. A previously study reported that activation of Wnt/β-catenin signaling is important for astrocytes migration (Yin et al., 2013). Another study found that upregulating Wnt/β-catenin signaling induced SCs migration (Y. Li et al., 2018). The present loss-of-function study showed that Wnt/β-catenin signaling induced SC migration to help heal facial nerve injuries.
In conclusion, we used multiple in vivo and in vitro approaches to highlight a novel mechanism of Foxc1 on the migration of SCs during FNI. We also provided relative evidence that Foxc1 regulated SC migration via CXCL12 release, leading to activation of the Wnt/β-catenin signaling. Our findings identified the underlying mechanism of the effects of Foxc1 on nerve regeneration, and provided support for the future use of gene therapy, cell therapy, and other basic biological methods for the treatment of facial nerve damages.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (grant no. 81974186 and no. 81671205 to Shiting Li; grant no. 81600278 to Wenzheng Xia), the Program from the Shanghai Committee of Science and Technology (grant no. 18XD1402700 to Shiting Li), and the Foundation for Interdisciplinary Research of Shanghai Jiao Tong University (grant no. YG2017MS68 to Jin Zhu).
AUTHOR CONTRIBUTIONS
W. Z. X., J. Z., and X. Y. W. have made substantial contributions to draft the manuscript, acquisition of data, analysis and interpretation of data. Y. D. T., P. Z., X. Y. W., B. W. C., X. Z., and W. C. Z. have been involved in analysis and interpretation of data. M. H. and S. T. L. have made substantial contributions to conception and design; been involved in revising it critically for important intellectual content; and gave their final approval of the version to be published. All authors edited the manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are included within the article.




