Histone H4 variant, H4G, drives ribosomal RNA transcription and breast cancer cell proliferation by loosening nucleolar chromatin structure
Abstract
The hominidae-specific histone variant H4G is expressed in breast cancer patients in a stage-dependent manner. H4G localizes primarily in the nucleoli via its interaction with nucleophosmin (NPM1). H4G is involved in rDNA transcription and ribosome biogenesis, which facilitates breast cancer cell proliferation. However, the molecular mechanism underlying this process remains unknown. Here, we show that H4G is not stably incorporated into nucleolar chromatin, even with the chaperoning assistance of NPM1. H4G likely form transient nucleosome-like-structure that undergoes rapid dissociation. In addition, the nucleolar chromatin in H4GKO cells is more compact than WT cells. Altogether, our results suggest that H4G relaxes the nucleolar chromatin and enhances rRNA transcription by forming destabilized nucleosome in breast cancer cells.
1 INTRODUCTION
Epigenetic aberration, including changes in histone posttranslational modifications (PTMs) and DNA methylation patterns, cause changes in gene expression patterns, which are important for maintaining the stability of the eukaryotic genome (Baylin, 2011; Cutter & Hayes, 2015; Di Cerbo & Schneider, 2013; Lo & Sukumar, 2008). In addition, changes in the expression pattern of several histone variants are also related to genome instability, as observed in cancer cells (Skene & Henikoff, 2013). Histone variants are substitutes for the core canonical histones and possess unique structural and functional features (Talbert & Henikoff, 2010). Histone variants exhibit some generic differences when compared with the canonical counterpart. (a) Canonical histones are replication-dependent and are expressed during the S-phase, whereas histone variants are replication-independent and are expressed constitutively throughout the cell cycle. (b) Canonical histone genes are highly conserved and typically multiple copies cluster along the chromosome, whereas histone variant genes are often single-copied and show a large sequence variation among species. (c) Canonical histones are intronless and their messenger RNAs (mRNAs) have a unique stem-loop structure at the 3′-end, while histone variant genes may have introns and generate transcripts with normal polyadenylated tails (Marzluff, Wagner, & Duronio, 2008).
Multiple histone variants were previously discovered for each linker and core histones. Compared with the linker histone H1 and core histones H2A, H2B, and H3, histone H4 was thought to be the only one that had no functional variants (Holmes et al., 2005). Two decades ago, a histone H4 gene (h4r) was reported in Drosophila that was cell-cycle independent and contained introns (Akhmanova, Miedema, & Hennig, 1996). However, h4r encodes a protein that is similar to its cell-cycle-dependent counterpart. More recently, we characterized the function of a previously uncharacterized hominidae-specific H4 variant—H4G (Long et al., 2019). H4G is the only histone H4 variant found in the human genome. As a hominidae-specific gene, it is present in the histone cluster 1 on chromosome 6 in humans. Unlike H4r, H4G only shares 85% amino acid sequence similarity to the canonical H4 (Long et al., 2019). Several transcriptomics analyses have shown elevated H4G expression in T-cell prolymphocytic leukemia and it was decreased in endometrioid carcinoma TOV112 cells upon tumor growth inhibitor BKM-570 (Durig et al., 2007; Jutras et al., 2010; Long et al., 2019).
We have previously reported that H4G is mainly expressed in breast cancer cell lines and breast cancer tissues in a tumor-stage-dependent manner. It is primarily localized to the nucleoli and its endogenous expression has been confirmed by mass spectrometry. In addition, in vitro and in cells experiments have shown that the nucleolar localization of H4G is mediated by the interaction between its α-helix 3 in the histone fold domain and the nucleolar histone chaperone NPM1 (Long et al., 2019; Okuwaki, Matsumoto, Tsujimoto, & Nagata, 2001; Swaminathan, Kishore, Febitha, & Kundu, 2005), which is also known as nucleophosmin and B23. In addition to nucleolar histone chaperone activity, NPM1 has many additional functions, such as nucleic acid binding, transcription processes, and centrosome duplication (Finn, Ellard, Eirín-López, & Ausió, 2012). Importantly, the absence of H4G in CRISPR-Cas9 knockout H4G MCF7 cell lines (H4GKOs) reduces the amount of rRNA. The lowered rRNA levels retards protein synthesis and leads to cell-cycle delay in H4GKOs. Furthermore, tumors formed by the H4GKOs cells were significantly smaller than that by the WT cells in mouse xenograft model (Long et al., 2019). These data suggested that H4G plays a role in driving breast cancer cell proliferation, probably through the upregulation of rRNA transcription. However, the molecular mechanism underlying the way in which H4G enhances rRNA transcription remains unknown.
This study shows that H4G is not stably incorporated into the nucleolar chromatin in vitro or in vivo, even with the help of the nucleolar histone chaperone NPM1. Alternatively, the H4G-containing nucleosome-like-structure rapidly dissociates from the DNA, and the nucleolar chromatin in the H4GKO cells is more compact compared with the WT. These results suggest that H4G competes with canonical histones to be loaded onto the nucleolar chromatin by NPM1 and then the rapid disassembly of the transient H4G nucleosome-like-structure helps to open-up the nucleolar chromatin. The resulting relaxed chromatin structure is likely to be related to an enhanced rDNA transcription and might be linked to the proliferative growth of breast cancer cells.
2 MATERIALS AND METHODS
2.1 Histone expression and purification
Except where indicated, recombinant histones were purified using the following method. In brief, the induced bacterial pellet was resuspended in wash buffer (50 mM Tris–HCl, pH 7.5, 100 mM NaCl, 1 mM EDTA, and 5 mM β-mercaptoethanol) and lysed by sonication. The inclusion bodies were resuspended and washed four times using the wash buffer with 1% Triton X-100 (vol/vol). Proteins were extracted by dimethyl sulphoxide and histone unfolding buffer (7 M guanidine hydrochloride, 20 mM Tris–HCl, pH 7.5, and 10 mM dithiothreitol) and dialyzed against urea buffer (7 M urea, 10 mM Tris–HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, and 5 mM β-mercaptoethanol) before HiTrap Q Sepharose FF (GE Healthcare) and HiTrap SP Sepharose FF (GE Healthcare) column purification on a fast protein liquid chromatography system. His-SUMO-H4 and His-SUMO-H4G were purified using Ni-NTA resin according to the manufacturer's instructions (Qiagen) under urea denaturing conditions, whereas His-NPM was purified under native conditions.
2.2 Histone transfer assay
The histone transfer assay was conducted as described, with modifications (Okuwaki et al., 2001). Briefly, the indicated histones were preincubated with His-tagged NPM1 for 30 min at 37°C and further mixed with 147-bp DNA fragments (either 601 or 5S rDNA nucleosome positioning sequences), followed by an additional 30 min of incubation at 37°C in buffer containing 25 mM Tris–HCl, pH 7.5, and 150 mM NaCl. The mixtures were resolved in 6% nondenaturing polyacrylamide gels. To assess the DNA-binding affinity of the His-tagged NPM1, the aforementioned histones–NPM1 mixture was instead mixed with 258-bp 5′-biotinylated 601 nucleosome positioning sequence and incubated at 37°C for 30 min in the same buffer. DNA-bound His-tagged NPM1 was pulled-down with BSA-blocked NeutrAvidin™ Agarose (Pierce). The precipitated product was then washed six times with Wash Buffer (0.1% NP-40, 25 mM Tris–HCl, pH7.5, and 150 mM NaCl). Recovered biotinylated DNA was purified and was resolved in 5% nondenaturing polyacrylamide gels. Precipitated His-tagged NPM1 and histones were visualized by western blot analysis.
2.3 Fluorescence recovery after photobleaching (FRAP)
FRAP experiments were conducted as described in Okuwaki et al. (2001). In brief, MCF7 cells transfected with the indicated plasmid(s) were grown on 35 mm confocal dishes (SPL), followed by setting on a confocal microscope (LSM710; Zeiss) with a Plan-Apochromat 63 × /1.4 oil objective in a heating chamber at 37°C. Cells with similar green fluorescent protein (GFP) or mCherry fluorescence signals were selected. The GFP or mCherry signal from the regions labeled by white circles were bleached using 100% transmission of a 488 nm Argon-ion laser for 100 iterations (scan speed, 8). Images were collected (512 × 512 pixels; zoom, ×4.1; scan speed, 10; pinhole, 1 airy unit; 488 nm Argon-ion laser with 2% output power for the GFP signal and 560 nm Diode-pumped solid-state laser with 2% output power for the mCherry signal) every 1.0 s. The ZEN2009 software (Zeiss) was used for the measurement of the fluorescence change at the bleached area. The relative fluorescence intensity was calculated by normalizing this readout with the initial fluorescence intensity of the bleached area at each time point. FRAP curves were fit to one-phase association exponential using the GraphPad Prism 7 software (GraphPad Software).
2.4 Isolation of nucleolar chromatin from MCF7 cells
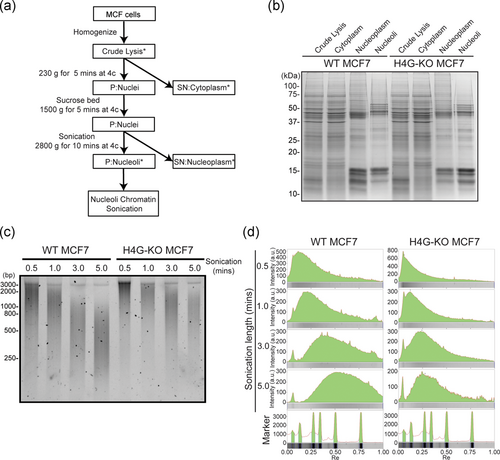
MCF7 cells (1.4 × 107 cells) were used for the isolation of nucleoli according to a method published previously (Hacot et al., 2010; O'Sullivan, Sullivan, & McStay, 2002). The cell pellet was suspended in 5 ml ice-cold hypotonic buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 5 mM β-mercaptoethanol, and protease inhibitor cocktail; Roche) and incubated on ice for at least 5 min, followed by 10 strokes from a pre-chilled Dounce homogenizer. Homogenized cells were centrifuged at 230 × g for 5 min at 4°C. The pellet was suspended in 3 ml of buffer S1 (0.25 M sucrose and 10 mM MgCl2), then placed on 3 ml of buffer S2 (0.35 M sucrose and 0.5 mM MgCl2). The supernatant was discarded after centrifugation at 1430 × g for 5 min at 4°C. The pellet was suspended in 3 ml of buffer S2 followed by sonication until no intact nuclei could be detected by a microscope. Sonicated nuclei were then transferred to 3 ml of buffer S3 (0.88 M sucrose and 10 mM MgCl2) and centrifuged at 2800 × g for 10 min at 4°C. The pellet containing the nucleoli was washed once with 3 ml of buffer S2 before further treatment. To determine the size range of nucleolar chromatin, nucleoli were resuspended in 200 μl of TE + sodium dodecyl sulfate (SDS) buffer (20 mM Tris, pH 8.0, 2 mM EDTA, and 2% SDS), followed by incubation at 37°C for 15 min. The resuspended nucleoli were sonicated with Q700 sonicator (QSonica) at 50% power for 0.5, 1, 3, and 5 min, respectively, and 50 μl of sonicated sample were collected at each time point. The sonicated nucleolar chromatin was treated with 50 μg of RNase A at 37°C for 30 min and 20 μg of proteinase K (NEB) at 37°C for 30 min. The DNA fragments were purified before their analyses using 5% native PAGE.
3 RESULTS AND DISCUSSION
3.1 H4G is unable to form stable nucleosomes, even with the assistance of NPM1 in nucleoli
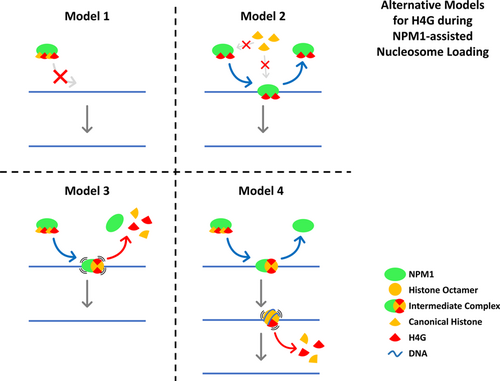
The histone H4 variant, H4G, was unable to load a nucleosome in vitro, which suggests that H4G likely has a distinct mode of action compared with H4, which may be attributed to its higher hydrophobicity (Long et al., 2019). However, the possibility exists that H4G might be able to form nucleosomes in the presence of histone chaperone NPM1, as H4G strongly interacts with this histone chaperone. NPM1 has been well-studied to act as a histone chaperone to assist the deposition of nucleosomes in vitro (Okuwaki et al., 2001; Okuwaki et al., 2012). In this regard, an in vitro histone transfer assay was conducted to assess whether NPM1 has the ability to incorporate H4G into a nucleosome (Okuwaki et al., 2001; Okuwaki et al., 2012; Swaminathan et al., 2005). In the absence of histone chaperones in the mixture, when histones and DNA are mixed together, they form large aggregates that do not enter the gel (Okuwaki et al., 2012). In contrast, if core histones are preincubated with increasing amounts of NPM1, free DNA and nucleosome bands can be detected where the amount of free DNA decreases and nucleosomes increase in a dose-dependent manner. However, with the exception of some tetrasome-like bands, no apparent nucleosome bands were observed when a mixture of histones containing His-SUMO-H4G was used (Figures 1a and S1). A similar result was obtained when using the 5S rDNA nucleosomal position sequences as a DNA template (Figure 1b). These results clearly indicate that H4G is either unable to form a nucleosome in vitro in the presence of NPM1 chaperone, or the nucleosome or nucleosome-like-structure that formed is highly unstable (Figure 1a,b). The lack of any visible nucleosome band in the H4G group can be explained by the following four possibilities: Model 1: Binding of H4G disables NPM1 and renders it unable to bind to DNA, Model 2: Binding of H4G blocks other histones from binding to the NPM1 complex, therefore nucleosome loading cannot initiate, Model 3: NPM1 can recruit H4G and other histones to initiate nucleosome loading on the DNA, but the process terminates prematurely because of the presence of H4G in the intermediate complex, or Model 4: NPM1 can deposit H4G nucleosome-like-structure onto the DNA; however, the structure dissociates very quickly since it is extremely destabilized compared with canonical nucleosome (Figure 2). Of note that, when these in vitro reconstitutions were carried out with a mixture of histones containing His-SUMO-H4G, more free DNA was observed in the reconstituted samples (Figure 1a,b). H4G was also found to pose no detrimental effect on the DNA-binding affinity of NPM1 (Figure 1c). In fact, the opposite was observed as slightly more NPM1 was pulled-down in the H4G samples. This directly dismisses model 1 as a possible mechanism since it requires H4G to reduce or disable the DNA-binding ability of NPM1. In addition, the intensities of nucleosome bands did not changed when extra H4G was added to the mixture of histones and NPM1, and the same trend of increased free DNA was observed in the extra-H4G group (Figure 1d,e). These results exclude model 2 and further reject model 1 since in both models, the presence of H4G prevents NPM1-assisted nucleosome loading from being initiated. Therefore, the histones–DNA mixture should behave as if there is no NPM1 added, and no free DNA bands or tetrasome-like bands should be observed. Also, the fact that canonical nucleosome loading is not affected by the addition of extra H4G indicates that free NPM1 must be constantly cycling in the NPM1-histones–DNA mixture, which is also not compatible with model 1 and model 2. This left us with model 3 and model 4, which remain equally likely.

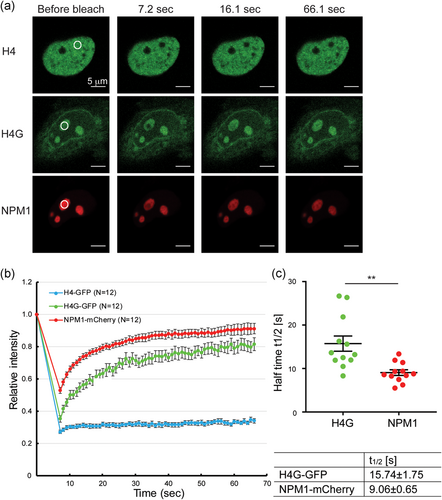
To discern between model 3 and model 4, we decided to conduct FRAP assays using MCF7 cells that had been transfected with GFP-tagged or mCherry-tagged proteins. The GFP fluorescence recovery rate of GFP-tagged canonical histones was very slow (over 8 hr) compared with GFP itself (a few seconds; Harada et al., 2018; Kujirai et al., 2016; Wiedemann et al., 2010; Figure 3a,b). This low recovery rate indicates that the canonical histones are immobile and stably bound to chromatin. Interestingly, the FRAP recovery rate of GFP-tagged H4G in nucleoli was fast, with a half time (t1/2) of fluorescence recovery within 20 s (Figure 3c). Although this was still slower than free GFP itself, this result indicates that H4G is not stably associated with the chromatin in nucleoli. Nucleolar proteins reportedly have different dynamic properties because of their specific functional roles in ribosome biogenesis. The FRAP rates of GFP-tagged UBF1, nucleolin, fibrillarin, Rpp29, and NPM1 are fast, with a t1/2 within 20 s, whereas the FRAP rates of the GFP-tagged ribosomal proteins S5 and L9 are relatively slower, with a t1/2 around 72 s (Chen & Huang, 2001). UBF and pre-rRNA processing factors, such as NPM1, move significantly faster than ribosomal proteins in nucleoli, which is consistent with the faster rates of transcription and processing of rRNA compared with the assembly of ribosomal subunits. In addition, the mobilities of fibrillarin, Rpp29, and NPM1 are usually similar with each other, indicating that they are involved in the processing of rRNA by forming an active complex. FRAP assays using MCF7 cells cotransfected with GFP-tagged H4G and mCherry-tagged NPM1 were also conducted because H4G and NPM1 had been shown to exhibit a high affinity of binding in our previous report (Long et al., 2019). The FRAP recovery half-life of H4G and NPM1 are 16 and 9 s, respectively (Figure 3c). These results also support that NPM1 interacts with H4G in cells and H4G is not involved in the formation of stable nucleolar chromatin. Furthermore, these results support model 4 over model 3 as the sequential release of NPM1 and H4G, and the formation of a transient H4G nucleosome-like-structure depicted in model 4 predicted that H4G should have a lower mobility and thus a longer FRAP recovery half-life than NPM1, which is exactly what have been observed (16 s vs. 9 s, p = .001679); while in model 3, the simultaneous release of NPM1 and H4G from the dissociation of the intermediate complex would suggest H4G is likely to diffuse faster and have a shorter FRAP recovery half-life due to its smaller size when compared with NPM1. Therefore, it is very likely that H4G is released on the nucleolar chromatin and forms temporary nucleosome-like-structure, which dissociates quickly.
3.2 H4G loosens nucleolar chromatin structure by interacting with NPM1 in nucleoli
Considering that H4G cannot be stably incorporated into nucleolar chromatin and that the interaction between H4G and NPM1 likely form transient nucleosome-like-structure (Figure 2, model 4), we decided to examine the chromatin configuration in nucleoli. To this end, the nucleolar chromatin of WT and H4GKO MCF7 cells was extracted, and then the size of the protected DNA after sonication was compared (Figure 4a–c; Long et al., 2019). The length of the DNA fragment obtained reflects the compaction state of chromatin. Larger DNA fragments can be observed if the nucleolar chromatin is more compact. Our analysis showed that the size of DNA fragments from WT MCF7 cells was smaller than that from H4GKO cells, regardless of the sonication time (Figure 4c,d). This suggests that the WT MCF7 cells, which express H4G, have a less compact nucleolar chromatin.
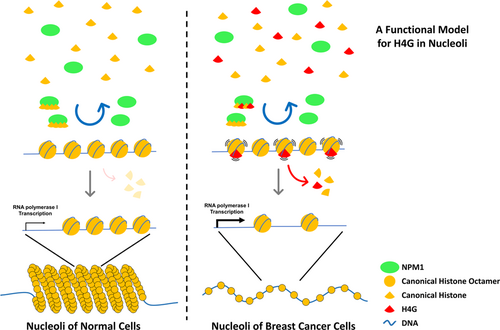
As described in the Introduction, histone variants are able to alter the accessibility of chromatin by affecting nucleosomal stability (Talbert & Henikoff, 2010). The changes in chromatin accessibility modulate the binding affinity of transcription factors and hence play an important role in gene expression regulation. NPM1 can mediate nucleosome assembly by forming homo- or hetero-pentamers with itself or with NPM3, which is essential for either the up- or downregulation of rDNA transcription in nucleoli (Herrera, Savkur, & Olson, 1995; Huang, Negi, Szebeni, & Olson, 2005; Savkur & Olson, 1998). Here, we discovered that the interaction between H4G and NPM1 indirectly loosens the nucleolar chromatin structure via the formation of destabilized H4G nucleosome-like structures that dissociate rapidly, thereby enhances rDNA transcription and accelerates protein synthesis, and eventually drives cell proliferation (Figure 5; Long et al., 2019). Because NPM1 interacts with many proteins that are involved in DNA replication and transcription, DNA repair, ribosome biogenesis, mitotic spindle formation, cell-cycle control, and apoptosis (Lindström, 2011), it would be worthwhile to study whether the interaction between H4G and NPM1 contributes to these processes. It would also be interesting to investigate whether other histone variants, histone mutations, or histone PTMs utilize a similar mechanism to modify chromatin structure in both nucleoli and the nucleus.
ACKNOWLEDGMENTS
This study was supported by grants from the Research Grants Council of the Hong Kong SAR (16104917, 26100214, C7029-15G, C7007-17G) to TI and by Canadian Institutes of Health Research (CIHR) grant (MOP−130417) to JA.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




