Elevated cell-free fetal DNA contributes to placental inflammation and antiangiogenesis via AIM2 and IFI16 during pre-eclampsia
Ning Li and Fei He contributed equally.
Abstract
Accumulated evidence has shown that pre-eclampsia (PE) is related to both maternal and utero-placental antiangiogenesis and inflammation. Remarkably, an elevated cell-free fetal DNA (cffDNA) level has been found in maternal circulation; however, it remains unclear whether this DNA can induce activation of cytosolic DNA sensor signaling pathways and lead to the development of PE. In this study, we found that trophoblast cells constitutively expressed the cytosolic DNA sensors, absent in melanoma 2 (AIM2) and interferon-inducible protein 16 (IFI16). The cffDNA and pro-inflammatory and antiangiogenic factors were present at higher concentrations in PE compared with the control group and correlated with the severity of PE. DNA stimulation significantly increased the AIM2 and IFI16 levels, consistent with the elevated AIM2 and IFI16 expression in women with PE, and elicited increased production of AIM2-mediated interleukin IL-8 (IL-8), IL-6 and CC chemokine ligand 2 (CCL2) and IFI16-mediated sEndoglin, sFlt-1 and CXCL10. Furthermore, enhancement of the inflammatory response was found to be induced by DNA exposure, but DNA exposure did not induce PE-like symptoms in pregnant mice. It is possible that elevated cffDNA could reflect the degree of placental damage and trigger cytosolic DNA sensor activation, which disrupts the immunity balance and, consequently, contributes to inflammatory and antiangiogenic responses. In conclusion, the results of this study suggest that circulating cffDNA levels are increased in preeclamptic women and act through AIM2 and IFI16 activation to promote the production of pro-inflammatory and antiangiogenic factors, which correlate with the severity of the disease, and may offer insights into the etiology and pathogenesis of PE.
1 INTRODUCTION
Pre-eclampsia (PE) is a complex pregnancy-specific hypertensive syndrome that affects 2–8% of pregnancies and is characterized by hypertension and proteinuria (Munoz-Hernandez et al., 2017; Young, Levine, & Karumanchi, 2010). Although the pathophysiologic mechanisms underlying this disease have not yet been fully elucidated, the presence of ineffective placentation resulting in placental hypoxia/ischemia seems to play a key role in the development of PE (Young et al., 2010). In fact, PE has been suggested to occur with placental dysfunction in early pregnancy, leading to increased release of trophoblastic debris, including DNA, RNA, adenosine, and uric acid (Gupta, Hasler, Holzgreve, Gebhardt, & Hahn, 2005). Thus, understanding the functions of these placental-derived microparticles is important for understanding disease progression.
Recently, it has been shown that cell-free fetal DNA (cffDNA) is increased in maternal circulation in association with pregnancy complications, including spontaneous abortion (Lim et al., 2013), PE (Contro, Bernabini, & Farina, 2017; Miranda et al., 2013), and preterm birth (Dugoff et al., 2016; Quezada, Francisco, Dumitrascu-Biris, Nicolaides, & Poon, 2015; van Boeckel, Davidson, Norman, & Stock, 2017). In a preliminary report from our group, we observed a strong relationship between elevated cffDNA and the severity of PE (Li et al., 2015). These findings have been attributed to accelerated apoptosis/necrosis of trophoblastic cells resulting from placental hypoxia/ischemia in women with PE (Martin, Krishna, Badell, & Samuel, 2014). Therefore, there is an urgent need to identify the function of the elevated cffDNA in maternal circulation, which may provide direct or indirect clues to the underlying physiology and pathology of PE.
cffDNA originates from the placenta via cell death, likely by apoptosis in normal pregnancy (Reddy et al., 2008) or necrosis during infection (Chu et al., 2014) and PE (Hahn, Huppertz, & Holzgreve, 2005; Reddy et al., 2008; van Boeckel, Davidson, Norman, & Stock, 2018) in the syncytiotrophoblast (STB) and cytotrophoblast (CTB) layers. cffDNA has been implicated in the regulation of various biological processes, including inflammation (Scharfe-Nugent et al., 2012), angiogenesis (Li et al., 2015) and oxidative stress (Sheller-Miller, Urrabaz-Garza, Saade, & Menon, 2017). Elevated cffDNA may act as a danger-associated molecular pattern (DAMP) and is recognized by intracellular DNA sensors. Absent in melanoma-2 (AIM2) and interferon-inducible protein-16 (IFI16), members of the HIN200 protein family, are mediators of innate immune responses to cytosolic double-stranded DNA (dsDNA) (Goubau, Rehwinkel, & Reis e Sousa, 2010). Since their function as cytosolic DNA sensors was disclosed, research on AIM2 and IFI16 has predominantly focused on their role in innate immunity during infection with DNA viruses or bacteria or can be mimicked experimentally by dsDNA transfection (Hornung et al., 2009; Unterholzner et al., 2010). The AIM2 inflammasome and IFI16 receptor are important for sensing bacterial and/or viral pathogens and triggering antimicrobial innate immunity (Jin et al., 2012; Kerur et al., 2011). With regard to cffDNA, although a previous study has reported that hypomethylated fetal DNA can activate TLR9 signaling in human PBMCs with elevated inflammation (Scharfe-Nugent et al., 2012), the biological implications of AIM2 and IFI16 during PE have not been characterized.
In the current study, we reveal the roles of AIM2 and IFI16 in placental inflammation and antiangiogenesis during PE. We show that circulating cffDNA levels are increased in PE and act through AIM2 and IFI16 activation to promote the production of pro-inflammatory cytokines and antiangiogenic factors that are essential biomarkers for PE.
2 MATERIALS AND METHODS
2.1 Participants
Human first-trimester placentas (n = 37), normal third-trimester placentas (n = 34), and severe PE placentas (n = 57) were obtained from elective terminations of normal pregnancies or cesarean deliveries. The study included 158 blood samples from established preeclamptic women during their third trimester (76 with mild PE and 82 with severe PE) together with 136 normotensive pregnant women. Written informed consent was obtained from all participants, and the use of human samples was approved by the Ethics Committees of the First Hospital of Jilin University, the Second Clinical Medical College of Jinan University, and Jilin Gynecology and Obstetrics Hospital.
PE was diagnosed as de novo gestational hypertension of ≥140/90 mmHg and ≥1 + on dipstick urinalysis or ≥0.3 g protein in 24 hr urine collection according to the criteria of the International Society for the Study of Hypertension in Pregnancy. Normal pregnancy was defined as a term delivery (≥37 weeks) without medical or obstetric complications. No participants in this study had a history of chronic hypertension, diabetes mellitus, heart disease, kidney disease, or other complications that may lead to vascular disorders, ischemia and/or hypoxia changes and no history of acute or chronic infectious diseases.
2.2 Circulating cell-free fetal DNA measurement
Blood samples were collected into tubes containing EDTA. Cell-free plasma samples were obtained by centrifugation of whole blood at 1,600g for 10 min, the supernatant was collected and centrifuged again at 16,000g for 10 min to remove all remaining cells. Total cell-free DNA (5 ml plasma) was extracted using a circulating cell-free DNA kit (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions and eluted in nuclease-free water (150 μl). To measure cffDNA, Ras-association domain family 1 isoform A (RASSF1A) and β-Actin sequences were simultaneously amplified by a duplex PCR assay. The enzyme-digested RASSF1A sequence was used to quantify cffDNA, and β-actin was used as a digestion control. The experiment was performed as previously described (Hindson et al., 2011; Manokhina, Singh, Penaherrera, & Robinson, 2014). Briefly, each sample (99 μl) was first treated with the methylation-sensitive restriction enzymes HhaI (60 U), BstUI (30 U) and HpaII (30 U) (New England Biolabs, Beijing, China) (120 μl total in 1 ×NEB buffer 4). The mixtures were incubated at 37°C for 2 hr, 60℃ for 2 hr, and then 65°C for 20 min. A second portion of the eluate (33 μl) was used in a no-digest control. Quantitative PCR was performed on an ABI 7500 system (Applied Biosystems, CA) in a quantitation-standard curve experiment. A TaqMan Fast Advanced Master Mix, TaqMan probe, and the primers were obtained from Life Technologies (Table S1). The standard curve was obtained using a human DNA quantification kit (Applied Biosystems, CA). All samples were analyzed in triplicate. A conversion factor of 6.6 pg of DNA per cell was used to express the results as genome equivalents per milliliter (GE/ml).
2.3 Placental villous explants and cell culture
The human first-trimester trophoblast cell line HTR-8/SVneo was cultured in DMEM/F12 (Invitrogen Life Technologies) supplemented with 10% fetal bovine serum (FBS; Gibco), 1 mmol/L sodium pyruvate, 2 mmol/L l-glutamine, and 100 U/ml penicillin/streptomycin (Invitrogen Life Technologies). HTR-8/SVneo cells were seeded onto 35 mm dishes (1 × 106) and incubated overnight to facilitate attachment. The cells were stimulated with poly(dA:dT)/LyoVec (Invivogen, San Diego, CA) or necrotic JEG3 DNA/LyoVec in Opti-MEM (Invitrogen Life Technologies) according to the manufacturer's instructions. The cells and supernatants were harvested for further tests. The trophoblast-derived choriocarcinoma cell line JEG3 was cultured in DMEM/F12 supplemented with 10% fetal bovine serum and 100 U/ml penicillin/streptomycin.
Human first-trimester placental villous explants of approximately 10 mg (wet weight, ten pieces per 35 mm dish) were cultured in phenol red-free DMEM/F12 containing 10% FBS and 100 U/ml penicillin/streptomycin. After culturing for 24 hr, the explants were transferred to serum-free Opti-MEM for 4 hr and subsequently transfected with DNA for 48 hr. Briefly, we mixed 1 μg DNA/150 μl LyoVec in a sterile 1.5 ml tube, homogenized gently and incubated at room temperature for 15 min. A DNA/LyoVec mixture was added into human first-trimester placental villous explants. To confirm the transfection efficiency, the placental villous explants were transfected with poly(dA:dT)-rhodamine (2 μg/ml; Invivogen, San Diego, CA) for 48 hr, LyoVec alone served as the negative control. Following treatment, the placental tissues were digested with trypsin and collagenase I, single cells were washed twice with Dulbecco's phosphate-buffered saline and analyzed using a flow cytometer. As shown in Figure S2, the results indicated that the transfection efficiency (%) is 69.36 ± 7.84 (60.2–81.5%).
2.4 sEndoglin, sFlt-1, and cytokine measurements
Blood samples were collected into tubes containing a clot activator and separator gel. The samples were centrifuged at 3000g for 10 min within 2 hr of collection, and the serum was then immediately stored in aliquots at − 80°C until further analysis. The serum samples and culture supernatants were analyzed using R&D ELISA kits for human soluble endoglin, soluble Flt-1, CC chemokine ligand 3 (CCL3)/MIP-1α, CCL11/eotaxin, chemokine ligand 1 (CXCL1)/GRO-α, IL-18, interferon-α (IFN-α), IFN-β, and IFN-γ or using BioLegend Max Pre-Coated kits for human IL-6, IL-8, CXCL10/IP-10, CCL2/MCP-1, CCL5/RANTES, IL-1β, and TNF-α.
2.5 Induction of cell necrosis
Chen, Stone, McCowan, and Chamley (2006) demonstrated that maternal vascular endothelial cells can phagocytose necrotic but not apoptotic trophoblasts and suggested a mechanism by which the endothelium can become activated during PE. In this study, JEG3 cells were induced to necrosis by three freeze-thaw cycles, as described previously (Chen et al., 2006). Briefly, JEG3 cells were frozen to −20°C for 1.5 hr, slowly thawed at 4°C for 4 hr, and then brought to room temperature for 1.5 hr. To measure the proportion of necrotic trophoblasts, the cells were quantified by annexin V–FITC/propidium iodide (BD Biosciences, San Jose, CA) staining followed by FACS analysis. Ninety-five percent of the cells were necrotic. The Genomic DNA Purification Kit (Promega) was used to extract necrotic DNA. The concentration and purity were measured by a Nanodrop 2000 (Thermo Fisher Scientific).
2.6 Real-time PCR
Total RNA was extracted from samples using TRI-Reagent (Sigma-Aldrich) according to the manufacturer's instructions. Reverse transcription PCR (RT-PCR) was performed using an RT reagent kit with a gDNA eraser (Takara, Kyoto, Japan). Quantitative real-time PCR was performed on an ABI 7500 system using SYBR Green PCR Master Mix (Roche Diagnostics, South San Francisco, CA). Each example was run in triplicate. The primers are listed in Table S2.
2.7 Western blot analysis
Trophoblast cells or placental samples were harvested and washed twice with PBS, and then homogenized in lysis buffer containing 1% Triton X-100, 150 mM NaCl, and 50 mM Tris–HCl (pH 7.4) in the presence of 1 mM phenylmethylsulfonylfluoride, 5 mg/ml aprotinin, 5 mg/ml leupeptin, 0.1 mM NaF, 10 μM E-64, and 5 mg/ml pepstatin A. Homogenates were centrifuged at 12,000 rpm for 20 min at 4°C, and the supernatants were collected and used for immunoblotting analysis. The antibodies are listed in Table S3.
2.8 Immunohistochemistry
Human first-trimester placental tissue sections (4 μm) were deparaffinized, rehydrated and subjected to an antigen retrieval step; human normal third-trimester and severe PE placental frozen sections (8 μm) were fixed in pre-cooled acetone for 10 min. After washing three times with PBS, the sections were blocked from endogenous peroxidase activity by 0.3% H2O2, and followed by blocking with 5% donkey serum for 1 hr at room temperature. To analyze AIM2 expression, the samples were incubated overnight at 4°C with AIM2 antibody (Abcam, Cambridge, MA; #ab180665) at a dilution of 1:400, rabbit IgG monoclonal antibody (1:400; Abcam; #ab172730) was used as an isotype control. After washing three times, specific staining was detected using an UltraSensitiveTM SAP IHC Kit and DAB Detection Kit (Maixin-Bio, China) according to the manufacturer's directions. The tissue sections were subsequently counterstained with hematoxylin (Sigma-Aldrich) before dehydration with ethanol and histosolving. The slides were mounted with Permount (Sigma-Aldrich) and visualized using light microscopy and analyzed using ImageJ2X software.
2.9 Animals
All mice experiments were conducted according to the experimental practices and standards approved by the Animal Welfare and Research Ethics Committee at Jinan University and were in accordance with the ethical guidelines of the International Association for the Study of Pain. C57bl/6J mice were provided by the Model Animal Research Center of Nanjing University.
Adult mice (8–12 weeks) were mated, and the appearance of a vaginal plug was designated as embryonic day (ED) 0.5. Pregnant mice were randomized into three groups and were treated daily (gestation days 7.5–12.5) with intraperitoneal injections of saline/TurboFect (in vivo Reagent, Thermo Fisher Scientific; Ctrl, n = 10), 300 μg/dam poly(dA:dT)/TurboFect (n = 10), and 300 μg/dam necrotic JEG3 DNA/TurboFect (n = 10). All solutions were prepared to a final volume of 200 μl per injection. On gestation day 14.5, the systolic blood pressure of pregnant mice was measured using the noninvasive tail-cuff method by a Visitech Systems unit (BP-2000). Systolic blood pressure was assessed 10 times, and three subsequent measurements with a variation of less than 5 mmHg were averaged to calculate maternal systolic blood pressure. The urinary total albumin content was measured using a Mouse Albumin ELISA Kit (Abcam), and urinary creatinine was measured using a Creatinine Parameter Assay Kit (R&D System, Minneapolis, MN), according to the manufacturer's instructions. The urine albumin concentrations are reported as the albumin/creatinine ratio (mg/g). Pregnant dams were killed via CO2 inhalation, and serum was collected for cytokine analysis using an R&D ELISA Kit for mouse soluble endoglin and soluble Flt-1, IL-1β, and CXCL10/IP-10 or a BioLegend Max Pre-Coated Kit for mouse IL-6, TNF-α, and CCL2/MCP-1.
2.10 Statistical analysis
All experiments were repeated independently in triplicate under identical conditions. Data are presented as the mean ± SD. A paired two-tailed Student's t-test or one-way or two-way analysis of variance with Tukey's HSD (Honestly Significant Difference) test was used to statistically analyze differences among groups. Calculations were conducted using GraphPad 8.0 (GraphPad Software, Inc., San Diego, CA). p ≤ .05 was considered statistically significant.
3 RESULTS
3.1 Clinical characteristics of the study population
The demographic and clinical parameters of women with a normal pregnancy and those with PE are summarized in Table 1. We collected samples from 294 pregnancies, 158 of which were from PE patients. Based on the severity of PE, these women were classified as having mild PE (MPE, n = 76) or severe PE (SPE, n = 82). There were no significant differences between the PE group and normal pregnant group (NP) regarding maternal age, pre-pregnancy body mass index (BMI), nulliparity, parity, and gestational age at the time of sampling (p > .05). None of the subjects were smokers in either the PE or control groups. As expected, the systolic blood pressure (SBP), diastolic blood pressure (DBP), and proteinuria of PE patients were significantly higher than those of the normal controls (p < .001). The gestational week at delivery was lower in women with severe PE compared with healthy and mild PE women (p < .001).
| Normal Pregnancy (n = 136) | Mild PE (n = 76) | Severe PE (n = 82) | p Value | |
|---|---|---|---|---|
| Maternal age (y) | 28.2 [24–33] | 30.4 [27–35] | 29.1 [26–34] | n.s. |
| Primipara, n (%) | 101 (74.3) | 59 (77.6) | 65 (79.3) | n.s. |
| Singleton pregnancy (%) | 100 | 100 | 100 | n.s. |
| Pre-pregnancy BMI (kg/m2) | 27.8 ± 2.7 | 27.4 ± 1.9 | 29.3 ± 3.1 | n.s. |
| Gestational age at sampling (weeks) | 31–36 | 31–36 | 31–36 | n.s. |
| Gestational age at delivery (weeks) | 39.5 ± 1.5 | 36.4 ± 2.4 | 34.8 ± 2.7 | p < .001 |
| SBP (mmHg) | 108.9 ± 15.9 | 149.1 ± 11.7 | 173.4 ± 13.3 | p < .001 |
| DBP (mmHg) | 71.6 ± 4.7 | 95.2 ± 5.8 | 114.6 ± 10.8 | p < .001 |
| Proteinuria | - | ≤ 1+ <3+ | ≥ 3+ | p < .001 |
| Male sex, n (%) | 69 (50.7) | 34 (44.7) | 48 (58.5) | n.s. |
- Categorical variables are represented by absolute frequencies and percentages (n, %). Noncategorical variables are represented by mean±SEM. P values are from comparison of control, mild pre-eclampsia and severe pre-eclampsia. SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index. n.s.: no significant.
3.2 AIM2 and IFI16 activation is triggered by dsDNA stimulation
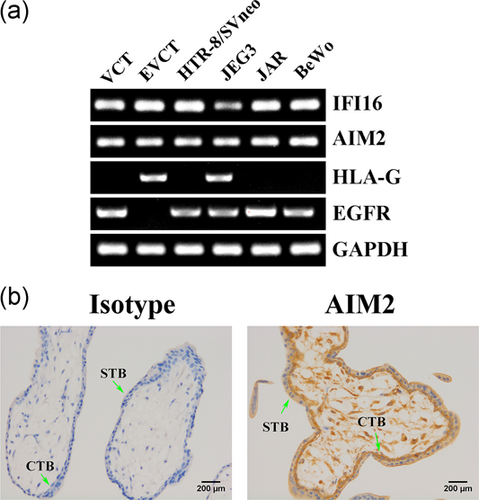
To investigate the role of cytosolic DNA sensors during PE, the expression patterns of AIM2 and IFI16 were evaluated. Human primary first-trimester extravillous cytotrophoblasts (EVCTs), villous cytotrophoblasts (VCTs), and four human trophoblast cell lines constitutively expressed AIM2 and IFI16, as demonstrated using semi-quantitative RT-PCR (Figure 1a). Immunohistochemistry analysis indicated that AIM2 was localized in both the STB and the CTB layers, but the level of AIM2 was higher in the CTB layer. In addition, all cells of the stromal core including stromal mesenchymal cells and the Hofbauer cells were AIM2 positive (Figure 1b). In our previous report, all CTBs and STBs expressed IFI16 (Li et al., 2015). Furthermore, compared with normotensive pregnant women, AIM2 (Figure 2a,b) and IFI16 (Li et al., 2015) were dramatically upregulated in the placentas of women with PE.
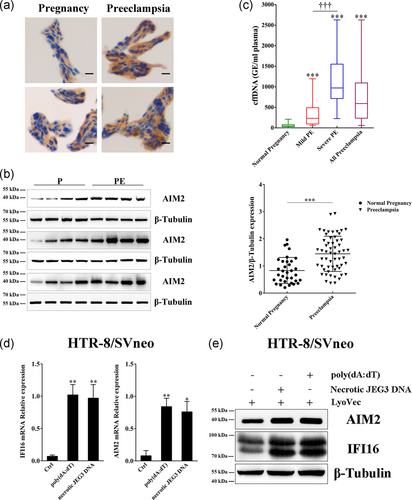
To explore the relationship between elevated cffDNA and AIM2 and IFI16 activation, we initially quantified cffDNA in maternal plasma using an enzyme-digested RASSF1A sequence. The results showed an almost 13-fold increase in the PE group over the control group (p < .001, Figure 2c). Moreover, both the MPE (5.6-fold) and SPE (20-fold) groups displayed a conspicuously higher level of cffDNA compared with the normal pregnancy group, and the SPE group had a level of cffDNA that was approximately 3.5-fold higher than that of the MPE group, confirming a significant difference between the two PE groups. This elevated cffDNA could act as a DAMP and might result in AIM2 and IFI16 activation. Therefore, we used an in vitro model to test this hypothesis in which HTR-8/SVneo cells were treated with synthetic dsDNA, poly(dA:dT), and necrotic JEG3 DNA. The AIM2 and IFI16 mRNA and protein levels were dramatically upregulated after DNA stimulation (Figure 2d,e), consistent with the observation of increased AIM2 and IFI16 expression in PE. Taken together, these results demonstrated that activation of AIM2 and IFI16 can be triggered in trophoblast cells in response to cffDNA stimulation.
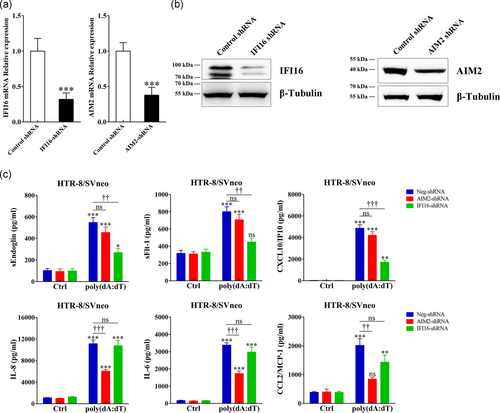
3.3 AIM2 and IFI16 are required for antiangiogenic and pro-inflammatory factors secretion
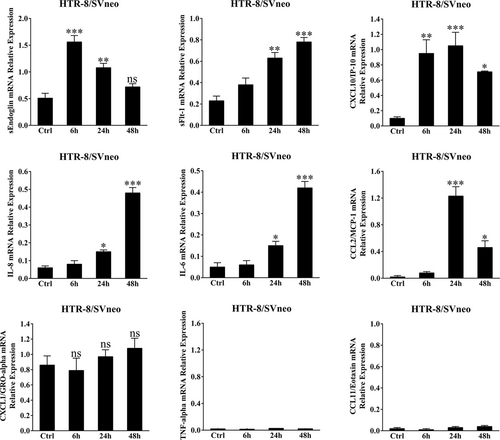
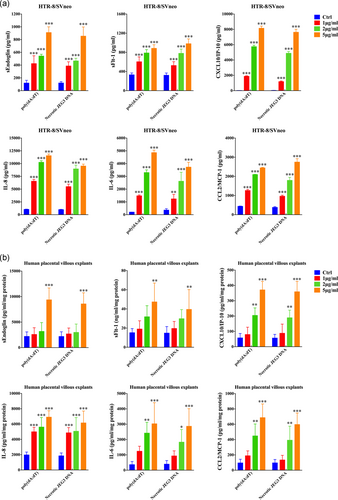
To show that cffDNA can be recognized by trophoblasts, we measured the production of antiangiogenic factors and pro-inflammatory cytokines/chemokines. As shown in Figure 3, poly(dA:dT) stimulation significantly increased the sEndoglin, sFlt-1, CXCL10, IL-8, IL-6 and CCL2 mRNA levels in HTR-8/SVneo cells. However, poly(dA:dT) stimulation did not influence the expression of CXCL1, TNF-α and CCL11, which had higher concentrations in the SPE group (Figure S1). Upon poly(dA:dT) or necrotic JEG3 DNA stimulation, strikingly increased sEndoglin, sFlt-1, CXCL10, IL-8, IL-6, and CCL2 secretion was observed in a dose-dependent manner (Figure 4a). In the study population, these mediators were dramatically increased in PE patients and seemed to correlate with the severity of the disease (Figure S1). Additionally, in an ex in vivo model, dsDNA stimulation induced a robust response in human placental villous explants (Figure 4b). To evaluate whether AIM2 and IFI16 are involved in production of cffDNA-induced antiangiogenic and pro-inflammatory factors, we suppressed AIM2 and IFI16 expression via transduction of AIM2- or IFI16-specific shRNA (Figure 5a,b). sEndoglin, sFlt-1 and CXCL10 secretion induced by poly(dA:dT) were significantly decreased in IFI16 knockdown cells, while IL-8, IL-6 and CCL2 production was inhibited in AIM2 knockdown cells (Figure 5c). Taken together, these results suggest that cffDNA induces the production of AIM2- or IFI16-dependent antiangiogenic factors and pro-inflammatory cytokines/chemokines in human trophoblast cells.
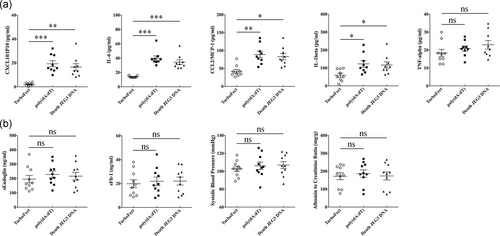
3.4 dsDNA exposure induces an inflammatory response in pregnant mice
There is substantial evidence that cffDNA is elevated before the clinical onset of PE (Levine et al., 2004). Therefore, we examined whether a cffDNA challenge could induce PE-like symptoms in pregnant mice. C57bl/6J dams were administered intraperitoneal injections of poly(dA:dT) or necrotic JEG3 DNA (300 μg/dams) once per day during gestation days 7.5–12.5. Notably, both types of DNA-induced secretion of the pro-inflammatory mediators IL-1β, IL-6, CCL2, and CXCL10 but did not induce TNF-α production (Figure 6a). However, no significant differences among groups were observed in regard to the sEndoglin and sFlt-1 concentrations, systolic blood pressure, and albumin to creatinine ratio (Figure 6b). These data indicated that elevated cffDNA induces enhancement of the inflammatory response but does not induce PE-like symptoms in pregnant mice.
4 DISCUSSION
The pathogenesis of PE starts early in pregnancy, with elevated cffDNA as a strong predisposing factor. However, the specific contribution of cffDNA is still under debate, as there is currently a lack of information regarding its mechanism of action. Trophoblasts express numerous Toll-like receptors (TLRs) and NOD-like receptors (NLRs), which are associated with preterm birth and PE (Cardenas et al., 2011; Koga, Izumi, Mor, Fujii, & Osuga, 2014; Scharfe-Nugent et al., 2012). Placental TLR3 and TLR7/8 activation contribute to PE in humans and mice (Chatterjee et al., 2012; Nakada et al., 2009). The NLRP3 inflammasome responds to crystalline cholesterol and uric acid, and induces IL-1β production during PE (Stodle et al., 2018). Accordingly, it is worth noting that increased expression of IFI16 (Li et al., 2015) and AIM2, which are cytosolic dsDNA sensors, may be associated with the recognition of and response to cffDNA.
In this study, we initially aimed to determine whether elevated cffDNA could be recognized by AIM2 and IFI16 and was sufficient to elicit pro-inflammatory and antiangiogenic responses. The major finding of this study is the upregulation of AIM2 and IFI16 activation and PE-associated factor production following exposure to dsDNA. Furthermore, knockdown of IFI16 inhibited DNA-induced sEndoglin, sFlt-1 and CXCL10 production, while IL-8, IL-6 and CCL2 secretion were dependent on AIM2. These findings demonstrated that elevated cffDNA induces pro-inflammatory and antiangiogenic factor production via IFI16 and AIM2 signaling.
The placenta is supposed to be, at least in part, a potential source of pro-inflammatory and antiangiogenic factors (Maynard & Karumanchi, 2011; Rusterholz, Hahn, & Holzgreve, 2007). Consistent with previous reports, we showed that PE patients had elevated concentrations of these factors compared with normal pregnancies and that these factors were associated with the severity of PE. sEndoglin and sFlt-1, which are antiangiogenic factors, impact the endothelium of the maternal systemic vasculature and prevent the remodeling of spiral arteries with reduced blood supply to the placenta (Caniggia, Grisaru-Gravnosky, Kuliszewsky, Post, & Lye, 1999; Levine et al., 2006). CXCL10 belongs to the CXC chemokine family and has pro-inflammatory and antiangiogenic properties. CXCL10 promotes the inflammatory response, including activation of T cells and migration of monocytes/macrophages and modulation of adhesion molecule expression (Taub et al., 1993). Angiolillo et al. (1995) demonstrated that CXCL10 inhibits the tube-like formation of human umbilical vein cells in a dose-dependent fashion. Recently, CXCL10 has been proposed to be a potential link between inflammation and antiangiogenesis in PE (Gotsch et al., 2007). IL-8 and CCL2 are known to be neutrophil, monocyte and macrophage chemoattractants. These cells can adhere to endothelial cells, invade sites of inflammation and release a variety of reactive oxygen species, enzymes and cytokines, leading to inflammation, endothelial cell damage and dysfunction, and oxidative stress in PE (Laskowska, Laskowska, Leszczynska-Gorzelak, & Oleszczuk, 2007; Szarka, Rigo, Lazar, Beko, & Molvarec, 2010; Yang et al., 2016). IL-6 is a classic multifunctional pro-inflammatory cytokine that regulates the immune response, acute phase reactions, hematopoiesis and endothelial dysfunction (Erzen, Sabovic, Sebestjen, Keber, & Poredos, 2007; Robertson, Seamark, Guilbert, & Wegmann, 1994; Saito, 2000); in fact, several studies have shown that IL-6 is involved in trophoblast invasion and proliferation, oxidative stress and hypertension, which play important roles in the pathogenesis and the severity of PE (Lockwood et al., 2008; Xiao et al., 2012). Here, we found that elevated cffDNA was a novel inducer of pro-inflammatory and antiangiogenic factors in trophoblast cells, suggesting that the cffDNA-AIM2/IFI16 axis may be particularly important in the development of PE.
Elevated circulating cffDNA occurs before the onset of PE (Zhong, Holzgreve, & Hahn, 2002), which could be ascribed to placental hypoxia/ischemia and apoptosis and/or necrosis of trophoblast cells (Ishihara et al., 2002; Martin et al., 2014). To date, the role of increased cffDNA in the etiology of PE has not been determined. In this study, we characterized the effects of cffDNA in pregnant mice. Notably, both poly(dA:dT) and necrotic JEG3 DNA induced the production of pro-inflammatory and antiangiogenic factors. However, different types of DNA were not able to induce PE-like symptoms. Conka, Konecna, Laukova, Vlkova, and Celec (2017) found that intraperitoneally injected human fetal DNA, mouse fetal DNA or mouse adult DNA did not induce PE-like symptoms in mice. Additionally, TLR3 activation during pregnancy causes mild PE, which is exacerbated by the absence of IL-10 or IL-4 (Chatterjee et al., 2011; Chatterjee et al., 2013). Therefore, it is possible that cffDNA-mediated AIM2 and IFI16 activation cannot directly induce PE-like symptoms without additional impairments of the immune system. Alternatively, it is possible that the increased cffDNA in women with PE may reflect placental damage and trophoblast apoptosis/necrosis. Elevated cffDNA triggers AIM2 and IFI16 activation, which induce programmed cell death (Chu et al., 2014), inflammatory responses and antiangiogenic mediator production. Subsequently, the increased amounts of placental-derived molecules are shed into maternal circulation, consistent with the observation that increasing concentrations of cffDNA, pro-inflammatory cytokines/chemokines and antiangiogenic factors correspond to the degree of disease severity. However, whether elevated cffDNA and the subsequent inappropriate inflammatory responses are a cause or a consequence of PE remains to be determined.
In conclusion, we describe a disturbance in the systemic and local immune balance during pre-eclampsia and suggest that elevated cffDNA can induce activation of placental AIM2 and IFI16 signaling, consequently contributing to the production of antiangiogenic and pro-inflammatory factors, which exacerbate the severity of pre-eclampsia symptoms and may be potential therapeutic targets; however, prospective studies must be conducted to determine the precise mechanisms.
ACKNOWLEDGMENTS
The study was supported by the National Natural Science Foundation of China (81700683), the Natural Science Foundation of Guangdong Province (2017A030310646 and 2016A020215207), and the Science and Technology Project of Shenzhen (JCYJ20180228164515747).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




