Novel factor Xa inhibitor, maslinic acid, with antiplatelet aggregation activity
Kyung-Min Kim and Jaehong Kim contributed equally to this study.
Abstract
As antithrombotic effects of maslinic acid (MA) have not yet been studied, MA-mediated downregulation of coagulation factor Xa (FXa) and platelet aggregation was studied. We show that MA inhibited the enzymatic activity of FXa and platelet aggregation, induced by adenosine diphosphate (ADP) and a thromboxane A2 (TXA2) analog, U46619 with a similar antithrombotic efficacy to rivaroxaban, a direct FXa inhibitor used as a positive control. Mechanistically, MA suppressed U46619- or ADP-induced phosphorylation of myristoylated alanine-rich C kinase substrate, and the expression of P-selectin, and activated PAC-1 in platelets. MA increased generation of nitric oxide, but downregulated excessive secretion of endothelin-1 in ADP- or U46619-treated human umbilical vein endothelial cells. In arterial and pulmonary thrombosis mouse model, MA showed prominent anticoagulant and antithrombotic effects. Our data suggest MA as a candidate molecule for a new class of drugs targeting anti-FXa and antiplatelet.
1 INTRODUCTION
Thrombosis is a leading cause of death worldwide and closely related to a series of cascades including adhesion, aggregation, and secretory functions of activated platelet, and activation of intrinsic and extrinsic coagulation systems, all of which are responsible for blood coagulation and fibrin formation (Gong, Qin, & Huang, 2011). The pivotal role that thrombin plays in thrombosis has been demonstrated and, therefore, development of inhibitors directly targeting thrombin has been the focus of early drug development efforts targeting thrombosis (Ostrem et al., 1998). Nonetheless, clinical trial studies showed that the steady production of thrombin from prothrombin was not hampered by direct thrombin inhibitors (Philippides & Loscalzo, 1996) and inhibition of high level of thrombin in vivo can lead to adverse effects such as undesirable anticoagulation events and increased complications from hemorrhage (Hahn & Bae, 2019). Therefore, inhibitory regulation of selective coagulation factors, upstream of thrombin is speculated important to reduce the bleeding risk. Because blood coagulation factor Xa (FXa) plays such an important role at the converging step between the extrinsic and intrinsic coagulation pathways (Gomez-Outes, Garcia-Fuentes, & Suarez-Gea, 2017), it has recently become an attractive molecular target for a new class of anticoagulants (Bauer, 2006). Additionally, it has been speculated that FXa inhibition may suppress the thrombin production, while keeping its basal activity required for primary hemostasis (Bauer, 2006).
Notably, platelet aggregation is also critically involved in a variety of thromboembolic diseases. Platelets are essential in hemostasis and also in the initiation and propagation steps of thrombus formation (Ruggeri, 2002). From large-scale randomized trials, it has been shown that inhibition of platelet functions improves patient outcomes in disease conditions including acute coronary syndromes, subsequent percutaneous revascularization procedures, and upper gastrointestinal bleeding. However, because of bleeding side effects and resistance problems, development of a novel class of antiplatelet agents is still required (Andre, 2004; Johansen, 2006).
Maslinic acid (MA, 2-α,3-β-dihydroxyolean-12-en-28-oic acid, Figure 1) is a compound of nature-derived triterpenoid from olive, known by the botanical name, Olea europaea: European olive and also in a variety of medicinal plants (Lozano-Mena, Sanchez-Gonzalez, Juan, & Planas, 2014; Reyes-Zurita, Rufino-Palomares, Lupianez, & Cascante, 2009). MA has been shown to have anti-inflammatory (Huang et al., 2011), antioxidant (Montilla et al., 2003), antimalarial (Moneriz, Mestres, Bautista, Diez, & Puyet, 2011), and antiprotozoan (De Pablos et al., 2010) activities. However, the antithrombotic effects of MA involving FXa and platelet have not yet been reported. Our goal of this study was to identify the antithrombotic activities of MA via analysis of FXa activity, blood clotting time, and platelet functions. We further characterized antithrombotic activities of MA in mouse models. To the extent of our knowledge, we report that MA has antithrombotic effects from downregulation of the functions of FXa and platelet for the first time.
2 MATERIALS AND METHODS
2.1 Cell culture and reagents
Human umbilical vein endothelial cells (HUVECs) were obtained from Cambrex Bio Science (Charles City, IA) and maintained as described previously (J. E. Kim et al., 2019; I. C. Lee & Bae, 2019; B. S. Lee, Lee, Yang, Ku, & Bae, 2019) and HUVECs at passages 3–5 were used in our studies. Tumor necrosis factor-α (TNF-α) and rivaroxaban (Riva) were from Abnova (Taipei, Taiwan) and Bayer HealthCare (Leverkusen, Germany), respectively. MA and collagen were obtained from Sigma-Aldrich (St. Louis, MO). U46619, was obtained from Calbiochem-Novabiochem Corp. (San Diego, CA). All of plasmin, FVIIa, FX, FXa, activated protein C, tPA, trypsin, and thrombin were obtained from Hematologic Technologies (Essex Junction, VT). For activated partial thromboplastin time (aPTT) and prothrombin time (PT) assays, reagents were obtained from Fisher Diagnostics (Middletown, VA). All of the chromogenic substrates (S-2222 for trypsin, S-2228 for tPA, S2238 for thrombin, S-2251 for plasmin, S-2366 for activated protein C, and S-2765 for FXa) were obtained from Chromogenix AB (Mölndal, Sweden). Anti-CD61-fluorescein isothiocyanate (FITC), anti-CD62PPE, anti-PAC-1, and anti-CD61-PE were from BD Pharmingen (BD Biosciences, San Diego, CA) and antitissue factor (TF) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
2.2 Animal care
Six- or 7-week-old male C57BL/6 mice (mean weight: 27 g) were obtained from Orient Bio Co. (Sungnam, Republic of Korea) and an acclimatization period of 12 days was given, as described (Jang, Kang, Mukherjee, & Yun, 2018; Y. Lee, Jeong, et al., 2018). The mice were handled for the studies in accordance with the Guidelines for the Care and Use of Laboratory Animals, approved by Kyungpook National University (Institutional Review Board [IRB] No. KNU 2017-101).
2.3 Preparation of human plasma and platelets
Human blood samples were collected from 10 fasting healthy volunteers (aged 24–28 years, six women and four men) without illness such as allergy, cardiovascular disorders, and carbohydrate or lipid metabolic disorders, and not undergone any drug treatment in the morning time. Our subjects did not take antioxidant food supplementation or addictive substances, and their diet was balanced. Blood samples were collected in sodium citrate (10.9 mM) and immediately spun down (1,300g for 15 min) to separate the plasma, which was pooled for further studies. Next, human platelets were prepared, as previously described (W. Lee, Ku, & Bae, 2015). In brief, platelet-rich plasma (PRP) was separated via spinning down blood samples at room temperature. Concentration of PRP was adjusted to 1 × 109 platelets/ml using a hemocytometer. The PRP pellet was washed once in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer with 1 mM CaCl2 and left at room temperature for additional 30 min. Then, for measurement of each clotting time point, 10 ml of the platelet samples were used. This study protocol (KNUH 2012-01-010) was approved by the IRB of Kyungpook National University Hospital (Daegu, Republic of Korea).
2.4 In vitro coagulation assay
One hour after administering dimethyl sulfoxide (DMSO) or MA, blood was withdrawn, and the plasma was obtained by centrifugation (2,000g for 10 min) at room temperature to measure the PT and aPTT using a thrombotimer (Behnk Elektronik, Norderstedt, Germany; T. H. Kim, Ku, & Bae, 2012). For the aPTT assay, micronized silica was used as an activator. In brief, 100 µl of platelet-poor plasma (PPP) was mixed with 100 μl of aPTT assay reagent for 1 min at 37°C, 100 µl of CaCl2 (20 mM) was added and the clotting times were recorded. For the PT assays, 200 μl of PT assay reagent was incubated for 10 min at 37°C, mixed with 100 μl of PPP for 3 min at 37°C and the clotting time was recorded.
2.5 In vitro platelet aggregation assay
Human PRP was incubated with MA dissolved in DMSO for the indicated time (1, 3, 5, or 10 min). Next, PRP was stimulated from addition of collagen (1 μg/ml), ADP (10 µM), U46619 (6 µM), or thrombin (3 U/ml) and an aggregometer (Chronolog, Havertown, PA) was used to analyze the degree of platelet aggregation.
2.6 Inhibition of FXa amidolytic activity
2.7 Inhibitory constant for FXa
Ki values were calculated from chromogenic substrate assays. In each assay, MA was used at a minimum of seven concentrations in duplicate to acquire an inhibition curve. Twenty-five microliters of each compound solution or inhibitor solution or blank buffer was mixed with 50 µl of the substrate. 25 μl of the enzyme solution was mixed just before when the plate was placed in the Labsystems IEMS microtiter plate reader (Cergy Pontoise, France) and left for 1 hr at 37°C before analysis. We spectrophotometrically at 405 nm monitored continuous production of p-nitroaniline yielded from hydrolysis of the substrate. The maximal initial reaction rates were calculated and expressed as millioptical density per minute. To calculate the Ki values, linear regression analysis was done for curve fitting (Dixon plot of 1/Vmax vs. inhibitor concentration).
2.8 Production of FXa on surface of HUVECs
Confluent HUVEC monolayers formed on 96-well culture plates were incubated with the indicated concentration of MA for 10 min and stimulated with TNF-α (10 ng/ml) in a serum-free medium for 6 hr. Then, the cells were incubated with FVIIa (10 nM) in buffer A (10 mM HEPES buffer [pH 7.45] with 11 mM glucose, 150 mM NaCl, and 4 mM KCl) supplemeted with 5 mM CaCl2 and 5 mg/ml bovine serum albumin for 5 min at 37°C with/without anti-TF immunoglobulin G (IgG; 25 µg/ml). FX was subsequently added to the mixture in a final reaction mixture volume of 100 µl and incubated for 15 min. The reaction was stopped by the addition of buffer A supplemented with 10 mM ethylenediaminetetraacetic acid, and the amount of FXa generated was measured with addition of S-2765. Changes in absorbance over 2 min were monitored spectrophotometrically at 405 nm. From a standard curve constructed with known concentrations of purified human FXa, initial rates of color development in the reactions were converted into FXa concentrations.
2.9 Cell viability assay
To measure cell viability, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used (W. Lee, Ku, et al., 2019). At a density of 5 × 103 cells/well, the cells were grown in 96-well plates. After 1 day, the cells were rinsed with fresh medium treated with MA. After 48-hr incubation, the cells were rinsed, 100 μl of MTT (1 mg/ml) was added, and incubated for 4 hr. Finally, for the purpose of solubilizing the formazan salt formed in the reaction, 150 μl of DMSO was added, and the amount of formazan salt formed was determined spectrophotometrically at 540 nm.
2.10 Western blot analysis
Same amounts of protein, from HUVEC extracts, were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrotransferred onto an Immobilon membrane (Millipore, Billerica, MA). The membranes were blocked and subsequently incubated with anti-phospho-myristoylated alanine-rich C kinase substrates (MARCKS) (Santa Cruz Biotechnology) for 1.5 hr at room temperature, and incubated with horseradish peroxidase-conjugated secondary antibody and ECL detected. We used anti-β-actin (1:1,000; Santa Cruz Biotechnology) as a loading control.
2.11 Measurement of intracellular Ca2+ mobilization
The level of intracellular Ca2+ ([Ca2+]i) in the platelets was analyzed as described previously (Wu, Wu, Wang, & Wu, 2007). In brief, platelets were loaded with Fura 2-AM (3 μM) at 37°C for 30 min, washed twice, suspended at a final concentration of 5 × 107 platelets/ml in Tyrode's solution without Ca2+. One minute before stimulation with platelet activators, 1 mM calcium was added to the suspension of platelets. The [Ca2+]i was calculated with an equation described by Grynkiewicz, Poenie, and Tsien (1985) by measuring the intensities of fluorescence signals (excitation/emission wavelengths: 339/500 nm) acquired from the platelets.
2.12 Measurement of PAC-1 and P-selectin expression
The expression levels of PAC-1 and P-selectin were measured using modifications of established methods (Frojmovic, Wong, & van de Ven, 1991). In brief, 0.5 ml of washed platelets (2 × 108 platelets/ml) were incubated with 20 μl of DMSO or MA (10–20 μM) for 3 min. After stimulation with ADP (10 μM) or U46619 (6 μM) for 6 min at 37°C, the platelets suspension was chilled at 4°C. For the determination of PAC-1 expression, activation-specific anti-PAC-1 and anti-CD61-PE in saturated concentrations were mixed with 50 μl of the sample, and then incubated for 25 min in the dark. For the determination of P-selectin expression, anti-CD62P-PE and anti-CD61-FITC in saturated concentrations were mixed with 50 μl of the sample, and then incubated for 25 min in the dark. Quench-diluted (<4-fold) samples were analyzed with a flow cytometer (BD Biosciences). Populations of platelets were gated on cell size with forward scatter versus side scatter and CD61 positivity to differentiate them from the noise and 5,000 events were analyzed per each sample. Fluorescence intensities from each channel were expressed as a mean value using BD Accuri C6 Software (BD Biosciences). Measurements were performed with samples from five different donors.
2.13 Quantification of nitrogen monoxide (NO) and endothelin-1 (ET-1)
The production of NO and ET-1 (R&D Systems, Minneapolis, MN) in the cell culture medium were quantified using a commercially available enzyme-linked immunosorbent assay kit.
2.14 Arterial thrombosis animal model
We established and used a FeCl3-induced thrombosis mouse model. Male C57BL/6 mice were fasted overnight, i.v. injections of indicated compound in 0.2 ml DMSO were given and anesthetized. Carotid artery of the animal was exposed, and a cotton thread (200 µm in diameter) saturated with FeCl3 solution (0.25 M) was applied to the adventitial surface for 5 min. After removal of the thread, saline solution was used to flush the wound. Formation of thrombus was monitored at 35°C with three-dimensional imaging. The rate and size of formation of thrombus were monitored, and we scored our findings as follows: 0, no thrombus; 1, small thrombus (75 × 50 µm length); 2, medium-sized thrombus (150 × 100 µm length); 3, large thrombus (300 × 200 µm length); and 4, >3 thrombi. The time taken from the endothelial injury with FeCl3 to occlusion of the carotid artery by a large thrombus was also recorded.
2.15 Acute pulmonary thrombosis induced by combined treatment of collagen and epinephrine in animal model
Mice were fasted overnight and divided into separate groups (10 animals each). MA was dissolved in DMSO and i.v. administered to each mouse and a mixture of collagen (500 µg/kg) and epinephrine (50 µg/kg) was injected into the tail vein of mice 1 hr after, to induce acute thrombosis as described previously (DiMinno & Silver, 1983; K. Kim, Bae, et al., 2012). Individual mouse was carefully examined for 15 min, to determine whether each mouse recovered from the acute thrombosis challenge or was paralyzed, or dead. To assess the degree of pulmonary thrombus formation, histologic analysis was performed on haematoxylin and eosin stained sections of lung tissues from five mice chosen at random. Five view fields were randomly chosen from within the left lobe in each section (one section per mouse), and the number of vessels larger than 20 μm in diameter with any detectable thrombus in each field were recorded. Thus, 25 fields for each group were examined in comparison. In each section, a total 60–80 vessels were examined with an imaging system (Leica, Germany). The findings were scored as number of thrombus per 25 mm2 lung field with some modifications according to the previous report (Satoh et al., 2017).
2.16 Statistical analysis
The data are shown as mean ± standard deviation (SD) value of five independent experiments performed in duplicate. When a one-way analysis of variance indicated a significant difference between different groups, Tukey's tests were performed as post-hoc analysis of the differences between the individual groups. p < .05 was regarded as statistically significant.
3 RESULTS
3.1 Effect of MA on clotting time in vitro and ex vivo
First, changes in the coagulation parameters in vitro, such as aPTT and PT, induced by MA were measured. We used rivaroxaban, a direct FXa inhibitor, as a positive control for our assays. MA significantly prolonged aPTT at doses ranging from 5 to 20 µM (Figure 2a). Rivaroxaban at 10.19 μM and MA at 7.54 μM, respectively, doubled the clotting time in our in vitro aPTT assays.
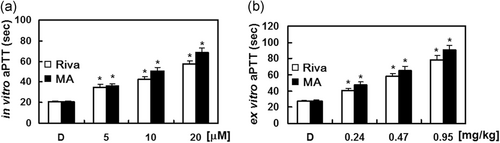
To validate the in vitro results in an ex vivo assay, i.v. injections of MA for 4 consecutive days were given to each mouse group (n = 5). Our results show that blood clotting time was significantly prolonged, in a concentration-dependent manner, by MA treatment (Figure 2b). Clotting time was doubled by treatment of MA at 7.89 μM and rivaroxaban at 9.96 μM, respectively, in ex vivo aPTT assays. On the basis of an estimated circulating blood volume of 72 ml/kg for mice (W. Lee, Lee, et al., 2018; W. Lee, Park, et al., 2018) and an average weight of 27 g for each mouse used in this study, the average blood volume was calculated to be 2 ml. Thus, each injection of MA (0.24, 0.47, or 0.95 µg/kg) yielded an estimated concentration of up to 5, 10, or 20 µM in the peripheral blood, respectively. Notably, we found that the PT was not changed in mice administrated with MA or rivaroxaban (data not shown).
3.2 Effect of MA on platelet aggregation in vitro
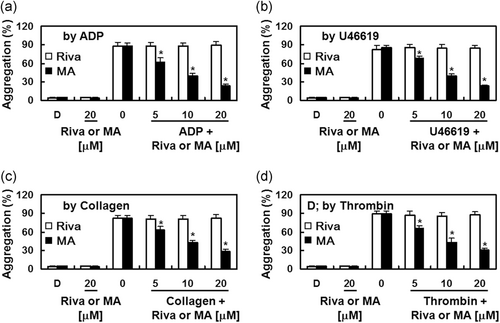
To determine whether MA inhibits platelet aggregation, agonist-induced platelet aggregation was analyzed with human PRP. While rivaroxaban did not affect the degree of platelet aggregation induced by various agonists (Jourdi et al., 2019; W. Lee, Ku, Kim, & Bae, 2017; Perzborn et al., 2010), MA significantly suppressed platelet aggregation induced by ADP (10 µM), collagen (1 μg/ml), thrombin (3 U/ml), and U46619 (6 µM) in a concentration-dependent manner (Figure 3).
3.3 Effects of MA on animal models of arterial and pulmonary thrombosis
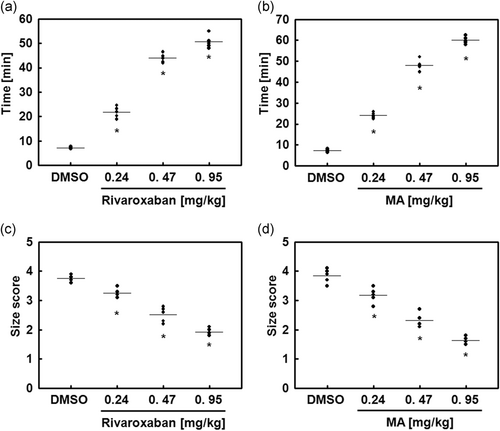
To analyze MA-mediated antithrombotic effects in vivo, we used the animal model of FeCl3-induced carotid artery thrombosis (Izuhara et al., 2008). The MA-mediated effects on the time and size of thrombus formation by FeCl3 are shown in Figure 4. FeCl3-induced endothelial injury resulted in the accelerated growth of large thrombi, which was significantly reduced with MA treatment. Additionally, the in vivo pulmonary thrombosis model, injected with a mixture of epinephrine and collagen, showed very serious pulmonary thrombosis with acute paralysis. MA treatment significantly reduced mortality and the scored thrombus formation in the mouse lung tissues than treatment of a mixture of collagen and epinephrine only (Figures 4 and 5, and Table 1).
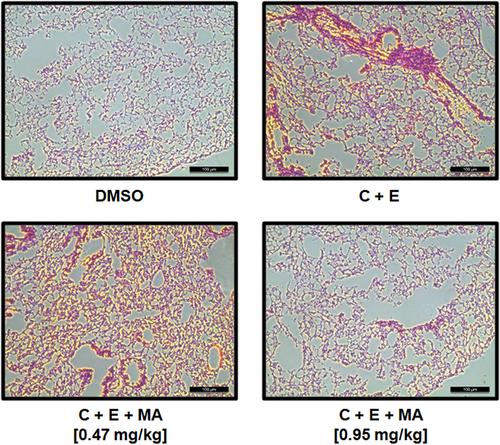
| In vivo pulmonary thrombosis model (mortality % and scored thrombus formation, n = 20) | ||||
|---|---|---|---|---|
| μM | Rivaroxaban | MA | ||
| % | Thrombi | % | Thrombi | |
| DMSO | 0 | 0 | 0 | 0 |
| C + E | 95 | 3.8 ± 0.2 | 95 | 3.9 ± 0.3 |
| 0.24 | 80 | 2.7 ± 0.1* | 70* | 2.4 ± 0.1* |
| 0.47 | 60 | 1.9 ± 0.1* | 60* | 1.7 ± 0.2* |
| 0.95 | 45* | 1.6 ± 0.2* | 40* | 1.4 ± 0.2* |
- Note: All inhibitors were dissolved in 0.2% dimethyl sulfoxide (DMSO).
- Abbreviations: DMSO, dimethyl sulfoxide; MA, maslinic acid; SD, standard deviation.
- a Values represent the means ± SD (n = 20).
- * p < 0.05 compared with C + E (collagen and epinephrine).
3.4 Effects of MA on catalytic activity and production of FXa in vitro
To investigate the mechanisms of MA-mediated regulation of coagulation and platelet aggregation, the effects of MA on the activity and production of FXa were determined. MA, in a concentration-dependent manner, suppressed the catalytic activity of FXa on S-2222 substrate. The Michaelis–Menten plots obtained for MA show a slope of 0.456 for the control without MA and slopes of 0.529 and 0.776 for MA at 10 and 20 µM, respectively (Figure 6a). The Lineweaver–Burk plots in Figure 6a (inset) show inhibition of FXa by MA, indicating a mixed type of inhibitory pattern. MA induced a decrease in the Vmax, with a concomitant increase in the Km value of FXa on S-2222. We determined that the Ki value of MA-mediated inhibition of FXa activity toward S-2222 was 3.90 µM (Table 2). Table 2 also shows a comparison of selectivity of MA for FXa with other representative human enzymes in coagulation cascade. We found that with a selectivity ratio (based on their respective Ki values) of >300 for FXa in comparison with other enzyme tested, MA is highly selective for FXa. Because in TNF-α-stimulated HUVECs, the activation of FX by FVIIa is dependent on T) expression (Hahn & Bae, 2019; W. Lee et al., 2017). We also characterized the effects of MA on the FVIIa-mediated activation of FX. TNF-α stimulation, given to HUVECs to induce TF expression, resulted in a 13.2-fold increase in the activation rate of FX by FVIIa (111.1 ± 9.1 nM) over that of the nonstimulated HUVECs (8.4 ± 0.9 nM; Figure 6b). Treatment of anti-TF IgG specifically abrogated the FX activation (19.2 ± 1.9 nM, Figure 6b). Our data indicate that pre-incubation with MA, concentration-dependently suppressed the FX activation (Figure 6b). Very recently, Petzold et al. (2020) showed that FXa could trigger platelet aggregation. Therefore, we determined the role of MA on FXa-mediated platelet aggregation. As shown in Figure 6c, MA significantly suppressed platelet aggregation induced by FXa.
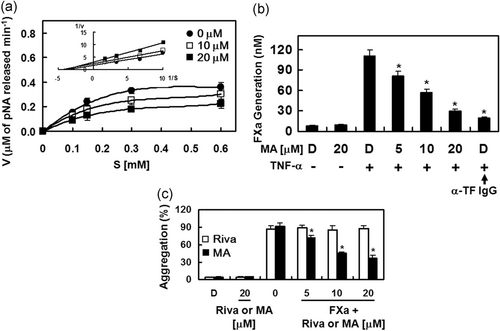
| MA | ||
|---|---|---|
| Enzyme | Kia | Ratiob |
| Factor Xa | 3.90 ± 0.15 | 1 |
| α-Thrombin | >300 | >100 |
| Trypsin | >300 | >100 |
| Plasmin | >300 | >100 |
| Protein Ca | >300 | >100 |
| tPA | >300 | >100 |
- Abbreviations: MA, maslinic acid; SD, standard deviation; tPA, tissue plasminogen activator.
- a Ki is represented by the mean ± SD (n = 5), μM.
- b Ratio = Ki enzyme/Ki factor Xa.
3.5 Effects of MA on activation of protein kinase C (PKC) and mobilization of intracellular calcium
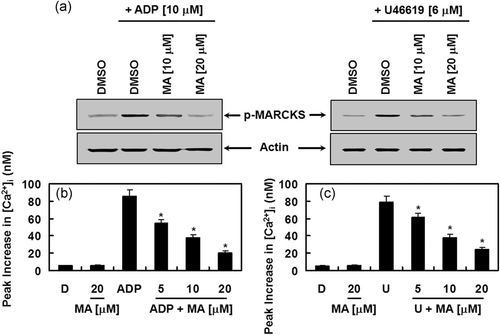
Next, the molecular mechanism by which MA regulated platelet aggregation was determined. Because both activation of PKC and increase in cytosolic Ca2 can induce platelet aggregation, we investigated the effects of MA on PKC activation by analyzing the phosphorylation level of MARCKS, which is a major phosphorylation substrate of PKC in human platelets (Elzagallaai, Rose, & Trifaro, 2000). Indeed, MA treatment inhibited ADP- and U46619-induced phosphorylation of MARCKS, indicating that PKC was inhibited from the MA treatment (Figure 7a). It is also well known that, upon activation of platelets with agonists, phosphatidylinositol 4,5-bisphosphate is hydrolyzed by phospholipase C (PLC) to inositol-1,4,5-triphosphate and diacylglycerol, which results in an increase in cytosolic Ca2+ and the activation of PKC, respectively (Wei, Schoenwaelder, Andrews, & Jackson, 2009). Synergistically, Ca2+ and PKC induce the granule secretion and activation of glycoprotein PAC-1 (GPIIb/IIIa) that functions as the final receptor for platelet aggregation (Wei et al., 2009). MA treatment suppressed ADP- and U46619-induced increases in [Ca2+]i (Figure 7b,c). Our data indicate that MA downregulated platelet aggregation via its suppression of PKC activation and [Ca2+]i.
3.6 Effects of MA on expression of P-selectin and PAC-1
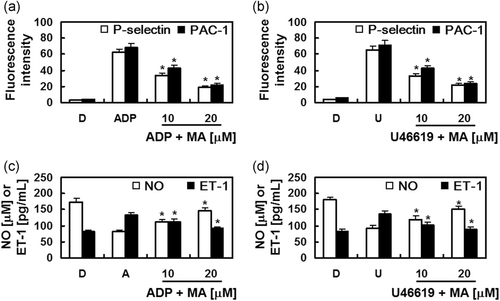
When platelets are activated, P-selectin is translocated to the external membrane and fibrinogen bridges PAC-1 between abutting platelets to induce aggregation (Merten & Thiagarajan, 2000). As shown in Figure 8a,b, the expressions of P-selectin and PAC-1 in platelets, induced by the ADP or U46619 was decreased by MA. Our data indicate that MA suppresses platelet aggregation by reducing the expressions of P-selectin and PAC-1 in platelets.
3.7 Effects of MA on NO and ET-1
NO, an important endogenous vasodilator, can protect the vascular wall by inhibiting platelet aggregation. NO is secreted by the vascular endothelium and ET-1, a potent vasoconstrictor, and produced from the vascular endothelium (Bredt & Snyder, 1994; d'Uscio, Barton, Shaw, & Luscher, 2000; Yanagisawa et al., 1988). Noting that adequate regulation of vasomotion from the controlled production of NO and ET-1 enables adequate maintenance of vascular homeostasis (Schmitz-Spanke & Schipke, 2000; Verhamme & Hoylaerts, 2006), the effects of MA on the levels of NO and ET-1 were determined to further provide a mechanistic understanding of the MA-mediated inhibition of platelet aggregation. Data showed that the increased production of ET-1 and the decreased secretion of NO, induced by ADP or U46619 were restored to baseline level with MA treatment (Figure 8c,d).
4 DISCUSSION
Many drugs targeting platelet aggregation are now available and frequently used to treat thrombotic disorders, either solely or in combination with other platelet inhibitor drugs. However, side effects from their prolonged use are frequently reported (Eikelboom, Hirsh, Spencer, Baglin, & Weitz, 2012; Ferraris, Ferraris, & Saha, 2011). Our finding that MA effectively suppressed ADP-, U46619-, collagen-, or thrombin-induced platelet aggregation suggest a novel potential of MA for the development of clinically acceptable drugs with fewer adverse effects. Also, it is expected that MA (with molecular weight of 472.7), smaller than other FXa inhibitors, provides substantial advantages including low production costs and low immunogenicity over larger anticoagulant molecules.
FXa shows a crucial role in the coagulation cascade (Dahlback, 2000). Noting that inhibitors directly targeting thrombin showed a very narrow therapeutic index, our hypothesis was that selective inhibition of FXa may facilitate a potentially promising strategy for new antithrombotic agents, especially considering the position of FXa at the converging step between the extrinsic and intrinsic coagulation pathways (Bauer, 2006; Loffredo, Perri, & Violi, 2015).
For determination of the efficacy of novel anti-thrombotic drugs, platelet aggregation and clotting time are the most commonly used parameters (Jauch et al., 2013). In our experiments, the anticoagulant effects of the MA were validated with aPTT and PT assays to evaluate its effects on the intrinsic and extrinsic coagulation pathways, respectively. We observed that MA treatment prolonged the aPTTs in mice than vehicle treatment and that MA treatment did not apparently increase PT. Currently, it is not known why MA treatment had an effect on aPTT, but not on PT. It is assumed that greater disruption of hemostasis with MA at anti-thrombotic doses or MA inhibition of intrinsic coagulation pathway-specific factors (factor XIa, XIIa, or VIIIa as a cofactor for FXa), but not the extrinsic coagulation pathway-specific factor VIIa was responsible. MA treatment also dose-dependently prolonged the ex vivo aPTT. MA also functions as a selective inhibitor of platelet aggregation, as shown from its inhibition of platelet aggregation induced by ADP, collagen, U46619, or thrombin treatment.
We showed that MA exhibited an anti-thrombotic activity as potent as that of rivaroxaban, in terms of inhibition of both the production and activity of FXa, increased blood clotting time, and delayed thrombogenesis and thrombogenic time. While rivaroxaban did not show inhibition of platelet aggregation induced by ADP or U46619, MA concentration-dependently inhibited the platelet aggregation. Our mechanistic studies revealed their antiplatelet functions mediated by the activation of PKC, intracellular Ca2+ mobilization, and the expression levels of P-selectin and PAC-1. Therefore, MA has antiplatelet potential by inhibition of a) activation and production of FXa, (b) cytosolic Ca2+ mobilization, (c) phosphorylation of MARCKS through the PKC pathway, (d) platelet aggregation, (e) coagulation times, and finally, (f) expression of P-selectin and PAC-1. In addition, improvement in the secretion of the vasodilator, NO, and inhibited production of the vasoconstrictor, ET-1 by MA were also observed. The coagulation event occurs by a mechanism that involves all of the activation, adhesion, and aggregation steps of platelets (Dahlback, 2000; Sim, Flaumenhaft, & Furie, 2005). Damages of the endothelial lining from an injury often initiate coagulation. Alterations in platelets and the exposure of subendothelial TF to FVII, which ultimately result in fibrin formation are initiated by blood exposure to the subendothelial space of blood vessels (Dahlback, 2000; Sim et al., 2005). Then, the expansion of the thrombus triggers the recruitment of additional platelets and amplification of the coagulation cascade by the activated intrinsic pathway, which includes FVIII, FIX, and the hemophilia factors (Dahlback, 2000). Platelets and endothelial cells provide thrombogenic surface, which is a significant step for amplification of the coagulation cascade. Therefore, the activation and altered behavior of platelets are the mechanistic targets of both pathways of blood coagulation and platelet aggregation, for the most important functions of the antithrombotic and antiplatelet effects.
Very recently, Petzold et al. (2020) showed that rivaroxaban reduced platelet aggregation by using blood obtained from patients under rivaroxaban treatment. Previous studies consistently have shown that rivaroxaban has been found not to affect platelet aggregation induced by collagen, ADP, thromboxane A2, or thrombin (Jourdi et al., 2019; W. Lee et al., 2017; Olivier et al., 2016; Perzborn et al., 2010; Perzborn, Heitmeier, & Laux, 2015), which was confirmed by our current study. One possible explanation for the difference is the source of blood sample. Clinical characteristics of blood sample in Petzold's study were from cardiovascular diseases such as thrombosis, hypertenstion, or diabetes. Once rivaroxaban is absorbed in the vascular blood system, it could interact with other medications, which may have resulted in the alternation of its original properties. And then, altered rivaroxaban might affect platelet aggregation by activating or inhibiting unknown platelet aggregation signaling pathways. Petzold et al. also identified FXa as potent platelet agonist, resulting in the platelet aggregation. In this study, it is shown that MA could suppress FXa-mediated platelet aggregation, indicating that MA exerts an antiplatelet effect through FXa-mediated signaling cascade.
In summary, we demonstrated that MA is a novel anti-FXa and antiplatelet compound from its inhibitory effect on intrinsic blood coagulation pathways. Our results may contribute to a novel design of treatment and prevention strategies for coagulation-related thrombotic disorders.
ACKNOWLEDGMENTS
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2020R1A2C1004131 and 2017M3A9G8083382) and also by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI15C0001).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




