Evaluating the mechanism underlying antitumor effect of interleukin 27 on B cells of chronic lymphocytic leukemia patients
Abstract
Chronic lymphocyte leukemia (CLL) is a B-cell malignancy resisted to apoptosis. Recently, some studies indicated that cytokines such as interleukin 27 (IL-27) can reduce B-cell proliferation. The aim of this study is to evaluate the mechanism underlying the proapoptotic effect of IL-27 on B cells of patients with CLL in comparison with B cells of normal subjects. The effect of IL-27 on the antitumor activity of natural killer (NK) and T cells was also evaluated. Peripheral blood mononuclear cells (PBMCs) were isolated from 35 patients with CLL and 15 normal subjects. B cells and PBMCs were cocultured with IL-27 and B cells apoptosis to evaluate proliferation. Both messenger RNA and protein expression of IL-27 and IL-27 receptor were determined using flow cytometry and real-time polymerase chain reaction analysis. To evaluate the apoptotic effect of IL-27 on B cells of patients with CLL, Annexin V-FITC and 7-AAD (BioLegend) fluorescent dyes were used. In addition, the IL-27 effect on activation of T cell and NK cell was determined by determining CD96 molecule expression. IL-27 and IL-27 receptor expression in patients with CLL was significantly lower than that of normal subjects (p < .05). IL-27 enhanced apoptosis of B cells in patients with CLL (p < .05) but this effect was not significantly observed in B cells of normal subjects (p > .05). Consequently, IL-27 reduced the proliferation of B cells and enhanced NK cell activity (p < .05). IL-27, through inducing apoptosis, can exert an inhibitory effect on cancer B cells of CLL patients with minimal effect on normal B cells.
1 INTRODUCTION
B chronic lymphocytic leukemia (CLL) is characterized by the expansion of B cells expressing CD5, CD19, and CD23, as well as low levels of surface immunoglobulins (IgM and IgD), CD21 and CD22, and CD79b, which accumulate in bone marrow, peripheral blood, and other lymphoid organs (Jurisic, Colovic, Kraguljac, Atkinson, & Colovic, 2008; Karan-Djurasevic et al., 2013; Kokhaei, Palma, Mellstedt, & Choudhury, 2005). CLL stems from the defect in intrinsic apoptosis pathways in cancer cells and numerous amount of survival signals such as chemokines and cytokines exist in their environment that enhance nuclear factor-κB and phosphoinositide 3-kinase/protein kinase B signals leading to overexpression of antiapoptotic proteins such as Bcl-2 family members (Billard, 2014).
Interleukin 27 (IL-27) is a heterodimeric cytokine which consists of two subunits, EBI3 and P-28 (Pflanz et al., 2002). It is a member of the IL-12 family and shows a similar structure and function with IL-12 cytokine. Coexpression of two subunits is necessary for the efficient physiological function of IL-27 (Pflanz et al., 2002). Antigen-presenting cells (APCs) like dendritic cells, trophoblast, and monocytes are the main source of IL-27 in human and mice (Larousserie et al., 2004; Pflanz et al., 2002). Recent studies have defined an antitumor role for IL-27 in several cancers, especially hematological malignancies (Cocco et al., 2010). Although IL-27 exerts distinct pro- and anti-inflammatory effects on different cell types, including macrophages, dendritic cells, B cells, and T cells, its apoptotic effect on tumor cells has been found to be dominant in in vivo studies (Fabbi, Carbotti, & Ferrini, 2017; Yoshimoto et al., 2008). Importantly, IL-27 can also lead to the differentiation of naïve T cells to T helper 1 (Th1) cells (Chen et al., 2000; Diveu et al., 2009) by phosphorylation of STAT1 and activation of the immune cell transcription factors T-bet and RoR-γt (Th1) as well as inhibition of GATA-3 (Th2) expression. Therefore, this cytokine can enhance Th1 dominance and subsequently prevent the progression of the tumor (Canale et al., 2011; Cocco et al., 2012).
IL-27 receptor is also present on B lymphocytes, including naive and memory B lymphocytes. Naïve B lymphocyte's response to IL-27 is stronger than memory B lymphocytes (Zorzoli et al., 2012), due to higher expression of IL-27 receptor in these cells (Akdis et al., 2011). IL-27 receptor simultaneously expresses in natural killer (NK) cells, CD8+ T cells, CD4+ T cells, monocytes, mast cells, and B cells (Pflanz et al., 2004). Moreover, IL-27 can exert an antitumor effect through activating NK cells and enhancing their toxicity (Liu et al., 2008). By this means, IL-27 in combination with IL-12 enhances cytotoxic T lymphocyte and NK cells activity (Salcedo et al., 2004), which can be because of the synergic effect of IL-27 and IL-12 in the production of interferon γ (IFNγ; Kamiya et al., 2004). It was reported that IL-27 indirectly suppressed cancer cell growth through angiogenesis inhibition in multiple myeloma and B-cell lymphoma (Cocco et al., 2012, 2010). Direct tumor killing of IL-27 was also determined in B acute lymphoblastic leukemia and acute myeloid leukemia (Canale et al., 2011; Palma et al., 2006; Zorzoli et al., 2012). Due to the anticancer role of this cytokine, evaluating the expression level of IL-27 and IL-27 receptor (WSX-1) in patients with CLL in comparison with control individuals provides scientific evidence to candidate IL-27 in clinical practice.
With this regard, the aim of this study is first to evaluate the expression of IL-27 and IL-27R expression at RNA and protein levels in peripheral blood mononuclear cells (PBMCs) and plasma of patients with CLL. In second, to evaluate the apoptotic effect of recombinant IL-27 on B cells, T cells, and NK cells of patients with CLL in comparison with normal subjects.
2 MATERIALS AND METHODS
2.1 Patient and samples
In this study, 35 new cases of patients with CLL and 17 normal subjects were enrolled. The CLL cancer was confirmed by oncology specialists in patients and disease characteristics were categorized according to Rai and Binet leukemia classification. Normal subjects as a control group were adjusted in age and sex with the CLL patient group. This study was approved by the ethics committee of the Semnan University of medical sciences. Patients received and signed the informed consent prepared by the Ethics Committee of Semnan University of Medical Sciences.
2.2 PBMC isolation and B-cell purification
Five milliliters of peripheral blood were taken from patients and normal subjects and transferred to a 10 ml tube. The blood samples were centrifuged in 1,000g for 10 min at 4°C. The serum sample was collected and deposit cells were used for lymphocyte separation. Deposit cells were diluted 1:1 with phosphate-buffered saline (PBS) and gently added on above of Ficoll Hypaque solution (Miltenyi Biotec, Bergisch Gladbach, Germany) in another 15 ml tube and then centrifuged at 600g for 20 min in 22°C. Finally, PBMCs were isolated from the middle layer of the cells in the tube. For B-cell isolation, PBMCs were washed twice with PBS and purified by positive selection of CD19 MACS beads (Miltenyi Biotec) according to the manufacturer's instructions.
2.3 Evaluating protein expression of IL-27R
Anti-IL-27 receptor-APC (WSX-1; R&D Systems), anti-CD19-PE (BD Biosciences), and anti-CD3-FITC (BD Bioscience) were used to evaluate IL-27 receptor expression on the surface of lymphocytes. 1 × 105 lymphocytes were loaded in flow cytometry tubes and antibodies were added to each tube according to manufactory instruction. Then, cells were incubated at 4°C for 30 min, and, finally, fluorescent intensity was quantified by flow cytometry using FACSCalibur (BD Biosciences). All data were analyzed by FlowJo software (Tree Star Inc., Ashland, OR).
2.4 Evaluating messenger RNA expression of IL-27 and IL-27R
To evaluate IL-27 and IL-27R messenger RNA (mRNA) expression in PBMCs of patients with CLL and normal subjects, total RNA was extracted from PBMC of patients with CLL and normal subjects by using the Bio Basic RNA Extraction Kit (Canada). After that, 2 µg of total RNA was revert transcript to complementary DNA (cDNA) using cDNA synthesis (Applied Biosystem) in accordance with company protocol. Primers for IL-27, IL-27R genes, and GAPDH gene, as an internal control, were designed using the exon junction approach (primer sequences are available in Table 1). The Quantitative Polymerase Chain Reaction (qPCR) assay was conducted on ABI 7900 (7900HT Real-Time PCR System). The real-time PCR thermal profile was set as follows: 2 min 50°C and 15 s 95°C and 40 cycles; 10 s 94°C and 30 s 60°C.
| Primer | Sequence |
|---|---|
| IL-27 receptor sense | 5′-GAG CCC CCT CCG AGT TAC-3′ |
| IL-27 receptor antisense | 5′-GGC TTC ATT TGG GTT TCT AGG-3′ |
| P-28 sense | 5′-CGC TTT GCG GAA TCT CAC-3′ |
| P-28 antisense | 5′-GGG CAT GGA AGG GCT GA-3′ |
| GAPDH sense | 5′-GGA AGG ACT CAT GAC CAC AG-3′ |
| GAPDH antisense | 5′-CAC GTT GGC AGT GGG GAC-3′ |
- Note: P-28 is a subunit of IL-27 dimer protein.
- Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL-27, interleukin 27.
2.5 Cell culture
To determine the effect of IL-27 on PBMCs and B cells of patients with CLL and normal subjects, these cells were cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% AB serum, 100 U/ml penicillin, 100 U/ml streptomycin, and 50 ng/ml IL-27 incubated in 37°C, 5% CO2 atmosphere, and 95% humidity for 24, 48, and 72 hr.
2.6 Viability test assay
To evaluate the direct effect of IL-27 on the survival of B cells and PBMCs cells, the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was conducted on pure B cells and PBMC of patients with CLL and normal subjects. At first, 1.5 × 105 PBMCs or B cells of patients with CLL and normal subjects were loaded in 96-well plates and 50 μg/ml of recombinant human IL-27 and incubated in 37°C, 5% CO2. Incubation time was set in three times as follows: 24, 48, and 72 hr. After incubation, the cell plate culture was centrifuged at 1000g, 4°C for 5 min and supernatant was discarded. Afterward, 50 µl cell culture media and 50 µl of MTT solution was added to each well and incubated in 37°C for 3 hr. Then, 150 µl of MTT solvent (dimethyl sulfoxide) was added to each well and optical density of each well was read at 590 nm by a plate reader (Stat Fax 2100 Microplate).
2.7 Evaluating the proapoptotic effect of IL-27 on PBMCs and pure B cells
To evaluate the proapoptotic effect of IL-27 on B CLL cells and PBMCs, apoptosis assay with Annexin V-FITC and 7-AAD (BioLegend) fluorescent dyes was conducted on PBMCs and pure B cells of patients with CLL and healthy subjects. In brief, 4.5 × 105 PBMC/well and pure B cells were cultured in 48-well plates supplemented with 50 ng/ml of recombinant human IL-27 and incubated in 5% CO2 at 37°C for 48 hr. The incubated cells were transferred to flow cytometry tube and then Annexin V and 7-AAD were added, according to manufacturing instruction. Finally, the apoptotic effect of IL-27 on nominated cells was quantified by flow cytometry using FACSCalibur (BD Biosciences). All data were analyzed by FlowJo software (Tree Star Inc).
2.8 Effect of IL-27 on NK cells and T cells activity
To evaluate the effect of IL-27 on the activity of NK cells and T cells activities, 3 × 105 PBMCs/well (96-well plates) of patients with CLL were cocultured with 25, 50, and 100 ng/ml of IL-27 and supplemented with human AB serum 10% and incubated in several time point as follows: 24, 48, and 72 hr. At the end of incubation time, all the cells were harvested and CD69 (anti—CD69-APC; BioLegend), as an activation marker, was evaluated in T cells (CD8+) and NK (CD56+) cells using flow cytometry analysis.
2.9 Statistical analysis
Data were analyzed and processed with SigmaPlot Version 16 (SPSS) on a Windows operating system. IL-27 apoptotic effect on B lymphocytes of patients and normal subjects was analyzed by the Mann–Whitney U test. Also, the comparison between IL concentration in different age groups and between patients and control subjects was performed using the Mann–Whitney U test. Data with p < .05 were considered as statistically significant.
3 RESULTS
3.1 Patients characteristics
In the present study, 35 patients with CLL and 23 normal subjects were included; 20 males and 15 females age 40–81 years old in the cancer group and 13 males and 10 females age 42–79 years old in the control group. The mean age of the patient and normal subjects in this study was 62.43 ± 10.16 and 60.28 ± 10.56, respectively, and approximately 70% of patients were in Scores III and IV Rai/Binet stage. The mean count of white blood cells of patients with CLL was 27.23 ± 14.49.
3.2 IL-27R expression in B and T cells
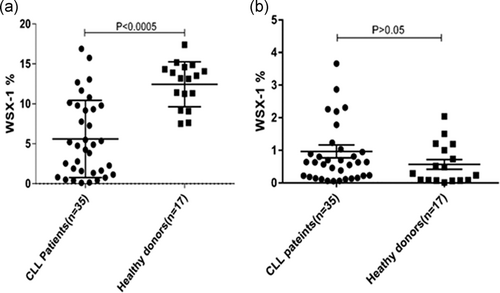
IL-27R expression on the surface of CD19+ B cells in patients with CLL was (6.92 ± 3.42) significantly (p < .005) lower than normal individuals (12.44 ± 2.81; Figure 1a). There was no significant difference (p > .05) between the expressions of IL-27 receptor on the surface of CD3+ T cells in patients with CLL (0.9 ± 0.81) in comparison with normal subjects (0.6 ± 0.53; Figure 1b).
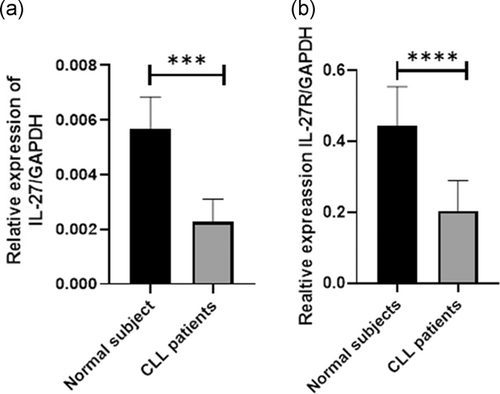
In this regard, IL-27 mRNA expression was evaluated in PBMC of patients with CLL and normal subjects. Similar to flow cytometry results, IL-27 expression in PBMC of patients with CLL was significantly (p < .05) lower (2.47-fold) than normal subjects (Figure 2a). In addition, IL-27R expression in patients with CLL was significantly (p < .05) lower (2.1-fold) than normal subjects (p < .05; Figure 2b).
3.3 The effect of IL-27 on lymphocytes survival
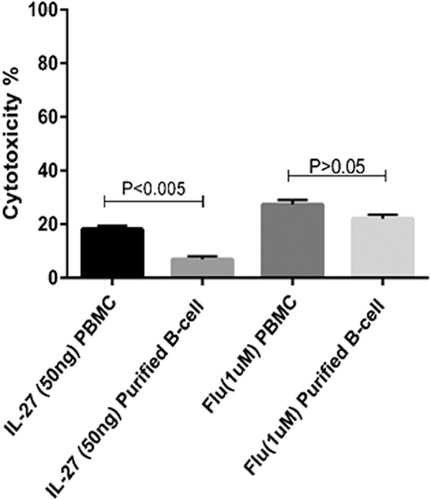
The IL-27 effect on the viability of PBMCs in CLL patients and healthy donors were assessed by MTT assay in 48 hr. The results indicated that inhibitory concentration (IC50) of IL-27 for 24 hr incubation is 50 ng/ml (p < .0005). Data indicated that 50 ng/ml of IL-27 reduced the viability of PBMCs similar to fludarabine that was used as a positive control (Figure 3). In addition, IL-27 exerted no significant cytotoxic effect (p > .05) on PBMC of normal subjects (Figure 3).
3.4 Apoptotic effect of IL-27 on PBMC and B cells of patients with CLL
Annexin V and 7-AAD were used for evaluating the apoptotic effect of IL-27 on PBMC and B cells of patients with CLL in comparison to normal subjects. These cells were cocultured with 25, 50, and 100 ng/ml of IL-27 in different incubation times from 24, 48, to 72 hr and the apoptotic effect of IL-27 was then assessed by flow cytometry analysis. Maximum apoptosis effect of IL-27 was seen in concentration of 50 ng/ml (IC50 = 50 ng/ml) in 48 hr incubation (p < .05; Figure 4). In addition, IL-27-induced apoptosis in PBMC of patients with CLL (62 ± 1.89%) in comparison with control PBMCs (50 ± 1.78%; Figure 4).
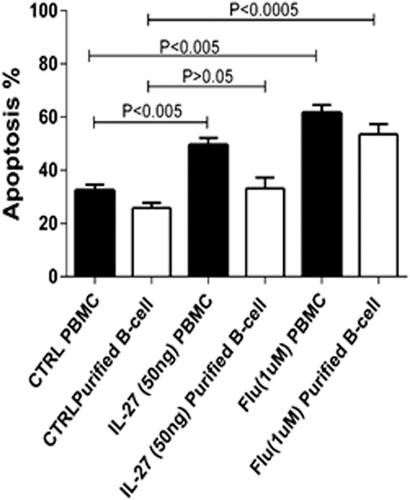
Purified B cells were cocultured with 50 ng/ml IL-27 for 48 hr. As shown in Figure 4, IL-27 significantly (p < .005) induced apoptosis in B cells of patients with CLL (33.4 ± 3%) in comparison with untreated (control) group (27.2 ± 2.1%; p < .0005). Fludarabine as a positive control drug had the maximum apoptotic effect (54 ± 3.3%) as shown in Figure 3.
3.5 Enhancement of T cell and NK cells activity by IL-27
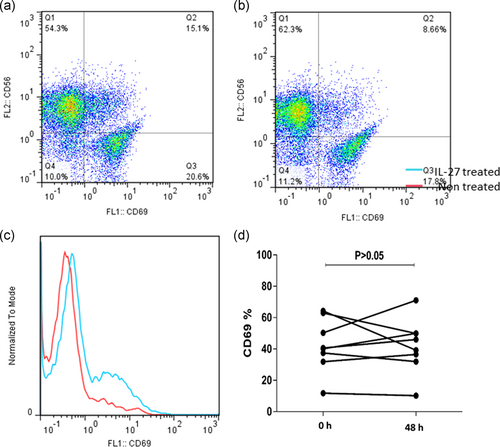
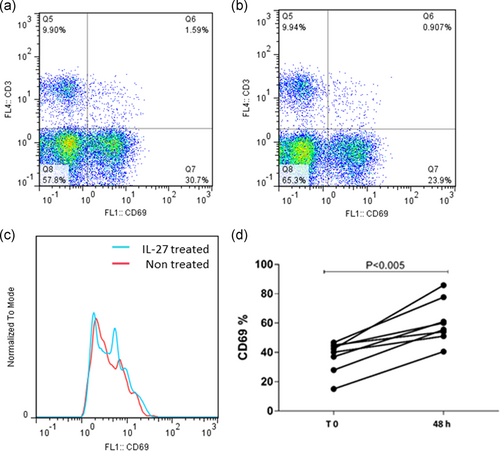
As mentioned above, PBMCs of patients with CLL were cocultured with different concentrations of IL-27 in different incubation times. The result of flow cytometry indicated that the maximum effect of IL-27 on CD69 expression in NK cells was identified in 48 hr with 50 ng/ml of IL-27 cytokine (p = .031; Figure 5). Also, the results indicated that CD69 expression in T cell was not significantly (p > .05) affected by IL-27 cytokine in different incubation times and concentrations (Figure 6).
4 DISCUSSION
The antitumor effect of IL-27 on various cancers, such as colon, melanoma, lung cancer, and head and neck cancers has been indicated in several investigations; however, its underlying mechanisms are unknown (Larousserie et al., 2006; Pflanz et al., 2002; Yoshimoto et al., 2008). A recent study has also shown the proapoptotic role for IL-27 on B lymphocytes (Canale et al., 2011). The purpose of the present study was to evaluate the proapoptotic effect of IL-27 on lymphocytes of patients with CLL in comparison with normal subjects. The result showed that IL-27 expression in PBMC of patients with CLL was lower than normal subjects. Moreover, IL-27R expression at mRNA/protein levels in patients with CLL was significantly lower than normal subjects. Of note, we indicated that IL-27 reduced the proliferation of B cells of patients with CLL through inducing apoptosis. This result is in line with the other study that showed IL-27 induced apoptosis in B cells of acute lymphoblastic leukemia patients (Cocco et al., 2012). In another study, a antitumor role for IL-27 on B cells of multiple myeloma cells was also shown through inhibition of angiogenesis and suppressing tumor growth (Cocco et al., 2010). WSX-1 is a subunit of IL-27 receptor which is related to the proapoptotic effect of this receptor (Di Meo et al., 2014). In this study, it was indicated that cancer B cells downregulate the expression WSX-1 mRNA and proteins to invade from its antitumor effects.
Furthermore, our results revealed that IL-27 could enhance the activity of NK cells, as seen by the elevation of CD69 expression in vitro after 48 hr incubation of treated NK cells. These results can be supported by the other study that showed IL-27 induced the activity of NK cells and CD8+ T cells through elevating IFNγ production (Liu et al., 2008). In addition, the results of the current study indicated that IL-27 exerted no significant effects on the expression of CD69 on the surface of T cells. Although CD69 is not the best marker of T cells and NK cells, it can represent early T cells and NK cells activity (Vuletic et al., 2015). In another study that conducted to investigate the serum level of IL-27 in multiple myeloma disease (Cocco et al., 2010), no significant difference was found in the serum levels of IL-27 between multiple myeloma patient in the early stages of the disease (Stage 1) in comparison with normal subjects, but in second and third phase of cancer progression, it was significantly decreased (Song et al., 2013). On the basis of “tumor escape” theory, tumor cells provide conditions for their growth and development by reducing the level of tumor growth-inhibiting factors (Pak, Barati, Shokrolahi, & Kokhaei, 2014). To sum up, it seems that IL-27 and its receptor are two growth inhibitory molecules that normal B cells produce them at high levels but cancer B cells express these molecules in the lower levels. By the results of the present study, the proapoptotic effect of IL-27 on normal B cells was very slight and neglectable, but this effect on cancer B cells was significant. In addition, it was demonstrated that IL-27 can enhance NK cell activity which can enhance immunosurveillance against CLL. Although IL-27 exerted antitumor activity in vitro against malignant immune cells from patients with CLL, further experimental and clinical studies are needed to confirm antitumor effect of this cytokine in in vivo condition.
ACKNOWLEDGMENTS
The authors would like to thank the Deputy of Research and Technology at Semnan University of Medical Sciences for supporting the study and in the compilation of the paper. This study was supported by Semnan University of Medical Sciences.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Parviz Kokhaei conceived and designed the study. Ehsan Manouchehri-Doulabi, Somaye Abbaspour, Shahrbano Rostami, conducted the experimental work and collected the data. Mohammad Faranoush and Farahnaz Ghahramanfard analyzed data. Fatemeh Pak and Mehdi Barati drafted the manuscript. Amir Abbas Momtazi-Borojeni. evaluated the data and critically revised the manuscript. All authors approved the final version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




