Immune-related long noncoding RNA signature for predicting survival and immune checkpoint blockade in hepatocellular carcinoma
Yaqiong Zhang and Liming Zhang contributed equally to this work.
Abstract
Long noncoding RNAs (lncRNAs) show multiple functions, including immune response. Recently, the immune-related lncRNAs have been reported in some cancers. We first investigated the immune-related lncRNA signature as a potential target in hepatocellular carcinoma (HCC) survival. The training set (n = 368) and the independent external validation cohort (n = 115) were used. Immune genes and lncRNAs coexpression were constructed to identify immune-related lncRNAs. Cox regression analyses were perfumed to establish the immune-related lncRNA signature. Regulatory roles of this signature on cancer pathways and the immunologic features were investigated. The correlation between immune checkpoint inhibitors and this signature was examined. In this study, the immune-related lncRNA signature was identified in HCC, which could stratify patients into high- and low-risk groups. This immune-related lncRNA signature was correlated with disease progression and worse survival and was an independent prognostic biomarker. Our immune-related lncRNA signature was still a powerful tool in predicting survival in each stratum of age, gender, and tumor stage. This signature mediated cell cycle, glycolysis, DNA repair, mammalian target of rapamycin signaling, and immunologic characteristics (i.e., natural killer cells vs. Th1 cells down, etc). This signature was associated with immune cell infiltration (i.e., macrophages M0, Tregs, CD4 memory T cells, and macrophages M1, etc.,) and immune checkpoint blockade (ICB) immunotherapy-related molecules (i.e., PD-L1, PD-L2, and IDO1). Our findings suggested that the immune-related lncRNA signature had an important value for survival prediction and may have the potential to measure the response to ICB immunotherapy. This signature may guide the selection of the immunotherapy for HCC.
1 INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common human malignancies and the fourth leading cause of death in human cancers (Bray et al., 2018). According to global cancer statistics, ∼841,080 new cases are diagnosed with HCC in 2018 and an estimated 781,631 deaths occur because of HCC in the world (Bray et al., 2018). Although early detection and management have achieved some advances, the rate of early diagnosis is low; most HCC cases are diagnosed at advanced-disease (Amin et al., 2017; Bruix, Reig, & Sherman, 2016). Moreover, up to 70% of HCC patients after resection present tumor recurrence at 5 years (Llovet et al., 2016). Thus, the 5-year survival rate of this disease remains very unsatisfactory (18%; Siegel, Miller, & Jemal, 2019). To improve survival and reduce the burden of HCC disease, it is important to identify novel biological molecular predictors and improve prediction of HCC prognosis.
Long noncoding RNAs (lncRNAs) is a common type of noncoding RNAs, with over 200 nucleotides in length. lncRNAs play an essential role in cancer development and progression (C. H. Li & Chen, 2016; Sanchez Calle, Kawamura, Yamamoto, Takeshita, & Ochiya, 2018). lncRNAs are involved in epigenetic regulation, gene expression, genetic imprinting, chromatin remodeling, transcription and posttranscriptional processes (Momen-Heravi & Bala, 2018; Zhang et al., 2019). lncRNAs play key roles in many cellular processes, such as cell cycle, cell differentiation, and DNA repair (X. Hu, Sood, Dang, & Zhang, 2018; Majidinia & Yousefi, 2016). Emerging evidence suggests that lncRNAs as regulators display an important influence in cancer immunity, such as antigen release, immune cell migration and infiltration, antigen presentation, and immune activation (Carpenter & Fitzgerald, 2018; Denaro, Merlano, & Lo Nigro, 2019). lncRNA LINK-A downregulates cancer cell antigen presentation via inactivation of protein kinase A signaling and intrinsic tumor suppression, suggesting that LINK-A associates with immunosuppression (Q. Hu et al., 2019). lncRNA SNHG1 regulates T cells regulatory (Treg) cell differentiation and impacts the immune escape in breast carcinoma (Pei, Wang, & Li, 2018). lncRNA-EGFR can act as an immunosuppressive activity through stimulating Treg cell differentiation, thereby promoting HCC immune evasion (Jiang et al., 2017). Recent studies suggest that some lncRNAs could serve as potential prognostic biomarkers in HCC (DiStefano, 2017; L. Wei et al., 2019). Some studies have reported the potential value of the lncRNA signature for predicting prognosis in HCC (Gu et al., 2019; Sun et al., 2019; Wu et al., 2018; Yan, Zhou, Guo, Li, & Wang, 2019) but most studies are not externally validated using independent data. In addition, the study regarding the immune-related lncRNA signature in HCC survival is lacking.
Using various cohorts of HCC patients, we first performed a comprehensive analysis to identify and validate a novel and robust molecular signature using the immune-related lncRNA classifier for HCC survival. Also, we investigated the prognostic value of this signature in various clinical groups, and the potential role of the immune-related lncRNA signature in immune checkpoint blockade (ICB) immunotherapy. This study was performed to provide more effective treatment and risk-stratification management for HCC.
2 MATERIALS AND METHODS
2.1 Sample information
Raw gene expression quantification data (HTSeq-Counts) for HCC were obtained from Genomic Data Commons Data Portal (The Cancer Genome Atlas [TCGA]). The mean gene expression levels with >1 in all samples were retained. The trimmed mean of M-values method of “edgeR” package was used to normalize the corresponding expression data. The normalized expression levels were further performed using the log2(x+1) transformation. Patients with HCC with incomplete follow-up information were excluded. The clinical features of patients with HCC were also derived. TCGA data was used as a training cohort. Finally, 368 cases with available expression data and the corresponding clinical and survival information were included.
The expression data of HCC and available clinical and survival data of patients with HCC were also derived from the Gene Expression Omnibus. Due to sufficient information, the GSE76427 dataset was collected from Illumina HumanHT-12 V4.0 expression beadchip. The normalized expression levels were performed using the “quantile” method of “lumi” package. Log2 transformation was conducted on this expression dataset. HCC cases with incomplete follow-up information were removed. GSE76427 dataset was utilized as an independent validation cohort. A total of 115 cases with available clinical and survival data were applied in this study.
In a word, the training set with 368 cases and an independent extra validation cohort with 115 cases for HCC were utilized in our study. Patients were confirmed that all informed consent was achieved. Overall survival (OS) was defined from the date of study enrollment until the date of death or the end of follow-up, and OS was used as the primary endpoint. The median follow-up time was 19.58 months (range: 0–122.5 months) in the training set and 13.92 months (range: 0.24–93.12 months) in the external validation cohort, respectively. The detailed clinical features of each cohort are listed in Table S1.
2.2 Immune-related lncRNAs
Immune metagenes were obtained from the publication (Charoentong et al., 2017), including 782 immune genes. The relationship was calculated based on the expression value between lncRNAs and immune genes. lncRNAs with Spearman's correlation coefficient with an absolute value of >0.4 and p < .0001 were set for further analysis.
2.3 Signature development of immune-related lncRNAs
To confirm the survival of immune-related lncRNAs, the risk score was designed to construct a unitive signature for HCC. First, univariate Cox proportional hazard regression analysis was conducted for 207 immune-related lncRNAs in the training set. Univariate Cox analysis with p < .05 was set for significant results. Final 35 immune-related lncRNAs were found. Second, the least absolute shrinkage and selection operator (LASSO) method was further used for significant results of the univariate Cox analysis (n = 35). The corresponding LASSO coefficient profiles and a partial likelihood deviance plot are shown in Figure S1, revealing the selected 21 immune-related lncRNAs. Eventually, a multivariate Cox proportional hazard regression model based on the two-step method was subsequently applied for these 21 immune-related lncRNAs. Final 10 immune-related lncRNAs and their corresponding coefficients were identified to build the prognostic signature in HCC. The formula used was as follows: Riskscore of immune-related lncRNAs = exp1 × β1 + exp2 × β2… + expi × βi. Here, expi was the expression value of each immune-related lncRNA in the sample, and β was the regression coefficient of the multivariate Cox regression model.
2.4 Role of immune-related lncRNA signature on the immunologic features
Gene set enrichment analysis (GSEA) was conducted to explore significant functional phenotypes between the two groups (high- and low-risk groups; Subramanian et al., 2005). In the current study, the gene sets of “h.all.v7.0.symbols.gmt [cancer hallmarks] and c7.all.v7.0.symbols.gmt [Immunologic signatures]” from the Molecular Signatures Database were analyzed using the GSEA software GSEA 4.0.3. Immunologic signatures collection, also called ImmuneSigDB, is consisted of gene sets that represent cell types, states, and perturbations within the immune system. Gene set permutations with 1,000 times were performed to achieve a normalized enrichment score for each analysis. A nominal p < .05 and a false discovery rate < 0.05 were considered significant results.
2.5 Tumor-infiltrating immune cells
CIBERSORT uses a deconvolution method to analyze the abundance of tumor-infiltrating immune cells (TIICs; Newman et al., 2015), including 22 TIIC subsets. The association of the immune-related lncRNA signature with 22 TIIC subsets was performed to explore whether this immune-related lncRNA signature may play a crucial role in immune infiltration in HCC.
2.6 Immune checkpoint blockade
Immune checkpoint gene expression may be correlated with treatment responses of immune checkpoint inhibitors (Goodman, Patel, & Kurzrock, 2017). We investigated six genes previously reported to be crucial targets of immune checkpoint inhibitors: programmed death 1 (PD-1) and its ligand 1 (PD-L1), and its ligand 2 (PD-L2), cytotoxic T-lymphocyte antigen 4 (CTLA-4), T-cell immunoglobulin domain and mucin domain-containing molecule-3 (TIM-3), and indoleamine 2,3-dioxygenase 1 (IDO1) in cancer (Kim et al., 2017; Nishino, Ramaiya, Hatabu, & Hodi, 2017; Zhai et al., 2018). To explore the possible role of our immune-related lncRNA signature in ICB therapy, and the correlation between these immune checkpoint inhibitors and our signature was analyzed in HCC.
2.7 Statistical analysis
The Wilcoxon signed-rank test was utilized to assess the association between our immune-related lncRNA signature and the clinicopathological characters of HCC. Spearman's correlation coefficients were calculated to investigate the potential relationship between our immune-related lncRNA signature and immune checkpoint inhibitors, and the corresponding signature score was conducted via a log2(x+1) transformation. Using the median risk score of the training set as the cut-off value, HCC patients were classified into two groups: low- and high-risk groups. Kaplan–Meier analysis with the logrank test was utilized to compare the survival difference between the low- and high-risk group. The univariate Cox proportional hazard regression analysis was performed to evaluate the prognostic value of our immune-related lncRNA signature. Multivariate analysis with Cox proportional hazard regression model was further conducted to investigate whether our immune-related lncRNA signature may be an independent prognostic factor in each cohort. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated. Time-dependent receiver-operating characteristic (ROC) analysis for survival prediction was applied to display the predictive performance of our immune-related lncRNA risk model. Data stratification analyses were also carried out based on age (≥60 and <60 years), gender (male and female), and disease stage (Stage 3–4 and Stage 1–2). All statistical analyses were performed using the R statistical environment (R version 3.5.1; Institute for Statistics and Mathematics, Vienna, Austria).
3 RESULTS
3.1 Immune-related lncRNAs in HCC
We extracted the list of immune genes from the publication (Charoentong et al., 2017). According to the significant associations between lncRNAs and immune genes (p < .0001), we identified 207 immune-related lncRNAs in this study (Table S2). Subsequently, after using univariate Cox regression analysis, LASSO method, and multivariate Cox regression model for these immune-related lncRNAs in the training set, the final 10 immune-related lncRNAs were identified.
3.2 Verification of the immune-related lncRNA signature in HCC survival
The 10 immune-related lncRNAs were LINC00623, MIR99AHG, SCARNA9, LINC00200, LINC01554, FIRRE, HPN-AS1, LINC00426, LINC01096, and PRKCQ-AS1. These 10 immune-related lncRNAs were combined to generate a molecular signature to predict HCC survival. An individual's risk score model was developed using the regression coefficients and the expression levels of ten immune-related lncRNAs. The corresponding formula is shown in Table S3. Using this 10 immune-related lncRNA signature, each patient obtained a risk score in each cohort of HCC.
Based on the cut-off value (0.9433), we divided the patients into two groups (the high-risk group and the low-risk group). The distribution of risk score of each HCC case is shown among the training set, the independent external validation cohort, and the whole cohort (Figure 1a), which demonstrated that patients in the low-risk group generally had better survival of HCC. In addition, Kaplan–Meier curves with the logrank test showed that the high-risk HCC patients had a closely shorter survival than the low-risk patients in the training set, the external validation cohort, and the whole cohort (Figure 1b; all P < .05). To further assess the accuracy of the ten immune-related lncRNA classifier in predicting survival of HCC at 1, 3, and 5 years, time-dependent ROC curves were analyzed. The area under the ROC (AUC) values were more than 0.75 at 1, 3, and 5 years in the training set, the external validation cohort, and the whole cohort (Figure 1c), suggesting an excellent capacity of our 10 immune-related lncRNA classifier for survival prediction.
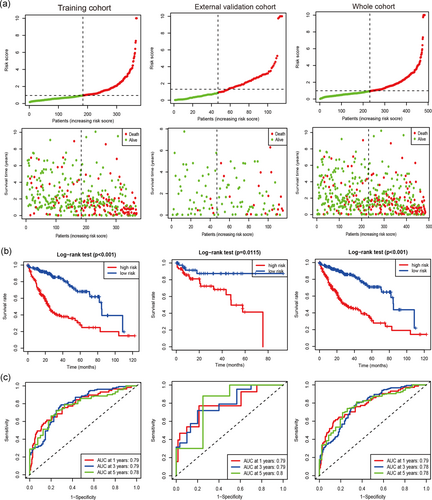
3.3 Our immune-related lncRNA signature correlates with disease progression
The association between our immune-related lncRNA signature and the clinicopathological characters of HCC was evaluated in the whole cohort (Figure 2). The results showed that our immune-related lncRNA signature was not correlated with age and gender but was significantly associated with advanced tumor stage (p = .004), histological grade (p = .007), invasion depth (T-stage; p < .001), vascular invasion (p = .004), and alpha-fetoprotein (p = .007) in HCC, suggesting that our immune-related lncRNA signature may play a crucial role in HCC progression.
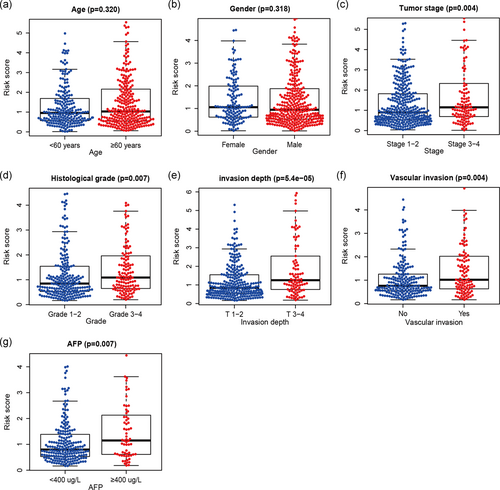
3.4 Our immune-related lncRNA signature as an independent factor in predicting survival
To examine whether the ten immune-related lncRNA signature was an independent prognostic factor in HCC, we conducted a Cox regression analysis. The univariate Cox regression analysis showed that the high-risk group of our immune-related lncRNA signature was closely associated with worse survival in the training set (HR = 3.696, 95% CI = 2.503–5.458; p < .001) and the external validation cohort (HR = 3.67, 95% CI = 1.247–10.81; p = .018; Table 1). After the adjustment of the available clinical and pathological parameters, multivariate Cox regression analysis was further performed. The results showed that our immune-related lncRNA signature was an independent prognostic factor for predicting survival in the training set (HR = 3.337, 95% CI = 2.191–5.084; p < .001) and the external validation cohort (HR = 4.352, 95% CI = 1.458–12.987; p = .008; Table 1).
| Univariate Cox model | Multivariate Cox model | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Training cohort | ||||
| Immune-related lncRNA signature (high- vs. low-risk) | 3.696 (2.503–5.458) | <.0001 | 3.337 (2.191–5.084) | <.0001 |
| Age (≥60 vs. <60 years) | 1.29 (0.901–1.847) | .164 | ||
| Gender (male vs. female) | 0.838 (0.583–1.205) | .341 | ||
| Histological grade (3–4 vs. 1–2) | 1.109 (0.766–1.606) | .584 | ||
| Tumor stage (3–4 vs. 1–2) | 2.442 (1.674–3.561) | <.0001 | 0.782 (0.107–5.698) | .808 |
| Invasion depth (T 3–4 vs. T1–2) | 2.57 (1.795–3.68) | <.0001 | 2.44 (0.334–17.839) | .379 |
| Vascular invasion (yes vs. no) | 1.307 (0.851–2.006) | .221 | ||
| AFP (≥400 vs. <400μg/L) | 1.058 (0.638–1.755) | .826 | ||
| Extra validation cohort | ||||
| Immune-related lncRNA signature (high- vs. low-risk) | 3.67 (1.247–10.81) | .018 | 4.352 (1.458–12.987) | .008 |
| Age (≥60 vs. <60 years) | 1.79 (0.735–4.357) | .2 | ||
| Gender (male vs. female) | 1.227 (0.282–5.344) | .785 | ||
| Tumor stage (3–4 vs. 1–2) | 2.336 (0.976–5.593) | .057 | 3.025 (1.247–7.342) | .014 |
- Abbreviations: AFP, alpha fetoprotein; CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; lncRNA, long noncoding RNA.
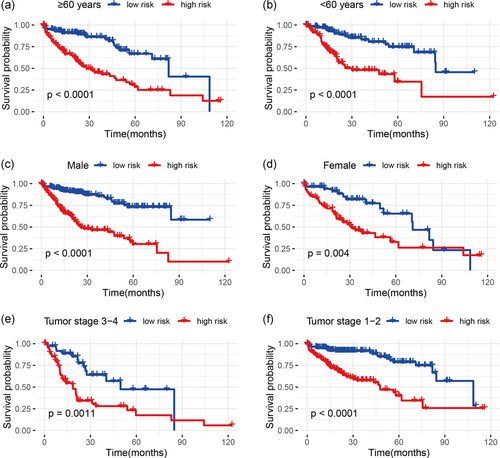
3.5 Stratification analyses
In the whole cohort, stratification analyses were performed according to the clinicopathological parameters of HCC (e.g., age, gender, and tumor stage). The results demonstrated that our immune-related lncRNA signature was still significantly correlated with worse survival in older (≥60 years) or younger (<60 years), male or female, and advanced-stage (Stage 3–4) or early-stage (Stage 1–2) patients (all p < .01; Figure 3), which suggested that our immune-related lncRNA signature-based risk stratification was still a powerful tool for predicting HCC survival in each stratum of age, gender, and tumor stage.
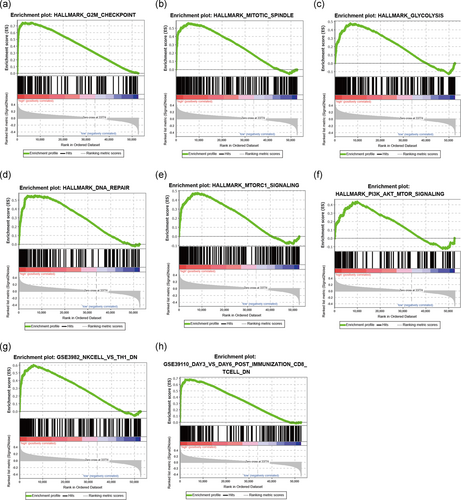
3.6 The immune-related lncRNA signature mediated cell cycle, glycolysis, DNA repair, mammalian target of rapamycin signaling, and immunologic characteristics
To explore insight into the potential molecular mechanisms of the immune-related lncRNA signature implicated in HCC progression, GSEA was carried out between the high- and low-risk groups. The results of cancer hallmarks showed that cell cycle (G2M phase and mitotic spindle), glycolysis, DNA repair, mammalian target of rapamycin (mTOR) signaling, and phosphatidylinositol-3-kinase/protein kinase B (Akt)/mTOR signaling and so forth were activated by the high-risk group of the immune-related lncRNA signature (Figure 4a–f and Table S4). In addition, the immune-related lncRNA classifier also regulated many immunologic signatures with more than 1,000 within the immune system, such as natural killer (NK) cells versus Th1 cells down, and postimmunization CD8 T cell down etc… (Figure 4g,h and Table S5), which suggested that the immune-related lncRNA signature involved in immunity-related regulation.
3.7 Correlation of the immune-related lncRNA signature with immune cell infiltration and ICB therapy-related molecule
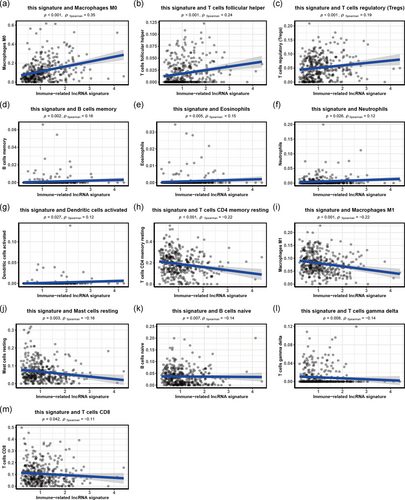
We found that many immunologic characteristics were regulated by this signature based on the above results. Thus, we further investigated whether this signature was associated with TIICs. This signature showed the most significant positive correlation with immune infiltration of macrophages M0 (r = .35; p < .001), T cells follicular helper (r = .24; p < .001), and Tregs (r = .19; p < .001), and the most significant negative correlation with immune infiltration of T cells CD4 memory resting (r = −.22; p < .001), macrophages M1 (r = −.22; p < .001), and Mast cells resting (r = −.16; p = .003; Figure 5). These findings strongly suggest that this immune-related lncRNA signature was associated with immune cell infiltration in HCC.
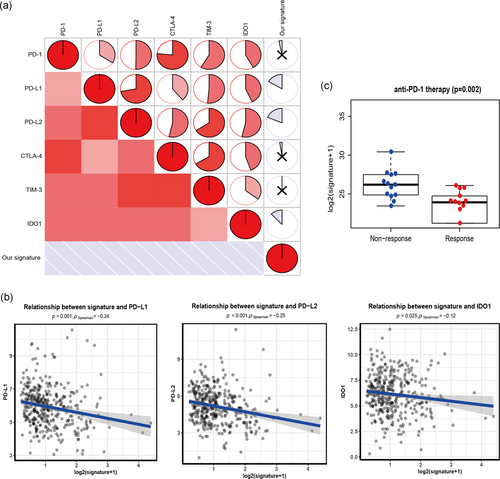
Tumor immunotherapy using ICB has become a promising shift in the treatment of advanced cancer, especially non-small-cell lung cancer and melanoma (Champiat et al., 2016; Nishino et al., 2017). We evaluated six key targets of immune checkpoint inhibitors: PD-1, PD-L1, PD-L2, CTLA-4, TIM-3, and IDO1 (Kim et al., 2017; Nishino et al., 2017; Zhai et al., 2018). We conducted the association between the immune-related lncRNA signature and ICB therapy-related genes to investigate the possible role of our immune-related lncRNA signature in the immunotherapy of ICB in HCC (Figure 6a). We found that the immune-related lncRNA signature was negatively related to PD-L1 (r = −.24; p < .001), PD-L2 (r = −.25; p < .001), and IDO1 (r = −.12; p = .025; Figure 6b), suggesting that the immune-related lncRNA signature may play a role in the evaluation and measurement of response to ICB immunotherapy in HCC. Because ICB treatment dataset was not available in HCC samples, ICB treatment data (GSE78220) were obtained from melanoma (Hugo et al., 2016) and one sample without sufficient survival information was excluded. This dataset including two groups (response vs. nonresponse to the therapy) was used to determine whether our signature was still effective for the response to ICB immunotherapy. The result demonstrated that the nonresponse group had higher immune-related lncRNA signature (p = .002; Figure 6c).
4 DISCUSSION
HCC is a highly heterogeneous malignant tumor from clinical and molecular standpoint, which is mainly owing to the unique molecular events such as genomic and genetic diversities (Cancer Genome Atlas Research Network, 2017; Schulze, Nault, & Villanueva, 2016). Studies identify that lncRNAs play crucial roles in the progression and prognosis of HCC and could become useful molecular therapeutic targets (DiStefano, 2017; L. Wei et al., 2019). lncRNAs involve in many functions such as various biological processes, gene activation, autophagy, metabolism, inflammation, and immune response, and so forth (Carpenter & Fitzgerald, 2018; Frankel, Lubas, & Lund, 2017; Majidinia & Yousefi, 2016; Mathy & Chen, 2017). Especially, emerging evidence suggests that lncRNAs act as regulators in controlling cancer immunity (Denaro et al., 2019). For instance, lncRNA-TIM-3 is shown to be associated with compromised antitumor immunity in HCC (Ji et al., 2018). Recently, studies suggest that immune-related lncRNAs may serve as new therapeutic targets and disease molecular biomarkers to provide potential support for clinic management and therapy in cancer (Heward & Lindsay, 2014; Xu et al., 2019; Y. Zhou, Zhu, Xie, & Ma, 2019). However, the potential role of the immune-related lncRNA signature as a useful therapeutic strategy is still unclear in HCC. Here, we identified an immune-related lncRNA signature and investigated its prognostic and predictive value, as well as its role in the measures of response to immune checkpoint therapy for HCC patients.
The immune-related lncRNA signature has been reported in several cancers, including glioblastoma, pancreatic cancer, glioma, and renal carcinoma, and these studies show that the immune-related lncRNA signature has important clinical implication for predicting patients' prognostication (Khadirnaikar et al., 2019; X. Li & Meng, 2019; C. Wei et al., 2019; M. Zhou et al., 2018). However, these studies have several limitations, such as relatively small sample sizes and lack of independent validation of other external cohorts, which may be limited for the prognostic and predictive capacity of these signatures. Considering the above issue, in our study, we used the training set and the independent extra validation cohort to identify an immune-related lncRNA signature in HCC prognosis. We found that our immune-related lncRNA signature involved in cell cycle, DNA repair, mTOR signaling, and immunologic characteristics. Our immune-related lncRNA signature in the high-risk group was correlated with HCC progression and worse survival. Moreover, this immune-related lncRNA signature was reliable for predicting prognosis, with a high AUC value (>0.75), and may have potential as a guide for measures of response to ICB immunotherapy in HCC.
With the rise of immune checkpoint inhibitors, ICB therapy as emerging targets for immunotherapy has shown therapeutic effects in human cancers (Llovet, Montal, Sia, & Finn, 2018; Pitt et al., 2016). Immunotherapy has afforded a new promising treatment option for HCC (Sim & Knox, 2018). However, less than one-third of patients show response to ICB therapy. These biomarkers such as immune checkpoint gene expression and mutational burden do not reliably predict benefit from ICB treatment. Thus, identification of biomarkers for the prediction of ICB immunotherapy responses is essential (Mushtaq et al., 2018; Ribas & Wolchok, 2018). Some lncRNAs associate with immune response and can predict response to immunotherapy response (Vishnubalaji, Shaath, Elango, & Alajez, 2019; Xu et al., 2018). In our study, the association analyses showed that PD-1, PD-L1, PD-L2, CTLA-4, TIM-3, and IDO1 were observed to be coexpressed (Figure 6a), which are in line with the previously reported coexpression of immune checkpoint genes in melanoma (Guo et al., 2019). In HCC, the immune-related lncRNA signature was found to be related to the ICB immunotherapy-related gene (i.e., PD-L1, PD-L2, and IDO1). Moreover, using melanoma data of ICB treatment, we found that patients in the nonresponse group had higher lncRNA signature. This suggested that the immune-related lncRNA signature can have a potential role for the measurement in response to HCC ICB immunotherapy. Recently, emerging evidence suggests that some lncRNAs act as regulators in controlling tumor immunity, such as antigen release and immune cell infiltration, and so forth (Carpenter & Fitzgerald, 2018; Denaro et al., 2019). In our study, we also found that our immune-related lncRNA signature was correlated with immune cell infiltration (i.e., macrophages M0, Tregs, T cells CD4 memory resting, and macrophages M1 etc.,) in HCC, which suggested that immune-related lncRNA signature may play a role in immune infiltration of HCC.
lncRNAs involve in various biological processes such as ell cycle and DNA repair (X. Hu et al., 2018; Majidinia & Yousefi, 2016), metabolism (Denaro et al., 2019), and immune regulation (Denaro et al., 2019), and so forth. Among some lncRNAs of our immune-related lncRNA signature, LINC00426 is associated with patients’ prognosis in lung cancer (Du, Sun, Gu, Zhang, & Zhang, 2019). PRKCQ-AS1 may be a potential biomarker for predicting survival in colorectal cancer (Shademan et al., 2019). LINC01096 involves in cell migration and invasion, and it can predict clinical outcomes in breast cancer (Wang et al., 2019). LINC01554 regulates glucose metabolism via inhibiting Akt/mTOR signaling. LINC01554 is downregulated and correlates with the progression and shorter survival in HCC (Zheng et al., 2019). FIRRE expression can predict prognosis in diffuse large B-cell lymphoma (Shi et al., 2019). FIRRE may play a key role in the posttranscriptional regulation of inflammatory genes in the innate immune system (Lu et al., 2017). In this study, we found that our immune-related lncRNA signature mediated cell cycle, DNA repair, metabolism (glycolysis), mTOR signaling, and immunologic function. Then, we constructed a novel multi-immune-related lncRNA signature for survival prediction in HCC, which suggested a strong predictive capacity for HCC patients’ prognosis. In addition, stratification analyses revealed that our immune-related lncRNA signature-based risk stratification was still powerful for predicting survival in each stratum of age (older or younger patients), gender (male or female), and tumor stage (early- and advanced-stage patients).
In summary, we identified that the ten immune-related lncRNA signature was correlated with HCC progression and prognosis, which can be applied as an independent prognostic molecular biomarker in predicting survival for HCC. This immune-related lncRNA signature also can stratify clinical subgroups in terms of survival. Furthermore, this immune-related lncRNA signature was related to immune cell infiltration and the potential ICB immunotherapy-related signature. Therefore, our study provided a potential approach to facilitate the personalized risk stratification and measure response to ICB immunotherapy of HCC, which might present valuable clinical applications in antitumor immunotherapy. However, further validation is suggested for this signature at different centers.
ACKNOWLEDGMENTS
This study was funded by the Science and Technology Projects of Taizhou Science and Technology Bureau, China (Grant no. 1801ky039 and 1801ky035). The authors gratefully acknowledge the Gene Expression Omnibus and The Cancer Genome Atlas.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Y. Z. and J. M. contributed to the conception and design of this study. Y. Z., L. Z., Y. X., X. W., Y. Z., and J. M. contributed to the drafting of the article and final approval of the submitted version. Y. Z., L. Z., Y. X., X. W., Y. Z., and J. M. contributed to data analyses and the interpretation and completion of the figures and tables. All authors read and approved the final manuscript.
ETHICS STATEMENT
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Gene Expression Omnibus and The Cancer Genome Atlas Human Subjects Protection and Data Access Policies, adopted by the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI). Informed consent was obtained from all individual participants included in the study.
Open Research
DATA AVAILABILITY STATEMENT
The data used during the current study are available from the corresponding author on reasonable request.All procedures performed in studies involving human participants were in accordance with the ethical standards of the Gene Expression Omnibus and The Cancer Genome Atlas Human Subjects Protection and Data Access Policies, adopted by the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI). Informed consent was obtained from all individual participants included in the study.




