OxLDL induces vascular endothelial cell pyroptosis through miR-125a-5p/TET2 pathway
Chen Jiaojiao and Wu Peng are co-first authors.
Abstract
Pyroptosis participates in the formation and development of atherosclerosis (As) by promoting inflammatory factor release and is closely related to the stability of atherosclerotic plaque. MicroRNAs can regulate the expression of target genes at the posttranscriptional level. Previous studies have shown that miR-125a-5p increases in hyperlipidemic–hyperglycemic conditions and is involved in apoptosis, but its specific role in pyroptosis and As remains unclear. We propose that miR-125a-5p may be implicated in oxidized low-density lipoprotein (oxLDL)-induced vascular endothelial cells (VECs) pyroptosis and therefore conducted the current study. We observed that miR-125a-5p can inhibit tet methylcytosine dioxygenase 2 (TET2) expression at the posttranscription level, resulting in abnormal DNA methylation, mitochondrial dysfunction, and increased reactive oxygen species production, activated nuclear factor-κB that induces activation of inflammasome and maturation, release of proinflammatory cytokines interleukin (IL)-1β and IL-18, and pyroptosis. Given the role of VECs in vascular physiology, oxLDL-induced VEC pyroptosis may promote the development of As. Our current study reveals a novel pathway associated with pyroptosis program regulation, which comprises miR-125a-5p and TET2 in VECs. Modulation of their expression levels may serve as a potential target for therapeutic strategies of As.
1 INTRODUCTION
Atherosclerosis (As) is the main cause of coronary heart disease and stroke (Frohlich & Al-Sarraf, 2013). In broad outline, As can be considered a form of chronic inflammation resulting from the interaction between modified lipoproteins, monocyte-derived macrophages, T cells, and the normal cellular elements of the arterial wall. This inflammatory process can ultimately lead to the development of complex lesions or plaques that protrude into the arterial lumen. Plaque rupture and thrombosis result in acute clinical complications of myocardial infarction and stroke (Glass & Witztum, 2001). Cell death can be observed throughout As and plays a crucial role in determining the fate of atherosclerotic lesion (Chang, Lin, Dong, & Li, 2013).
Pyroptosis is one manner of programmed cell death (PCD), is characterized by rapid plasma membrane rupture, with consequent release of cellular contents and proinflammatory mediators, including interleukin (IL)-1β and IL-18, and is closely related to the occurrence and development of As (Hoseini et al., 2018; Man, Karki, & Kanneganti, 2017; Xu, Zheng, Hu, & Wang, 2017). Tet methylcytosine dioxygenase 2 (TET2) is an epigenetic regulatory enzyme that catalyzes the oxidation of 5-methylcytosine (5mc) in DNA to 5-hydroxymethylcytosine (5hmc) and also exerts noncatalytic actions (Ko et al., 2010). Abnormal methylation of DNA is closely related to cell death (Kontaki & Talianidis, 2010; Lehmann-Werman et al., 2016). Our previous study showed that overexpression of TET2 features protective effects on oxidized low-density lipoprotein (oxLDL)-induced vascular endothelial cell (VEC) dysfunction through upregulating the cystathionine γ-lyase (CSE)/hydrogen sulfide system by DNA demethylation of the CSE gene promoter (Peng et al., 2017). oxLDL can inhibit autophagy by downregulating TET2 (G. H. Li et al., 2015). Autophagy, apoptosis, and pyroptosis are all belong to PCD. Therefore, we hypothesize that TET2 may participate in oxLDL-induced VEC pyroptosis.
MicroRNAs (miRNAs) are endogenous, small noncoding RNAs. Mature miRNAs approximate 22 nucleotides in length and can regulate the expression of target genes at the posttranscriptional level (Z. L. Zeng, Lin, et al., 2018). Through bioinformatics analysis, we recognized that TET2 is the target gene of miR-125a-5p. Thus far, the role of miR-125a-5p in the cardiovascular system (Gareri et al., 2017; Tiedt et al., 2017), tumor (Naidu et al., 2017), apoptosis (Tong et al., 2015), and inflammation (Banerjee et al., 2013) has been reported. Moreover, the expression of miR-125a-5p is increased in hyperlipidemic (Simionescu, Niculescu, Sanda, Margina, & Sima, 2014). A previous study revealed that downregulation of apo(a) expression by miR-125b-5p by targeting Ets1 in HepG2 cells is related to As (J. F. Zeng, Zeng, et al., 2018). Nevertheless, whether miR-125a-5p is involved in VEC death upon oxLDL exposure and its relation to TET2 downregulation remain to be clarified.
The current study aimed to investigate the hypothesis that downregulation of TET2 by miR-125a-5p promotes oxLDL-induced pyroptosis in VECs. We first examined the effect of oxLDL on the pyroptosis of VECs and intracellular TET2 and miR-125a-5p expression. Then, we further determined whether miR-125a-5p and TET2 regulate oxLDL-induced dysfunction of VECs via the pyroptosis system and investigated the underlying mechanism in this process.
2 MATERIALS AND METHODS
2.1 Cell culture and transfection
Human umbilical vein endothelial cells were obtained from Science Cell Research Laboratories (Carlsbad, CA). The cells were grown in endothelial cell medium (Sciencell Research Laboratories, Carlsbad, CA) supplemented with 10% fetal bovine serum at 37°C with 5% CO2 and 95% air. Overexpression/short hairpin RNA (shRNA) lentivirus of TET2 and the negative control (NC) were synthesized by GeneChem (Shanghai, China). MiR-125a-5p inhibitor and NC were purchased from RiboBio (Guangzhou, China). Cells were treated for 24 hr with different concentrations of oxLDL. VECs were transfected with overexpression/shRNA lentivirus of TET2 and miR-125a-5p inhibitors or NC when the cell density reached 50–70%. For experiments involving pharmacological inhibitors, total reactive oxygen species (ROS) scavenger N-acetyl-l-cysteine (NAC) and nuclear factor-κB (NF-κB) inhibitor (BAY 11–7082) were purchased from TCI Chemicals (Shanghai, China). Endothelial cells were pretreated with NAC (5 mM) or BAY 11–7082 (20 µM) for 2 hr and subsequently treated by oxLDL 100 µg/ml (Yiyuan, China) for 24 hr in the presence of inhibitors.
2.2 Measurement of intracellular ROS levels
The level of intracellular ROS was determined on the basis of the oxidative conversion of cell-permeable 2′,7′-dichlorofluorescein diacetate (DCFH-DA; KeyGen, Jiangsu, China) to fluorescent dichlorofluorescein upon reaction with hydroxyl radical, hydrogen peroxide, or peroxynitrite. Briefly, cells were incubated with control media (phosphate-buffered saline [PBS]) or oxLDL in the presence or absence of antioxidants NAC for 24 hr. Then, the cells were washed twice with cold PBS (pH 7.4) and incubated with DCFH-DA at room temperature for 30 min in the dark. VECs added 100 μg/ml oxLDL after 24 hr transfected with miR-125a-5p inhibitors, then perform ROS determination. Since TET2 has green fluorescence, we use ROS probes (DHE; KeyGen) for inspection. Dihydroethidine is an indicator of peroxide, which is oxidized in the cytoplasm and can enter the DNA of the cell, giving the nucleus a bright red fluorescence. The cells were incubated with DHE at a final concentration of 50 μM for 30 min and then washed three times with serum-free medium. The fluorescent signal was recorded by using fluorescence microscopy (Olympus Microscope IX3; Olympus, Tokyo, Japan).
2.3 Cell death assay
Pyroptotic cell death was evaluated with lactate dehydrogenase (LDH) release and Hoechst 33342/propidium iodide (PI) staining. For LDH release, cell culture supernatants were collected, and LDH activity was detected using the LDH assay kit (Nanjing Jiancheng Biology Engineering Institute, Jiangsu, China). Briefly, 25 µl cell supernatant and 25 µl substrate were mixed together and incubated at 37°C for 15 min. Then, 25 µl of 2,4-dinitrophenylhydrazine was added into the samples and incubated at 37°C for 15 min. Finally, 250 µl of 0.4 mol/l NaOH solution was added and incubated with the mixture at room temperature for 5 min. Absorbance was measured at 450 nm on a spectrophotometric microplate reader. For Hoechst 33342/PI double staining, VECs were cultured in six-well plates at a density of 5 × 105 cells/well. Then, the cells were transfected with TET2 lentivirus, miR-125a-5p inhibitor, or treated with drugs. Next, the cells were stained with 10 µl Hoechst 33342 solution at 37°C in the dark for 10 min, followed by staining with 5 µl PI at 37°C in the dark for 10 min. The stained cells were observed under a fluorescence microscope (Olympus Microscope IX3).
2.4 RNA extraction, retrotranscription, and real-time polymerase chain reaction (PCR)
MiRNA quantification was determined by using Bulge-Loop™ miRNA qRT-PCR Primer Set (one reverse-transcription (RT) primer and a pair of quantitative PCR primers for each set) specific for miR-125a-5p, which is designed by RiboBio (Guangdong, China). MiRNA expression was defined based on Ct, and relative expression levels were calculated as 2−[(Ct of miR-125a-5p) – (Ct of U6)] after normalization with reference to the expression of small nuclear RNA U6. The extracted RNA was pretreated with RNase-free DNase, and 500 ng of RNA from each sample was used for cDNA synthesis primed with the specific miRNA RT primer (Supporting Information Table 3).
2.5 Luciferase reporter assays
Luciferase reporters containing wild-type or mutated miR-125a-5p plasmid were constructed using psi-CHECK2 vectors (Promega, Madison, WI). The luciferase vector (100 ng) containing the pcDNA3.1-miR-125a-5p plasmid was cotransfected with miR-125a-5p mimic, miR-125a-5p inhibitor, or NC (scramble sequence of miR-125a-5p; Supporting Information Table 1) into human embryonic kidney 293 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with 10 ng of Renilla luciferase reporter used as an internal control. After 48 hr, the cells were collected and lysed. Luciferase activities were determined using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions.
2.6 Western blot analysis
Cells were washed twice with chilled PBS and lysed with radioimmunoprecipitation assay buffer and phenylmethanesulfonyl fluoride (94:6) on ice for 30 min and centrifuged (9,500g, 15 min). Nuclear proteins and cytoplasmic protein were extracted by Nuclear and Cytoplasmic Protein Extraction Kit (KeyGen) according to the manufacturer’s instructions. The total proteins were extracted, after which their concentrations were determined using a BCA Kit (CWBio, Peking, China). Briefly, protein samples of 20 µg/well were loaded in 8–12% sodium dodecyl sulfate polyacrylamide gel and transferred onto a nitrocellulose filter membrane and subsequently blocked by 5% nonfat milk dissolved in PBS for 2 hr. The membrane was probed with primary antibodies against TET2 (1:1,000; Abcam, MA), caspase-1 (1:500; Proteintech, Rosemont), IL-1β (1:500; Proteintech), NLRP3 (1:500; ABclonal, MA), NF-κB p65 (1:1,000; Abcam), NF-kB p65 (phospho S53, 1:1000; Abcam), IkBα (1:1,000; Abcam), Histone H3 (1:5,000; Abcam) and diluted in Tris-buffered saline with Tween® 20 containing 2.5% skim milk buffer at 4°C overnight. Membranes were then washed five times in PBS with Tween 20 and incubated with fluorescence-conjugated antirabbit IgG secondary antibody at room temperature for 1.5 hr (1:2,000). β-Actin (Proteintech) was used as an internal control. Western blot bands were quantified using the Quantity One Software (California, CA) by measuring band intensity (area × optical density).
2.7 Immunofluorescence
Immunofluorescence staining was performed to detect the expressions of caspase-1, 5hmC, and NF-κB p65 in endothelial cells. Briefly, the cells were fixed with 4% paraformaldehyde for 30 min, penetrated by 0.1% Triton X-100 for 30 min, and then blocked with 5% goat serum for 1 hr. Subsequently, the cells were incubated with anti-caspase-1 (1:250; Abcam), anti-5hmC (1:200; Proteintech), or NF-κB p65 (1:250; Abcam) antibody at 4°C overnight, followed by incubation with Alexa Fluor-conjugated secondary antibody (Invitrogen) in the dark for 1 hr. The nuclei were stained by 4′,6-diamidino-2-phenylindole (Beyotime, Shanghai, China) for 5 min. The cells were observed under a fluorescence microscope (Olympus Microscope IX3, Tokyo, Japan).
2.8 Enzyme-linked immunosorbent assay (ELISA)
IL-1β secretion in VECs detected by ELISA. In brief, VECs were cultured in 24-well plates. Following treatment, cellular supernatants were collected and 100 μl of undiluted sample was used for ELISA analysis (NeoBioscience, Guangzhou, China). All of the measurements were performed according to the manufacturer’s protocol.
2.9 Bioinformatics analysis
We obtained genomic and miRNA sequences from miRDB (http://mirdb.org/miRDB/), which predicts miRNA targets in various species. MiR-125a-5p binding sites were identified using the TargetScan (http://www.targetscan.org/) and miRNA (http://www.microrna.org/microrna/) web servers. Hybridization minimum free energy was calculated using the RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/bibi/Tools.html) website. We use the following websites for CpG island analysis: MethPrimer (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi) and UCSC (http://genome.ucsc.edu/cgi-bin/hgGateway).
2.10 Mitochondrial membrane potential detection
In mitochondrial dysfunction (MDF), the transmembrane potential (Δψ) is disrupted, resulting in the change in the concentration of 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) entering the mitochondria. The use of flow cytometry to detect the intensity of green/red fluorescence can reflect the mitochondrial membrane potential and indirectly reflect mitochondrial function. Briefly, the treated cells were washed twice with PBS and centrifuged at 2,000 rpm for 5 min. Cells were suspended in 500 µl of JC-1 working solution and incubated at 37°C in a 5% CO2 incubator for 15 min. Centrifugation was performed at room temperature (2,000 rpm, 5 min); cells were harvested and resuspended in 500 µl of 1× incubation buffer. Finally, detection was achieved by flow cytometry (Ex = 488 nm; Em = 530 nm).
2.11 Statistical analysis
The data in this study are shown as means ± SEM differences among groups were analyzed using one-way analysis of variance accompanied with Newman–Keuls multiple comparisons test (GraphPad Prism version 5.0, CA). Two-tailed Student’s t test was used for comparison between two groups; p < 0.05 was considered statistically significant.
3 RESULTS
3.1 oxLDL induces VEC pyroptosis
oxLDL is a well-known atherogenic factor (Glass & Witztum, 2001). VECs are the barriers between the blood and the vascular wall, and damage to VECs is considered the starting point of As lesions (Sitia et al., 2010). To elucidate the relationship between oxLDL and pyroptosis, we used VECs for in vitro experiments. In VECs incubated with different concentrations of oxLDL (0, 25, 50, and 100 µg/ml) for 24 hr, the expressions of caspase-1, NLRP3, and proinflammatory cytokines (IL-1β) significantly increased (Figure 1a–c). We also used immunofluorescence to examine the effect of different concentrations of oxLDL on the expression of caspase-1 in VECs (Figure 1d). During pyroptosis, pores can be formed in the cell membrane and cause the release of cellular contents and positive staining of dead cells, as can be determined by PI staining (Miao, Rajan, & Aderem, 2011). To further characterize oxLDL-induced pyroptosis of VECs, we double-stained Hoechst 33342 and PI in VECs. Our results showed that oxLDL-induced pore formation and membrane rupture, as indicated by the increased extensive PI-positive staining of cells (Figure 1e,f). To clarify the effect of different concentrations of oxLDL on the death of VECs, we used LDH release assay. The results suggest that oxLDL upregulates the release of LDH in a concentration-dependent manner (Figure 1g). These results show that oxLDL concentration-dependently induces the pyroptosis of VECs. The optimum oxLDL concentration is 100 µg/ml. Thus, we used this concentration in our follow-up experiments.
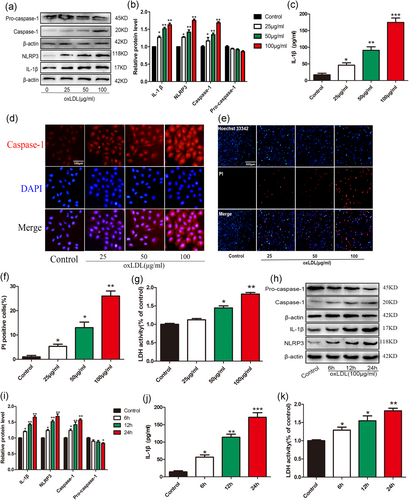
Oxidized low-density lipoprotein (oxLDL) triggered pyroptosis in vascular endothelial cells (VECs). (a,b) The protein levels of caspase-1, NLRP3 and interleukin (IL)-1β were upregulated in VECs after treatment with different concentration oxLDL for 24 hr, as indicated by western blot results, β-actin was used as an internal control *p < 0.05, **p < 0.01 versus control group.. (c) IL-1β production in the medium was increased with the elevated treatment concentration of oxLDL. *p < 0.05, **p < 0.01, ***p < 0.001 versus control group (d) Caspase-1 (red) fluorescence intensity were increased with the increase of the concentration of oxLDL. The nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Original magnifications, ×200; scale bar = 100 μm. (e,f) The percentage of propidium iodide (PI; red)-positive cells were increased in VECs after treatment with oxLDL. Original magnifications, ×100; scale bar = 500 μm. *p < 0.05, **p < 0.01 versus control group. (g) The relative lactate dehydrogenase (LDH) release was elevated in oxLDL-treated endothelial cells. *p<0.05, **p < 0.01 versus control group. (h,i) The protein levels of caspase-1, NLRP3, and IL-1β were upregulated in VECs after treatment with 100 μg/ml oxLDL for different time, as indicated by western blot results, β-actin was used as an internal control. *p < 0.05, **p < 0.01 versus control group. (J) IL-1β concentration in the medium was increased with the prolonging of oxLDL treatment time. *p < 0.05, **p < 0.01, ***p < 0.001 versus control group. (k) The relative LDH release was elevated after treatment with 100 μg/ml oxLDL for different time. *p < 0.05, **p < 0.01 versus control group. All results are expressed as the mean ± SD of three independent experiment [Color figure can be viewed at wileyonlinelibrary.com]
VECs were incubated with 100 µg/ml oxLDL for 0, 6, 12, and 24 hr to investigate whether oxLDL can induce pyroptosis of VECs in a time-dependent manner. Western blot results (Figure 1h–j) showed that the protein expression of NLRP3, caspase-1, and IL-1β significantly upregulated in VECs after 24 hr of incubation with oxLDL. With prolongated incubation time, the expressions of NLRP3, caspase-1, and IL-1β increased. The strongest effect was observed in the 24 hr group. To clarify the effect of different treatment times of oxLDL on the death of VECs, we used LDH release assay. The results suggest that oxLDL upregulates the release of LDH in a time-dependent manner (Figure 1k). The above results indicate that oxLDL can induce pyroptosis of VECs in a time- and concentration-dependent manner.
3.2 oxLDL downregulates TET2 expression in a concentration-dependent manner
TET2 acts as a DNA demethylase and can inhibit As to mediate the protective effects of hydrogen sulfide on the oxLDL-induced dysfunction of VECs (Peng et al., 2017). Our previous study showed that TET2 upregulates the level of autophagy against ApoE−/− mice As lesions (Peng et al., 2016), whereas the absence of TET2 accelerates the development of As (Fuster et al., 2017). Autophagy and pyroptosis are both associated with PCD and play important roles in atherosclerotic lesions. Therefore, we speculate that TET2 may be involved in oxLDL-induced VECs pyroptosis. Thus, we investigated the changes in TET2 during oxLDL-induced VECs pyroptosis, and the results suggest that oxLDL downregulated TET2 in a concentration-dependent manner (Figure 2).
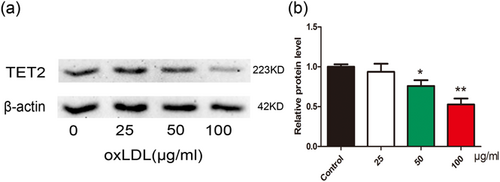
Oxidized low-density lipoprotein (oxLDL) inhibits tet methylcytosine dioxygenase 2 (TET2) expression in vascular endothelial cells (VECs). (a,b) The protein levels of TET2 was downregulated in VECs after treatment with different concentration oxLDL for 24 hr, as indicated by western blot results, β-actin was used as an internal control. *p < 0.05, **p < 0.01 versus control group. All results are expressed as the mean ± SD of three independent experiment [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Pyroptosis is curbed when TET2 is overexpressed
We conducted further experiments to further clarify the effect of TET2 on oxLDL-induced VECs pyroptosis. First, we tested the TET2 overexpression and interference efficiency. Immunofluorescence showed that the transfection efficiency was greater than 70% (Supporting Information Figure S1). The western blot results showed that TET2 overexpression and interfering lentivirus can significantly increase or reduce TET2 protein expression levels, respectively (Figure 3a,b). Further, we examined the effect of TET2 overexpression on VECs pyroptosis treated by oxLDL. As a result, in the VECs treated with oxLDL, overexpression of TET2 can inhibit the expression of pyroptosis-related proteins (NLRP3, caspase-1, and IL-1β; Figure 4a–c,e), reduce the release of LDH (Figure 4d), significantly reduce the number of PI-positive cells (Figure 4f,g). These results indicate that TET2 inhibits pyroptosis in VECs treated with oxLDL.
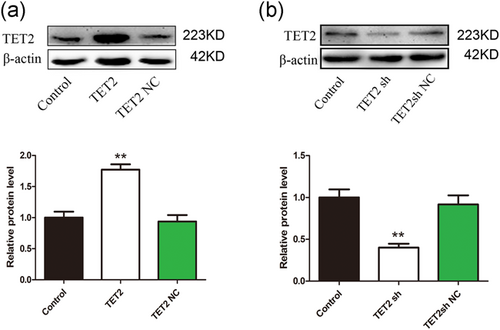
Tet methylcytosine dioxygenase 2 (TET2) overexpression/interference efficiency detection. TET2 overexpression (a)/interfering (b) lentivirus can significantly increase or reduce TET2 protein expression levels, respectively, as indicated by western blot results. β-actin was used as an internal control. **p < 0.01 versus the control group. All results are expressed as the mean ± SD of three independent experiment [Color figure can be viewed at wileyonlinelibrary.com]
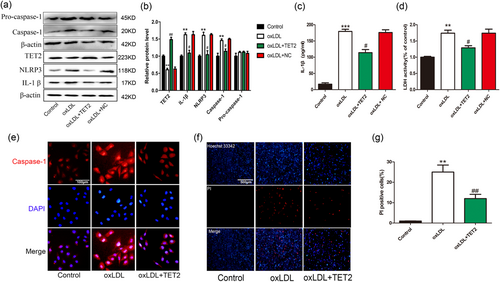
Overexpression tet methylcytosine dioxygenase 2 (TET2) inhibits vascular endothelial cells (VECs) pyroptosis induced by oxidized low-density lipoprotein (oxLDL). (a,b) The protein levels of caspase-1, NLRP3, and IL-1β in oxLDL-treated VECs were decreased and TET2 is increased after transfection of TET2 overexpression lentivirus. NC: negative control. **p < 0.01 versus control group; #p < 0.05, ##p < 0.01 versus oxLDL treatment group. (c) The interleukin (IL)-1β levels in the medium in oxLDL-treated VECs were decreased after transfection of TET2 overexpression lentivirus. ***p < 0.001 versus control group; #p < 0.05 versus oxLDL treatment group. (d) Pyroptotic cell death was determined by LDH release, and the relative LDH activity was suppressed by overexpression of TET2. **p < 0.01 versus control group; #p < 0.05 versus oxLDL treatment group. (e) Caspase-1 (red) fluorescence intensity were declined after transfection with TET2 overexpression lentivirus. The nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Original magnifications, ×200; scale bar = 100 μm. (f,g) Propidium iodide (PI)-positive cells were reduced by overexpression of TET2 in the presence of oxLDL. Original magnifications, ×100; scale bar = 500 μm. **p < 0.01 versus control group; ##p < 0.01 versus oxLDL treatment group. All results are expressed as the mean ± SD of three independent experiment [Color figure can be viewed at wileyonlinelibrary.com]
3.4 oxLDL increases the methylation level of VECs and can subside via overexpression of TET2
TET2, as a demethylase, is an important participant in the epigenetic modification. Epigenetic modification abnormalities are closely related to cell function and fate. Thus, we hypothesized that TET2 mitigate the oxLDL-induced pyroptosis of VECs may be associated with DNA methylation. oxLDL leads to DNA hypermethylation (including global DNA and mitochondrial DNA [mtDNA]). Subsequently, overmethylation of DNA causing MDF leads to an increase in ROS production. ROS increase causes NLRP3 inflammasome activation and promotes VEC death. Therefore, we examined the overexpression of TET2 on DNA methylation. 5hmC is a hydroxylated form of 5mc and represents the level of demethylation of DNA. Using 5hmC antibody for immunofluorescence, the results showed that oxLDL can reduce the level of 5hmC. Overexpressing TET2 can increase the level of 5hmC (Figure 5a), indicating that oxLDL can increase DNA methylation and overexpress TET2 can inhibit DNA methylation. However, the role of epigenetic regulation in oxLDL-induced pyroptosis of VECs requires more in-depth exploration, which we will explain in the discussion.
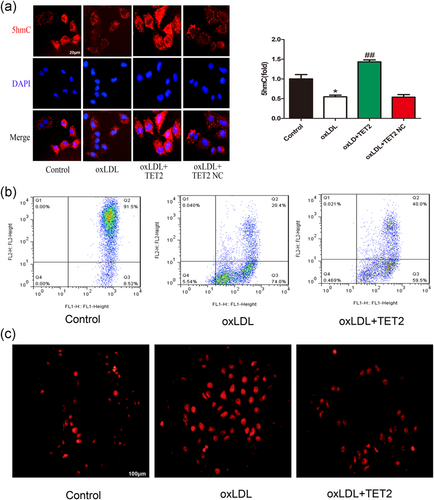
Overexpression of tet methylcytosine dioxygenase 2 (TET2) improve mitochondrial function and reduce reactive oxygen species (ROS) generation caused by oxidized low-density lipoprotein (oxLDL). (a) Immunofluorescence was used to detect intracellular 5- hydroxymethylcytosine (hmC) levels. oxLDL significantly reduced intracellular 5hmC (red) levels compared with the control group. Overexpression of TET2 increased intracellular 5hmC levels. The nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Original magnifications, ×400; scale bar = 20 μm. *p < 0.05 versus control group; ##p < 0.01 versus oxLDL treatment group. All results are expressed as the mean ± SD of three independent experiment. (b) The mitochondrial membrane potential of VECs was significantly inhibited in cells treated with oxLDL compared with control groups and transfected with TET2 overexpression lentivirus can improves oxLDL-induced mitochondrial membrane potential abnormalities, as assayed by flow cytometry. (c) oxLDL increased intracellular ROS level of VECs and this increase was inhibited by TET2 overexpression. Original magnifications, ×200; scale bar = 100 μm [Color figure can be viewed at wileyonlinelibrary.com]
3.5 Overexpression of TET2 can improve mitochondrial membrane potential abnormalities and reduce ROS generation caused by oxLDL
Epigenetic modifications (including methylation modifications) play a crucial role in cell survival. Studies have shown that nuclear and mtDNA methylation can affect mitochondrial function through S-adenosyl methionine (Wallace & Fan, 2010). Numerous studies have shown that mitochondria are closely related to cell death, and the disruption of mitochondrial transmembrane potential (Δψ) is considered to be one of the earliest times of the apoptotic cascade. This disruption occurs before the appearance of apoptotic features in the nucleus (chromatin condensation and DNA fragmentation). Once the mitochondrial transmembrane potential collapses, apoptosis becomes irreversible. Pyroptosis is similar to chromatin condensation and DNA fragmentation. Thus, we examined the effect of oxLDL, overexpression of TET2 on mitochondrial membrane potential. Flow cytometry results showed that oxLDL significantly increased the ratio of Q3 district/Q2 district compared with the control group, indicating that oxLDL may cause mitochondrial membrane potential abnormalities. Overexpression of TET2 can reduce the ratio of Q3 district/Q2 district compared with the oxLDL-treated group (Figure 5b), indicating that the overexpression of TET2 may extenuate Δψ abnormalities induced by oxLDL. These results indicate that oxLDL-induced pyroptosis of VECs is associated with MDF.
3.6 Pyroptosis is alleviated by ROS elimination
The mitochondrial membrane potential partially reflects mitochondrial function; the production of ROS mainly originates from mitochondria, and MDF can lead to increased ROS production (Desoti et al., 2012). ROS are important mediators for inflammasome activation (Guo, Callaway, & Ting, 2015). To elucidate the possible role of ROS in oxLDL-induced pyroptosis in VECs, we first detected the changes in ROS levels in VECs treated with oxLDL (100 µg/ml). Our results demonstrated that oxLDL treatment dramatically increased ROS levels, TET2 overexpression can inhibit the production of ROS (Figure 5c), which is consistent with its improved mitochondrial function. oxLDL-induced ROS also was buffered by NAC, a ROS inhibitor (Figure 6a). NAC also inhibited oxLDL-induced protein levels of NLRP3, caspase-1, and inflammatory cytokine IL-1β (Figure 6b–d,f). NLRP3 is the most important part of the inflammatory platform in pyroptosis. The N-terminal pyrin segment of NLRP3 serves as a scaffold to nucleate apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC), which contains a pyrin domain and a caspase activation and recruitment domain (CARD). Through its pyrin domain, ASC interacts with sensor molecules, and the CARD domain interacts with procaspase-1 and initiates procaspase-1 self-cleavage to form a caspase-1 mature body. On the one hand, activated caspase-1 recognizes the inactive IL-β and IL-18 precursors and converts them into mature inflammatory cytokines. In contrast, caspase-1 cleaves gasdermin D (GSDMD) to form 31-kDa-sized amino-terminal products (GSDMD-N) that mediate membrane pore formation. After membrane pore formation, the release of inflammatory factors is promoted, and cells swell, finally inducing pyroptosis (Afonina, Zhong, Karin, & Beyaert, 2017). Thus, the above result suggests that NLRP3 inflammasome activation depends on ROS generation. Additionally, NAC pretreatment reduced the number of host 33342 and PI double-positive cells (Figure 6g,h) LDH release in oxLDL-treated VECs (Figure 6e), suggesting the importance of ROS in oxLDL-induced VECs pyroptosis.
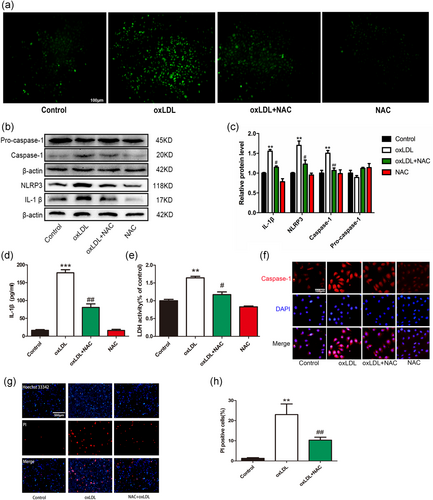
Pretreatment with reactive oxygen species (ROS) scavenger mitigated oxidized low-density lipoprotein (oxLDL)-induced vascular endothelial cells (VECs) pyroptosis. (a) oxLDL increased intracellular ROS level of VECs and this increase was inhibited by N-acetyl-cysteine (NAC, 5 mM). Original magnifications, ×200; scale bar = 100 μm. (b,c) NAC pretreatment suppressed the upregulation of caspase-1, interleukin (IL)-1β, and NLRP3 protein levels in the presence of oxLDL. **p < 0.01 versus control group; #p < 0.05; ##p < 0.01 versus oxLDL treatment group. (d) The IL-1β levels in the medium in oxLDL-treated VECs were decreased after pretreatment with NAC. ***p < 0.001 versus control group; ##p < 0.01 versus oxLDL treatment group. (e) Pyroptotic cell death was determined by lactate dehydrogenase (LDH) release, and the relative LDH activity was decreased when pretreated with NAC. **p < 0.01 versus control group; #p < 0.05 versus oxLDL treatment group. (f) Caspase-1 (red) fluorescence intensity were decreased when pretreatment with NAC. The nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Original magnifications, ×200; scale bar = 100 μm. (g,h) The percentage of propidium iodide (PI)-positive cells was declined after pretreatment with NAC in oxLDL-treated VECs. Original magnifications, ×100; scale bar = 500 μm. **p < 0.01 versus control group; ##p < 0.01 versus oxLDL treatment group. All results are expressed as the mean ± SD of three independent experiment [Color figure can be viewed at wileyonlinelibrary.com]
3.7 NF-κB involved in the protective effect of TET2 on oxLDL-induced VEC pyroptosis
Numerous studies have confirmed that ROS can activate NF-κB, NF-κB phosphorylation accounts for NLRP3 priming and the secondary step of activation (Luo et al., 2017). Thus, we further analyzed the effect of TET2 on NF-κB activity. Transfection of TET2 overexpression lentivirus led to the inhibition of IkBα degradation and NF-κB p65 nuclear translocation, whereas transfection with TET2 shRNA lentivirus significantly promoted IkBα degradation and NF-κB p65 nuclear translocation in oxLDL-treated VECs (Figure 7b–e). Furthermore, we examined the effect of TET2 on NF-κB p65 phosphorylation. Our results showed that TET2 overexpression can reduce the levels of phosphorylated Ser536 of NF-κB p65 (p-p65), which corresponds to its active form, and instead, TET2 sh lentivirus can increase the phosphorylation of NF-κB p65 (Figure 7a). Thus, these data point to an inhibitory role of TET2 in NF-κB activation in oxLDL-treated VECs and may partly explain why TET2 inhibits oxLDL-induced VECs pyroptosis.
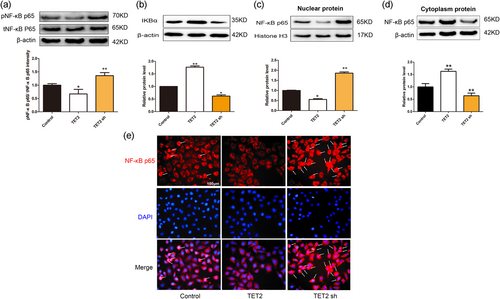
Effects of tet methylcytosine dioxygenase 2 (TET2) on nuclear factor-κB (NF-κB) activation in oxidized low-density lipoprotein (oxLDL)-induced dysfunction of vascular endothelial cells (VECs). VECs were transfected with or without TET2 overexpression/TET2 short hairpin RNA (shRNA) lentivirus in the presence of oxLDL for 24 hr. (a) Phosphorylated NF-κB p65 and total NF-κB p65 were evaluated by western blot analysis in each group of cells. ∗p < 0.05, ∗∗p < 0.01 versus control group. (b–d) The levels of IkBα protein and nuclear/cytoplasm NF-κB p65 protein were evaluated by western blot analysis in each group of cells. ∗p < 0.05; ∗∗p < 0.01 versus control group. All results are expressed as the mean ± SD of three independent experiment. (e) Distribution of NF-κB p65 protein expression was detected by immunostaining in each group of cells. NF-κB p65-positive staining is red. NF-κB p65 accumulation in the nuclei of the cells shows pink. 4′,6-Diamidino-2-phenylindole (DAPI) staining is blue. Original magnifications, ×200; scale bar = 100 μm [Color figure can be viewed at wileyonlinelibrary.com]
To further verify the role of NF-κB in oxLDL-induced VEC pyroptosis, we treated NF-κB inhibitor BAY 11–7082 (20 µM) for 2 hr and then added oxLDL (100 µg/ml) for 24 hr. Western blot detected NLRP3, caspase-1, and IL-1β protein expressions levels. The results show that NF-κB inhibition can decrease the expression of NLRP3, caspase-1, and proinflammatory cytokine IL-1β (Figure 8a–d) and LDH release (Figure 8e). BAY pretreatment also reduced the number of PI-positive cells (Figure 8f,g). These results indicate that oxLDL-induced endothelial cell pyroptosis requires NF-κB activation.
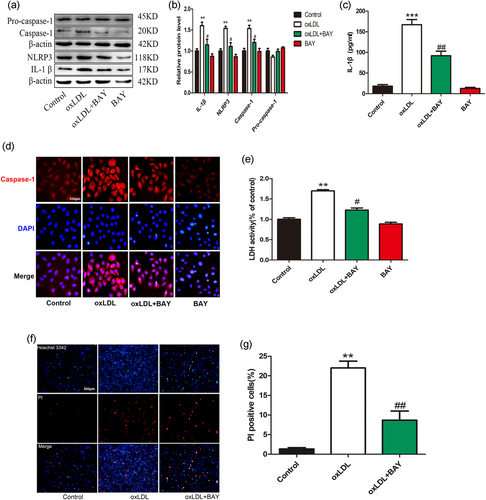
oxLDL-induced vascular endothelial cells (VECs) pyroptosis requires nuclear factor-κB (NF-κB) activation. (a,b) BAY pretreatment suppressed the upregulation of caspase-1, interleukin (IL)-1β, and NLRP3 protein levels in the presence of oxidized low-density lipoprotein (oxLDL). **p < 0.01 versus control group; #p < 0.05 versus oxLDL treatment group. (c) The IL-1β levels in the medium in oxLDL-treated VECs were decreased after transfection of BAY. ***p < 0.001 versus control group; ##p < 0.01 versus oxLDL treatment group. (d) Caspase-1 (red) fluorescence intensity were decreased when pretreatment with BAY. The nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Original magnifications, ×200; scale bar = 100 μm. (e) Pyroptotic cell death was determined by lactate dehydrogenase (LDH) release, and the relative LDH activity was suppressed by BAY pretreatment. **p < 0.01 versus control group; #p < 0.05 versus oxLDL treatment group. (f, g) The percentage of propidium iodide (PI)-positive cells was declined after pretreatment with BAY in oxLDL-treated VECs. Original magnifications, ×100; scale bar = 500 μm. **p < 0.01 versus control group, ##p < 0.01 versus oxLDL treatment group. All results are expressed as the mean ± SD of three independent experiment [Color figure can be viewed at wileyonlinelibrary.com]
3.8 Prediction and verification of the miR-125a-5p target site in the TET2 3′-untranslated region (3′-UTR)
Next, we further explored the molecular mechanism of downregulation of TET2 by oxLDL. The miRNA usually binding to the 3′-UTR of target messenger RNAs (mRNAs) to inhibit mRNA translation at the posttranscriptional level may participate in the downregulation of TET2 by oxLDL. Through bioinformatics analysis, we observed that miR-125a-5p can bind to the 3′-UTR of TET2 mRNA. First, we analyzed the conservation of miR-125a-5p using the miRDB database. The results showed that miR-125a-5p is highly conserved among various species, suggesting that miRNAs are important in the evolution of species and in modulating target gene expression (Figure 9a). Then, the TargetScan, MicroRNA, and RNAhybrid databases were utilized to predict the miR-125a-5p target sites in the 3′-UTR of TET2 transcripts. The results indicated that miR-125a-5p can bind to the TET2 3′-UTR. The TargetScan and MicroRNA websites identified a binding site for miR-125a-5p within the TET2 3′-UTR (Figure 9b). In addition, a free energy score of −30.1 kcal/mol (human) was calculated in both humans and mice on RNAhybrid (Figure 9c,d), showing that miR-125a-5p may form a stable association with TET2 mRNA. Previous studies have found that miR-125a-5p is increased (Simionescu et al., 2014), while TET2 is reduced in hyperlipidemic (Peng et al., 2016, 2017). Suggesting a negative association between miR-125a-5p and TET2. Based on the support of bioinformatics and existing literature, we investigated the relationship between miR-125a-5p and TET2 in oxLDL-induced VEC pyroptosis. We performed a luciferase reporter gene assay and noted that miR-125a-5p mimic can inhibit the expression of TET2, whereas miR-125a-5p inhibitor can increase the expression of TET2 (Figure 9e–g). These data indicated that miR-125a-5p can regulate TET2 expression at the posttranscriptional level by targeting the TET2 3′-UTR.
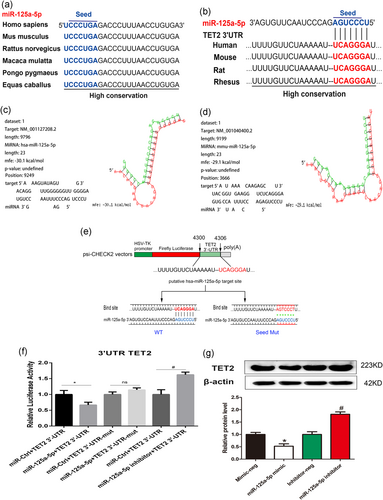
MiR-125a-5p inhibits tet methylcytosine dioxygenase 2 (TET2) expression by directly targeting the TET2 3′-untranslated region (3′-UTR). (a) MiR-125a-5p has similar sequences and is highly conserved among various species (miRDB). (b) TargetScan and microRNA databases were used to identify the putative site at which miR-125a-5p binds to the TET2 3′-UTR in multiple species. (c,d) The RNAhybrid database was used to evaluate the schematic alignment between the miR-125a-5p seed region and the TET2 3′-UTR target site. (e) Schematic depiction of the pFLuc-3′-UTR-TET2 luciferase reporter construct containing the 3′-UTR of the human TET2 gene; the predicted miR-125a-5p binding site is indicated (4,300 and 4,306). Show below is the wild-type miRNA binding site (UCAGGGA), along with the corresponding complementary sequence in the miR-125a-5p miRNA; the sequence of the mutated binding site (AGTCCCT) is also shown. (f) HEK 293T cells were transfected with the indicated constructs; 2 days after transfection, luciferase activity was measured and is expressed relative to cells transfected with the respective control miRNA. *p < 0.05 versus mimic-Ctrl group (mimic-neg group). ns, not significant. #p < 0.05 versus inhibitor-Ctrl group (inhibitor-neg group). (g) Cells were transfected with miR-125a-5p mimic (100 nM), miR-125a-5p inhibitor (100 nM), or their respective controls for 24 hr. Western blot analysis was then used to detect TET2 protein levels. *p < 0.05 versus mimic-neg group; #p < 0.05 versus inhibitor-neg group. All results are expressed as the mean ± SD of three independent experiment [Color figure can be viewed at wileyonlinelibrary.com]
3.9 oxLDL upregulates miR-125a-5p expression and inhibits miR-125a-5p mitigation of oxLDL-induced VEC pyroptosis
Existing research findings show that circulating miR-125a-5p levels increased in hyperlipidemic (HL) and/or hyperglycemic (HG) conditions, suggesting the contribution of these miRNAs to the atherosclerotic process (Simionescu et al., 2014). However, the relationship between oxLDL and miR-125a-5p in VEC-related pyroptosis remains unclear. Therefore, we examined the effect of different concentrations of oxLDL on the expression of miR-125a-5p in VECs by PCR. The results showed that the expression of miR-125a-5p increased with the increase in oxLDL concentration (Figure 10a). To further clarify the effect of miR-125a-5p on oxLDL-induced VEC pyroptosis, we conducted further experiments regarding the inhibition of miR-125a-5p. The result showed that miR-125a-5p inhibitor significantly reduced the expression of miR-125a-5p and miR-125a-5p mimic significantly increased the expression of miR-125a-5p (Figure 10b,c). Furthermore, transfection of miR-125a-5p inhibitor can reduce the expression of pyroptosis-related proteins (NLRP3, caspase-1, and IL-1β; Figure 10d,e,f,h) and the release of LDH (Figure 10g), significantly reduce the number of PI-positive cells (Figure 10i,j). These results demonstrate that inhibition of miR-125a-5p mitigates pyroptosis of VECs induced by oxLDL.
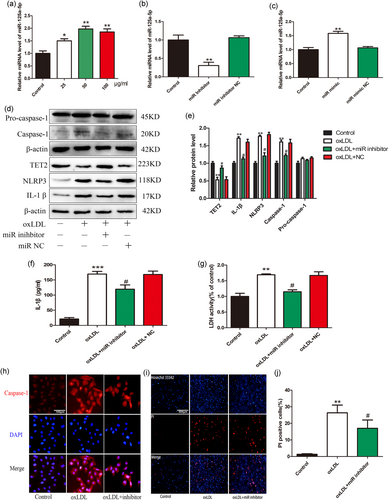
MiR-125a-5p inhibition reduces vascular endothelial cells (VECs) pyroptosis induced by oxidized low-density lipoprotein (oxLDL). (a) oxLDL upregulated miR-125a-5p expression in a concentration-dependent manner, as indicated by Bulge-LoopTM miRNA qRT-PCR results, the U6 gene was used as an internal control, *p < 0.05, **p < 0.01 vs. control group. (b) miR-125a-5p inhibitor significantly reduce the expression of miR-125a-5p, **p < 0.01 versus control group. (c) miR-125a-5p mimic significantly increased the expression of miR-125a-5p, **p < 0.01 versus control group. (d,e) The protein levels of caspase-1, NLRP3, and interleukin (IL)-1β in oxLDL-treated VECs were decreased while the TET2 expression was increased after transfection of miR-125a-5p inhibitor. NC: negative control. *p < 0.05 versus control group; **p < 0.01 versus control group; #p < 0.05 versus oxLDL treatment group. (f) The IL-1β levels in the medium in oxLDL-treated VECs were decreased after transfection of miR-125a-5p inhibitor. NC: negative control. ***p < 0.001 versus control group; #p < 0.05 versus oxLDL treatment group. (g) Pyroptotic cell death was determined by lactate dehydrogenase (LDH) release, and the relative LDH activity was suppressed by transfection of miR-125a-5p inhibitor. **p < 0.01 versus control group. #p < 0.05 versus oxLDL treatment group. (h) Caspase-1 (red) fluorescence intensity were declined after transfection with miR-125a-5p inhibitor. The nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Original magnifications, ×200; scale bar = 100 μm. (i,j) propidium iodide (PI)-positive cells were reduced by inhibition of miR-125a-5p in the presence of oxLDL. Original magnifications, ×100; scale bar = 500 μm. **p < 0.01 versus control group; #p < 0.05 versus oxLDL treatment group. All results are expressed as the mean ± SD of three independent experiment [Color figure can be viewed at wileyonlinelibrary.com]
3.10 MiR-125a-5p inhibitor reduces ROS production by improving DNA methylation and mitochondrial function
To determine whether miR-125a-5p inhibitor inhibits oxLDL-induced VEC pyroptosis consistent with overexpression of TET2, we examined the effects of miR-125a-5p inhibitor on DNA methylation, mitochondrial function, and ROS production. The results showed that miR-125a-5p inhibitor partially abolished the inhibition of DNA demethylation by oxLDL (Figure 11a), improved mitochondrial function (Figure 11b), reduced ROS production (Figure 11c). However, miR-125a-5p inhibitor transfection had almost no effect on oxLDL-caused DNA hypermethylation, abnormal membrane potential and ROS production, when transfection of the TET2 sh lentivirus simultaneously. This indirectly suggests that miR-125a-5p inhibitor improves mitochondrial function and inhibits ROS production, at least in part dependent on increased expression of TET2.

MiR-125a-5p inhibitor reduces reactive oxygen species (ROS) production by improving DNA methylation and mitochondrial function. (a) Immunofluorescence was used to detect intracellular 5-hydroxymethylcytosine (hmC) levels. Oxidized low-density lipoprotein (oxLDL) significantly reduced intracellular 5hmC (red) levels compared with the control group. Inhibition of miR-125a-5p increased intracellular 5hmC levels. However, miR-125a-5p inhibitor transfection had almost no effect on oxLDL-caused DNA hypermethylation, when transfection of the tet methylcytosine dioxygenase 2 (TET2) sh lentivirus simultaneously. The nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Original magnifications, ×400; scale bar = 20 μm. *p < 0.05 versus control group, #p < 0.05, versus oxLDL treatment group. All results are expressed as the mean ± SD of three independent experiment. (b) The mitochondrial membrane potential of vascular endothelial cells (VECs) was significantly inhibited in cells treated with oxLDL compared with control groups and transfected with miR-125a-5p inhibitor can improves oxLDL-induced mitochondrial membrane potential abnormalities, However, miR-125a-5p inhibitor transfection had almost no effect on oxLDL-caused abnormal membrane potential, when transfection of the TET2 sh lentivirus simultaneously, as assayed by flow cytometry. (c) oxLDL increased intracellular ROS level of VECs and this increase was inhibited by miR-125a-5p inhibitor transfected. However, miR-125a-5p has almost no effect on oxLDL-induced oxLDL production when simultaneously transfected with TET2 sh lentivirus. Original magnifications, ×200; scale bar = 100 μm [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
The data presented in the current study demonstrates that oxLDL-induced miR-125a-5p expression and inhibition of TET2 cause abnormal DNA methylation, increased ROS generation and NF-κB nuclear transposition, activation of NLRP3 inflammasome, inflammatory response, and subsequent pyroptosis in the setting of As. Inhibition of miR-125a-5p or overexpression of TET2 inhibited oxLDL-induced NLRP3 and caspase-1 activation and inflammatory cytokine IL-1β secretion. Further experiments revealed that the oxLDL-NLRP3-caspase-1-pyroptosis pathway is activated by ROS and NF-κB activation, as ROS scavengers (NAC) and NF-κB inhibitor BAY 11–7082 prevented endothelial cell pyroptosis. Previous studies on miR-125a-5p have been performed mainly in the tumor-related fields, such as hepatocellular carcinoma (G. Li, Zhang, Gong, & Huang, 2017), lung cancer (Jiang et al., 2010), and gastric cancer. In the cardiovascular system, miR-125a-5p is highly expressed in VECs; (Cao et al., 2018) reported that miR-125a-5p expression is increased in HL and/or HG state and accompanied by elevated IL-1β, which may promote As. However, in another study, Chen et al. (2009) revealed that miR-125a-5p inhibitors increase lipid phagocytosis of monocytes–macrophages and monocyte–macrophage inflammatory factors (IL-2, IL-6, transforming growth factor β, and tumor necrosis factor α) secretion. As a miRNA may target multiple genes, its role is complex, and a single gene change is inadequate to explain its role in protection or promotion of As. Unfortunately, thus far, no in vivo study reported the role of miR-125a-5p in As. Thus, in vivo experiments are needed to determine the specific role of miR-125a-5p in As.
DNA methylation modification is a form of epigenetic modification that occurs primarily in CpG dinucleotides and is often associated with gene suppression. The expression of TET2 and 5hmC in human atherosclerotic plaques is significantly lower than that in normal blood vessels. Both in vitro and in vivo experiments have shown that TET2 inhibits As (Peng et al., 2017). Global DNA methylation modification involves the methylation of nuclear and mitochondrial DNA. Although controversy has surrounded the epigenetic modifications of mtDNA in the past, mitochondrial epigenetic modifications have regained the attention of researchers since the discovery of the epigenetic modification products 5mc and 5hmc in mtDNA in 2011. The D-loop contains the promoter sites of both mtDNA strands; thus, the D-loop is important for transcriptional regulation of mtDNA, whereas the two epigenetic modification products, 5mc and 5hmc, are present in the D-loop, suggesting that both methylation and demethylation may occur on the D-loop (Shock, Thakkar, Peterson, Moran, & Taylor, 2011). Except for tissue-specific mitochondrial DNA methyltransferase 1 (mtDNMT1), which can bind to the D-loop of mtDNA, DNMT1, DNMT3a, and DNMT3b are also present in the mitochondria (Bellizzi et al., 2013). Therefore, mtDNA gene expression is inevitably modified and regulated by methylation. In our study, change in global DNA methylation was detected, and no specific detection was observed in mtDNA methylation. Possibly, the change in mtDNA methylation caused by TET2 downregulation is more closely related to mitochondrial function change and VEC pyroptosis. Further studies will be required to delineate the role of mtDNA methylation in MDF.
Several risk factors of As have been identified; these factors include hyperlipidemia, hypertension, diabetes, smoking, and aging. Cell death and inflammation are fundamental characteristics in the initiation and development of As (Chang et al., 2013). Pyroptosis is an inflammatory form of cell death and has been implicated in cardiovascular diseases (X. Li et al., 2014). The pathophysiologic mechanisms by which oxLDL promotes vascular diseases, particularly As, are manifold and complex. Endothelial cells play a critical role in maintaining the integrity of the vessel wall, and endothelial cell injury is an initiator of As (Davies, 2009). In agreement with a previous study, in this study, we noted that endothelial cells displayed characteristic features of pyroptosis in VECs with oxLDL, as evidenced by increased levels of caspase-1, NLRP3, IL-1β, and PI-positive cells. Wu et al. (2018) showed that ROS is fundamental in NLRP3 inflammasome activation. Similar to the research results of Wu et al., our study showed that oxLDL promoted ROS production, and this oxidative stress may possibly be an upstream mechanism for NLRP3 inflammasome activation, which can be neutralized by NAC, a ROS inhibitor. The antioxidant agent NAC diminished both inflammasome activation and inflammatory cytokine maturation and subsequently, the pyroptotic death induced by oxLDL in VECs.
Collectively, our results provide the first evidence that the proatherosclerotic property of oxLDL is primarily conferred by its ability to cause VEC pyroptosis via upregulation of miR-125a-5p-inhibited TET2 and increased NF-κB activation, activation of NLRP3 and caspase-1, and ultimately result in pyroptosis of endothelial cells. Hence, pyroptosis is possibly a cellular mechanism underlying the detrimental effect of oxLDL on As, with the production of ROS and activation of NLRP3 as upstream mediators. Targeting miR-125a-5p and its downstream TET2, through regulation of DNA methylation and improvement of mitochondrial function, may be considered a new approach for alleviating atherosclerotic lesions induced by oxLDL.
Our study features a few limitations. First, the specific role of miR-125a-5p in As and the effect of TET2 on pyroptosis of VECs still need to be tested in vivo. A second limitation of our study is that we provided no clarification on whether the effect of TET2 on VECs pyroptosis was achieved by the methylation of specific genes. We used online websites to analyze NLRP3, caspase-1, IL-1β, and GSDMD and observed that only GSDMD contain CpG islands (Supporting Information Figure S2). However, we failed to detect the effects of TET2 on the methylation level of GSDMD and we did not detect methylation level on mtDNA. The research on epigenetic modifications, especially the regulation of mitochondrial DNA methylation, will be the focus of future studies on TET2 in VECs exposed to oxLDL.
5 CONCLUSION
In this study, we revealed that miR-125a-5p mediates oxLDL-induced pyroptosis in VECs by downregulating TET2 and increasing NF-κB activation, activating NLRP3 and caspase-1, and ultimately causing pyroptosis of VECs. Furthermore, after TET2 downregulation, abnormal DNA methylation will occur, and subsequently, MDF will induce ROS production, which activates NLRP3 inflammasome, leading to the activation of caspase-1. Activated caspase-1 triggers pore formation of the membrane, DNA fragmentation, and release of mature IL-1β and IL-18 from cells, causing a sterile inflammation response and further contributing to pyroptotic cell death and subsequently promoting As (Figure 12).
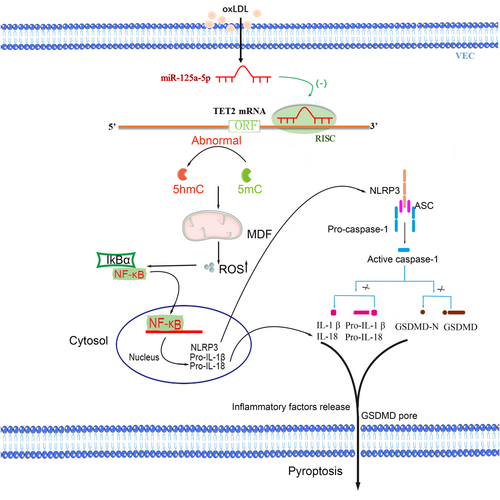
Proposed model of oxidized low-density lipoprotein (oxLDL)-induced vascular endothelial cells (VECs) pyroptosis. oxLDL increases miR-125a-5p expression, miR-125a-5p inhibits tet methylcytosine dioxygenase 2 (TET2) expression by targeting 3′-untranslated region (3′-UTR) of TET2 messenger RNA (mRNA), resulting in abnormal mDNA methylation levels and mitochondrial dysfunction (MDF) induces the production of reactive oxygen species (ROS), which activates nuclear factor-κB (NF-κB) and subsequent NLRP3 inflammasome, leading to the activation of caspase-1. In contrast, activated caspase-1 converts prointerleukin (IL)-1β and pro-IL-18 into mature inflammatory cytokines. In contrast, caspase-1 cleaves gasdermin D (GSDMD) generate 31 kDa-sized amino-terminal products (GSDMD-N) that triggers pore formation of the plasma membrane, DNA fragmentation and release of IL-1β and IL-18 from cells, causing a sterile inflammation response, further contributing to the pyroptosis and subsequent promoting atherosclerosis. 5hmc: 5-hydroxymethylcytosine; 5mc: 5-methylcytosine [Color figure can be viewed at wileyonlinelibrary.com]
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 81070221) and the Project of Hunan Provincial Health and Family Planning Commission (B2016139, B2016133); Project of Hunan Provincial Department of Education (16C1392).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




