RETRACTED: Molecular insights into development of Trichoderma interfusants for multistress tolerance enhancing antagonism against Sclerotium rolfsii Sacc
Abstract
The current study aimed at developing diverse Trichoderma fusants for fungicides, drought, and salt tolerance with enhanced antagonistic activity against Sclerotium rolfsii Sacc. Trichoderma virens NBAII Tvs12 (mycoparasitic) and Trichoderma koningii MTCC796 (multistress tolerant) were used as parental strains for development of interspecific protoplast fusants. A total of 36 stable fusants were used for mycoparasitism, fungicides, and abiotic stresses (drought and salt) tolerance. The results revealed 20 homozygous progenies showing characteristics of either one parental strain and 14 heterozygous mutants depicting traits of both parental strains. A novel concept of inhibition coefficient was established using growth-related key parameters that represent the pathogen biology and the biocontrol-related biophysics of Trichoderma fusants. The results indicated a differential inhibition coefficient of the test pathogen and the highest (92.88%) inhibition coefficient of S. rolfsii was observed by interstable fusant Fu21. It also grew better under fungicides and abiotic stress (drought and salt) conditions. The molecular characterization and heterozygosity analysis evidenced the highest observed heterozygosity (0.5441) and gene flow (0.3872) in stable heterozygous Fu21. Principal coordinates analysis exhibited 62.7% of total variability. The ecofriendly heterozygous Trichoderma fusant (Fu21) might be useful for biocontrol of stem rot disease under adverse conditions or as a part of integrated disease management.
1 INTRODUCTION
Trichoderma species have been known since 1930s to show antifungal activity and for use as biocontrol agents against plant pathogenic fungi namely, Sclerotium rolfsii (Hirpara, Harsukh Gajera, Hirpara, & Baljibhai Golakiya, 2017a), Fusarium species (Sivan & Chet, 1989), Pythium species (Naseby, Pascual, & Lynch, 1999) and Rhizoctonia species (Gajera, Hirpara, Katakpara, Patel, & Golakiya, 2016) through various mechanisms such as antibiosis, competition, suppression, mycoparasitism, induced resistance, hypovirulence, and predation (Cray et al., 2016). Our previous findings identified the antioxidant and antifungal constituents of potential Trichoderma for their biocontrol activity against stem rot disease (S. rolfsii) in groundnut (Hirpara, Gajera, Bhimani, & Golakiya, 2016). Trichoderma species (Ascomycetes, Hypocreales) are of economical importance as sources of antibiotics, enzymes, as plant growth promoters, commercial biofungicides, and degraders of xenobiotica (Hatvani et al., 2006; Savitha, Sadhasivam, & Swaminathan, 2010).
Application of chemical pesticides (carbendazim, thiram, tebuconazole, mancozeb, copperoxychloride, copper oxide, copper hydroxide, hexaconazole, propiconazole etc.) as part of the chemical management of stem rot disease is extensively used in farming systems. The combined approach of chemical fungicides at a lower concentration and the biological control agent Trichoderma could be efficient for plant protection with reduced environmental pollution (Basamma, 2008). Therefore, there is an emerging need for development of Trichoderma strains that can tolerate chemical fungicides, which would be a prerequisite for their application in an integrated disease management (IDM) strategy combining biological and chemical means of control (Gajera, Savaliya, Hirapara, Patel, & Golakiya, 2016).
All the components of a live cell after experimental removal of the cell wall are known as the protoplast (Hawksworth, Kirk, Sutton, & Pegler, 1995; Lalithakumari, 2000). Protoplast fusion has been reported in many species of Ascomycetes where the sexual cycle is difficult and also in Deuteromycetes where only the asexual cycle occurs. Genetic manipulation in genus Trichoderma can effectively be achieved through fusion of protoplasts (Lalithakumari & Mathivanan, 2003). The fungal protoplast fusion approach (intraspecific, intergeneric, and interspecific hybridization) is an imperative tool in genetic and physiological research through which potential strains with desirable characteristics could be obtained with minimum interruption in their physiology and biocontrol efficiency (Prabavathy, Mathivanan, Sagadevan, Murugesan, & Lalithakumari, 2006). The fusant with the ability for mycoparasitism and having the ability to tolerate pH, salt, drought, temperature, fungicides is most effective to control a wide range of pathogens (Hamari et al., 1997; Hatvani et al., 2006; Peberdy, 1989).
With this background, the study aimed to (a) develop an interspecific Trichoderma fusant using mycoparasitic biocontrol Trichoderma virens NBAII Tvs12 and the multistress tolerant Trichoderma koningii MTCC796 strain, (b) assess the multi stress tolerance capacity of the parental Trichoderma strain and their fusants for fungicides (carbendazim, Bavistin 50% WP; tebuconazole, Raxil 100% WP; thiram, Seedon 75% SD; and mancozeb, Mount-45 75% WP) and abiotic stresses (salt, sodium chloride (NaCl) and water, PEG), (c) evaluate biocontrol-related antagonistic activity using biophysical parameters to resolve the inhibition coefficient of Trichoderma fusants against pathogen S. rolfsii causing stem rot disease in groundnut, and (d) assess the molecular diversity, heterozygosity and conformation of fusants for genetic structure (homozygous or heterozygous) using gene specific SSR markers.
2 EXPERIMENTAL PROCEDURES
2.1 Sources of microbial strains
Based on our previous findings (Hirpara et al., 2016), the mycoparasitic Trichoderma strains T. virens NBAII Tvs 12 inhibiting the maximum growth of S. rolfsii was chosen as the parental strain P1. A total of six Trichoderma strains viz., Trichoderma harzianum NBAII Th1, T. harzianum Local, T. viride NBAII Tv23, T. virens NBAII Tvs12, Trichoderma harzianum NBAII Tha1, and T. koningii MTCC796 collected either from the Microbial-Type Culture Collection (MTCC), Chandigarh or the Indian-Type Culture Collection (ITCC, indicating accession number with NBAII) New Delhi and utilized for screening for multistress tolerance as per the method described later under Section 2.6. As a result, T. koningii MTCC796 showed the highest redial growth under adverse conditions (36.5 mm for mixed fungicides, carbendazim, tebuconazole, thiram, mancozeb; and 36 mm for drought, PEG 6000; and salt, NaCl) and hence was selected as a parental strain P2 to develop diverse fusants. The pathogen S. rolfsii was isolated from the infected groundnut plant by the hyphal tip method (Sinclair & Dhingram, 1985) and maintained on potato dextrose agar (PDA). The isolated pathogen strain was sent for identification and deposition at ITCC, Division of Plant Pathology, IARI, New Delhi and they identified the pathogen as S. rolfsii with the identity number 9107.13.
2.2 Protoplast formation
About 1.5 ml of conidial suspension (5 × 108 conidia/ml) of Trichoderma strains (P1 = Tvs12 and P2 = MTCC796) was inoculated into 50 ml of PDB and incubated on a rotary shaker at 120 rpm at 28 ± 1°C for 18 hr. The culture was harvested and the mycelia were collected by filtration. About 100 mg of wet mycelia were washed twice with sterile distilled water and 0.1 M phosphate buffer (pH 5.8) followed by incubation with Novozyme 234 (Sigma-Aldrich, St. Louis, MO) at 10 mg/ml concentration prepared in phosphate buffer containing 0.7 M KCl (osmotic stabilizer). The enzyme mycelium mixture was then incubated with mild shaking at 30°C temperature and the release of protoplasts was monitored regularly with a phase contrast microscope. After 3 hr, protoplast preparation was filtered using a sterile cotton wad followed by centrifugation at 3000 rpm at 4°C for 5 min. The aqueous supernatant was discarded and the pallet of protoplasts was resuspended immediately in buffer-osmotic stabilizer solution (El-Bondkly, 2006; El-Bondkly & Talkhan, 2007).
2.3 Examination of protoplast
Counting of protoplasts was carried out using two methods: (a) direct counting by haemocytometer observed under a phase contrast microscope and (b) counting of protoplast after Gram's crystal violet staining (1:4 dilutions) containing 0.3 M sucrose as an osmotic stabilizer. The protoplast suspension was centrifuged, diluted, and then stained with crystal violet (Savitha et al., 2010). The numbers of protoplasts were determined using a current formula (cells/ml) = the average count per square × the dilution factor × 104 (Kim, Ryu, & Lee, 1983).
2.4 Protoplast fusion
The protoplast fusion was performed by using the method of Kowsari, Motallebi, and Zamani (2014) with some modifications. Polyethylene glycol (PEG; MW, 6,000; Sigma) prepared in Sorbitol, Tris–HCl, CaCl2 (STC) buffer (0.6 M sorbitol; 10 mM Tris–HCl; 10 mM CaCl2, pH 6.5) was used as a fusogen agent. One milliliter of 30% wt/vol PEG solution was mixed with an equal volume of protoplast suspension (1 × 106 protoplasts/ml). Then the fusion mixture was incubated at 28 ± 1°C (bio-oxygen demand [BOD] incubator) for 15 min and the mixture was diluted with 1 ml of STC buffer. The PEG present in the fusion reaction mixture was washed away twice using STC buffer, and the fused protoplasts were collected by centrifugation for 10 min at 100 rpm, suspended in STC buffer (Hatvani et al., 2006).
2.5 Regeneration of fused protoplasts and fusants stability
The fused protoplasts were assessed for their ability to regenerate on agar medium as actively growing fungal colonies. The protoplast fusant suspension was diluted and 1 × 103 protoplasts were plated in a protoplast regeneration minimal medium (PRMM) containing (NH4)2SO4 (2.8 g), KH2PO4 (4 g), urea (600 mg), CaCl2·2H2O (600 mg), MgSO4 (200 mg), glucose (40 g), ZnSO4·H2O (2.8 mg), FeSO4·7H2O (10 mg), MnSO4·H2O (3.2 mg), CoCl·6H2O (4 mg), agar (20 g), and sucrose (100 g; Toyama, Yamaguchi, Shinmyo, & Okada, 1984). The regeneration of protoplasts and formation of colonies were observed on culture plates incubated at room temperature and individual colonies developed on plates were counted. The regenerated fusant colonies were transferred onto PDA plates (Kowsari et al., 2014). Nonfusion protoplasts of both the parental strains served as a control. The stability of derived fusants was evaluated using successive subculturing of fusion products (106 spores) on PDA for three generations (Fahmi, Al-Talhi, & Hassan, 2012). The numbers of produced colonies from each fusant strain were counted. Before subculturing, the slow growing homo or heterokaryotic colonies were subjected to nuclear fusion by exposure to ultraviolet for 2 min and cultivated on minimal medium (MM) supplemented with d-camphor followed by further incubation at 28 ± 1°C for 7 days to obtain stable mutants (Ogawa, Ohara, & Toyama, 1988). The parental strains (P1 and P2) were also incubated on MM agar as a control to check the diplodization pattern. Conidial morphology was observed under a phase contrast microscope. The formation of intra and inter fusants as homozygous and heterozygous mutants was confirmed by colony morphology, pigmentation, mycelial growth pattern, sporulation, multistress tolerance, mycoparasitism, and molecular heterozygosity estimated by gene specific SSR markers.
2.6 Fungicides and abiotic stress tolerance
The poison plate method was used to screen out true nuclear fusants having multistress tolerant capacity (Nene & Thapliyal, 1982). Minimum inhibitory concentrations (MIC) of four fungicides carbendazim 50 WP (5 ppm a.i.), tebuconazole 100 WP (500 ppm a.i.), thiram 75SD (1,000 ppm a.i.), Mancozeb 75 WP (3,000 ppm a.i.) were taken and mixed into PDA. Both parental Trichoderma strains (P1 and P2) and 36 fusants were inoculated on a PDA plate containing fungicides. However, 11.9% of PEG (MW, 6,000; − 0.2 MPa or −2 bar osmotic stress for drought tolerant) and 100 mM NaCl (−0.45 MPa or −4.5 bar water potential for salt tolerant) were also tested using PDA media for both parental strains and fusants. The plates were incubated for 15 days at 28 ± 1°C and radial growth was measured to screen the fusants for abiotic stress tolerance. Each experiment was run in triplicate, the standard deviation calculated and expressed as a bar in a graphical presentation.
2.7 Antagonistic activity
The screening of Trichoderma fusants and their parental strains against pathogen S. rolfsii was carried out as described by Dennis and Webster (1971) and Hirpara et al. (2016). Interactions between Trichoderma and S. rolfsii were carried out by inoculating culture media using a dual culture technique. The antagonist Trichoderma fusants and pathogen S. rolfsii were cultivated in 20 ml of PDA for 7 days. Two discs of 5-mm diameter, one with Trichoderma fusant mycelium and another with S. rolfsii mycelium were taken from the actively growing culture plates and placed opposite each other (70-mm apart) on a PDA plate at the same time for antagonism. For control, the medium was inoculated only with S. rolfsii. Plates were sealed using parafilm, incubated at 28 ± 1°C (BOD incubator) and assessed daily over a 12-day period. The experiment was conducted in triplicate and the standard deviation was calculated for expression of the inhibition coefficient, as described below.
2.8 Determination of growth rates and inhibition coefficient
where A is the pathogen S. rolfsii growth rate (mm/day); B the percentage of growth rate of value A over control; C the radial growth rate of S. rolfsii between the sites of inoculation of the fungal pathogen and biocontrol agent Trichoderma expressed as percentage over control; D the growth rate of pathogen S. rolfsii in zone of mixed culture (mm/day); and E the percentage of growth rate of value D over control.
2.9 Scanning electron microscopy (SEM) of best T. fusant and parental strains
A detailed representation of the initiation of interaction structures between the putative fusant and S. rolfsii and development of coiling was obtained at 10 days after inoculation (DAI) by observation under SEM. The Trichoderma fusant having the highest inhibitory coefficient and their parents (P1 and P2) were chosen for visualization under SEM. Mycelial samples were collected from the interaction zone and fixed with vapors of glutaraldehyde and osmium tetroxide (3:1) for 24 hr, air dried for 48 hr, and coated with gold using a sputter coater. The mycoparasitism of the best fusant on hyphal cells of the test pathogen were observed under SEM (Gajera, Bambharolia, Patel, Khatrani, & Golakiya, 2012).
2.10 Molecular variability and conformation of fusants compared with their parents
The genomic DNA of Trichoderma fusants (36), parents (2), and pathogen S. rolfsii were isolated using the cetyl trimethylammonium bromide (CTAB) method described by Narayanasamy and Saravana (2009) and subjected to simple sequence repeats (SSR) analysis using 44 gene specific SSR markers (Supporting Information Table S1). Polymerase chain reaction amplification was performed in 15 µl of total reaction volume, which contained: 1.5 µl 10× Taq buffer with MgCl2 for a final concentration of 1.5 mM, 1.2 µl 10 mM/µl deoxynucleoside triphosphate mix, 1.2 µl (0.2 mM) of forward and reverse primers, 0.3 µl 1 U/µl Taq polymerase and 1.2 µl (25 ng) of genomic DNA. A total volume to 15 µl per reaction was maintained using nuclease free double-distilled water (9.6 µl; Gajer & Vakharia, 2010; Hirpara, Gajera, Hirpara, & Golakiya, 2017b). The reactions were placed in a thermal cycler (VeritiTM; Applied Biosystem, Foster City, CA) and the reaction conditions are depicted in Supporting Information Table S1 (Baek, Howell, & Kenerley, 1999; Baranski, Klocke, & Nothnagel, 2008; Buensanteai, Mukherjeea, Horwitz, Dangott, & Kenerley, 2010; Carsolio, Gutiurrez, Jimtnez, Montagut, & Herrera-Estrella, 1994; Chowdappa, Nirmal kumar, Mohan kumar, & Krishna, 2012; Djonovic, Pozo, & Kenerley, 2006; Gajera, Bambharolia, Hirpara, Patel, & Golakiya, 2015; Geistlinger, Zwanzig, Heckendorff, & Schellenberg, 2015; Kheiri, Motallebi, Zamani, & Delj, 2014; Klemsdal, Clarke, Hoell, Eijsink, & Brurberg, 2006; Limon et al., 2004; Loc et al., 2011; Mendoza-Mendoza et al., 2007; Mukherjee, Mukherjee, & Kale, 2007; Prameela Devi, Prabhakaran, Kamil, Borah, & Pandey, 2012; Ramot, Viterbo, Friesem, Oppenheim, & Chet, 2004; Steyaert, Marlene, Jaspers, Carpenter, & Ridgway, 2004; Wiest et al., 2002). The amplification products were separated as per manufacturer protocol and guide lines using capillary electrophoresis genetic analyzers (30110 QIAxcel Advanced; Qiagen, Hilden, Germany). The bands were recorded for presence and absence according to codominant markers across the parental Trichoderma strains and their fusion products.
The data generated by scoring of SSR profiles of different primers (gene specific) were used to construct a similarity matrix using Jaccard's coefficients and the value of the similarity was utilized to determine cluster analysis (Rohlf, 1998). The clustering pattern was derived using sequential agglomerative hierarchical nonoverlapping. The unweighted pair group method analysis was used to construct a dendrogram for estimation of Nei's coefficients based genetic similarity on between fusants and parent group and within group using NTSYS-2.02. Bootstrap analysis using 1,000 resamplings was carried out to test robustness of the nodes (Pavlicek, Heda, & Fleg, 1999).
Popgene software (Population Genetics, Howard Towner, Loyola Marymount University, CA) was used to determine Nei's unbiased genetic distance among fusants and their parents along with that of the pathogen (S. rolfsii). The SSR phenotype matrix was analyzed on the basis of several population genetics indices such as numbers of polymorphic loci, %polymorphism, genetic diversity or heterozygosity (H = Nei's genediversity), observed numbers of alleles (na), effective numbers of alleles (ne), observed homozygosity (Ho), expected homozygosity (He), observed heterozygosity (Heo), expected heterozygosity (Hee) and Shannon's information index (I), total gene diversity (Fit), gene diversity within populations (Fis), coefficient values of genetic differentiation (Fst = Dst/Fit), and gene flow estimation (Nm 0.25 × (l – Fst)/Fst) and diversity among populations (Dst) using POPGENE program version 1.32 (Nei, 1978). The GenALEx software with three hierarchical levels (individual, population, and their groups) was used for molecular variance (AMOVA) analysis using data of gene specific SSR markers (Excoffier, Smouse, & Quattro, 1992; Peakall & Smouse, 2001). GenALEx software was also used to compute the principal coordinate analysis (PCA) that represents the relationship of the distance matrix elements based on their first three principal coordinates.
3 RESULTS AND DISCUSSION
3.1 Isolation of protoplasts and development of fusants
The mycelium of T. virens NBAII Tvs12 (P1) and T. koningii MTCC796 (P2) were lysed with enzymes Novozyme 234 and the result showed the lysis of cell wall followed by release of protoplast (Figure 1a,b). Swelling and rounding up of cell were observed initially. After 2 hr, the Trichoderma mycelium started lysing and almost complete digestion of the mycelial cell wall and protoplasts or spheroplasts was obtained at 3 hr of incubation of the parental strain with the enzyme (Figure 1c,d). The concentrations of lysing enzymes significantly affected the release of protoplasts from fungal mycelia. The lysis of mycelium was confirmed at 10 mg/ml enzyme concentration, where, standard protoplasting and fusion occurred. However, at a higher enzyme concentration (20 mg/ml), protoplasts bust immediately after release and partially disintegrated. The protoplasts just released out of the mycelium were tiny in size but after 3 hr they slowly enlarged to a spherical structure. When the protoplasts and PEG solution were mixed, they were stuck together and protoplasts pairings were observed (Figure 1e,f). After 15 min of incubation, in the place of contact of both the protoplasts, plasma membranes were dissolved and fusion of protoplasmic contents took place and finally, the fused protoplasts became single and large with round or oval shaped structures (Figure 1g).
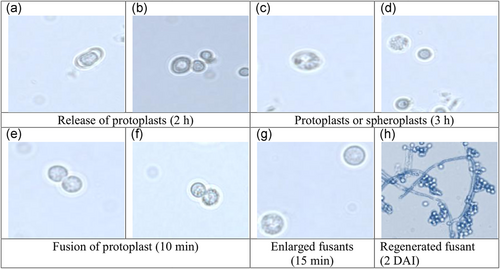
Protoplast fusion stages: (a,b) release of protoplasts from parent strains Trichoderma virens NBAII Tvs12 (P1) and Trichoderma koningii MTCC796 (P2), respectively, by means of enzymatic action, (c,d) release of protoplast from parent strains Tvs12 and MTCC796, respectively, (e,f) fusion of protoplast of parental strains after treatment with PEG, (g) enlargement of fusion product and (h) regeneration of fusant on PRMM media. PEG: polyethylene glycol; PRMM: protoplast regeneration minimal medium [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Regeneration of fused protoplasts and fusants stability
The fused protoplasts started to regenerate at 2 days (Figure 1h), the mycelium was developed after 3 days on PRMM media. The fusants and parental strains colonies exhibited fast mycelial growth after 3 days. Moreover, protoplasts that presented in clumps did not remain viable and failed to germinate into mycelial colonies. A total of 42 colonies were regenerated after protoplast fusion. The experiment of fusant stability was conducted by subculturing of the obtained fusants on PRMM up to three generations. The results showed that some of fusants were unstable and lost mycelial growth through cycles of mitotic division. So, as a result, a total of 36 fast growing stable fusants were obtained and designated as Fu1 to Fu36 (Figure 2a).
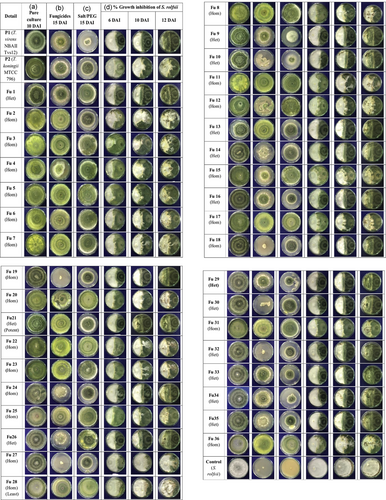
Screening of protoplast fusion products in comparison to their parental native Trichoderma strain on PDA media. (a) Pure culture of parents and regenerated stable fusants (10 DAI). (b) Fungicide tolerance (carbendazim 50 WP, 5 ppm a.i.; tebuconazole 100 WP, 500 ppm a.i.; thiram 75 SD, 1000 ppm a.i.; mancozeb 75 WP, 3000 ppm a.i.; 15 DAI). (c) Abiotic stress tolerance (salt 100 mM NaCl and drought 11.9% PEG 6000; 15 DAI). (d) Antagonism activity in dual culture (left side: test pathogen; right side: Trichoderma parents and fusants). Control: pathogen Sclerotium rolfsii (9107.13); DAI: days after inoculation; Fu1 to Fu36: 36 Trichoderma protoplast fusants; P1:Trichoderma virens NBAII Tvs12; P2: Trichoderma koningii MTCC796; PDA: potato dextrose agar; PEG: polyethylene glycol [Color figure can be viewed at wileyonlinelibrary.com]
The protoplast fusants obtained in the current study were further subjected to growth on MM agar to stimulate nuclear fusion before subculturing. The frequency of diplodization from homo/heterokaryone fusants can be enhanced by treatment of d-camphore. During incubation (7 days, 28°C), the conidia were almost able to germinate on the MM agar and heterokaryotic fusants segregated having the characteristics of both types of parental conidiam, while, homokaryotic fusants showed segregation pattern similar to either one parental strain. Thus, the resulting strains that grew on MM agar were regarded as homozygous/heterozygous mutants. It was observed that, the conidial size of heterozygous mutants was found to be higher than that of haploid parental strains (Supporting Information Figure S1). The 14 heterozygous interstrain fusants produced larger conidia representing both nuclei, which were functional. Prototrophy was fully restored and rapid growth permissive on the media. This, surely, would be the case when stable heterozygous mutants are produced by karyogamy. Furuya, Ishige, Uchida, and Yoshino (1983) obtained stable diploids from Aspergillus oryzae by protoplast fusion between healthy parents and its protease improved mutant. Similar to current study, they examined the mutant strain obtained by fusion of a single nuclei protoplast showing elevation of conidial size compared with their haploid parents.
Pe'er and Chet (1990) obtained the highest frequency of intra-specific protoplasts from T. harzianum through Novozyme 234 at 10 mg/ml concentration with 0.6 M KCl and 33% PEG. Savitha et al. (2010) regenerated an intergeneric hybrid by protoplasmic fusion between Graphium putredinis and Trichoderma harzianum using 50% PEG (fusogen agent) with CaCl2 for effective protoplast fusion. The use of PEG at higher concentrations caused shrinking and busting of protoplasts. However, 30% PEG in STC buffer was used for effective pairing of protoplast fusion in the current study.
Fahmi et al. (2012) developed interspecific protoplast fusants to enhance Trichoderma biocontrol activity against the phytopathogens Pythium ultimum, Rhizoctonia solani, and Fusarium oxysporum. Fu7 showed the highest ability of growth inhibition of phytopathogens during the presence of the fungicide used under study and it was concluded that protoplast fusion is an important tool for improvement of biocontrol activity of Trichoderma against plant diseases under the IDM strategy. El-Bondkly (2006) studied intergeneric protoplast fusion in fungi and observed that some of fusants were unstable mutants, some heterokaryones and new recombinants, which were different from the parental culture.
3.3 Morphological descriptors of fusants
Colony morphology was used for identification of intergeneric, intraspecific and interspecific fusion products, especially if the species differ greatly in colony morphology (Savitha et al., 2010). The parental colony of NBAII Tvs12 (P1) was greenish white in color and formed a discrete, ring like, circular zone on the PDA plate with a dense mycelium (Figure 2a). However, MTCC796 the P2 parental strain was observed in dark greenish yellow color and completely covered the surface of the culture plate with a thick green dense mycelium. Interestingly, 36 fusant colonies appearing on the third day after inoculation on PDA were morphologically observed as a white, very thick greenish yellow, sparse yellowish brown and a thick dense green mycelium. The conidiation of parental strains was a ring-like zone for NBAII Tvs12 (P1) and a circular zone for MTCC796 (P2), but their fusion products were observed with a ring like zone, circular zone and small discrete irregular ring-like zone conidiation appearance. The pigmentation of parental strains was greenish white and dark greenish yellow but the pigment of their fusants ranged from dark green, off-white to yellow. The intensity of yellow pigmentation in fusant strains was high as compared with that of the parents. Genetic interaction at the metabolic level was taking place during protoplast fusion, which may have resulted in the pigment variation in fusant colonies. The modification of pigment during protoplast fusion was also observed in other fungal species (Kevei & Peberdy, 1977).
3.4 Screening for fungicides and abiotic stress tolerance
The growth rate of Trichoderma parents and their 36 fusants was determined in the presence of fungicides as well as drought (PEG-6000) and salt (NaCl) conditions up to 15 DAI (Figure 2b,c). The protoplast fusion products were observed with significant differences in radial growth at a MIC of fungicides (carbendazim [5 ppm a.i.] + tebuconazole [500 ppm a.i.] + thiram [1000 ppm a.i.] + mancozeb (3000 ppm a.i.) and under abiotic stress (11.9% of PEG [drought] + 100 mM NaCl [salt]) conditions (Figure 3). Many fusants exhibited a considerable degree of tolerance. Result indicated that out of 36, 27 fusants were found to be viable under multistress conditions of which, 19 mutants, namely, Fu1, Fu2, Fu3, Fu4, Fu5, Fu6, Fu7, Fu8, Fu11, Fu12, Fu17, Fu20, Fu21, Fu22, Fu23, Fu25, Fu28, Fu31, and Fu36 were found to be faster growing than both the parental strains under fungicides, drought and salt stresses. Out of 36 stable fusants, 20 homozygous colonies showing characteristics of either one parental strain and 14 heterozygous mutants depicting traits of both parental strains such as mycoparasitism, fungicides and abiotic stress (drought and salt) tolerance (Figures 2b,c and 3).
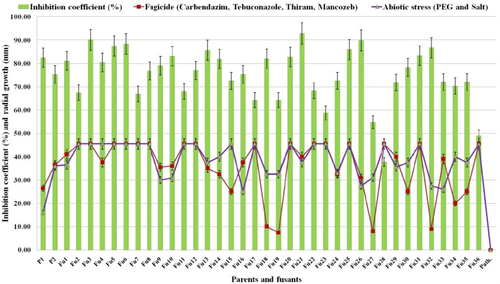
Inhibition coefficient of Sclerotium rolfsii (%) and radial growth of Trichoderma fusants (mm) cultured in PDA plates supplemented with a mixture of fungicides (carbendazim + tebuconazole + thiram + mancozeb) as well as abiotic stress (drought [PEG 6000] + salt [NaCl]) tolerance compared with their parental strains. Bar indicates standard deviation between three replications. Fu1–Fu36: Trichoderma fusants; P: pathogen Sclerotium rolfsii (9107.13); P1: Trichoderma virens NBAII Tvs12; P2: Trichoderma koningii MTCC796; PDA: potato dextrose agar [Color figure can be viewed at wileyonlinelibrary.com]
Hatvani et al. (2006) developed fusion products resistant to methyl benzimidazole-2-yl carbamate (MBC) and tebuconazol. They determined the sensitivity of two cold-tolerant Trichoderma strains (Trichoderma atroviride and T. harzianum) against 16 fungicides that are commonly used in agriculture, as a result, seven fungicides: carbendazim, thiram, mancozeb, imazalil, tebuconazole, captan, and copper sulfate inhibited growth of the Trichoderma strains significantly with 0.4, 50, 50, 100, 100, 100, and 300 µg/ml minimal inhibitory concentrations, respectively. The tebuconazole resistant mutants of parental strains would be useful as a potential candidate for IDM.
3.5 Inhibition coefficient of S. rolfsii during antagonism with T. fusants and parental strains
The 36 Trichoderma fusants along with parental strains were examined for antagonism ability against the test pathogen S. rolfsii up to 12 DAI using a dual culture assay (Figure 2d). In all the dual culture plates tested, at the contact zone, a concave oriented curve was obtained towards the pathogen (Figure 2d). The colony growth of antagonistic fusants was observed in a faster rate than the pathogenic fungi at the time the contact zone was obtained with the curvature in the same PDA plate depends on growth rate of both the colonies (Hayat & Christias, 2010; El-Refai, Said, Assawah, & Draz, 2013). However, if antagonistic fusants and pathogen have the same growth rate then the inhibition zone would be observed in a straight line when both the fungi came into contact (Petrescu, Sesan, & Oprea, 2012).
Inhibition coefficients of test pathogen were derived using different key parameters (Table 1). They represent the biology of the pathogen and biological control-related biophysics of Trichoderma. The results indicated differential values of the inhibition coefficient against the test pathogen. In the presence of P1 (NBAII Tvs12), P2 (MTCC796) and the potential antagonistic fusant (Fu21), the growth rate of the pathogen S. rolfsii was severely restricted to 11.8, 10.4, and 5.0 mm/day as compared with the control that was 15.7 mm/day (Table 1). This was, in part at least, achieved by the motility of the P1 that were capable to fill the available space rapidly, near the fungal colony where P2 was able to maintain a zone of inhibition. Both the parental strains (P1 and P2) were able to restrict the S. rolfsii growth and contain its diminutive colony (Figure 2d).
| No | S. rolfsii growth rate (mm/day) (A) | Growth rate A as a percentage of control (B) | Growth of S. rolfsii as a percentage of the distance between site of inoculation (C) | Time until contact between S. rolfsii and biocontrol agent (days) | S. rolfsii rate in zone of mixed culture (mm/day) (D) | Growth rate A as a percentage of control (E) | Inhibition coefficient (%) |
|---|---|---|---|---|---|---|---|
| P1 | 11.8 ± 1.64 | 37.5 ± 2.2 | 33.85 ± 2.42 | 4.2 ± 1.73 | −6.14 ± 1.16 | −55.14 ± 1.44 | 82.50 ± 1.78 |
| P2 | 10.4 ± 1.90 | 66.2 ± 2.3 | 25.94 ± 2.26 | 3.9 ± 1.68 | −0.14 ± 0.00 | −1.28 ± 1.67 | 47.40 ± 1.62 |
| Fu1 | 6.8 ± 3.35 | 43.5 ± 2.9 | 31.77 ± 3.11 | 4.1 ± 2.03 | −6.29 ± 1.30 | −56.42 ± 1.84 | 81.16 ± 2.50 |
| Fu2 | 12.4 ± 2.18 | 78.8 ± 2.3 | 3.43 ± 1.65 | 3.0 ± 1.09 | 6.43 ± 1.90 | 57.71 ± 1.98 | 55.57 ± 2.00 |
| Fu3 | 8.0 ± 1.00 | 51.2 ± 1.8 | −1.95 ± 1.87 | 2.9 ± 1.04 | 1.14 ± 0.71 | 10.26 ± 2.08 | 78.25 ± 1.49 |
| Fu4 | 9.4 ± 1.86 | 59.6 ± 2.1 | 18.66 ± 1.94 | 3.0 ± 2.39 | 0.14 ± 0.23 | 1.28 ± 2.15 | 68.44 ± 1.66 |
| Fu5 | 9.2 ± 1.96 | 58.9 ± 2.1 | 6.68 ± 1.55 | 3.6 ± 1.62 | −1.00 ± 0.00 | −8.98 ± 2.24 | 75.56 ± 1.57 |
| Fu6 | 8.9 ± 1.95 | 57.0 ± 1.9 | 2.52 ± 1.72 | 3.8 ± 2.31 | −0.14 ± 0.04 | −1.28 ± 2.32 | 76.45 ± 1.59 |
| Fu7 | 9.9 ± 1.08 | 63.0 ± 1.8 | 18.16 ± 2.05 | 3.9 ± 1.93 | 7.00 ± 1.48 | 62.84 ± 2.38 | 54.97 ± 1.75 |
| Fu8 | 10.6 ± 1.85 | 67.8 ± 2.1 | 19.46 ± 1.60 | 4.0 ± 1.74 | 0.14 ± 0.00 | 1.28 ± 2.45 | 64.84 ± 1.61 |
| Fu9 | 5.7 ± 1.13 | 36.2 ± 1.7 | 29.59 ± 1.68 | 4.2 ± 1.76 | −3.00 ± 1.18 | −26.93 ± 2.51 | 79.08 ± 1.64 |
| Fu10 | 5.8 ± 0.84 | 37.0 ± 1.6 | 18.18 ± 1.95 | 4.3 ± 2.45 | −2.86 ± 1.06 | −25.65 ± 2.56 | 83.08 ± 1.60 |
| Fu11 | 9.3 ± 1.63 | 59.3 ± 1.6 | 21.81 ± 1.80 | 3.9 ± 1.03 | 8.43 ± 1.32 | 75.66 ± 2.61 | 56.03 ± 1.79 |
| Fu12 | 10.3 ± 1.55 | 65.3 ± 1.8 | 22.75 ± 1.86 | 3.8 ± 1.51 | −0.14 ± 0.00 | −1.28 ± 2.65 | 65.04 ± 1.58 |
| Fu13 | 5.4 ± 1.42 | 34.3 ± 2.0 | 25.27 ± 2.29 | 4.1 ± 1.61 | −5.29 ± 1.35 | −47.45 ± 2.69 | 85.68 ± 1.96 |
| Fu14 | 6.1 ± 1.34 | 38.6 ± 1.9 | 23.90 ± 1.56 | 4.2 ± 2.16 | −3.86 ± 0.52 | −34.62 ± 2.72 | 81.91 ± 1.69 |
| Fu15 | 10.8 ± 1.77 | 68.8 ± 2.1 | 28.95 ± 2.09 | 3.6 ± 1.73 | 0.14 ± 0.00 | 1.28 ± 2.75 | 60.64 ± 1.74 |
| Fu16 | 6.8 ± 2.16 | 43.1 ± 2.4 | 37.19 ± 2.47 | 3.1 ± 1.67 | −4.14 ± 0.60 | −37.19 ± 2.76 | 75.32 ± 2.07 |
| Fu17 | 11.4 ± 1.90 | 72.7 ± 2.3 | 40.77 ± 2.59 | 3.2 ± 2.43 | 1.29 ± 0.00 | 11.54 ± 2.76 | 52.30 ± 1.91 |
| Fu18 | 6.0 ± 1.18 | 38.1 ± 1.9 | 27.34 ± 1.84 | 3.1 ± 1.85 | −4.57 ± 0.85 | −41.04 ± 2.73 | 82.01 ± 1.71 |
| Fu19 | 7.8 ± 1.27 | 49.7 ± 1.7 | 48.00 ± 1.79 | 3.0 ± 1.74 | −1.86 ± 1.57 | −16.67 ± 2.74 | 64.25 ± 1.82 |
| Fu20 | 9.6 ± 1.10 | 61.1 ± 1.7 | 11.80 ± 1.76 | 3.9 ± 1.86 | 0.00 ± 0.04 | 0.00 ± 2.68 | 70.84 ± 1.46 |
| Fu21 | 5.0 ± 1.60 | 31.5 ± 1.9 | 18.95 ± 1.58 | 2.9 ± 2.01 | −7.29 ± 1.61 | −65.40 ± 2.69 | 92.88 ± 1.87 |
| Fu22 | 10.1 ± 1.77 | 64.3 ± 2.1 | 19.15 ± 1.67 | 3.2 ± 1.75 | 5.71 ± 2.18 | 51.30v2.70 | 56.36 ± 2.08 |
| Fu23 | 10.9 ± 2.17 | 69.4 ± 2.2 | 37.87 ± 2.56 | 3.9 ± 1.24 | 5.71 ± 2.05 | 51.30 ± 2.71 | 46.83 ± 2.34 |
| Fu24 | 7.7 ± 1.17 | 48.8 ± 1.7 | 34.19 ± 1.99 | 3.0 ± 1.09 | −3.29 ± 0.46 | −29.49 ± 2.66 | 72.59 ± 1.59 |
| Fu25 | 9.5 ± 2.79 | 60.6 ± 2.5 | 16.56 ± 2.92 | 3.5 ± 1.45 | −2.71 ± 0.00 | −24.37 ± 2.62 | 74.01 ± 2.17 |
| Fu26 | 5.6 ± 1.62 | 35.9 ± 1.9 | 20.18 ± 1.61 | 3.9 ± 2.87 | −6.86 ± 0.94 | −61.55 ± 2.62 | 89.89 ± 1.74 |
| Fu27 | 7.3 ± 1.20 | 46.8 ± 1.8 | 36.08 ± 2.15 | 3.8 ± 2.45 | 6.71 ± 1.08 | 60.27 ± 2.61 | 54.79 ± 1.76 |
| Fu28 | 14.2 ± 1.92 | 90.4 ± 2.1 | 49.58 ± 2.95 | 3.9 ± 2.09 | 10.14 ± 0.69 | 91.05 ± 2.55 | 25.80 ± 2.05 |
| Fu29 | 7.9 ± 2.01 | 50.5 ± 2.2 | 51.41 ± 2.52 | 2.9 ± 1.66 | −7.00 ± 1.56 | −62.84 ± 2.42 | 71.80 ± 2.13 |
| Fu30 | 6.4 ± 1.60 | 41.0 ± 1.9 | 29.47 ± 2.13 | 3.1 ± 1.78 | −3.57 ± 0.85 | −32.06 ± 2.26 | 78.24 ± 1.75 |
| Fu31 | 9.8 ± 2.01 | 62.2 ± 2.3 | 15.68 ± 2.03 | 3.0 ± 1.34 | −1.43 ± 0.04 | −12.82 ± 2.20 | 71.41 ± 1.71 |
| Fu32 | 5.2 ± 1.64 | 32.8 ± 2.0 | 22.12 ± 1.82 | 2.9 ± 1.09 | −4.86 ± 0.81 | −43.60 ± 2.18 | 86.74 ± 1.69 |
| Fu33 | 7.7 ± 1.62 | 48.8 ± 1.9 | 48.75 ± 2.16 | 2.8 ± 3.01 | −6.14 ± 1.73 | −55.14 ± 2.13 | 71.99 ± 1.91 |
| Fu34 | 7.4 ± 1.78 | 46.9 ± 1.9 | 42.03 ± 2.17 | 2.7 ± 2.46 | −3.29 ± 0.90 | −29.49 ± 1.92 | 70.31 ± 1.73 |
| Fu35 | 7.7 ± 2.84 | 48.8 ± 2.2 | 42.98 ± 2.88 | 2.7 ± 1.68 | −4.86 ± 0.91 | −43.60 ± 1.74 | 72.02 ± 2.11 |
| Fu36 | 11.9 ± 1.84 | 76.0 ± 1.6 | 36.56 ± 1.62 | 3.9 ± 1.33 | 10.00 ± 0.81 | 89.77 ± 1.50 | 37.02 ± 1.47 |
| Control | 15.7 ± 2.23 | 99.9 ± 0.0 | 99.93 ± 0.01 | 0.0 ± 0.00 | 11.14 ± 0.49 | 100.03 ± 1.28 | 0.04 ± 0.80 |
- Note. Values are expressed as mean ± standard deviation between three replications.
The parental strain NBAII Tvs12 (P1) and Fu21 confirmed potent inhibitory activity against S. rolfsii, which was visible even from the initial assessment (one day after inoculation). By contrast, the inhibitory activity of the MTCC796 strain (P2) was only observed once the antagonist and pathogen colonies came into contact (Figure 2d). For the purpose of biocontrol, wide coverage of plant surfaces with the antagonistic agent is required (Elad & Kapat, 1999; Elad, Chet, & Henis, 1983), in practice, it is implausible that every mycelial cell or spore of a pathogen comes into contact with one or more cells of the Trichoderma (Gajera et al., 2015). So for this reason, 40% of substantial weighting was given to the ability to inhibit the fungal pathogen before contact (values A–C; Table 1). This said entire coverage of the plant pathogen through mycoparasitism by antagonist is rarely attained in practice by the antagonist Trichoderma. Where coverage with the Trichoderma biocontrol is incomplete, efficacy of control is further reduced when hyphae of the fungal plant-pathogen grow in between or away from cells/colonies of the biocontrol agent. For this reason, 20% of weightage was put on this final component of the inhibition coefficient (values D and E; Table 1). In the event that the Trichoderma biocontrol agent showed mycoparasitism, the value E (fungal growth rate in the zone of mixed culture) would automatically be negative with a higher value, such as for Fu21 (−65.40) followed by Fu29 (−62.84), Fu26 (−61.55), Fu33 (−55.14), P1 (−55.14) and Fu32 (−53.60). This was reflected by a higher value for the inhibition coefficient, such as Fu21 (92.88) followed by Fu26 (89.89), Fu32 (86.74) and P1 (82.50). For those biocontrol agents that maintain the zone of inhibition, the value E were automatically be zero or around zero, namely, P2 (−1.28), Fu4 (1.28), Fu6 (−1.28), Fu8 (1.28), Fu12 (−1.28), Fu15 (1.28), and Fu20 (0.00; Table 1). For fusants in which the pathogen over grew on the antagonist, a higher positive E value were obtained as Fu28 (91.05), Fu36 (89.77), Fu11 (75.56), Fu2 (57.71), and Fu27 (60.27) which resulted in a lower inhibition coefficient value, such as Fu28 (25.80), Fu36 (37.02), and Fu2 (47.04; Table 1).
The potent Fu21 is the only candidate mutant that produced the highest inhibition coefficient (92.88%) against the pathogen S. rolfsii in dual cultures and grew efficiently under fungicides and abiotic (drought and salt) stresses indicating better antagonistic activity than its parent during adverse conditions (Figure 3).
The spatial dynamics of the interactions that were observed in the current study (Figures 2 and 3 and Table 1) have parallels with the findings of interactions between antagonistic bacterial strains and the fungi F. oxysporum pathogen (Leong, Pettersson, Rice, Hocking, & Schünrer, 2011) and the growth inhibition activities of Bacillus subtilis secreted metabolites against F. oxysporum (Magan & Lacey, 1984; Pryor, Siebert, Gibson, Gossett, & Walker, 2007). Pan and Bhagat (2007) used the dual culture method for screening of Trichoderma isolates against five soil borne pathogen namely, F. oxysporum, R. solani, Macrophomina phaseolina, S. rolfsii, and Pythium species and the study revealed that Trichoderma isolates not only differ in their activity against different phytopathogens but antagonists themselves differed in the action against any single pathogen, and required 3–7 days after incubation to attain the S1 phase (antagonist completely overgrew on pathogen). This may be due to the variability in antagonistic isolates isolated from diverse ecological niches. The pathogens Pythium species and R. solani were highly affected by all Trichoderma isolates (T. harzianum, T. virens, and T. viride), whereas M. phaseolina and S. rolfsii were comparatively less inhibited.
3.6 Scanning micrograph of potent Trichoderma fusant and parental strains
The interaction effect of parents and the potent fusant with S. rolfsii were examined under SEM at 10 DAI (Figure 2d). The parent P1 (NBAII Tvs12) overgrew on the pathogen and a coil-like structure around the pathogen hyphae was observed (Figure 4a). However, the P2 (MTCC796) strain inhibited the growth of the pathogen through antibiosis. The secreted compounds of P2 into PDA efficiently degraded the pathogen mycelia (Figure 4b). The best strain Fu21 completely overgrew on the S. rolfsii pathogen with mycoparasitism as observed in microscopy observations and the contact zone revealed that the parasitic Trichoderma hyphae reached and grew over the mycelial surface of the test pathogen with surround coiling, hook like structure and formation of spores over S. rolfsii mycelia and later it formed apressoria-like structures without penetrating the S. rolfsii cell wall. The S. rolfsii invaded hyphae looked disintegrated and disrupted under SEM (Figure 4c). Gajera et al. (2012) obtained the pathogen specific mechanism of Trichoderma antagonists for biocontrol activity. The strain T. koningii MTCC796 was able to overgrow on M. phaseolina, coiling around hyphae and form apressoria and a hook-like structure. Contrarily, our study revealed that the T. koningii MTCC796 (P2) degrade S. rolfsii mycelia by formation of an inhibition zone, which suggested that the T. koningii MTCC796 adopted the pathogen dependant mode of action for biological control activity.
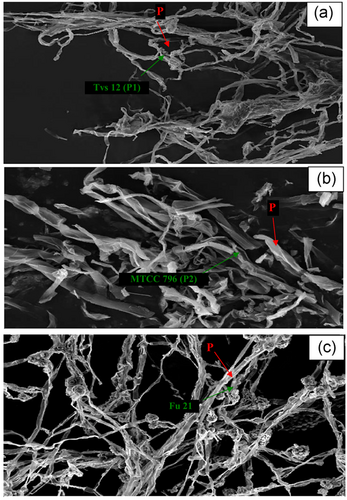
Scanning electron micrograph depicting evidence of mycoparasitism or pathogen Sclerotium rolfsii (P) mycelium degradation pattern of antagonist (a) Trichoderma virens NBAII Tvs12 (P1), (b) Trichoderma koningii MTCC796 (P2) and (c) potent Trichoderma fusant (Fu21) at 10 days after inoculation [Color figure can be viewed at wileyonlinelibrary.com]
3.7 Molecular variability and relationship among fusants and their parents
In the current study, molecular variability was analyzed across 36 fusants, parents (P1 = NBAII Tvs12 and P2 = MTCC796) and the pathogen (S. rolfsii) using 44 gene-specific SSR markers (Figure 5). The codominant marker analysis was carried out for diversity analysis and molecular conformations of fusants using SSR data. The values of the effective and observed allele for the best fusant (Fu21) were found to be 2.73 and 4.00, respectively (Table 2). Zhang, Blair, and Wang (2008) observed that the average number of alleles was found to be 6.07 with 2.64 effective numbers of alleles in the common bean. The average observed and expected homozygosity values in the combined population of 36 fusants, parents and pathogen were found to be 0.7892 and 0.3499, respectively. The mean of the observed and expected heterozygosity values for all populations were 0.2108 and 0.6501, respectively and the average Nei's expected heterozygosity (gene diversity across alleles) value per population was found to be 0.6453.

Gene specific SSR markers amplified across Trichoderma fusants and their parents. Fu1–Fu36: Trichoderma fusants; M: DNA size marker (50–1.5 bp); P1: Trichoderma virens NBAII Tvs12; P2: Trichoderma koningii MTCC796; P: pathogen Sclerotium rolfsii (9107.13) [Color figure can be viewed at wileyonlinelibrary.com]
| Locus | Naa | Neb | Ic | Obs Homd | Obs Hetd | Exp Home | Exp Hete | Neif |
|---|---|---|---|---|---|---|---|---|
| P1 | 4.00 | 1.6666 | 0.7665 | 0.8529 | 0.1471 | 0.5971 | 0.4029 | 0.4000 |
| P2 | 4.00 | 2.7418 | 1.1148 | 0.8088 | 0.1912 | 0.3600 | 0.6400 | 0.6353 |
| Fu1 | 4.00 | 2.5710 | 1.0665 | 0.7206 | 0.2794 | 0.3844 | 0.6156 | 0.6111 |
| Fu2 | 4.00 | 2.9109 | 1.1441 | 0.8235 | 0.1765 | 0.3387 | 0.6613 | 0.6561 |
| Fu3 | 4.00 | 2.9294 | 1.1471 | 0.8088 | 0.1912 | 0.3365 | 0.6635 | 0.6586 |
| Fu4 | 4.00 | 2.8927 | 1.1400 | 0.8235 | 0.1765 | 0.3408 | 0.6592 | 0.6543 |
| Fu5 | 4.00 | 2.7714 | 1.1162 | 0.8529 | 0.1471 | 0.3561 | 0.6439 | 0.6392 |
| Fu6 | 4.00 | 3.0292 | 1.1634 | 0.8382 | 0.1618 | 0.3252 | 0.6748 | 0.6699 |
| Fu7 | 4.00 | 3.1989 | 1.2282 | 0.8235 | 0.1765 | 0.3075 | 0.6925 | 0.6874 |
| Fu8 | 4.00 | 3.2598 | 1.2512 | 0.7794 | 0.2206 | 0.3016 | 0.6984 | 0.6932 |
| Fu9 | 4.00 | 2.8437 | 1.1438 | 0.7647 | 0.2353 | 0.3464 | 0.6536 | 0.6488 |
| Fu10 | 5.00 | 2.7073 | 1.1398 | 0.7206 | 0.2794 | 0.3647 | 0.6353 | 0.6306 |
| Fu11 | 5.00 | 3.0411 | 1.1966 | 0.7794 | 0.2206 | 0.3239 | 0.6161 | 0.6712 |
| Fu12 | 5.00 | 2.9900 | 1.2010 | 0.7941 | 0.2059 | 0.3295 | 0.6705 | 0.6655 |
| Fu13 | 4.00 | 2.8837 | 1.1830 | 0.7794 | 0.2206 | 0.3419 | 0.6581 | 0.6532 |
| Fu14 | 4.00 | 2.8792 | 1.1516 | 0.6165 | 0.3235 | 0.3425 | 0.6575 | 0.6527 |
| Fu15 | 5.00 | 2.9266 | 1.2152 | 0.8088 | 0.1912 | 0.3368 | 0.6632 | 0.6583 |
| Fu16 | 4.00 | 2.7450 | 1.1461 | 0.7941 | 0.2051 | 0.3596 | 0.6404 | 0.6357 |
| Fu17 | 5.00 | 3.3447 | 1.2959 | 0.8382 | 0.1618 | 0.2938 | 0.7062 | 0.7010 |
| Fu18 | 4.00 | 2.5103 | 1.0914 | 0.8382 | 0.1618 | 0.3939 | 0.6061 | 0.6016 |
| Fu19 | 4.00 | 2.9036 | 1.1986 | 0.8235 | 0.1765 | 0.3395 | 0.6605 | 0.6556 |
| Fu20 | 4.00 | 3.2759 | 1.2470 | 0.7647 | 0.2353 | 0.3001 | 0.6999 | 0.6947 |
| Fu21 | 4.00 | 2.7385 | 1.0953 | 0.4559 | 0.5441 | 0.3605 | 0.6395 | 0.6348 |
| Fu22 | 5.00 | 3.3410 | 1.2878 | 0.7941 | 0.2059 | 0.2941 | 0.7059 | 0.7007 |
| Fu23 | 5.00 | 3.3666 | 1.3058 | 0.7794 | 0.2206 | 0.2918 | 0.7082 | 0.7030 |
| Fu24 | 4.00 | 2.6767 | 1.1324 | 0.7647 | 0.2353 | 0.3690 | 0.6310 | 0.6264 |
| Fu25 | 5.00 | 3.0094 | 1.2456 | 0.7500 | 0.2500 | 0.3273 | 0.6727 | 0.6677 |
| Fu26 | 4.00 | 2.7192 | 1.1208 | 0.8382 | 0.1618 | 0.3631 | 0.6369 | 0.6322 |
| Fu27 | 4.00 | 2.6559 | 1.1351 | 0.7353 | 0.2647 | 0.3719 | 0.6281 | 0.6235 |
| Fu28 | 4.00 | 2.8756 | 1.1507 | 0.7647 | 0.2353 | 0.3429 | 0.6571 | 0.6522 |
| Fu29 | 4.00 | 2.4800 | 1.0722 | 0.7647 | 0.2353 | 0.3988 | 0.6012 | 0.5968 |
| Fu30 | 4.00 | 2.5103 | 1.0786 | 0.7206 | 0.2794 | 0.3939 | 0.6061 | 0.6016 |
| Fu31 | 4.00 | 3.4559 | 1.3055 | 0.8235 | 0.1765 | 0.2841 | 0.7159 | 0.7106 |
| Fu32 | 4.00 | 2.7240 | 1.1612 | 0.7794 | 0.2206 | 0.3624 | 0.6376 | 0.6329 |
| Fu33 | 4.00 | 2.8092 | 1.1581 | 0.7941 | 0.2059 | 0.3512 | 0.6488 | 0.6440 |
| Fu34 | 4.00 | 2.6280 | 1.1119 | 0.8529 | 0.1471 | 0.3759 | 0.6241 | 0.6195 |
| Fu35 | 4.00 | 2.6521 | 1.1242 | 0.8971 | 0.1029 | 0.3724 | 0.6276 | 0.6229 |
| Fu36 | 4.00 | 3.2993 | 1.2563 | 0.7647 | 0.2353 | 0.2979 | 0.7021 | 0.6969 |
| Pathogen | 5.00 | 2.6690 | 1.1996 | 0.9853 | 0.0147 | 0.3700 | 0.6317 | 0.6253 |
| Mean | 4.23 | 2.8625 | 1.1613 | 0.7892 | 0.2108 | 0.3499 | 0.6501 | 0.6453 |
| St. Dev. | 0.42 | 0.3267 | 0.0922 | 0.0770 | 0.0770 | 0.0508 | 0.0508 | 0.0505 |
- a Observed number of alleles.
- b Effective number of alleles.
- c Shannon's information index.
- d Observed homozygosity and heterozygosity.
- e Expected homozygosity and heterozygosity.
- f Nei's (1973) expected heterozygosity.
Heterozygosity can be considered as a measure of molecular variability. Observed heterozygosity (Ho) is the chance for heterozygous individuals in the said population samples and expected heterozygosity (He) is the share of an individual heterozygous from any of the loci. In the current study, the highest Ho values were obtained with locus Fu21 (Ho = 0.5441) and Fu21 was a potent fusant inhibiting the highest growth of S. rolfsii. The 14 true-type heterozygous fusants (Fu1, Fu9, Fu10, Fu13, Fu14, Fu16, Fu21, Fu26, Fu29, Fu30, Fu32, Fu33, Fu34, and Fu35) were found with Ho in the range of 0.1029–0.5441. The Ho indicated low values in relation at He values in all loci, and indicated an excess of homozygosity. Mean Ho per locus in fusants and their parents was found to be higher than those in Perseguini et al. (2011), Blair, Díaz, Hidalgo, Díaz, and Duque (2007) and Zhang et al. (2008). This would be possibly due to the greater diversity of fusants and parents used in this study.
Total gene diversity (Fit) for 36 fusants, parents (P1 = NBAII Tvs 12 and P2 = MTCC796) and the pathogen (S. rolfsii) was found to be in the range of 0.1857 (Fu21) to 0.9765 (Pathogen). The diversity among populations (Dst) was found to be in the range of 0.2777 (P1) to 0.3524 (P2) for parents and 0.2927 (Fu35) for fusants (Table 3 ). Among all of the population, the coefficient of gene differentiation (Fst) was observed to be the highest in S. rolfsii (0.7207) and the lowest in the potent fusant 21 (0.3923) while the reverse trend was found for gene flow (Nm) where the highest value was obtained with Fu21 (0.3872) and the lowest for the pathogen (0.0954).
| Locus | Fit | Fis | Dst | Fst | Nm |
|---|---|---|---|---|---|
| P1 | 0.6493 | 0.3716 | 0.2777 | 0.4418 | 0.3158 |
| P2 | 0.7733 | 0.4209 | 0.3524 | 0.6085 | 0.1609 |
| Fu1 | 0.5769 | 0.1265 | 0.4504 | 0.5157 | 0.2348 |
| Fu2 | 0.8022 | 0.4712 | 0.3310 | 0.6259 | 0.1494 |
| Fu3 | 0.7922 | 0.3641 | 0.4281 | 0.6733 | 0.1213 |
| Fu4 | 0.8014 | 0.4394 | 0.3620 | 0.6456 | 0.1372 |
| Fu5 | 0.8616 | 0.6252 | 0.2364 | 0.6307 | 0.1464 |
| Fu6 | 0.8134 | 0.3869 | 0.4265 | 0.6957 | 0.1093 |
| Fu7 | 0.7885 | 0.4227 | 0.3658 | 0.6336 | 0.1446 |
| Fu8 | 0.7189 | 0.2940 | 0.4249 | 0.6018 | 0.1655 |
| Fu9 | 0.6720 | 0.0923 | 0.5797 | 0.6386 | 0.1415 |
| Fu10 | 0.5564 | 0.0615 | 0.4949 | 0.5273 | 0.2242 |
| Fu11 | 0.6968 | 0.2200 | 0.4768 | 0.6113 | 0.1590 |
| Fu12 | 0.7354 | 0.3476 | 0.3878 | 0.5944 | 0.1706 |
| Fu13 | 0.7001 | 0.3332 | 0.3669 | 0.5503 | 0.2043 |
| Fu14 | 0.5544 | 0.0945 | 0.4599 | 0.5079 | 0.2422 |
| Fu15 | 0.7851 | 0.3631 | 0.4220 | 0.6626 | 0.1273 |
| Fu16 | 0.7305 | 0.3476 | 0.3829 | 0.5869 | 0.1760 |
| Fu17 | 0.8085 | 0.3532 | 0.4553 | 0.7040 | 0.1051 |
| Fu18 | 0.7561 | 0.3016 | 0.4545 | 0.6507 | 0.1342 |
| Fu19 | 0.7202 | 0.3066 | 0.4136 | 0.5966 | 0.1691 |
| Fu20 | 0.7467 | 0.3054 | 0.4413 | 0.6354 | 0.1435 |
| Fu21 | 0.1857 | −0.3400 | 0.5257 | 0.3923 | 0.3872 |
| Fu22 | 0.7674 | 0.2527 | 0.5147 | 0.6887 | 0.1130 |
| Fu23 | 0.7522 | 0.2974 | 0.4548 | 0.6473 | 0.1362 |
| Fu24 | 0.7139 | 0.3419 | 0.3720 | 0.5653 | 0.1922 |
| Fu25 | 0.6225 | 0.2008 | 0.4217 | 0.5276 | 0.2238 |
| Fu26 | 0.8081 | 0.4613 | 0.3468 | 0.6437 | 0.1384 |
| Fu27 | 0.6061 | 0.1309 | 0.4752 | 0.5467 | 0.2073 |
| Fu28 | 0.6660 | 0.2630 | 0.4030 | 0.5467 | 0.2073 |
| Fu29 | 0.6943 | 0.2928 | 0.4015 | 0.5677 | 0.1903 |
| Fu30 | 0.6340 | 0.1889 | 0.4451 | 0.5487 | 0.2056 |
| Fu31 | 0.7882 | 0.4884 | 0.2998 | 0.5859 | 0.1767 |
| Fu32 | 0.7110 | 0.2207 | 0.4903 | 0.6292 | 0.1473 |
| Fu33 | 0.6973 | 0.2927 | 0.4046 | 0.5720 | 0.1871 |
| Fu34 | 0.7845 | 0.3752 | 0.4093 | 0.6550 | 0.1317 |
| Fu35 | 0.8414 | 0.5487 | 0.2927 | 0.6486 | 0.1355 |
| Fu36 | 0.7496 | 0.3823 | 0.3673 | 0.5946 | 0.1704 |
| Pathogen | 0.9765 | 0.9149 | 0.0616 | 0.7239 | 0.0954 |
| Mean | 0.7207 | 0.2966 | 0.4241 | 0.6030 | 0.1646 |
- Note. Fit: total gene diversity; Fis: gene diversity within populations; Fst: coefficient of gene differentiation (Fst = Dst/Fit); Nm: gene flow (Nm = 0.25(1−Fst)/Fst); Dst: diversity among populations (Dst = Fit − Fis).
Our results indicated high levels of Nm in the potent fusant (Fu21) which would account for low differentiation between populations and indicates the good genetic purity of Fu21 (Table 3 ). It should be noted that indirect estimates of Nm values must be interpreted with caution and this data therefore should be viewed as general indicators of the magnitude of genetic exchange. Blair et al. (2007) reported that there was higher Nm between the Nueva Granada race and the Peru race (2.45) but lower Nm with Chile race and previous two races (1.22 and 1.49, respectively). While among the Colombian genotype groups, Nm was found to be higher and averaged 2.38 between race Peru and three subgroups of race Nueva Granada. Zhang et al. (2008) showed that the Nm was 2.87 within the Andean group and 0.09 within the Mesoamerican group and ranged from 0.23 to 0.41 between gene pool groups. Gene flow across gene pools was 0.86 or below.
The overall diversity amongst 36 fusants, parents (P1 = NBAII Tvs12 and P2 = MTCC796) and the pathogen (S. rolfsii) is represented in bidimensional graphics by using PCA (Figure 6). In the graphical form, the first, second, and third axis displayed overall 62.70% of total variability. A potent fusant with respect to stress tolerance (fungicide and abiotic) having the highest inhibition coefficient (92.88%) against the S. rolfsii was Fu21 and it was more diverse compared with the parent P2, the least potent fusant Fu28 and the S. rolfsii pathogen. Some of the 14 true homokaryon fusants (Fu1, Fu2, Fu3, Fu4, Fu5, Fu7, Fu11, Fu13, Fu14, Fu15, Fu20, Fu22, Fu30, Fu31) were found to be clustering near the parents and rest of the 13 true fusants (Fu6, Fu8, Fu17, Fu21, Fu23, Fu24, Fu25, Fu26, Fu28, Fu33, Fu34, Fu35, Fu36) were observed to be more diverse and clustered far away from their parents. The most potent (Fu21) and least potent (Fu28) fusants were diverse and found away from parents.
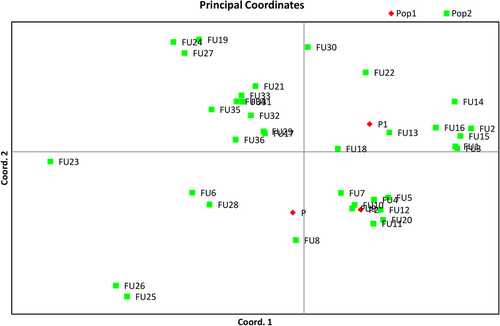
Principal coordinate analysis of 36 fusants, two parents and one pathogen (Sclerotium rolfsii ). Pop 1: two parents and one pathogen (S. rolfsii); Pop 2: 36 Trichoderma fusants [Color figure can be viewed at wileyonlinelibrary.com]
The SSR based dendrogram for molecular variability between fusants, parents and the pathogen S. rolfsii was observed in the range of 0.24–0.90 (Figure 7). The S. rolfsii (24%) was out grouped from Trichoderma fusants and their parents by minimum similarity with 100% boot strapping value. The parents P1 (NBAII Tvs12) and P2 (MTCC796) out grouped into two groups carrying several fusants in each cluster and shared 32.5% similarity with 84% boot strapping value. The 14 heterozygous strains (inter fusants) except Fu16 out grouped from the parent P1 and P2 clusters but were found nearer to the P1 cluster and shared 79.2% similarity with P1. However, these mutants had 32.5% similarity with P2 cluster. The mycoparasitic potent and diverse heterozygous fusant Fu21 was found nearer to the P1 cluster with 72.3% similarity. However, the least homozygous fusant (Fu28) was observed in the P2 cluster. Benchimol et al. (2007) evaluated Andean and Mesoamerican genotypes (P. vulgaris L.) using microsatellites markers and observed that genetic distances in Mesoamerica ranged from 0.37 to 0.71 having a mean value of 0.57 ; genetic distances for Andean ranged from 0.47 to 0.75 with a mean value of 0.63, which was approximately similar to that of our results.
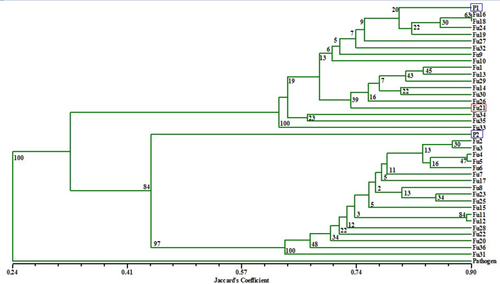
UPGMA-based dendrogram depicting phylogenetic relationships between Trichoderma fusants and their parents using gene specific SSR data. Fu1–Fu36: Trichoderma protoplast fusants; P1: Trichoderma virens NBAII Tvs12; P2: Trichoderma koningii MTCC796; pathogen: Sclerotium rolfsii (9107.13); SSR: simple sequence repeats; UPGMA: unweighted pair group method analysis [Color figure can be viewed at wileyonlinelibrary.com]
3.8 Gene specific conformation of fusants compared with their parents
Molecular characterization of 36 fusants (Fu1–Fu36) and their parents using gene specific microsatellite markers indicated the occurrence of novel fragments, which may be due to recombination events between parents P1 (NBAII Tvs12) and P2 (MTCC796; Figure 5). The data confirmed the occurrence of nuclei fusion of parental strains and produced stable fusants. Therefore, the present result reflects the occurrence of nuclear fusion-associated protoplast fusion between two different species of Trichoderma strains leading to the formation of hybrids. Therefore, we could recommend protoplast fusion within species and between different species to obtain recombinant strains. This could be obtained without sexual reproduction and get the Trichoderma species with the desired characteristics.
This result was in agreement with that of Savitha et al. (2010), who reported that confirmation of formation of fusants was done by genetic markers such as the restriction digestion pattern and random amplified polymorphic DNA and the mycelial protein pattern. Mendoza-Mendoza et al. (2007) studied MAP kinase TVK1 that regulates hydrophobicity, conidiation and the expression of genes that encode cell wall-related proteins in T. virens. The MAP kinase Tvk1 was found to play a major role in control of conidia production. Clear differences in the gene expression pattern amplified by AH-con1F/R markers were shown during conidiation, suggesting the different functions of the corresponding proteins in these phenomena. Our study also showed morphological variations in fusants corresponding to parents, antagonist activity against S. rolfsii and amplification intensity of biocontrol-related gene specific SSR markers.
4 CONCLUSIONS
To develop a useful antagonistic strain showing multi stress tolerance and mycoparasitic activity, two parental Trichoderma, mycoparasitic strain T. virens NBAII Tvs12 (P1) and the multistress tolerant strain T. koningii MTCC796 (P2) were subjected to protoplast fusion. The fused protoplasts were regenerated and 36 stable fusants (20 homozygous and 14 heterozygous mutants) derived after diploidization and successive subculturing. Morphological variations were exhibited by the fusants and among those, 27 fusants were obtained viable under multistress conditions (fungicides, drought, and salt stress) of which, 19 fusants were found fast growing as compared with parent strains. Results of biocontrol activity against pathogen S. rolfsii indicated that heterozygous Fu21 exhibited the highest inhibition coefficient (92.88%) of the test pathogen with elevated multistress tolerance capacity. Molecular characterization of two parental strains (NBAII Tvs12 and MTCC796), 36 fusants and pathogen S. rolfsii was carried out using biocontrol gene specific SSR and codominant marker analysis performed for heterozygosity and molecular variability among the fusants and their parents. The Nei's diversity indices and Shannon's index results led to the same conclusion that higher genetic variability was found in the potent fusant (Fu21). The study addressed development of diverse Trichoderma fusants for fungicides, drought and salt tolerance, gene specific confirmation for heterozygous and homozygous mutants, molecular diversity among Trichoderma fusants, parents and pathogen and identified potent fusants for biocontrol activity against pathogen S. rolfsii. Protoplast fusion is a powerful technique for development of multistress tolerant Trichoderma enhancing biocontrol activity against pathogen S. rolfsii causing stem rot in groundnut. Furthermore, the potent fusant (Fu21) derived from the current study could be utilized to confirm the bioefficacy of Fu21 for in vivo inhibition of S. rolfsii causing stem rot in groundnut by formulating an effective bioformulation with a low dose of fungicides as part of IDM under adverse conditions (abiotic stresses: drought and salt).
AUTHOR CONTRIBUTIONS
D. G. H. carried out the experiment, microbial work, molecular analysis, and working out the results; H. P. G. was responsible for interpretation of data and writing of the manuscript. A. K. P. and Z. A. K. were responsible for helping with the microbial analysis; and B. A. G. was liable for the idea and coordination of the experiment. All authors contributed critically to the drafts and gave final approval for publication.




