Identification of intracellular peptides associated with thermogenesis in human brown adipocytes
Abstract
Objectives
Currently, brown adipose tissue (BAT) is a therapeutic target in obesity and diabetes, but the mechanism of BAT activation remains unclear. Because increasing emphasis has been placed on the role of intracellular peptides in biological processes, we conducted a study to gain insight into the mechanism of BAT activation by using a peptidomic approach and then attempted to identify peptides that are capable of activating BAT.
Methods
In the present study, we generated the peptidomic profile of the intracellular peptides in brown adipocytes treated with forskolin (FSK) using a peptidomic approach. Then, the differentially expressed peptides were evaluated via Gene Ontology (GO) enrichment, KEGG pathway, and protein–protein interaction (PPI) network analysis. Finally, we selected candidate peptides for further validation via assessing the expression levels of UCP-1 and PGC-1α in brown adipocytes exposed to the peptides.
Results
A total of 4,370 peptides were identified, of which 951 were upregulated and 379 were downregulated after FSK treatment. Bioinformatic analysis demonstrated that the ECM-receptor interaction GO term was the most enriched and that collagen alpha-related proteins exhibited the highest degree of PPI. Four peptides separately derived from TSC22 domain family protein 1 (T22D1), bromodomain and WD repeat-containing protein 1 (BRWD1), protein piccolo (PCLO), and collagen alpha-1 (III) chain (CO3A1) increased the expression levels of UCP-1 and PGC-1α.
Conclusions
ECM-receptor interaction may play an important role in the process of FSK-stimulated BAT activation, and the pT22D1tide, pBRWD1tide, pPCLOtide, and pCO3A1tide peptides potentially promote BAT thermogenesis.
1 INTRODUCTION
Obesity is characterized by theaccumulation of triacylglycerol in adipocytes and the excessive expansion of adipose tissue mass. Obesity confers an increased risk for many severe diseases, including type 2 diabetes, cardiometabolic diseases, hypertension, osteoarthritis, and cancer, all of which reduce the quality of life and lifespan (Olshansky et al., 2005). The worldwide prevalence of overweight and obesity is rapidly growing, and the World Health Organization (WHO) estimates that approximately 500 million adults are obese (Finucane et al., 2011). Currently, obesity affects both medical and social services and has stirred public controversy and concern.
Because the exact mechanism of obesity pathogenesis remains obscure and the existing therapeutic approaches are universally disappointing, research on the etiology and treatment of obesity has burgeoned in recent years. Brown adipose tissue (BAT), regarded as the principal tissue responsible for adaptive thermogenesis during non-shivering cold exposure, is recognized to be present and active only in human newborns. However, emerging evidence suggests that BAT also exists in adults (Cannon & Nedergaard, 2004; Cypess et al., 2009; Nedergaard, Bengtsson, & Cannon, 2007). In contrast to white adipose tissue (WAT), which synthesizes and stores excess energy, BAT emphasizes the consumption of chemical energy for the generation of heat (Harms & Seale, 2013; Nedergaard & Cannon, 2010; Rosen & Spiegelman, 2014; Sidossis & Kajimura, 2015). Evidence from rodent studies indicates that BAT activity is essential for rodents in the defense against cold exposure and the counteraction of obesity, insulin resistance, and glucose intolerance induced by a high-fat diet (Harms & Seale, 2013; Nedergaard & Cannon, 2010; Rosen & Spiegelman, 2014; Sidossis & Kajimura, 2015). Thus, BAT activity represents a novel target for strategies aimed at increasing the metabolic rate, and the current search for novel molecules involved in BAT thermogenesis provides a new direction for obesity therapy.
The development of the peptidomic approach provides a deep understanding of the endogenous peptides involved in physiological and pathological processes. Asprosin is the C-terminal cleavage product of profibrillin and is secreted by white adipocytes during fasting. Asprosin activates the G protein-cyclic adenosine monophosphate (cAMP)-PKA pathway and modulates hepatic glucose release (Romere et al., 2016). The pNaKtide peptide derived from the nucleotide-binding domain of the Na/K-ATPase α subunit attenuates oxidant stress and ameliorates an obesity phenotype (Sodhi et al., 2015) in mice. Given the evidence for roles of endogenous peptides in the performance of a large variety of functions in the endocrine, respiratory, cardiovascular, immune, and nervous systems (Neijssen et al., 2005; Sokos, Nikolaidis, Mankad, Elahi, & Shannon, 2006; Takeda, Sato, Rakugi, & Morishita, 2011), we reasoned that intercellular peptides may play a functional role in the process of BAT activation. However, to date, few studies have addressed this possibility.
Forskolin (FSK), isolated from the roots of the Coleus forskohlii plant, is commonly used as a tool in the investigation of cAMP-dependent transduction pathways and brown adipocyte physiology (Seale et al., 2008). FSK directly activates adenylyl cyclase (AC) through its catalytic subunit, resulting in elevated intracellular levels of cAMP. In addition, the activation of β-adrenergic signaling cascades drives the expression and activity of PGC-1α that contributes to the physiological activation of BAT thermogenesis (Cao et al., 2004; Harms & Seale, 2013). Therefore, the detection of altered peptide expression in brown adipocytes before and after treatment with FSK promises to provide novel insight into the role of intracellular peptides in BAT activation and their potential as therapeutic targets for obesity.
In the present study, we generated the intracellular peptide profiles of BAT before and after treatment with FSK by peptidomic analysis and subjected the peptides with a significant difference in expression to bioinformatic analysis. As a result of these analyses, we found that ECM-receptor interaction may play an important role in the process of FSK-stimulated BAT activation. The candidate peptides predicted to have a potential function in BAT activation were selected and chemically synthesized for further identification in vitro. Exposure to four peptides separately derived from TSC22 domain family protein 1 (T22D1), bromodomain and WD repeat-containing protein 1 (BRWD1), protein piccolo (PCLO), and collagen alpha-1(III) chain (CO3A1) increased the expression level of the thermogenic genes UCP-1 and PGC-1α in BAT. Our findings indicate an important role for intracellular peptides in the regulation of BAT activation.
2 MATERIALS AND METHODS
2.1 The isolation, culture, and differentiation of primary brown adipocyte
The primary pre-adipocytes used in the study were isolated from the human fetal interscapular BAT. The experimental procedure was approved by the Human Research Ethics Committee of Nanjing Maternity and Child Health Care Institute (permit number [2015]110) and the parental donors all had signed a written informed consent.
The methods were carried out following the previous description (Seiler et al., 2015). In brief, the obtained fetal interscapular BAT was immediately transported in Dulbecco’s modified Eagle medium (DMEM, Thermo Fisher Scientific, Waltham, MA) containing 10% fetal bovine serum (Gibco, Grand Island, NY) and 1% penicillin/streptomycin solution (Sciencell Research, Laboratories, Carlsbad, CA). Then the tissue was minced and transferred to a 1% collagenase (Sigma-Aldrich, St. Louis, MO), 1% bovine serum albumin and 1% phosphate-buffered saline (Thermo Fisher Scientific, Waltham, MA) containing solution for 45 min at 37°C with gentle inversion. The homogenate was then filtrated through a nylon mesh strainer (BD Falcon, San Jose, CA). The filtrates were collected and centrifuged (500g, 5 min) to generate a brown preadipocyte cell pellet. The cells were cultured in DMEM containing 10% fetal bovine serum supplied with 20 ng/ml hbFGF (R&D, Minneapolis, MN) and incubated at 37°C, 5% CO2. Brown adipocyte differentiation was induced to differentiate when cells reached confluence. The medium used in differentiate was an adipogenic cocktail including DMEM/F12 (Gibco, Grand Island, NY), 0.5 mM IBMX, 0.5 mM dexamethasone, 10 μg/ml transferrin, 1 nM T3, 17 μM pantothenate, 1 μM rosiglitazone, 33 μM biotin and 100 nM insulin. After 4 days of differentiation, the medium was changed to a maintenance medium containing DMEM/F12, 1 nM T3 and 100 nM insulin (all reagents used here were purchased from Sigma-Aldrich, St. Louis, MO).
2.2 Peptides extraction and purification
The crude peptides extracted from brown adipocytes were performed as described (Che et al., 2001; Cunha et al., 2008). In brief, Post-treatment of FSK for 6 hr, the human brown adipocytes (1 × 108 cells) were first washed with phosphate buffered saline for four times by centrifugation at 1,000g for 3 min. Then resuspended the pellet with 10 ml deionized water and subjected it to a water bath (80°C, 20 min) to inactivate proteases. After cooling down the samples on ice, HCL was added with the final concentration of 10 mM. Then samples were treated with ultrasonic treatment of three times (20 pulses, 4 Hz). After that, double centrifugations were performed. First, the homogenate was centrifuged (1,500g, 30 min, 4°C) and the supernatants were collected in ultracentrifuge tubes. Second, the ultracentrifuge tubes were placed into Optima™ L-100 XP ultracentrifuge (Beckman) and centrifugation (100,000g, 30 min, 4°C). The supernatants were collected and transferred to centrifugal filter devices for filtration through a molecular weight (MW) cut-off of 5 KDa (Millipore). The filters were washed with 0.5 ml H2O before use. The filtrates of peptides were then desalted and concentrated with C18 solid phase extraction (Strata C18-E, 55 μm, Phenomenex). At last, the obtained samples were dried in a vacuum centrifuge and store at −80°C. The diverse peptides abundance from different samples were analyzed by LC-MS/MS.
2.3 Isotopic labeling and LC-MS/MS analysis
The isotopic labeling was performed following the previous description (Morano, Zhang, & Fricker, 2008). Control group and FSK treatment group were labeled with different isotopic tags. The peptides mixture after labeling with isotopic were subjected for LC-MS/MS analysis using a Synapt G2 mass spectrometer and a NanoAcquity capillary liquid chromatography system (Waters Co., Milford, MA). The peptides were first separated with an LC packings C18 column (Acclaim PepMap, 75 μm × 150 mm) using an Ultimate 3000 nano-LC system (Eksigent Technologies, Dublin, CA) following the previous strategy (Liu et al., 2016). The obtained elutions were subjected to MS analysis. The MS data spectra were analyzed using Mascot database (http://www.matrixscience.com) against the SwissProt sequence database (http://www.expasy.org/tools/) considering the variable modifications including phosphorylation, deamidation, carbamylation, methionine oxidation, sulfation, acetylation, oxidized.
2.4 RNA isolation and quantitative reverse transcription polymerase chain reaction
Total RNAs from brown adipocyte lysates were isolated using TRIzol reagent (Life Technologies, Carlsbad, CA). Then mRNAs were subjected to reverse transcription to synthesized cDNAs using the PrimeScript RT Master Mix (Takara, Dalian, China). The next quantitative polymerase chain reaction (qPCRs) were conducted to determine the expression levels of specific mRNAs using SYBR Premix Ex Taq II (Takara). The relative mRNA level was normalized to peptidyl-prolyl cis-trans isomerase A (PPIA).
2.5 Peptide preparation and treatment
All peptides used in the present study were chemically synthesis in conjunction with an N-terminal cell-penetrating peptide (GRKKRRQRRRPPQQ) from ShangHai Science Peptide Biological Technology Co., LTD (Shanghai, China). The peptides were dissolved in water and stored at −80°C. When treatment, the peptide solution were added to the culture medium of brown adipocytes to a final concentration of 50 µM.
2.6 Bioinformatics analysis
The Online tool (http://web.expasy.org/compute.pi/) was used to characterize the general feature of peptides including MW and isoelectric point (pI). The differentially expressed peptides and corresponding precursor proteins were sent for gene ontology (GO) and KEGG pathway analysis via DAVID database (https://david.ncifcrf.gov/tools.jsp). The protein–protein interaction (PPI) network was generated using STRING (https://string-db.org/) coupled with Cytoscape soft (version 3.2.1).
2.7 Statistical analysis
We utilized SPSS 17.0 software (SPSS, Inc., Chicago, IL) for statistical analysis. Values are represented as the mean ± standard deviation. The student's t-test was used to determine the differences between groups and that with p < 0.05 was considered as a statistically significant difference.
3 RESULTS
3.1 Effect of FSK treatment on the peptidomic profile of brown adipocytes
To generate the intracellular peptide profiles of BAT before and after treatment with FSK, we first examined the impact of FSK treatment on the function of BAT. Brown adipocytes differentiated for 6 days were treated with 10 μm FSK for 3, 6, and 12 hr (h). Then, we conducted a qPCR analysis to determine the expression levels of the specific thermogenic genes encoding uncoupling protein 1 (UCP-1) and peroxisome proliferator-activated receptor gamma coactivator (PGC-1α). Notably, upon FSK stimulation, the brown adipocytes responded with a dramatic induction of UCP-1 and PGC-1α gene expression. Treatment with FSK for 6 hr resulted in the most apparent difference, an approximately 120-fold increase in UCP-1 mRNA and an approximately 110-fold increase in PGC-1α compared to the control (Figures 1a,b). This result indicated that FSK significantly elevated BAT thermogenesis and that 6 hr may be the optimum duration of treatment of subsequent experiments. Then, the samples of differentiated brown adipocytes treated with FSK were prepared and sent for peptidomic analysis. A total of 4,370 peptides were identified, of which 951 were upregulated and 379 were downregulated after FSK treatment, with a p-value < 0.05. The detailed information for all identified peptides is listed in Supporting Information Table S1. From these peptides, we selected 645 peptides that exhibited a greater than 2-fold change for the generation of a clustered heat map, which compactly displays a large number of peptides and allows the alterations in expression levels to be visualized (Figure 1c,d). The heat map demonstrated that FSK treatment dramatically influenced the level of peptides in brown adipocytes and implied that certain peptides may be involved in the process of BAT activation.
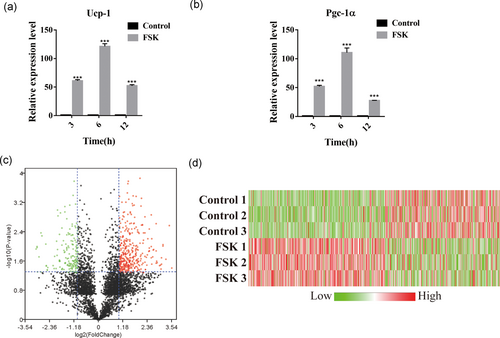
Effect of FSK treatment on the peptidomic profile of brown adipocytes. (a,b) Relative abundance of Ucp-1 and Pgc-1α mRNA in human brown adipocytes after treatment with FSK for the indicated times (3, 6, and 12 hr). (c) The volcano plots displaying the raw p-values for peptides versus fold change observed when the peptide abundance in the untreated group was compared to that in the FSK-treated group by a peptidomic approach. The plots with a p-value < 0.05 and a fold change > 2 are marked with red (high expression in the FSK-treated group) and green (low expression in the FSK-treated group). (d) Heat map showing significantly differentially expressed peptides. A positive fold change (red) corresponds to a higher expression level after FSK treatment, and a negative fold change (green) corresponds to a lower expression level after FSK treatment. ***p < 0.0001, Student’s t-test. The data are presented as the mean ± SD. FSK: forskolin [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Characteristics of peptides with significant changes in expression level in response to FSK
To analyze the general features of the peptides with significant expression level changes in response to FSK, we conducted a statistical analysis and classified these peptides on the basis of MW and pI (Figures 2a–c). We observed that most peptides were concentrated in the MW range of 0.5–1.5 kDa and the pI range of 5.0–7.0 or 8.0-12.0. Because proteolytic cleavage of precursor proteins is the major source of peptides, the diverse distribution of amino acids at the cleavage sites at the amino terminal end (N-terminal) and carboxyl-terminal end (C-terminal) of these peptides was analyzed (Figure 2d). The result of this analysis showed that leucine (L) was the most common amino acid at the C-terminal end of the precursor peptides and the identified peptides, whereas lysine (K) was dominant at the N-terminal cleavage site of the identified peptides, and glycine (G) was the most common amino acid at the N-terminal end of the cleaved peptide. Next, we attempted to determine the precursor proteins that generated multiple peptides. We observed that mucin-19 (MUC19) and E3 ubiquitin-protein ligase RNF213 (RNF213) generated more peptides compared to the other precursor proteins; these two precursor proteins generated eight peptides and six peptides, respectively.
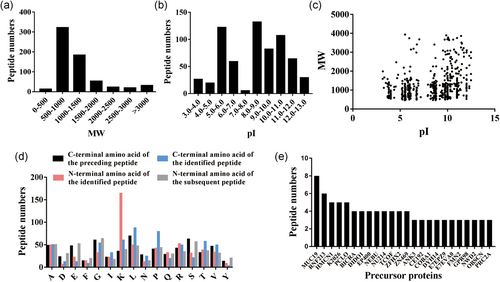
Characteristics of peptides with significant changes in expression level in response to FSK. (a) The molecular weight (MW) distribution of all identified peptides. (b) The isoelectric point (pI) distribution of all identified peptides. (c) Scatter plot of MW versus pI for all identified peptides. (d) Distribution of the specific four cleavage sites in all identified peptides. (e) The numbers of peptides derived from the corresponding precursors. FSK: forskolin [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Bioinformatic analysis of the precursor proteins of differentially expressed peptides
To explore the biological function of the peptides with a significant difference in expression in response to FSK treatment, we performed GO and KEGG pathway analysis to reduce complexity and highlight biological processes involved in BAT activation. In the biological process enrichment analysis, we observed that the precursor proteins of the differentially expressed peptides were mostly enriched in the following GO terms: ECM-receptor interaction, protein digestion, and absorption and PI3K-Akt signaling pathway, which implicated these proteins in the modulation of glucose homeostasis (Figure 3a). In the KEGG pathway analysis, hepatic fibrosis/hepatic stellate cell activation and the intrinsic prothrombin activation pathway were the most enriched pathways (Figure 3b). However, PPARα/RXRα activation and cAMP-mediated signaling were also enriched in the KEGG pathway analysis (Figure 3b). In addition, PPI network mapping was introduced to view the interactions among the precursor proteins that generated the differentially expressed peptides. Interestingly, we found that most nodes in the network were occupied by collagen alpha related proteins such as collagen alpha-4 (IV) chain (COL4A4), collagen alpha-1 (II) chain (COL2A1) and collagen alpha-2 (IV) chain (COL4A2). Similar to the results of the GO analysis, collagen alpha-related proteins that belonged to the ECM-receptor interaction category exhibited a higher degree of involvement in the interaction network (Figure 3c). The above result suggested a potential role for ECM-receptor interaction in the FSK-stimulated activation of brown adipocytes.
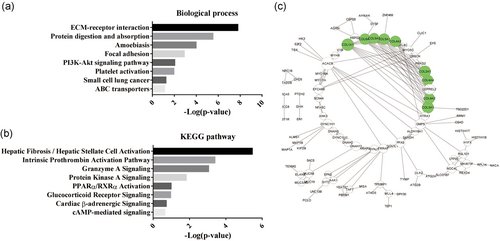
Bioinformatic analysis of the precursor proteins of the differentially expressed peptides. (a) The biological process enrichment from the Gene Ontology (GO) analysis. (b) The KEGG pathway analysis. (c) The protein–protein interaction (PPI) network. The green circles denote collagen alpha-related proteins [Color figure can be viewed at wileyonlinelibrary.com]
3.4 Selection of candidate peptides with a potential function in BAT activation
To select candidate peptides that had a potential function in brown adipocyte activation for further investigation, we listed the top 50 upregulated and downregulated peptides (Table 1, Figure 4a,b). The maximal fold changes of the up- and down-regulated peptides were 11.59 and 8.18, respectively. Considering that FSK treatment was more likely to activate or increase the expression level of downstream molecules and contribute to BAT activation, we preferentially selected the candidates from the data set of upregulated peptides. More scrutiny was given to the peptides with a greater significant difference and a higher fold change. Finally, the peptides derived from T22D1, bromodomain and WD repeat-containing protein 1 (BRWD1), protein piccolo (PCLO), and collagen alpha-1 (III) chain (CO3A1) were selected for further confirmation.
| Peptide | MW | Fold change | p-value | Precursor protein |
|---|---|---|---|---|
| Upregulated | ||||
| KAPGTK | 600.36 | * | 0.016 | RL14 |
| SAVSSSGSPA | 848.39 | * | 0.000 | T22D1 |
| KKLIDSGVGLAP | 1196.71 | * | 0.008 | F5H1F7 |
| RIYQDTKVDIPSLIQTIRRVVKGRPGPRKQ | 3517.05 | * | 0.007 | PMGT2 |
| VGSVVHR | 752.43 | * | 0.002 | G5E953 |
| RHGGA | 496.25 | * | 0.001 | A0A087WWH3 |
| SVGCQVPKPEVIFSL | 1601.85 | 11.59 | 0.038 | ZN484 |
| LEKLLYKPQAG | 1258.73 | 10.71 | 0.003 | BRWD1 |
| ASLITAIPITQ | 1126.66 | 9.49 | 0.022 | UNC80 |
| KGSVPPAAA | 796.44 | 8.63 | 0.009 | PCLO |
| MGKVL | 546.32 | 8.59 | 0.009 | GPR98 |
| TQAIRTGT | 846.46 | 8.20 | 0.030 | SKT |
| DGGFLDLNGESMEADV | 1667.70 | 8.19 | 0.020 | E9PG32 |
| LDELIRKRGAAAKGRLN | 1880.11 | 8.04 | 0.041 | PDIP3 |
| KTLTMIALILTQKN | 1586.94 | 7.89 | 0.031 | TTF2 |
| GGGARP | 513.27 | 7.73 | 0.032 | GAREL |
| AMVATAATLVRR | 1258.72 | 7.12 | 0.027 | M0QZD8 |
| KKLMH | 655.38 | 6.91 | 0.025 | E9PNQ6 |
| KTLKVC | 690.41 | 6.82 | 0.028 | CREL2 |
| KSKEI | 603.36 | 6.80 | 0.002 | DYH14 |
| LVPPS | 511.30 | 6.78 | 0.013 | H3BPE1 |
| TKATAP | 587.33 | 6.28 | 0.012 | BCAR1 |
| IVLYT | 607.36 | 6.22 | 0.012 | ZGRF1 |
| WLKDGVP | 813.44 | 6.10 | 0.019 | HMCN1 |
| LGKLPAGGVL | 923.58 | 5.91 | 0.006 | PRC2A |
| KTVLQRPLSLIQGP | 1548.94 | 5.86 | 0.030 | RENT1 |
| VVGAGGAGL | 699.39 | 5.84 | 0.010 | SDHA |
| LRTELATYPGI | 1232.68 | 5.82 | 0.001 | H0YJ66 |
| CIEPDV | 674.29 | 5.78 | 0.020 | URB2 |
| LDQTVVPILLQGL | 1407.83 | 5.48 | 0.003 | DPY30 |
| QFGKSVQKKTMVLGTPVK | 1975.13 | 5.43 | 0.013 | A0A0A6YYA3 |
| SAASPAVALKALEFQ | 1501.81 | 5.43 | 0.013 | LNX2 |
| AGAAVRL | 656.40 | 5.43 | 0.011 | CELR3 |
| QTFLLV | 719.42 | 5.39 | 0.013 | A0A087WSX5 |
| ESLAAI | 602.33 | 5.17 | 0.006 | SCN4A |
| LSSRL | 574.34 | 5.08 | 0.016 | H3BPE1 |
| RPSLRLLSEKI | 1310.80 | 5.03 | 0.032 | REXO4 |
| KVPGG | 456.27 | 4.90 | 0.019 | A0A0A0MTR7 |
| PLGPPPLRRDAPQGRLHPQPPRVLPTSPLDIA | 3457.94 | 4.77 | 0.041 | CAD15 |
| QGPRGDKGETGERGAA | 1584.76 | 4.77 | 0.041 | CO3A1 |
| KTRDGL | 688.39 | 4.69 | 0.027 | ANK2 |
| KPAACKT | 717.38 | 4.67 | 0.018 | RIMS1 |
| TTLLL | 559.36 | 4.66 | 0.002 | PRC2A |
| QLSCGTA | 678.30 | 4.66 | 0.004 | C163B |
| KTEIINK | 844.50 | 4.47 | 0.007 | CE152 |
| KDRVILAL | 926.59 | 4.45 | 0.004 | JKIP1 |
| KSTTVAV | 704.41 | 4.44 | 0.050 | UN13B |
| KGLTGRVFISKTAR | 1532.92 | 4.44 | 0.050 | KI21A |
| KTAAAA | 531.30 | 4.44 | 0.050 | OTUD4 |
| LLPNAL | 639.40 | 4.40 | 0.008 | ZFHX2 |
| Downregulated | ||||
| TKSVVLEK | 902.54 | −8.18 | 0.01 | TBCD4 |
| PGGGRVK | 669.39 | −7.95 | 0.01 | MTAP2 |
| PLGFLVPQAALK | 1252.75 | −7.91 | 0.01 | UNC4 |
| ATGSIIL | 673.40 | −7.75 | 0.05 | C9JCP7 |
| CKLLFIFLLGH | 1302.75 | −7.42 | 0.02 | AP2A1 |
| LSNAQLH | 781.41 | −6.99 | 0.00 | PHC1 |
| LSVLNP | 641.37 | −6.90 | 0.05 | CE192 |
| KHKPG | 565.33 | −6.68 | 0.00 | E7EVA0 |
| RLSVG | 530.32 | −5.90 | 0.01 | MUC19 |
| GLPPSTKKSGQKPVLDV | 1750.00 | −5.80 | 0.03 | DC1L1 |
| RGKVPGPG | 766.44 | −5.79 | 0.03 | DGKZ |
| AAHKSLAGA | 824.45 | −5.62 | 0.04 | QSOX2 |
| PIISTLTT | 844.49 | −5.62 | 0.04 | A0A0A0MTL6 |
| SGGVAICK | 733.38 | −5.54 | 0.04 | A0A0A0MSD0 |
| FDLLVN | 719.39 | −4.65 | 0.03 | Q5T3Q7 |
| VPPPPKETLP | 1073.61 | −4.46 | 0.04 | SON |
| PERASSPGLN | 1026.51 | −4.42 | 0.02 | ZN469 |
| PGLSQ | 500.26 | −4.21 | 0.04 | RENT1 |
| RQLEGAEINKSLLALK | 1782.04 | −4.11 | 0.01 | KIF2B |
| RAECLLHPLPPSADDNLKTP | 2186.12 | −4.03 | 0.02 | NPB11 |
| HQLNQILR | 1020.58 | −3.96 | 0.00 | MED14 |
| RAATL | 530.32 | −3.96 | 0.01 | K1683 |
| KTRGLEEIPVFDISEKTVNGIE | 2473.31 | −3.92 | 0.00 | RIF1 |
| KKRPAPRAPSASPLALHASRLS | 2310.34 | −3.75 | 0.02 | MILK1 |
| KSKFSR | 751.43 | −3.75 | 0.02 | CAC1C |
| PSPLV | 511.30 | −3.74 | 0.02 | ULK2 |
| TLIPDSLPVAPGR | 1334.76 | −3.74 | 0.02 | PF21B |
| EVDLTNPKT | 1015.52 | −3.70 | 0.00 | H0Y9T6 |
| RGPPGPAGANGAP | 1117.56 | −3.67 | 0.02 | CO3A1 |
| KTRQDIID | 987.53 | −3.64 | 0.04 | DOCK5 |
| KKTSITQQP | 1029.58 | −3.63 | 0.00 | A0A087WUH9 |
| QTSIAVCKLQA | 1160.62 | −3.46 | 0.04 | TTF2 |
| VPPGP | 465.26 | −3.42 | 0.00 | LIMA1 |
| VQGVPAAP | 737.41 | −3.42 | 0.01 | H7BXZ5 |
| LYGPAGLGKTTLAQK | 1516.86 | −3.42 | 0.04 | J3KN39 |
| RPPGLRLALA | 1062.67 | −3.40 | 0.04 | SHSA8 |
| VRRGPG | 640.38 | −3.30 | 0.01 | MON1A |
| PAVSPLPVRGGHV | 1284.73 | −3.23 | 0.00 | NPT2A |
| KTLSSNSDKLLNEA | 1518.79 | −3.23 | 0.04 | LAMA3 |
| GSKGLKGGGGGP | 970.52 | −3.20 | 0.01 | ZN687 |
| LCVGIV | 602.35 | −3.16 | 0.01 | A0A0A0MTR7 |
| SKPRLSFSTKP | 1246.70 | −3.13 | 0.01 | KCNK2 |
| PGMLR | 572.31 | −3.11 | 0.00 | A0A0D9SG19 |
| TTFGRC | 683.31 | −3.11 | 0.00 | BRAT1 |
| KKTAF | 593.35 | −3.10 | 0.03 | F5H269 |
| SSLPPPASSKH | 1106.57 | −3.09 | 0.03 | MD13L |
| FGGPL | 489.26 | −3.07 | 0.03 | E9PLL1 |
| AAVTVPEPPPEPE | 1331.66 | −3.02 | 0.01 | SON |
| RHGLSALSAPGLP | 1274.71 | −3.00 | 0.02 | DUS8 |
| QEDKDDSVV | 1033.46 | −2.95 | 0.05 | DC1L1 |
- Note. FSK: forskolin; MW: molecular weight.
- * Refers to no signal of the given peptide was detected before FSK treatment.
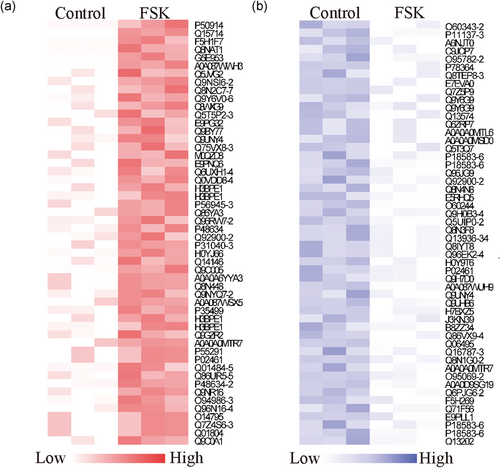
Selection of candidate peptides with a potential function in BAT activation. (a,b) The heat map showing the top 50 upregulated peptides (left panel) and the top 50 downregulated peptides (right panel). BAT: brown adipose tissue [Color figure can be viewed at wileyonlinelibrary.com]
3.5 Validation of candidate peptide function
To validate the function of the candidate peptides in BAT activation, we used the peptides generated from the chemical synthesis approach. These peptides were named pT22D1tide, pBRWD1tide, pPCLOtide, and pCO3A1tide in accordance with the names of their precursor proteins. After the treatment of brown adipocytes with the corresponding peptides, the expression levels of UCP-1 and PGC-1α were determined by qPCR. We observed that all peptides represented different degrees of thermogenesis. The pT22D1tide and pCO3A1tide peptides exhibited a more significant effect on the elevation of UCP-1 and PGC-1α mRNA levels, whereas pBRWD1tide and pPCLOtide increased the mRNA levels only slightly (Figure 5a–d). However, these data provided a preliminary suggestion that the four peptides pT22D1tide, pBRWD1tide, pPCLOtide, and pCO3A1tide may improve thermogenesis in brown adipocytes.
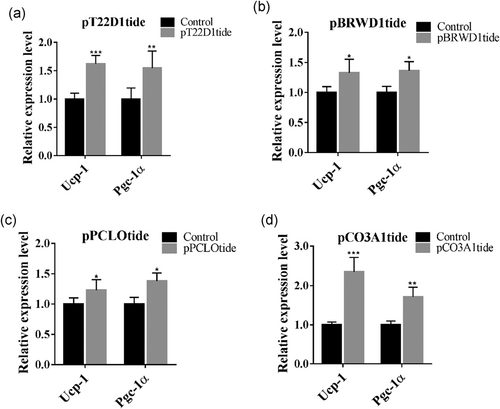
Validation of candidate peptide function (a–d). The effect of the four candidate peptides on the mRNA expression levels of the thermogenic genes Ucp-1 and Pgc-1α. *p < 0.05, **p < 0.01, ***p < 0.01, Student's t-test. The data are presented as the mean ± SD
4 DISCUSSION
Since the existence of thermogenically active BAT in adult humans was established, BAT activation has been suggested to serve as a potential target for obesity therapy. Recent research highlights the urgent need for an intensive study into the mechanism of BAT activation. However, the mechanisms regulating thermogenesis in BAT remain obscure, resulting in the limited application of BAT activation to obesity therapy. In this study, we attempted to explore the underlying mechanism of BAT activation via an intracellular peptidome approach. Brown adipocytes were treated with FSK and then sent for intracellular peptide analysis. The intracellular peptide profile of brown adipocytes was mapped, and a total of 4,370 peptides were identified, of which 1,330 exhibited a significant difference in expression. Finally, four peptides derived from T22D1, BRWD1, PCLO, and CO3A1 were identified as having a potential effect on thermogenesis in BAT. Our study presents the peptide alterations in brown adipocytes during FSK treatment and provides novel insight relevant to therapeutic interventions for obesity.
Intracellular peptides, as portions of endogenous peptides, have been suggested to function in diverse physiological and pathological processes such as cardiovascular diseases, immune system function, insulin resistance and obesity (Berti et al., 2012; Gao et al., 2017; Dengjel et al., 2005; Li, Hashim, & Anandsrivastava, 2006). In the field of metabolism, some intracellular peptides have been shown to function in obesity and diabetes. The intracellular peptides identified in the adipose tissue of rats, DBI peptide, LDBI peptide, and VGN peptide, are capable of increasing insulin-stimulated glucose uptake in 3T3-L1 adipocytes treated with palmitate (Berti et al., 2012). Metformin, a classic drug for the treatment of type 2 diabetes, dramatically alters the intracellular peptidomic profile of human primary visceral adipocytes. In addition, four peptides derived from ATP binding cassette subfamily A member 1 (ABCA1), apolipoprotein B (APOB) and CREB-binding protein (CBP) are predicted to have potential anti-obesity functions (Gao et al., 2017). In our data, four peptides derived from distinct precursor proteins were identified to function in elevating the expression levels of PGC-1α and UCP-1, thereby exhibiting a potential pro-thermogenic effect. These results demonstrate a clear association between intracellular peptides and adipocyte metabolism.
Extracellular matrix (ECM) components including collagens and fibronectin provide mechanical support for tissues. The ECM is able to interact with transmembrane receptors and regulate a variety of cellular activities, including adhesion, migration, proliferation, and differentiation. Aberrant ECM remodeling in WAT is recognized to be initiated during the progression of obesity. Collagen VI (Col6a3) genetic knockout mice exhibit symptoms of systemic metabolic dysfunction when fed a high-fat diet (Khan et al., 2009). Additionally, ECM deposition in the WAT of ob/ob mice is suppressed by treatment with metformin; this suppression contributes to enhanced systemic insulin sensitivity through the suppression of transforming growth factor-β (TGF-β)/Smad3 signaling (Luo et al., 2016). Interestingly, microfibril-associated glycoprotein 1 (MAGP1), a component of ECM microfibrils, relieves the inhibitory effect of TGF-β on PPAR- and PRDM-16-related coactivation of PGC-1α as well as on UCP-1 transcription and thus on thermogenesis (Craft et al., 2014). All these discoveries highlight the vital function of ECM in modulating metabolic homeostasis. In our study, ECM-receptor interaction was significantly enriched in the KEGG enrichment analysis, and the majority of the PPI network nodes were relevant to collagen-related proteins. We observed that a peptide derived from collagen alpha-1 (III) chain (CO3A1) significantly elevated UCP-1 and PGC-1α mRNA levels (Figure 5d). These results provoke us to hypothesize that peptides derived from ECM-related proteins may act as important players in BAT activation and thermogenesis.
We observed that UCP-1 and PGC-1α mRNA levels are increased dramatically following treatment with FSK for 6 hr (UCP-1: 120-fold; PGC-1α: 110-fold, Figures 1a,b). However, compared to FSK treatment, exposure to the four peptides we selected as candidates elevated the expression levels of these proteins only slightly (UCP-1: 2.3-fold; PGC-1α: 1.7-fold, Figure 5), suggesting that some other peptides may be implicated in the process of BAT activation. It raises the question of whether there exist other peptides with a more powerful ability to increase BAT thermogenesis. Meanwhile, the detection of an increase in UCP-1 expression was insufficient to conclude the BAT was activated after peptide treatment. Except for thermogenic genes expression, more evidence such as mitochondrial respiratory function or mitochondrial DNA copy number assay was essential for BAT activity evaluation in exposure to these peptides. Nevertheless, all of these peptides promoted the expression of thermogenic genes, representing a potential function in BAT activation. Further investigation is needed to obtain a definitive answer.
The results of the present study show that FSK treatment significantly alters the intracellular peptidomic profile of brown adipocytes. The functional clustering indicates that ECM-receptor interaction may play an important role in FSK-stimulated BAT activation. In addition, four peptides were identified by their function in elevating the expression of thermogenic genes through the initial screening. Clearly, our study provides insight into the mechanism of BAT activation, and this insight is of considerable therapeutic importance with the growing population of obese individuals in current society and the elevated risk of related complications.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant No. 81700744, 81770866, 81770837, 81601333, 81670773, 81330067, 81701491), the 333 high level talents training project of Jiangsu Province, the Jiangsu Provincial Medical Youth Talent (QNRC2016109, QNRC2016108), Nanjing Technological Development Program (201715054, 201803013), Jiangsu provincial key research and development program (BE2016619, BE2018616), the science and technology development Foundation of Nanjing Medical University (2016NJMUZD062, 2016NJMUZD060), the Nanjing Medical science and Technique Development Foundation (YKK16199, YKK17180).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




