Hereditary breast cancer; Genetic penetrance and current status with BRCA
Abstract
The most important cause of developing hereditary breast cancer is germline mutations occurring in breast cancer (BCs) susceptibility genes, for example, BRCA1, BRCA2, TP53, CHEK2, PTEN, ATM, and PPM1D. Many BC susceptibility genes can be grouped into two classes, high- and low-penetrance genes, each of which interact with multiple genes and environmental factors. However, the penetrance of genes can also be represented by a spectrum, which ranges between high and low. Two of the most common susceptibility genes are BRCA1 and BRCA2, which perform vital cellular functions for repair of homologous DNA. Loss of heterozygosity accompanied by hereditary mutations in BRCA1 or BRCA2 increases chromosomal instability and the likelihood of cancer, as well as playing a key role in stimulating malignant transformation. With regard to pathological features, familial breast cancers caused by BRCA1 mutations usually differ from those caused by BRCA2 mutations and nonfamilial BCs. It is essential to acquire an understanding of these pathological features along with the genetic history of the patient to offer an individualized treatment. Germline mutations in BRCA1 and BRCA2 genes are the main genetic and inherited factors for breast and ovarian cancer. In fact, these mutations are very important in developing early onset and increasing the risk of familial breast and ovarian cancer and responsible for 90% of hereditary BC cases. Therefore, according to the conducted studies, screening of BRCA1 and BRCA2 genes is recommended as an important marker for early detection of all patients with breast or ovarian cancer risk with family history of the disease. In this review, we summarize the role of hereditary genes, mainly BRCA1 and BRCA2, in BC.
1 INTRODUCTION
When compared with other types of cancer, breast cancer (BC) is the primary cause of female deaths, as well as being the most common malignancy. It includes an incidence of 25.2% (1.38 million) of all new cancer cases reported, accounting for 14.7% (522,000) of all cancer death reports among women and 6.4% in both genders (Ferlay et al., 2015). Generally, BC is classified into two types of familial and sporadic cases. Familial breast cancer (FBC) is diagnosed when other female relatives have also had cancer, and may occur in up to 50% of women in a large family. Conversely, sporadic BC occurs in individuals with an absence of family history (Nathanson, Wooster, & Weber, 2001). Mostly, genetic and environmental factors determine the incidence of BC and can be placed on a spectrum, from highly environmental to highly genetic. For example, hereditary breast cancer (HBC) is a subtype of FBC with a strong genetic component. In fact, it is a genetic heterogeneity that is passed down under an autosomal dominant inheritance fashion and can be recognized clinically, by dominant inheritance, early onset (when two or more generations of women have BC before menopause) or severity of the disease (van der Groep, Bouter, & van der Zanden, 2006). Also, BC in males and BC involving the ovary or the fallopian (uterine) tube in females is characterized as HBC (van der Groep et al., 2006; Figure 1).
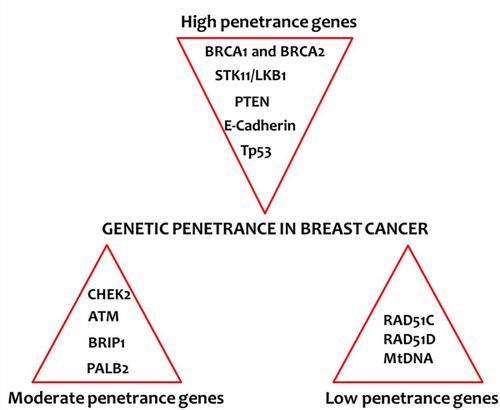
Genetic penetrance in breast cancer: List of genes associated with high, moderate and low penetrance groups. mtDNA: Mitochondrial DNA; PTEN: phosphatase and tensin homolog [Color figure can be viewed at wileyonlinelibrary.com]
HBC has been recognized as a syndrome by Lynch and Krush (1971) and it has been seriously discussed in medical literature from the 19th century. In most cases where a close family member has had BC, the probability of developing cancer is elevated dramatically. An increase in the number of such relatives also increases BC risk for an individual (Newman, Austin, & Lee, 1988). First-degree relatives of a woman diagnosed with BC face twice the risk of this cancer in comparison to the general population (Newman et al., 1988), which highlights the importance of heredity (Peto & Mack, 2000). It was indicated that, among 38 studies, the relative risk of developing BC would be 2.1 if an individual has a first-degree relative with BC (J. Hall et al., 1990).
Approximately 27% of BC is closely associated with hereditary factors (Peto & Mack, 2000). Nevertheless, 5–10% of BC is strongly hereditary, while only 4–5% occurs because of relatively high-penetrance genes that are transmitted in an autosomal dominant fashion (J. Hall et al., 1990; Newman et al., 1988). The genetic starting point of HBC is normally based on a hereditary germline mutation that occurs in one of the alleles of high-penetrance genes, followed by a reduction in heterozygosity of somatic tissues. The high-penetrance susceptibility genes of BRCA1 and BRCA2 account for about 20–25% of this risk (Diez et al., 2011). The BRCA proteins take a vital part in repairing the breaks of the double-stranded DNA, which can be done by homologous recombination (HR; Cass et al., 2003). BRCA defective cells are therefore highly susceptible to DNA damage and carcinogenesis (Cass et al., 2003). According to the literature, one-third of the inherited BCs are a result of dominant mutations of the BRCA gene. In 1994, the main susceptibility genes for breast cancer (BRCA1 and BRCA2) were cloned (Wooster et al., 1994), after which commercial genetic testing was developed. Together, these events led to a global advancement in the study of the genetics of hereditary cancer.
2 GENETIC PENETRANCE AND BC
Genetic biomarkers of cancer risk can be categorized by two primary criteria, penetrance and population frequency. Penetrance refers to the estimate that a specific condition, in this case cancer, will occur in the presence of a specific genotype. The penetrance of mutations in BRCA genes determines the probability of BC occurrence among women who have a significant mutation in BRCA (Nelson, Huffman, Fu, & Harris, 2005). In the following equation, D+ represents an individual with cancer, D− represents an individual who does not have cancer, and G indicates an individual with a significant mutation of BRCA. Accordingly, penetrance is represented by . Therefore, according to the Bayes rule, penetrance is: .
Biomarkers of cancer risk can be considered as low penetrance (1.00 ≤ RR < 1.5), moderate penetrance (1.5 ≤ RR < 5.0), and high penetrance (RR ≥ 5.0; Foulkes, 2008). The number of cases in which BC was resulted from genetic polymorphisms and genes with low-penetrance (regarding environmental interactions) is considerably larger than the number of BC hereditary cases resulted from mutations of high-penetrance genes, such as BRCA1 (Seal et al., 2006). For example, in a general population, only about 1 in 400 to 1 in 800 people will have a mutation in their BRCA1 or BRCA2 genes. Only about 5% of BCs occur due to high penetrance genes (J. Hall et al., 1990; Newman et al., 1988). A greater number of these cases are caused by medium-penetrance genes (Seal et al., 2006). Specific genes and their clinical and molecular characteristics are detailed below.
2.1 High penetrance genes
The BRCA1 and BRCA2 genes belong to this category. The risk of BC facing the carriers of these mutations is 10–20 times higher (RR ≥ 10) than those without BRCA mutations (Seal et al., 2006). BRCA1 and BRCA2 belong to the tumor suppressor gene family as they have the ability to perform DNA double-strand break (DSB) repair, which helps to fix damaged DNA. In a study on BC among 70-year-old non-Ashkenazi Jewish women or women regardless of their ethnicity, it has been estimated that the number of mutations occurring in BRCA1 and BRCA2 vary from 35% to 84% (A. C. Antoniou et al., 2002; A. Antoniou et al., 2003). In 52% of families with multiple cases of this disease, BC was found to be connected to BRCA1, while in 32% of them BC was caused by BRCA2. Also, in 84% of families with breast and ovarian cancer, BC was associated to BRCA1 and 14% of those families were linked to BRCA2 (A. Antoniou et al., 2003). There are a number of other genes associated with some rare syndromes, described as high penetrance.
Another key partner in the high-penetrance gene category is serine/threonine kinase 11/liver kinase B1 (STK11/LKB1). Mutations in STK11/LKB1 can result in the syndrome called Peutz–Jegher, during which hamartomatous polyps appear in the small intestine and pigmented macules of the buccal mucosa, lips, fingers, and toes. These mutations are connected with a risk of about 20% for breast carcinomas. Cowden syndrome (adenomas and follicular cell carcinomas of the thyroid gland, polyps, and adenocarcinomas of the gastrointestinal tract, and ovarian carcinoma) results from mutations in phosphatase and tensin homolog (PTEN), a dual-specificity phosphatase, and are connected with a risk of 20–30% for BC. Li–Fraumeni syndrome (LFS) is caused by germline mutations in TP53. This syndrome leads to very early appearance of BC. More than 70% of LFS families have inherited TP53 mutations. Individuals suffering from LFS cannot normally react to radiation at low dose rates with malfunctioning apoptosis. The significance of recognizing this syndrome is because women with LFS have a higher risk of second primary malignancies thus must not undergo radiotherapy. Only <0.1% of BC cases are caused by LFS, however, in cases with mutations in TP53, there is a BC risk of 18–60 times higher than the general population among individuals younger than 45 (Malkin, 1994). High risk of BC is also associated with E-cadherin (CDH1), the cause of HDGC or hereditary diffuse gastric cancer syndrome. Finally, in male BC cases in the zinc finger and among families with medical evidence of androgen insensitivity, there are two germline mutations in the androgen receptor gene, which are known to increase susceptibility to this cancer. Nevertheless, it has been suggested that these mutations are rarely considered as the cause of male BC, since no additional mutations have been identified after analyzing the larger series. What matters is that the mutations that occur in these genes are the cause of an insignificant number of heritable BC cases. Moreover, all of these susceptibility alleles are quite infrequent in the general population.
2.2 Moderate penetrance genes
In a significant portion of individuals with BC, or ovarian or tubal cancer, with a history of hereditary diseases in their families, high-penetrance gene mutations cannot be demonstrated. The reason for this might be related to germline mutations in genes, such as checkpoint kinase 2 (CHEK2), ataxia telangiectasia mutated (ATM), BRCA1-interacting protein 1 (BRIP1), and partner and localizer of BRCA2 (PALB2; Seal et al., 2006). All of these genes contribute to mechanisms of DNA repair. These groups of genetic variants associated with BC have a minor allele frequency (MAF) of 0.005–0.01 and RR of 2- to 4-fold, with moderate effects on cancer risk (Swift, Reitnauer, Morrell, & Chase, 1987). These mutations are not completely penetrant, meaning that some family members who inherit a cancer susceptibility mutation do not develop cancer in their lifetime.
ATM, a protein kinase with gene penetrance of approximately 15%, contributes to repairing DSBs. For many years, it was proposed that ATM heterozygotes are a cause of increase in BC risk (Swift et al., 1987). However, it has been proved recently that ATM heterozygotes in women older than 50 leads to an increase in the relative risk of BC, up to 2.23 (Kelsey & Wiencke, 1998). CHEK2 is responsible for producing ;a protein that controls the process of repairing breaks in double-stranded DNA by adding a phosphate group into BRCA1 and p53. A particular germline mutation related to CHEK2, 1100delC, gives a relative risk of 2.34 for BC (Kelsey & Wiencke, 1998). Despite variations in the occurrence of this mutation among populations, 1100delC occurs in 0.2–1% of populations in Europe and 4.2% of BC families.
BRIP1 encodes a helicase that, in cooperation with BRCA1, plays a vital role in checkpoint control, thus making individuals with mutations in this gene susceptible to BC (Seal et al., 2006). BRIP1 mutations give a relative risk of 2.0 for breast carcinoma. Carriers of biallelic mutations in BRIP1 are at risk of a condition known as FANC-J, which stands for Fanconi anemia complementation group J. The phenotype of patients with FANC-J differs from those with biallelic mutations in BRCA2 in that they have a lower rate of solid tumors in children. Furthermore, PALB2 encodes a protein that facilitates DNA repair by stabilizing and localizing BRCA2. BC risk caused by mutations of PALB2 is approximately 2.3 (Seal et al., 2006). Similar to biallelic mutations in BRCA2, it has also been indicated that biallelic mutations in PALB2 lead to FANC-N. These genes commonly encode proteins that are responsible for degradation or activation of carcinogens. Although the relative BC risk caused by these mutations happens to be modest in the population, high-risk alleles are highly frequent. Therefore, they are likely to take part in the development of BC in a much larger proportion of patients than the high-penetrance genes (Kelsey & Wiencke, 1998).
2.3 Low penetrance genes
Germline mutations in high- or moderate-risk genes are not solely responsible for genetic susceptibility to BC. Actually, the final level of risk in individuals suffering from other mutations or in the general population will be determined by a series of single-nucleotide polymorphisms (SNPs) or low-penetrance variants. Thus, efforts are underway to identify additional genes or SNPs, including those found in BC. Some of these SNPs are known to serve as modifiers for BRCA1 and BRCA2. With regard to these genes (BRCA1 and BRCA2), approximately 90 validated SNPs have been recognized (Kelsey & Wiencke, 1998). Recently, a study on lifetime BC risk revealed that only five of the SNPs in BRCA2 mutation carriers were required to determine the risk of developing BC, and these SNPs raised it from 45% to 95% (A. C. Antoniou et al., 2010). Mutations in RAD51C and RAD51D genes of the RAD51 group have been detected in breast or ovarian cancer kindred, but not in families that exclusively suffer from BC (A. C. Antoniou et al., 2010). In addition, it has been reported that the mitochondrial DNA (mtDNA) is an important regulator in BC pathogenesis. Reduction in the mtDNA content or copy numbers decreases in mitochondrial gene expression, and germline or somatic mtDNA mutations lead to an increased risk of BC (Weber, Ridderskamp, Alfert, Hoyer, & Wiesner, 2002).
3 BRCA GENES
With their crucial parts in repairing DNA breaks, BRCA1 and BRCA2 are two of the most important susceptibility genes for cancer (O'donovan & Livingston, 2010). The proteins encoded by these genes take part in repairing DSBs by the mechanism of HR (Diez et al., 2011). Both BRCA1 and BRCA2 proteins have been implicated in important cellular functions, including transcriptional regulation, development of embryologic, and DNA-damage repair (Fridlich, Annamalai, Roy, Bernheim, & Powell, 2015). Yoshida and Miki (2004) demonstrated that the BRCA proteins interact with a number of regulatory proteins (Yoshida & Miki, 2004; Table 1). However, the mechanisms by which BRCA1 and BRCA2 conduct these functions and potential differences between them still remain largely an unknown.
| DNA repair | ATM, CHK2, ATR, BRCA2, RAD51, RAD50/MRE11/NBS1, BASC, PCNA, H2AX, c-Abl |
|---|---|
| Transcription | RNA polymerase II holoenzyme (RNA helicase A, RPB2, RPB10α), HDAC1, HDAC2, E2F, CBP/p300, SWI/SNF complex, CtIP, p53, androgen receptor, ATF1, STAT1, estrogen receptor α, c-Myc, ZBRK1 |
| Cell cycle | RB, CDK2, p21, p27, BARD1 |
| Others | BAP1, BIP1, BRAP2, importin α |
- Yoshida and Miki (2004).
Subsequent studies revealed the involvement of BRCA1 and BRCA2 in complexes that activate the repair of DSBs, and initiate the HR, linking the maintenance of genomic integrity to tumor suppression (Fridlich et al., 2015). A recent study has demonstrated that BRCA1 contributes to the regulation of c-Abl activity (Li et al., 2015). The c-Abl is implicated as a DNA damage repair and a regulator of transcription. These findings suggest a route by which BRCA1 and BRCA2 affects cellular responses to DNA-damage, distinct from a direct role in DNA repair or a role in checkpoint control of cell cycle. The BRCA1 has been implicated in the transcriptional regulation of several genes activated in response to DNA damage (Wu, Lu, & Yu, 2010). These genes were also shown to regulate transcription in a purified in vitro system. Studies using microarray technology have shown that the p53-responsive cell cycle progression inhibitor and the stress-response factors (such as p21 and GADD45) are stimulated by overexpression of BRCA1 (C. J. Braun et al., 2008; Fischer, 2017). Cell cycle checkpoints play an important role in cell survival by preventing the propagation of DNA damage through cell cycle progression before DNA repair. Recent studies using cells defective for different DNA damage-responsive proteins have indicated that both ATM and BRCA proteins are required for effective S phase and G2/M-phase checkpoints (C. C. Chen et al., 2017; Fridlich et al., 2015). In the near future, numerous other proteins that bind to BRCA proteins will probably be identified, leading to the discovery of new functions. As a result, if an individual inherits a mutation in one of these genes along with loss of heterozygosity, the risk of malignant transformation is increased by making cells susceptible to chromosomal instability. This increases the chance of BC development (Cass et al., 2003). The first BC predisposition gene, BRCA1 (MIM# 113705), was found to confer high-penetrance susceptibility for ovarian cancer, as well as BC. The BRCA1 gene, which is situated on chromosome 11q21, comprises 22 coding exons and two noncoding exons. It spans 80 kilobase (kb) of the genomic DNA and contains a 7.8 kb transcript with a complementary DNA encoding 1863 amino acids (Peto & Mack, 2000). BRCA2 (MIM# 600185) is also a susceptibility gene for BC, which is situated on chromosome 13q12-13. It contains 26 coding exons, spanning 70 kb of the genomic DNA with an 11.4 kb transcript, as well as coding for a protein with 3418 amino acids (384-kDa nuclear protein; Cass et al., 2003).
3.1 Mutations in BRCA
Mostly, BRCA1 and BRCA2 dysfunction arises from germline mutations, promoter methylation, and somatic mutations. Mutations in BRCA1 and BRCA2 occur in splice sites located in the whole coding region. Most of the mutations in these genes are minor additions or deletions leading to nonsense mutations, frameshift mutations, and splice site mutations, causing premature termination mutation in proteins. There are presently about 12,000 carriers of BRCA1 mutation or unclassified variant, and about 11,000 BRCA2 carriers identified. They comprise 1700 and 2000 unique mutations for BRCA1 and BRCA2, respectively. Out of 1700 unique BRCA1 mutations, 858 have been introduced as “clinically significant” or “pathologic,” which are known to lead to an increase in cancer risk by producing dysfunctional proteins, or probably no protein at all. The majority of these mutations are frameshift mutations, resulting in truncated proteins. Almost 2% of BRCA1 pathogenic mutations are caused by missense mutations. About 15–27% of mutations are caused by large deletions (deletion of one exon, for instance) or large insertion or duplications (Mazoyer, 2005). To detect mutations, usually splice sites and the coding region of these genes received regular screening. Nevertheless, many large genomic deletions and rearrangements within both genes have been recently detected, and these were unable to be identified using traditional polymerase chain reaction-based techniques (Mazoyer, 2005).
3.2 Frequency of BRCA mutations
The most common BRCA1 mutations are as follows: 185delAG (16.5%), 5382insC (8.8%) and the missense alteration C61G (1.8%; Wang et al., 2012). The most repeatedly reported BRCA2 mutations can be listed as: 6174delT (9.6%), K3326X (2.6%), 3036del4 (0.9%), and 6503delTT (0.8%). Wang et al. (2012) performed a comprehensive meta-analysis review on the distribution and frequency of common mutations in BRCA1 and BRCA2 (Wang et al., 2012). These mutations were found to be associated with BC risk in 29 epidemiological studies (Wang et al., 2012), out of which 20 common germline mutations were revealed. In addition to this, four of the BRCA1 mutations (5382insC, 3819del5, 4153delA, and 185delAG) and two of the BRCA2 mutations (4075delGT and 5802del4) were consistently named in various publications. In the case of the BRCA1 mutations, the overall frequency for 5382insC equaled 0.09, the frequency of 185delAG was 0.07, the frequency of 3819del5 was 0.02 and the frequency of 4153delA was 0.06. With regard to BRCA2 mutations, the overall frequency of 4075delGT equaled 0.02 and the frequency of 5802del4 was 0.07 (Wang et al., 2012).
3.3 BRCA1/BRCA2 ratio
In studies reporting high-risk clinical databases, mutations of the BRCA1 gene are almost always more frequent than mutations in BRCA2 (M. J. Hall et al., 2009). In contrast, one study has shown that mutations in BRCA2 outnumber those in BRCA1 (Nelson-Moseke et al., 2013). The largest published analysis of genetic test results of commercially tested patients found that among 46,276 patients, 5,795 harbored a deleterious BRCA gene mutation (M. J. Hall et al., 2009). Of these, 58% carried a deleterious BRCA1 mutation and 42% of patients carried a deleterious BRCA2 mutation.
3.4 Double heterozygosity (DH)
After the first report of DH in BRCA1 and BRCA2 in 1997 (Ramus et al., 1997), few cases have been detected in publications and most of these were of Ashkenazi Jewish ancestry (Nomizu et al., 2015). Nomizu et al. (2015) identified a particularly rare case of familial BC with harmful mutations in the BRCA1 and BRCA2 genes (DH; Ramus et al., 1997). It is reported that in women, the risk linked with mutations in each of the genes is >80%, compared with only 6% in men, by the time the carrier reaches 70 years of age. Since BRCA1 and BRCA2 genes are situated on separate chromosomes, 13q and 17q, respectively, each gene is separately passed on to the next generation with a probability of 50%. Therefore, it is natural that either gene is transmitted to the next generation.
3.5 Founder mutations and geographical distribution of carriers
Despite the fact that numerous pathogenic mutations for BRCA1 have been identified, the phenotype and prevalence of these mutations differ depending on race and country (Liede & Narod, 2002). As a result of founder effects, substantial differences are observed in mutation frequencies of various populations (Kooshyar, Nassiri, Mahdavi, Doosti, & Parizadeh, 2013). This means that founder mutations are more frequent in some populations. For instance, in contrast to global frequency of BRCA mutations, in Iran 185delAG and 5382insC mutations are very rare (Roa, Boyd, Volcik, & Richards, 1996). However, in the Ashkenazi Jewish population, three founder mutations, 5382insC and 187delAG in BRCA1, as well as 6174delT in BRCA2, have been well characterized and estimated to be in 2.5% of the population (Fodor et al., 1998; Johannesdottir et al., 1996). Approximately 98–99% of reported mutations in this population are these three founder mutations (Thompson & Easton, 2004). All over Europe, the BRCA1 mutation, 5382insC, is frequent. It first appeared in countries surrounding the Baltic region some 38 generations earlier. In Iceland, BRCA1 mutations are infrequent and 999del5 BRCA2 founder mutation accounts for most ovarian and/or BC cases. About 0.4–0.6% of the population of Iceland exhibit one BRCA2 founder mutation known as 999delTCAAA (Thompson & Easton, 2004). Swedish populations carry six mutations, such as 1201del11 and 3172ins5 in the BRCA1 gene, which are accountable for 75% of mutations in a unit for clinical screening (Johannesdottir et al., 1996). The 2594delC mutation in Danish populations is responsible for 18% of all BRCA1 mutations (Thompson & Easton, 2004) and seven mutations are accountable for 35% of carriers.
3.6 Position effect
A relatively high frequency of cancer patients have three mutated domains of the BRCA1 protein, including a region encoded by exons 11–13, the RING domain (exons 2–7), and the BRCT domain (exons 16–24). The amino acids that are encoded by exons 11–13, include protein-binding domains for a variety of proteins. The RING domain, however, serves as an E3 ubiquitin ligase. Furthermore, the BRCT domain (exons 16–24) is a phosphoprotein binding domain focused on proteins phosphorylated by the ATM and ataxia Rad3-related protein (ATR) kinases (Thompson & Easton, 2004). For both genes of BRCA1/2, approximately half the mutations were detected in exon 11 (which constitutes approximately 60% of the coding regions of each gene). There is some evidence of variation of cancer risk by position of mutation within each gene. In a study on 356 families, the association between position of mutations and breast/ovarian cancer ratio was analyzed to improve the early findings. In this study, mutations of the central part of the gene, including spanning nucleotides 2401–4190, were related to significantly lower BC risks and/or higher ovarian cancer risks when compared with mutations in other parts of the gene (Evans et al., 2008). It has been proposed that certain BRCA mutations may pose a differential risk for future development of BC. Moreover, a linear trend of increasing BC risk is associated with increasing downstream location of BRCA1 mutation and an increased risk associated with mutations in the outer area of the BRCA2 ovarian cancer cluster region (OCCR; Evans et al., 2008). However, BRCA1 or BRCA2-associated cancers based on mutation location showed not much difference in tumor characteristics.
3.7 Variant of uncertain significance (VUS)
A BRCA genetic test can yield three possible results: positive (a pathogenic mutation is found), negative (no mutation detected or a variant of no clinical significance—a polymorphism) or a VUS (Vears, Sénécal, & Borry, 2017). A VUS is detected in 12–13% of patients tested for BRCA1 and BRCA2 mutation status. A VUS is also a change in the gene sequence with unknown consequences on the gene product function or risk of causing disease. Some of these variants behave as low-penetrance gene mutations and should not be managed in the same way as a highly penetrant mutation. The unclassified variants are reported as following: the missense mutation in exon 11 of the BRCA2 gene (unclassified variant), change in exon 13 of BRCA2 (rare polymorphism), heterozygous for BRCA2 c.9098 C>T, p.Thr3033lle (clinical significance unknown), and BRCA1 VUS S1187N (3679G→A)- BRCA1 VUS S1187N (3679G→A), as well as the substitution of valine for isoleucine at amino acid position 2672 of the BRCA2 protein (Eccles, Copson, Maishman, Abraham, & Eccles, 2015).
3.8 Lifetime risk with mutation in BRCA
There has been an increase observed in BC risk among females who suffer from mutations in BRCA1 and BRCA2 genes. Females with a pathogenic BRCA1 mutation have 60–85% cumulative risk of BC development, as well as 40–60% ovarian cancer risk. Females with a pathogenic BRCA2 mutation are exposed to 50–60% BC risk and 30% risk of ovarian cancer (Kelsey & Wiencke, 1998). They account for 45% of families who have numerously experienced cases of BC and also for 90% of families experiencing both ovarian and BC (Swift et al., 1987).
In pooled pedigree data from 22 studies, BC relative risk for carriers of a BRCA1 mutation decreased with age. For individuals younger than 40, this risk was >30 times reduced, and for those older than 60, the risk was 14 times less significant. The relative risk for BRCA2, however, was approximately 11 times higher for all patients older than 40, and was not much higher for younger ages (Nelson et al., 2005). Evans et al. (2008) reviewed 385 families with mutations in BRCA1 or BRCA2 genes and evaluated the penetrance in women who are mutation carriers for ovarian and BC (Evans et al., 2008). They showed BC penetrance in people aged 70 and 80 years was 68% and 79.5%, respectively for BRCA1, and 75% and 88% for BRCA2. For the same age groups, the risk for ovarian cancer was 60% and 65% for BRCA1, and 30% and 37% for BRCA2. Analysis performed on BRCA1 and BRCA2 penetrance in mutation carriers at 70 years of age suggested that the mean cumulative meta-analytic BC risk was 57% for BRCA1 mutation carriers and 49% for BRCA2; compared with 40% ovarian cancer risk for BRCA1 mutation carriers and 18% for BRCA2 mutation carriers (S. Chen & Parmigiani, 2007).
3.9 Risk for men
BRCA gene mutations are inherited as autosomal dominant. There are equal chances of both male and female offspring to inherit the mutated genes from their parents. As a result, men may also be carriers of the mutations with an increased risk of malignancy among male mutation carriers. Male BC is an exceptionally rare type of cancer that is poorly understood with obscure risk management strategies. BRCA1 and BRCA2 gene mutations are associated with 16% and 76% of male BC, respectively, which is among high-risk families with ovarian or BC (Nelson et al., 2005). Male BRCA mutation carriers, compared with noncarriers, show higher risk for prostate, gastric, breast, hematologic, and pancreatic cancers (S. Chen & Parmigiani, 2007). BRCA2 mutation carriers younger than 65 exhibit an increased risk of pancreatic and prostate cancers.
3.10 Genotype–phenotype associations and phenotypic differences between BRCA1 and BRCA2 cases
Certain pathological and clinical qualities are associated with hereditary BRCA1 or BRCA2 mutations for BC (S. Chen & Parmigiani, 2007). FBCs carrying BRCA1 mutations normally show different pathological features from carriers of BRCA2 mutations and nonfamilial BCs. Some data suggest that carriers of BRCA2 exhibit signs of cancer at older ages compared with their BRCA1 counterparts (Liede, Karlan, & Narod, 2004). Patients with BRCA1-related tumors show a negative status for steroid receptor, positivity of p53 and HER-2/neu, high proliferation, minor tumor differentiation (Phillips, 2000), overexpression of epidermal growth factor receptor (EGFR; Lynch & Krush, 1971) and positivity in cytokeratin 5/6 (Foulkes et al., 2004). However, it seems that there is no clinically significant genotype–phenotype correlation in BRCA2-associated BC (Senst et al., 2013). Also, there is evidence that there is an increase in BC risk among women who have had a paternally inherited mutation in their BRCA1 gene, versus women with a maternally inherited mutation. BC risk in women carrying a mutation in the BRCA2 gene was not affected by parental origin of mutation (Senst et al., 2013).
In addition, ductal carcinoma in situ (DCIS), a type of BC, is known to be rather rare among carriers of BRCA1 mutation. There are a number of features associated with these types of cancers, for instance increased pushing margins, mitotic frequency, and high nuclear pleomorphism, indicating medullary carcinoma (Foulkes et al., 2004). BRCA2 gene mutations result in a significantly higher risk of BC in men than BRCA1 mutations (Basham et al., 2002). Consequently, BRCA1 and BRCA2 cases can be classified with certainty using a decision tree with age, Ki67 antigen, and EGFR (van der Groep et al., 2006). BRCA1 tumors are characterized by an increase in cell proliferation expression as well as markers related to cell cycle. Such tumors have proven to be very proliferative based on proliferation determined by Ki67 labelling index (Ki67 > 65%; Cortesi et al., 2000). Furthermore, van der Groep et al. (2006) suggested that, in 82% of females <54-year-old who had a combination of high proliferation (Ki67 expression >25%) and EGFR positivity, a BRCA1 mutation could be predicted (van der Groep et al., 2006).
3.11 Genome-wide association study (GWAS) and SNPs associated with BC
High-, low-, and moderate-penetrance genes have been detected in studies of family linkage; however, they could not detect any other major genes and rare variants in families without BRCA mutations (Kelsey & Wiencke, 1998). High-penetrance susceptibility genes are only responsible for around 25% of cases with familial aggregation, thus they are hardly sufficient to describe the genetic variability observed in BC. However, it is more useful when there are many frequent low-penetrance susceptibility alleles present in populations, each minimally contributing to BC risk (Hunter, Thomas, Hoover, & Chanock, 2007). Investigation and recent recognition of low-penetrance allelic variants, mostly by the GWAS approach, served a vital function in BC etiology. GWAS is a research strategy used to identify genetic associations between SNPs and diseases, traits, or variation (Easton et al., 2007). The SNPs of genes associated with BC can be used as a potential tool for improving cancer diagnosis and treatment planning. In recent years, research using this method focused on identifying the majority of BC susceptibility alleles and determining their effects (Orr & Chanock, 2008). Based on the International HapMap Project on the human genome, around 10 million common SNPs with MAF >5% have been identified (Orr & Chanock, 2008). Most SNPs in a specified region are inherited together, which provides an extended field for GWAS. On the contrary, GWAS analyzes the relationship between genetic variants at different loci on varying chromosomes in a huge number of patients compared with control subjects, in an effort to detect new alleles of susceptibility to BC (Gold et al., 2008). The GWAS approach is a novel method to detect lower penetrance alleles, not discoverable by studies of genetic linkage. Individually, these alleles do not increase BC risk significantly (only ≤1.3 times); however, it is critical to remember the cumulative effect of these susceptibility alleles (Orr & Chanock, 2008). These studies use SNPs to assess disease associations in the human genome that are based on linkage disequilibrium patterns (Gold et al., 2008).
In recent years, four GWAS projects, the Memorial Sloan–Kettering Cancer Center, DeCode Islanda, Cancer Genetic Markers of Susceptibility, and Breast Cancer Association Consortium, identified many new risk alleles for BC (Fanale et al., 2012; Orr & Chanock, 2008). Studies by each institution were performed in three steps: first, common SNPs were detected in control subjects and patients, then SNPs were examined to know which ones were common among most case and control subjects, finally, new alleles of susceptibility for BC were identified (R. Braun & Buetow, 2011). Antoniou et al. (2010) detected nine common susceptibility polymorphisms for BC with increased risk in BRCA2 mutation carriers, including: MAP3K, FGFR2, TOX3, LSP1, 5p12, 6q25.1, 1p11.2, 2q35, and SLC4A7 (Antoniou et al., 2010), while only three of these SNPs (2q35, 6q25.1, and TOX3) exhibited increased risk for BC in BRCA1 mutation carriers. Nowadays, there are 19 SNPs at 18 loci identified that are associated with BC susceptibility (O'donovan & Livingston, 2010). Nevertheless, there are many undiscovered susceptibility variants, which is why a large percentage of BC familial risk is unexplained (Lynch & Krush, 1971). One recent study suggested that these SNPs (18 of which are validated) cause varying risks for women with BRCA2 mutations, but not for those with BRCA1 mutations. This information about SNPs is useful in helping women with BRCA2 mutations determine their best treatment option (Foulkes et al., 2004). In 2012, three novel SNPs were identified (rs4973768, rs4132417, and rs6504950) and published (R. Braun & Buetow, 2011).
Although validated susceptibility SNPs may be usefully used for predicting BC risk for women with BRCA mutations, some are in doubt about these methods. This is because it is not completely understood how new allelic variants cause BC susceptibility. Moreover, there were inconsistencies between such studies (R. Braun & Buetow, 2011) because of sample-size differences, genetic heterogeneity, or population stratification in terms of the setting of various genotyping platforms and different algorithms to filter data (Fanale et al., 2012). A newly developed multi-SNP GWAS approach called Pathways of Distinction Analysis (PoDA) utilizes data from GWAS, as well as pathway gene and gene SNP associations, to detect pathways that distinguish case subjects from control subjects (Modica et al., 2007). PoDA further improves the analytical possibilities in prediction of BC risk using GWAS.
3.12 Genetic testing
Based on its purpose, genetic testing can be categorized as research investigation or genetic counseling tests. Studies of BRCA mutations are classified under the three following groups: analyzing the whole coding region of both or either of the genes, partial screening of either or both genes, and screening for common or founder mutations. Instead of full screening, it is more cost efficient and convenient to use mutation-specific screening or phased screening. However, it is only plausible to use such methods in populations with a high percentage of known founder mutations. Since a large number of research studies have not investigated large rearrangement (LR) mutations, there is a possibility in some populations that common founder mutations are still unidentified. In this situation, analyzing the entire coding region of high penetrance genes seems to be necessary. Genetic testing and counseling to detect mutations of BRCA genes among high risk subjects are globally accessible and applicable, especially in Europe and the United State, as are instructions and guidance for those who are fit for genetic testing (Modica et al., 2007).
Finally, there is a possibility that mutations in BRCA are overlooked in the process of screening, even after comprehensive sequencing. Furthermore, BRCA mutation analysis is cumbersome, expensive, and may be impractical as a screening method for detection in all patients. On the contrary, patients with BRCA promoter methylation and other mechanisms of loss are not identified using current approaches. Therefore, we need multiple procedures, such as mutation analysis, hypermethylation studies, and immunohistochemistry (IHC) methods. IHC is an easy and inexpensive technique that is available to most pathologists and has been incorporated as a routine test for screening for other hereditary syndromes, like Lynch syndrome in colorectal cancer, and more recently, endometrial cancer (Garg et al., 2013). It seems that BRCA1 IHC can be a useful method to stratify subjects for germline genetic testing. Also, it is used to identify other mechanisms of BRCA1 dysfunction in high-grade ovarian carcinomas (Visvanathan et al., 2009). In addition, recent advances in next generation sequencing will lower testing costs and time, as well as moderating the testing criteria.
3.13 Therapy and prevention
Available evidence suggests BRCA-associated cancers are not unavoidable. It is practically possible to identify BRCA germline mutations in fetal cells from chorionic villus sampling (11th week) or amniocentesis (16th week; Kriege et al., 2004). Furthermore, chemoprevention and prophylactic surgery are effective in reducing cancer risk for carriers of BRCA mutations (King et al., 2001).
During the past 25 years, BC mortality rates in North America and many European countries have significantly dropped due to prompt detection using mammography (Modica et al., 2007). Despite the benefits of mammography, it is not cost efficient in many developing countries (Visvanathan et al., 2009). With respect to the high risk of developing BC, it is recommended that carriers of BRCA1 and BRCA2 mutations begin annual mammograms and bilateral breast magnetic resonance imaging (MRI) screening at the age of 25–30 (King et al., 2001). That being said, mammography may not be sensitive enough to detect lesions in women under the age of 35 and only detects about 40–50% of lesions (Lynch & Krush, 1971). MRI has greater sensitivity than mammography, and the combination of mammography and MRI identify 70–100% of cancer malignancies in susceptible females (Kriege et al., 2004). Unfortunately, MRI has a rather limited sensitivity with regard to identifying in situ ductal carcinoma, which may cause complications when screening individuals who carry a BRCA2 mutation.
3.14 Chemoprevention and treatment
Some studies have proposed that chemoprevention could prove beneficial in carriers of BRCA1 and BRCA2 (Murphy & Fornier, 2010). In most breast tumors, the modified expression of estrogen receptors (ERs) facilitated the development of several targeted agents to be developed, including estrogen antagonists (Fulvestrant), selective ER modulators (Tamoxifen), and aromatase inhibitors (Anastrazole, Letrozole, and Exemestane; King et al., 2001). Similarly, overexpression of wild-type human epidermal growth factor receptor 2 (HER2) receptors gave rise to the utilization of treatment methods in HER2 amplified BC, for example, using Trastuzumab and Lapatinib (Farmer et al., 2005). Tamoxifen is an antiestrogen drug that reduces ER-positive tumor incidence, while it is ineffective in cases of ER-negative tumors. BRCA1-related BC is very likely to be ER-negative, while BRCA2-associated disease is often positive. Tamoxifen has proven to be effective in reducing BC risk in high-risk women by 50% (Murphy & Fornier, 2010), and prevents contralateral BC in women carrying a BRCA1 or BRCA2 mutation (Farmer et al., 2005). Poly (ADP-ribose) polymerase (PARP) inhibitors are another drug used for BRCA predispositions. Single-strand DNA breaks are repaired by an enzyme called PARP, using the mechanism of base excision repair (Murphy & Fornier, 2010). In BRCA1 or BRCA2 mutant cells, double-stranded breaks cannot be repaired by PARP, so inhibiting the enzyme will allow the cells to undergo apoptosis, rather than producing dysfunctional proteins (Farmer et al., 2005).
4 CONCLUSION
HBCs, a type of FBC with complex molecular interactions, are usually associated with a germline inherited mutation in one of the alleles of high-penetrance genes, as well as loss of heterozygosity in the patient's somatic tissues. Most of this risk is caused by mutations occurring in BRCA1 and BRCA2, two high-penetrance susceptibility genes. To present date, approximately 1700 unique mutations of the BRCA1 have been identified in literatures, of which, 858 have been confirmed as being pathologic. 185delAG, 5382insC, and the missense alteration in C61G, are all known to be highly frequent among mutations of BRCA1. This frequency, along with phenotypes associated with BRCA mutations, varies significantly in BC patients from different age groups and ethnic backgrounds. Interestingly, a pathogenic BRCA1 mutation leads to a condition where women will have a 60–85% cumulative risk of BC, and a 40–60% risk of ovarian cancer. Also, male patients with BC who belong to families that are at a high-risk of developing breast or ovarian cancer, exhibit a high proportion of BRCA1 and BRCA2 mutations. Clinically, patients with BRCA1-related tumors show negative steroid receptor status, high proliferation, p53 and HER-2/neu positivity, and poor tumor differentiation. In recent years, there has been a tremendous increase in the use of GWAS. They are a useful tool in identifying gene interaction pathways, as well as SNP associations with BRCA mutations and other genes in BCs. Furthermore, germline mutations in a number of genes, for instance CDH1, ATM, PTEN, and TP53, lead to an increase in BC risk. However, the frequency of mutations occurring in such genes is so insignificant that they are accountable for a limited proportion of cases with inherited BC. Therefore, according to the conducted studies, BRCA1 and BRCA2 genes have a significant effect on the incidence progression of BC and these genes can be titled as a molecular indicator in early diagnosis BC. In fact, the screening of BRCA1 and BRCA2 genes is recommended as a diagnostic method and an important marker in the early stages of breast and ovarian cancer.
ACKNOWLEDGMENTS
The author thank Griffith University and Ferdowsi University of Mashhad for their support during this project.




