Toll-like receptor 3 agonist poly I:C reinforces the potency of cytotoxic chemotherapy via the TLR3-UNC93B1-IFN-β signaling axis in paclitaxel-resistant colon cancer
Abstract
Type I interferon (IFN) signaling in neoplastic cells has a chemo-sensitizing effect in cancer therapy. Toll-like receptor 3 (TLR3) activation promotes IFN-β production, which induces apoptosis and impairs proliferation in some cancer cells. Herein, we tested whether the TLR3 agonist polyinosinic: polycytidylic acid (poly I:C) can improve chemotherapeutic efficacy in paclitaxel (PTX) resistant cell lines. Human colon cancer cell lines HCT116, SW620, HCT-8 (sensitive to PTX), and HCT-8/PTX (resistant to PTX) were treated with poly I:C and the cell viability was measured. Results showed that poly I:C specifically impaired the cell viability of HCT-8/PTX by simultaneously promoting cell apoptosis and inhibiting cell proliferation. In addition, when TLR3 was overexpressed in HCT-8/PTX cells, we found that TLR3 contributed to the production of IFN-β that reduced cell viability, and poly I:C preferentially activated the TLR3-UNC93B1 signaling pathway to mediate this effect. Moreover, cotreatment of poly I:C and PTX acted synergistically to induce cell apoptosis of HCT-8/PTX via upregulating the expression of TLR3 and its molecular chaperone UNC93B1, assisting in the secretion of IFN-β. Notably, a combination of poly I:C and PTX synergistically inhibited the PTX-resistant tumor growth in vivo without side effects. In conclusion, our studies demonstrate that poly I:C reinforces the potency of cytotoxic chemotherapeutics in PTX-resistant cell line through the TLR3-UNC93B1-IFN-β signaling pathway, which supplies a novel mechanism of poly I:C for the chemotherapy sensitizing effect in a PTX-resistant tumor.
1 INTRODUCTION
To date, chemotherapy remains the major clinical method for tumor treatment. However, the issue of drug resistance remains dismal. Novel strategies with promising results are in a particularly urgent need. It has been reported that an optimal chemotherapeutic response is mediated by type I interferon (IFN) signaling in neoplastic cells, and toll-like receptor (TLR3), as an innate immunity receptor, is an optimal signal transduction of this process (Sistigu et al., 2014). TLR3 is expressed both in immune cells and nonimmune cells, such as myeloid dendritic cells (Matsumoto et al., 2003), endothelial cells (Kaiser, Kaufman, & Offermann, 2004), and epithelial cells (Royer et al., 2017) with pleiotropic biological effects. On one hand, TLR3 detects double-stranded RNA (dsRNA) in endosomes, leading to activation of NF-κB for rapid and abundant production of IFNs and proinflammatory cytokines to initiate innate immunity for antiviral responses (Lucifora et al., 2018; Szeles et al., 2015). On the other hand, it also directly induces apoptosis in multiple cancer cell types to facilitate tumor elimination (Salaun, Coste, Rissoan, Lebecque, & Renno, 2006). Therefore, the capacity to mediate immune activation and tumor cell apoptosis has rendered TLR3 as an attractive target in cancer therapy (Cheng & Xu, 2010).
UNC93B1 is a highly-conserved transmembrane protein resident in the endoplasmic reticulum (ER) (Kashuba et al., 2002). It has been reported to be involved in innate immune responses with clinical importance. UNC93B1 physically interacts with the nucleotide-sensing TLRs such as TLR3 via their transmembrane domains, and UNC93B1 delivers them from ER to endosomes for downstream signaling (Brinkmann et al., 2007). Recent studies have shown that TLR3 responds to exogenous nucleic acids in endosomes, where proteolytic processing is tightly regulated by UNC93B1 (Garcia-Cattaneo et al., 2012; Qi, Singh, & Kao, 2012). Stimulation by TLR3 agonist polyinosinic: polycytidylic acid (poly I:C) upregulates the transcription of UNC93B1 for IFN production, whereas the deficiency of UNC93B1 in herpes simplex virus encephalitis patients impaired cellular IFN -α/β and -γ for an antiviral response (Casrouge et al., 2006). In addition, a unique TLR3-UNC93B1 interrelationship has been presented where the TLR3 agonist upregulates endogenous UNC93B1 expression, which in turn, translocates TLR3 to plasma membranes and potentiates activation of TLR9 in human umbilical vein endothelial cells (Pohar, Pirher, Bencina, Mancek-Keber, & Jerala, 2013). Furthermore, an extended role of UNC93B1 has been described to participate in the initiation of signal pathways to induce cell death (Harris & Coyne, 2015). Poly I:C can also be detected by cytoplasmic receptors, such as MDA-5 and RIG-1, which trigger an antiviral response by producing type I IFNs (Huang et al., 2017). In addition, poly I:C has been previously reported to induce cell apoptosis (Bianchi, Pretto, Tagliabue, Balsari, & Sfondrini, 2017) and acted synergistically with cytotoxic agents to induce toxicity in some cancer cells (Jiang, Wei, & Tian, 2008; Van et al., 2012). Recently, poly I:C treatment in combination with PD-L1 blockade has been shown to efficiently invigorate the immune checkpoint inhibition response in glioblastomas (De Waele et al., 2018). Our previous studies have also confirmed that tumor treatment can benefit from the sequential utilization of poly I:C with cisplatin (Ding et al., 2017). Paclitaxel (PTX) is a diterpenoid compound which exhibits antineoplastic effects against various types of cancer. However, the resistance of cancer cells to PTX remains to be elucidated. Based on the important contribution of poly I:C to activate innate immunity and induce cell apoptosis, we try to test the hypothesis that poly I:C may improve the chemotherapeutic efficacy in PTX-resistant colon cancer. In the present study, we demonstrate that poly I:C reinforces the potency of cytotoxic chemotherapeutics in PTX-resistant colon cancer cell line via TLR3-UNC93B1-IFN-β signaling axis, which reveals a new mechanism of poly I:C for the chemotherapy sensitizing effect in the PTX-resistant tumor.
2 MATERIALS AND METHODS
2.1 Cell culture and reagents
The human colon cancer cell lines HCT116, HCT-8, SW620, and HCT-8/PTX were cultured in complete Dulbecco's modified Eagle medium (Gibco, Life Technologies, Carlsbad, CA) with 10% fetal bovine serum and 1% v/v penicillin and streptomycin (Gibco, Life Technologies, Carlsbad, CA) at 37°C in a 5% CO2 incubator. For maintaining the drug resistance of HCT-8/PTX cell line, a proper concentration of PTX (Sigma-Aldrich, St. Louis, MO) was added into medium once every 2 weeks. Neutralizing antibody against TLR3.7 was purchased from Thermo Fisher Scientific (Waltham, MA) and recombinant human IFN-β protein was purchased from (R&D Systems, Minneapolis, MN) .
2.2 Cell viability
Cell viability was measured using a Cell Counting Kit-8 (CCK-8) assay. Briefly, cells were seeded at a density of 5 × 103 cells per well in 96-well plates in quintuplicate and incubated in a 5% CO2 atmosphere at 37°C before treatment. Culture medium was replaced with fresh medium containing the indicated concentrations of poly I:C (InvivoGen, San Diego, CA) alone or in combination with PTX for another 48 hr. Then the supernatant was discarded and fresh medium containing 10 μl of 2-(2-Methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazole monosodium salt (WST-8) reagent from CCK-8 kit (Dojindo, Kumamoto, Japan) was added. After incubated for another 4 hr at 37°C, the absorbance of samples was determined with a multi-well spectrophotometer (BioTek, VT) at 450 nm. Data were analyzed using CompuSyn software and the combination index (CI) values were calculated based on the median-effect principle and the Chou–Talalay equation to determine the synergistic effect (Chou, 2010). The combined effects of poly I:C and PTX were summarized as follows: CI < 1 indicates synergistic effects and CI, 0.1–0.3 and CI, 0.3–0.7 represent a strong synergism and synergism, respectively.
2.3 Isolation of total RNA and quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from differently treated cells using TRIzol (Invitrogen, Carlsbad, CA). The collected messenger RNA (mRNA) was then reverse-transcribed to complementary DNA (cDNA) using HiScript II 1st Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd, Nanjing, China) according to the instructions. Then SYBR Green PCR Kit (Bio-Rad, Hercules, CA) was used to measure target genes with an Applied Biosystems StepOne-Plus real-time PCR system (Applied Biosystems, Foster City, CA). Finally, the method was used to calculate the relative expression ratio of target genes. The sequences of reverse transcription-polymerase chain reaction (qRT-PCR) primers are provided in Table 1.
| Gene | Primer sequence |
|---|---|
| GAPDH | 5′-GCACCGTCAAGGCTGAGAAC-3′ 5′-TGGTGAAGACGCCAGTGGA-3′ |
| UNC93B1 | 5′-TGCTCACCTACGGCGTCTA -3′ 5′-GATGGGAGTCACGTTGATGC -3′ |
| TLR3 | 5′-TTGCCTTGTATCTACTTTTGGGG-3′ 5′-TCAACACTGTTATGTTTGTGGGT -3′ |
| IFN-β | 5′-ATGACCAACAAGTGTCTCCTCC -3′ 5′-GGAATCCAAGCAAGTTGTAGCTC -3′ |
| RIG-1 | 5′-CTGGACCCTACCTACATCCTG-3′ 5′-GGCATCCAAAAAGCCACGG-3′ |
| MDA-5 | 5′-GCCCGCTACATGAACCCTG-3′ 5′-CAGCAATCCGGTTTCTGTCTT-3′ |
- Note. GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IFN: interferon; PCR: polymerase chain reaction; TLR3: toll-like receptor 3
2.4 Plasmids transfection and RNA interference
For TLR3 overexpression, the plasmids (pCMV-C-Flag and pCMV-C-Flag-TLR3) were constructed and purchased from Generay (Shanghai, China). Cell transfection was performed by lipofectamine 3000 (Invitrogen) according to the manufacturer's instructions.
For RNA interference, small interfering RNA (siRNA) against TLR3 and UNC93B1 siRNAs and negative control siRNAs were purchased from Ribobio (Guangzhou, China) and the HCT-8/PTX cells were transfected with either TLR3/UNC93B1 siRNA or their negative control siRNAs with lipofectamine 3000 following the manufacturer's protocol. After 48 hr, the knockdown of target genes was confirmed by qPCR.
2.5 Flow cytometric analysis
Cell apoptosis was detected using annexin V-fluorescein isothiocyanate (FITC) analysis. Briefly, cells were seeded overnight and treated with poly I:C at an indicated concentration for another 48 hr. Then the cells were harvested and washed twice with PBS. Following 15 min incubation with annexin V-FITC and proliferation index (PI) in binding buffer at room temperature in the dark, cell apoptosis was detected by FACS Calibur flow cytometry (FCM) (BD Biosciences, San Jose, CA). The early apoptotic cells (annexin V positive only) plus late apoptotic cells (annexin V and PI positive) were quantified.
For cell proliferation analysis, cells were pretreated with eBioscience™ Cell Proliferation Dye eFluor™ 670 (Invitrogen) according to the manufacturer's instructions at a concentration of 10 μM. After the last wash with phosphate buffer saline (PBS), cells were cultured in the dark. Overnight, cells were further treated with various concentrations of poly I:C for 48 hr. Then cells were harvested, washed twice with cold PBS, and detected by FACS Calibur FCM. The PI of cells was analyzed using Mod Fit LT 3.0 software.
2.6 Western blotting
Cell samples with indicated treatments were lysed in an ice-cold radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotechnology, Haimen, China) and collected supernatant was quantitated with a bicinchoninic acid (BCA) Protein Quantification Kit (Thermo Fisher Scientific). Equal amounts of proteins were then fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad). After blocked with 5% bovine serum albumin (BSA) at room temperature for 1 hr, the samples were immunostained with primary antibodies (anti-MDA-5 and RIG-1 antibodies; Cell Signaling Technologies (CST, Danvers, MA); anti-TLR3, Novus, Littleton, CO; anti-GAPDH antibody, Thermo Fisher Scientific) at 4°C overnight. Then secondary antibodies (Thermo Fisher Scientific) were incubated at room temperature for 1 hr. Finally, target protein expression was visualized using ECL Plus Western blotting (WB) detection reagents (Millipore, Bedford, MA).
2.7 Enzyme-linked immunosorbent assay for IFN-β in cell culture media
IFN-β in cell culture supernatant was measured via an enzyme-linked immunosorbent assay using a commercial kit (R&D) according to the manufacturer's instructions. Briefly, after HCT-8/PTX cells were treated with poly I:C, PTX, or both for 24 hr, cell culture medium supernatant was collected and centrifuged at 2,000×g for 10 min to remove cell debris. Aliquots (50 µl) diluted samples were then measured according to the manufacturer's instructions and the final data were obtained from detection of optical density (OD) at 450 nm.
2.8 Nude mice xenograft tumor assay
All animal work was approved by the Animal Care Committee of Nanjing University in accordance with Institutional Animal Care and Use Committee guidelines. All the efforts were made to minimize the animal suffering.
For the nude mice xenograft tumor assay, 2 × 107 HCT-8/PTX cells suspended in 100 μl of sterile PBS were injected subcutaneously into the right flank of female BALB/c nude mice (6–8 weeks). When tumors reached an average size of 100 mm3, mice were randomized into three groups for different treatments (n = 8): Control (5% glucose injection), PTX alone (10 mg/kg, intravenous injection), and a combination of poly I:C (25 µg every mouse, intraperitoneal injection) with PTX (10 mg/kg, intravenous injection) every 2 days for about 1 week. Body weight of mice and two perpendicular diameters (L and W) of tumors were recorded until the end of the experiment; tumor size was calculated according to the formula: V (volume) = (LW2)/2, where “L” represents the largest length and “W” represents the smallest width.
2.9 Immunohistochemical staining
Immunohistochemical assay was carried out on histological sections of 4% formalin-fixed and paraffin-embedded slides. After deparaffinized in xylene and hydrated in descending dilutions of ethanol, antigen retrieval was obtained according to the manufacturer's protocol of primary antibodies and endogenous peroxidase was blocked by 3% H2O2 for 10 min. Then the slides were covered with 5% goat serum at room temperature. After 1 hr, the slides were incubated with primary antibodies of TLR3 (Novus) or Ki67 (Abcam, Cambridge, UK) at 4°C overnight. Next, HRP-conjugated second antibodies (Abcam) were incubated for 1 hr at room temperature and slides stained with 3.3′-diaminobenzidine (Beyotime Biotechnology) for 5 min were followed. Finally, the cell nucleus was counterstained with hematoxylin (CST) and then the slides were subjected to morphometric analysis.
2.10 Statistical analysis
All experiments were repeated at least three times. One-way analysis of variance followed by Student's t test was used to analyze the statistical significance among groups, and the statistical difference was defined as significant for *p < 0.05, very significant for **p < 0.01 and *** for p < 0.001.
3 RESULTS
3.1 Poly I:C specifically impairs the viability of the PTX-resistant cell line HCT-8/PTX
First, the response of different colon cancer cell lines to PTX were characterized. From CCK-8 result, we observed that SW620 and HCT116 were much more sensitive to PTX than HCT-8 (Figure 1a), whereas HCT-8/PTX was far more resistant to PTX, with an IC50 about 50-fold greater than HCT-8 IC50 values (Figure 1b and Figure 2a). A further characterization analysis of these paired cell lines (HCT-8 and HCT-8/PTX) is depicted in Figure 1c–e, where HCT-8/PTX showed a feature with higher expression of drug resistance genes of ABCB1, MRP, and BCRP and the protein of P-gp. In addition, the accumulation of Rho-123 in HCT-8/PTX cell line was much weaker than that in HCT-8 cells (Figure 1f,g). Next, Figure 2b displayed that PTX-sensitive cell lines (SW620 and HCT116) stimulated with poly I:C resulted in minimal change in cell viability. While the parent cell line HCT-8 showed a moderate but not significant response to poly I:C, the survival of HCT-8/PTX was significantly lessened in a concentration-dependent manner. Moreover, poly I:C directly induced cell apoptosis and inhibited cell proliferation of HCT-8/PTX in a dose-dependent manner (Figure 2c–f), both of which accounted for the decreased cell viability mentioned above. Collectively, these data indicate that poly I:C specifically impairs the viability of the PTX-resistant cell line, HCT-8/PTX.
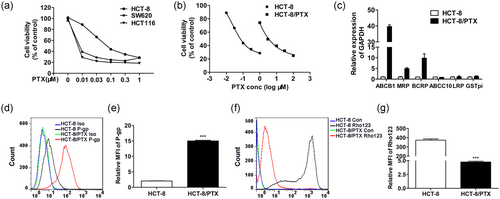
Characterization of HCT-8 and HCT-8/PTX cells. (a) Different colon cancer cell responses to PTX following treatment of 48 hr. (b) HCT-8/PTX cells were more resistant to PTX than HCT-8 cells. (c) The drug resistance genes expression was analyzed in HCT-8 and HCT-8/PTX cells. (d,e) HCT-8/PTX cells showed much higher expression of P-gp than HCT-8. (f,g) The accumulation of Rho-123 in HCT-8/PTX cells was much weaker than it in HCT-8 cells. PTX: paclitaxel [Color figure can be viewed at wileyonlinelibrary.com]
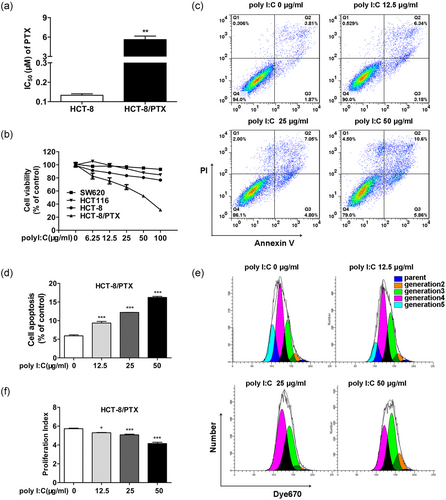
Poly I:C specifically impairs the viability of the PTX-resistant cell line HCT-8/PTX. (a) The IC50 of PTX was calculated by the cell viability of HCT-8/PTX and its parent cell line HCT-8 for a 48 hr treatment of PTX. (b) Poly I:C specifically impaired cell viability of the PTX-resistant cell line HCT-8/PTX compared to other colon cancer cells. (c, e) Poly I:C promoted apoptosis and inhibited proliferation of HCT-8/PTX cells in a concentration-dependent manner. (d, f) Quantitative analysis of the early and late apoptosis rate and proliferation index in HCT-8/PTX cells of (c) and (e). PI, proliferation index; Poly I:C: polyinosinic: polycytidylic acid; PTX: paclitaxel [Color figure can be viewed at wileyonlinelibrary.com]
3.2 TLR3 contributes to IFN-β secretion to decrease the viability of poly I:C-treated HCT-8/PTX cells
Type I IFN mediates antitumor effects by inducing apoptosis or affecting proliferation in tumor cells (Chawla-Sarkar et al., 2003). From the results of qPCR, we observed that after dsRNA treatment, mRNA fold change of IFN-β in PTX-sensitive colon cell lines was blunt in comparison with the PTX-resistant cell line HCT-8/PTX (Figure 3a). Compared with the control group, the secretion of IFN-β in the medium was remarkably increased in HCT-8/PTX cells treated with poly I:C (Figure 3b). In addition, cell survival was further impaired by exogenous IFN-β in a concentration-dependent manner, indicating that IFN-β was indeed responsible for the decreased viability of HCT-8/PTX cells (Figure 3c). Next, as the crucial determinant in a responsive state of exogenous nucleotides, the expression level of dsRNA receptors was detected by WB. As our data indicated in Figure 3d, the expression level of dsRNA receptors such as TLR3, MDA-5, and RIG-1 in PTX-sensitive cell lines HCT116 and SW620 exhibited little variation after poly I:C stimulation. Nevertheless, the expression level of MDA-5 and RIG-1 was both markedly upregulated in HCT-8/PTX and its parent cell line HCT-8. Though the varied expression of TLR3 was not detected in WB, the results of FCM indicated that TLR3 was present both inside and on the cell surface in this paired cell lines, and its expression level was increased upon poly I:C stimulation (Figure 3e). To make a further comparison among the varied expression of dsRNA receptors, we found that despite the expression of MDA-5 and RIG-1 in poly I:C-treated HCT-8 cells were much more enhanced than that in HCT-8/PTX, the transcription of IFN-β seemed impervious to this variation. Thus, we assumed that TLR3 might be responsible for the secretion of IFN-β in HCT-8/PTX cells. As indicated, overexpression of TLR3 receptors led to amplified responsiveness to poly I:C with accelerated IFN-β expression, and significantly decreased cell viability (Figure 3f–h). Next, the TLR3-neutralizing antibody TLR3.7 was used to explore whether TLR3 receptor on cell membranes played a role in promoting cell apoptosis. Pretreatment with monoclonal antibodies blocking (mAb) cell-surface TLR3 receptors recovered the cell viability upon poly I:C stimulation in HCT-8/PTX cells which confirmed this assumption (Figure 3i). Engaged TLR3 receptor with the production of IFN-β, the activation of NF-κB is indispensable for TLR3-mediated apoptosis (Alexopoulou, Holt, Medzhitov, & Flavell, 2001). Following dsRNA stimulation, the elevated phosphorylated P65 was also detected in HCT-8/PTX (Figure 3j). Taken together, these data indicate that TLR3 contributes to the enrichment of IFN-β which induces cell apoptosis in poly I:C-stimulated HCT-8/PTX cells.
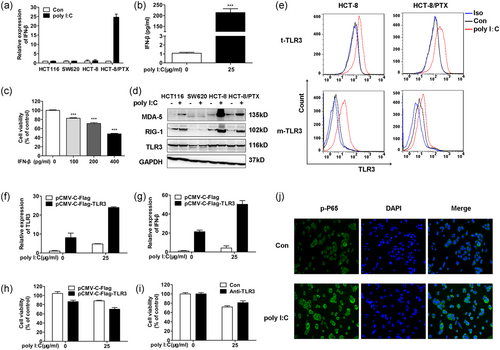
TLR3 contributes to IFN-β secretion to decrease the viability of poly I:C treated HCT-8/PTX cells. (a) Poly I:C specifically enhanced the transcriptional level of IFN-β in HCT-8/PTX than other colon cancer cells. (b) The secretion level of IFN-β was measured after treatment of HCT-8/PTX cells with 25 μg/ml poly I:C for 24 hr. (c) Exogenous IFN-β inhibited cell viability of HCT-8/PTX in a concentration-dependent manner. (d) The protein expression level of dsRNA receptors in different colon cancer cells was measured upon poly I:C stimulation for 24 hr. (e) The total and cell membrane expression levels of TLR3 (namely t-TLR3 and m-TLR3) were upregulated upon poly I:C stimulation in both HCT-8 and HCT-8/PTX cells. (f,g) The upregulations of TLR3 and IFN-β in HCT-8/PTX cells were determined by qPCR following transfection with a plasmid encoding TLR3 and poly I:C treatment. (h) Overexpression of TLR3 further accelerated the decreased viability of HCT-8/PTX cells upon poly I:C stimulation. (i) The impaired viability of HCT-8/PTX cells treated with poly I:C was restored by the neutralizing antibody TLR3. (j) Activated NF-κB signal pathway upon poly I:C stimulation was characterized by the phosphorylation of P65. dsRNA: double-stranded RNA; IFN: interferon; poly I:C: polyinosinic: polycytidylic acid; PTX: paclitaxel; TLR3: toll-like receptor 3 [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Poly I:C prefers TLR3-UNC93B1 signaling axis for IFN-β production in HCT-8/PTX cells
Although the expression level of TLR3 was increased in poly I:C-stimulated HCT-8, no upregulation of IFN-β or the correlated cell viability decline was detectable. The previous study has been demonstrated that IFN-β production is dependent on UNC93B1, a trafficking chaperone responsible for translocating TLR3 from ER to endosomes for signal priming (Lee et al., 2013; Qi et al., 2012). Therefore, the role of UNC93B1 as a contributor to IFN-β secretion and the decrease of cell viability was investigated. As data indicated in Figure 4a, poly I:C specifically contributed to the upregulation of UNC93B1 in HCT-8/PTX other than HCT-8. Furthermore, Figure 4b as well indicated that the expression level of UNC93B1 observed in poly I:C-stimulated HCT-8/PTX was comparative to that transfected with plasmid DNA coding TLR3, and was larger in the combined treatment group. This elevated effect confirmed that the expression level of UNC93B1 could be reinforced by TLR3. In addition, transfection of HCT-8/PTX cells with TLR3 resulted in augmented response to poly I:C where the production of IFN-β was increased and the cell viability was decreased (Figure 3f–h). This indicated that poly I:C induced IFN-β production through TLR3-UNC93B1 signaling axis in HCT-8/PTX cells. Then, we further explored whether the production of IFN-β was in a UNC93B1-dependent manner. To our surprise, the expression level of IFN-β was higher in response to poly I:C, along with a further decreased cell viability upon gene silence of UNC93B1(Figure 4c-e). Knockdown of TLR3 showed a similar result (Figure 4f-h). Therefore, we speculated that other dsRNA receptors, such as MDA-5 or RIG-1 might be responsible for the more plentiful expression of IFN-β. As expected, the transcription level of both MDA-5 and RIG-1 was upregulated upon poly I:C stimulation (Figure 4i-l) in accordance with the former protein level in Figure 3d and RIG-1 might play an essential role in mediating the increased IFN-β, as downregulation of either UNC93B1 or TLR3 further increased IFN-β expression under the treatment of poly I:C. In sum, these data suggest that the production of IFN-β is preferentially mediated through TLR3-UNC93B1 signaling in poly I:C-stimulated HCT-8/PTX cells and it is at least partially via the upregulation of UNC93B1, which might alter TLR3 localization to endosomes for IFN-β production.
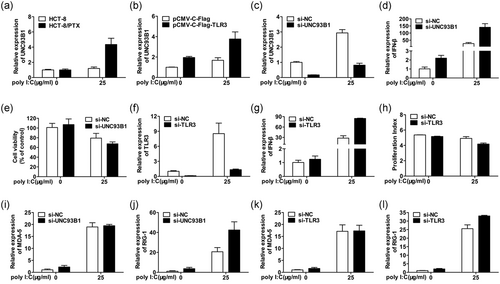
Poly I:C prefers TLR3-UNC93B1 signaling axis for IFN-β production in HCT-8/PTX cells. (a) mRNA expression of UNC93B1 was markedly elevated in poly I:C stimulated HCT-8/PTX cells compared with that in its parent cell line HCT-8. (b) The gene expression level of UNC93B1 was upregulated by transfected TLR3 and further enhanced by the followed treatment with poly I:C in HCT-8/PTX cells. (c,d) The gene expression level of IFN-β was further upregulated under the condition of UNC93B1 knockdown and followed by the treatment with poly I:C. (e) The silence of UNC93B1 further impaired HCT-8/PTX cell viability. (f,g) The gene expression level of IFN-β was further upregulated under the condition of TLR3 knockdown and followed by the treatment with poly I:C. (h) HCT-8/PTX cell proliferation was further inhibited by TLR3 silencing followed by poly I:C stimulation as indicated by the proliferation index (PI). (i-l) The mRNA expression level of MDA-5 and RIG-1 is quantified by qPCR in HCT-8/PTX cells under the condition of UNC93B1 or TLR3 knockdown followed by poly I:C treatment. IFN: interferon; mRNA: messenger RNA; poly I:C: polyinosinic: polycytidylic acid; PTX: paclitaxel; qPCR: quantitative PCR; TLR3: toll-like receptor 3
3.4 Combination of poly I:C with PTX synergistically intensifies the TLR3-UNC93B1 signaling for IFN-β production in HCT-8/PTX cells
As shown in Figure 5a, HCT-8/PTX cell viability was inhibited by PTX in a concentration-dependent manner, and poly I:C at different concentrations effectively interrupted the cell growth with an additive effect. Then studies of combined effect were followed and results showed that the combined treatment with ploy I:C and PTX significantly decreased cell viability over individual treatment (Figure 5b). Data analyzed by CompuSyn software with CI values indicated that the combination was synergistic with a strong synergism (CI, 0.1–0.3), except the lowest concentration combination group only displayed synergism (CI, 0.66; Figure 5c). Next, the underlying mechanism correlating to decreased cell viability was explored. As data showed that the PTX per se did not alter either the expression level of TLR3, UNC93B1, or IFN-β (Figure 5d–f). While administered with poly I:C, PTX acted synergistically with it following the boosted expression level of TLR3-UNC93B1 and secretion of its downstream molecular IFN-β Figure 5g, which was responsible for cell death described above. In keeping with the results shown above, the protein level of TLR3 in the WB experiment was not associated with the varied transcriptional level of TLR3, which might be related to the fact that TLR3 was processed with proteolysis, thus, inhibiting detection (Figure 5h).
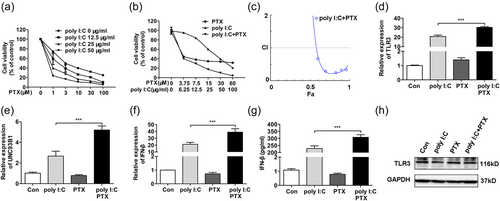
Combination of poly I:C with PTX synergistically intensifies the TLR3-UNC93B1 signaling for IFN-β production in HCT-8/PTX cells. (a) Poly I:C at different concentrations enhanced the toxicity of PTX in HCT-8/PTX cells. (b) Poly I:C and PTX synergistically decreased the viability of HCT-8/PTX cells. (c) Fa-CI plot showed CI values for each fractional effect of HCT-8/PTX cells. The curves were generated with CompuSyn software. CI < 1, CI = 1, and CI > 1 indicates synergistic, additive, and antagonistic effects, respectively. (d–f) The combination of poly I:C (25 μg/ml) and PTX (3 μM) synergistically contributed to the mRNA expression of TLR3, UNC93B1, and IFN-β in HCT-8/PTX cells. (g,h) The combination of polyI:C (25 μg/ml) and PTX (3 μM) on the secretion of IFN-β and protein expression of TLR3 in HCT-8/PTX cells. CI: combination index; IFN: interferon; mRNA: messenger RNA; poly I:C: polyinosinic: polycytidylic acid; PTX: paclitaxel; TLR3: toll-like receptor 3 [Color figure can be viewed at wileyonlinelibrary.com]
3.5 Poly I:C augments the cytotoxicity of antineoplastic agents in a PTX-resistant mouse model
To investigate whether the combination of poly I:C and PTX has the same elimination effect on PTX-resistant tumors in vivo, a heterologous HCT-8/PTX tumor model was established. As our data showed, while the single treatment of PTX seemed only a little effective, the combined treatment of PTX and poly I:C significantly inhibited or even abrogated tumor growth (Figure 6a,b). In addition, TLR3 was upregulated and the expression of Ki67 was decreased in the combined treatment group in comparison with those in the control or single PTX treatment group (Figure 6d–g). Moreover, the mean body weight in the combined treatment groups was not significantly changed which suggested that the combined treatment of poly I:C and PTX may not cause serious adverse effects in vivo (Figure 6c).
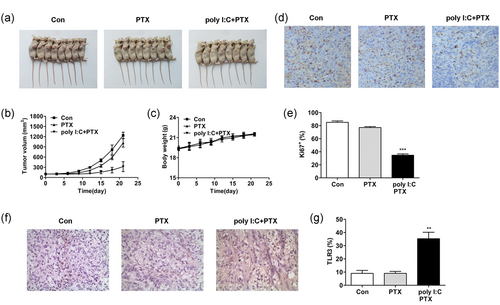
Poly I:C augments the cytotoxicity of antineoplastic agents in a PTX-resistant mouse model. (a,b) Poly I:C in combination with PTX significantly inhibited tumor formation in PTX-resistant tumors. (c) The treatment of poly I:C and PTX resulted in no differences in the body weight of tumor-bearing mice. (d) The cell proliferation in PTX-resistant tumors was significantly impaired by the combined treatment with poly I:C and PTX, as indicated by immunohistochemistry using Ki67. (e) Statistical analysis of Ki67 expression in (d). (f) Poly I:C and PTX synergistically promoted TLR3 expression in the tumor tissue. (g) Statistical analysis of TLR3 expression in (f). Poly I:C: polyinosinic: polycytidylic acid; PTX: paclitaxel; TLR3: toll-like receptor 3 [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
In our study, we found that poly I:C specifically reduced the viability of the PTX-resistant cell line HCT-8/PTX compared with other colon cancer cells. The decreased cell viability was correlated with IFN-β production in a TLR3-dependent mechanism. Further study results demonstrated that UNC93B1, responsible for the production of INF-β by trafficking TLR3 from ER to endosomes, could be upregulated by TLR3 and poly I:C preferentially activated the TLR3-UNC93B1-IFN-β signaling axis to promote HCT-8/PTX cell death. In addition, the combination of poly I:C and PTX synergistically inhibited cell proliferation of HCT-8/PTX with augmented TLR3-UNC93B1 signaling for IFN-β expression both in vitro and in vivo. Thus, our findings demonstrate that TLR3 can be as a regulator of UNC93B1, which in turn governs the IFN-β responsiveness to PTX-resistant tumor treatment.
In our study, we observed that exogenous poly I:C had no effect on cell survival in PTX-sensitive cell lines SW620 and HCT116, which was consistent with previous reports (Bugge et al., 2017; Nojiri et al., 2013). As our results indicated, the nonresponsiveness phenotype of these cell lines might be the loss of poly I:C-induced upregulation of dsRNA receptors and disrupted type I IFN production and sensing. It was noteworthy that poly I:C markedly decreased the cell viability of HCT-8/PTX other than HCT-8 by simultaneously inducing cell apoptosis and inhibiting cell proliferation. Thus, it was intriguing to identify the specific mechanism. Previous studies have shown that TLR3 directly triggers apoptosis in human cancer cells and type I IFN response mainly contributes to this effect (Bianchi et al., 2017). Furthermore, cells defective in fundamental and induced expression of TLR3 would result in a dsRNA-unresponsive phenotype with an inactivated NF-κB pathway and impaired induction of IFN-β (Sun & Leaman, 2004). Congruently, in our study, we observed that upon poly I:C stimulation, the cell viability of HCT-8/PTX was decreased accompanied by INF-β production in a TLR3-dependent manner. However, what interested us was that while IFN-β responded well to poly I:C in HCT-8/PTX with upregulated TLR3, it was not altered in HCT-8 with an almost constant cell viability. Furthermore, though our qPCR and FCM experiment showed that both mRNA and protein expression levels of TLR3 were upregulated in HCT-8/PTX cells and its parent cell line upon poly I:C stimulation, the synthetic antibody used in our study with recognition amino acids 55–85 of human TLR3 as the immunogen did not detect the variation. This indicated that TLR3 might be subject to complex regulation through cleavage for its activation. As the dsRNA receptor, TLR3 has been acknowledged to respond to exogenous nucleic acids in endosomes with proteolytic cleavage (Duffney et al., 2018; Garcia-Cattaneo et al., 2012). UNC93B1, as an important molecular chaperone, has been demonstrated to physically interact with its client proteins including TLR3, and deliver them from ER to endosomes where additional cleavage takes place for signal priming (Kim et al., 2013; Lee et al., 2013). Moreover, one report (Garcia-Cattaneo et al., 2012) demonstrates that a deficiency in UNC93B1 leads to impaired cellular IFN-α/β antiviral responses in a genetic etiology for HSE. Based on the pivotal contribution of UNC93B1 to the responsiveness of IFN-β, we proposed a role of it in endosome localization of TLR3 for its cleavage and functional activation. As our results indicated, though the constitutive expression level of UNC93B1 in HCT-8 cells was approximately equal to HCT-8/PTX cells, it was exclusively upregulated upon poly I:C stimulation in the latter with associated IFN-β production. Pohar et al. (2013) make a point that stimulation by exogenous poly I:C, as well as TLR3, can upregulate the transcription of UNC93B1 with a contribution to IFN signaling (Casrouge et al., 2006). Notably, this is in line with our observations where the expression of UNC93B1 in HCT-8/PTX cells transfected with TLR3 was upregulated to the same level seen in poly I:C-treated cells, and it was superimposed by the simultaneous treatment of both, along with dramatically increased IFN-β and decreased cell viability. All the points above, as well as our results seem to corroborate the notion that TLR3 stimulation upregulates UNC93B1 resulting in the accumulation of IFN-β, which in turn further contributes to TLR3 expression. Thus, a positive feedback loop is formed. It is reasonable to conclude that a TLR3-UNC93B1-IFN-β signaling feedback loop leads to the decreased viability observed in HCT-8/PTX cells. In addition, there is a recent report that UNC93B1 is required for secretion, as well as effects on the plasma localization of TLR3 in some cells (Qi et al., 2010). Nevertheless, in our experiment, cell surface TLR3 expression was unaltered in HCT-8/PTX with UNC93B1 knockdown (data not shown). Moreover, though TLR3 was accumulated on the cell membrane of poly I:C-treated HCT-8 cells, the expression of UNC93B1 remained unchanged. This implied that UNC93B1 might not participate in TLR3 trafficking to the cell membrane in this paired cell lines. Whether this difference is due to the diverse cell lines or the complex regulation of TLR3 remains to be determined.
In addition, mAb blockade of cell-surface TLR3 interrupted the dsRNA-mediated cell viability decrease verified that TLR3 on the cell surface indeed participated in the recognition of dsRNA and triggered downstream signals leading to IFN-β production. This result is in agreement with the former reports, where extracellular TLR3 is essential for IFN-β induction (Duffy et al., 2007; Matsumoto, Kikkawa, Kohase, Miyake, & Seya, 2002). However, though our study indicated that cell surface TLR3 might play a role in TLR3 responses, internalization by UNC93B1 into endosomes leading to downstream signaling might be more important for cell apoptosis, as TLR3 was also expressed on the cell surface of HCT-8, of which the expression of IFN-β was not altered. It should be noted that we could not completely exclude the possibility that RIG-1 and MDA-5 contribute to the recognition of poly I:C internalized by endocytosis for IFN-β production. As our experimental results showed, poly I:C also elevated the expression levels of RIG-1 and MDA-5 along with IFN-β production, and this effect was stronger with impaired TLR3-UNC93B1 signaling in HCT-8/PTX cells. Following a comprehensive analysis of experimental results in the parent cell line, where there was a little alteration in the production of IFN-β and more enhanced expression levels of both RIG-1 and MDA-5, we proposed that poly I:C preferred TLR3-UNC93B1 signaling rather than RIG-1 or MDA-5 for IFN-β production leading to cell death in HCT-8/PTX. Therefore, the different responses to poly I:C in these two cell lines might be that upon poly I:C stimulation, TLR3 on HCT-8/PTX cell surface detects NF-κB activation, and UNC93B1 localized in endosomes is undergoing proteolysis for further concurrent signal priming. However, HCT-8 cells are defective in upregulation of UNC93B1, or they are not activated by poly I:C. Thus, the nonfunctional UNC93B1 fails to exit ER or physiologically interact with TLR3 for trafficking, and consequently, does not initiate IFN-β production. Additional studies are necessary to identify whether the role of UNC93B1 in trafficking cell membrane TLR3 to endosomes for IFN-β secretion is a universal phenomenon in PTX-resistant cell lines. We also found that poly I:C could specifically decrease the viability of A2780/PTX cells other than PTX-sensitive ovarian cancer cells; TLR3 was exclusively expressed on the surface of A2780/PTX cells, but not on its parent cell line, A2780, or even ES-2 and SKOV3 cells. The upregulation of IFN-β in A2780/PTX cells upon poly I:C stimulation accompanied by increased expression of UNC93B1 (data not shown).
With attempts to optimize practical results, combination chemotherapy regimens for neoplastic cell treatment are frequently favored over single agents. Recent studies display that poly I:C in combination with anticancer agents like cycloheximide, 5-fluorouracil, or PTX for chemo-immunotherapy appears to offer promising effects (Jiang et al., 2008; Matijević, Kirinec, & Pavelić, 2011; Park et al., 2012). Our results also indicated the efficiency of poly I:C in killing cancer cells. Further, the anticancer effects of poly I:C combined with PTX in HCT-8/PTX cell line for a chemo sensitive effect were explored. Our data showed that poly I:C strengthened the chemo sensitivity to PTX via the TLR3-UNC93B1-IFN-β signaling pathway. A positive result was also demonstrated in a PTX-resistant mouse model. In conclusion, our findings indicate that the UNC93B1-dependent production of IFN-β regulates cell viability by TLR3-dependent pathways in PTX-resistant colon cancer cell HCT-8/PTX, which further broadens the potential utility of poly I:C as a chemo-sensitization strategy for treating PTX-resistant patient populations.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (No. 81572354 to Tingting Wang and 81572557 to Shuli Zhao) and The State Key Laboratory of Reproductive Medicine (SKLRM-K201507 to Shuli Zhao).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




