Altered plasma proteins released from platelets and endothelial cells are associated with human patent ductus arteriosus
Abstract
Patent ductus arteriosus is the third most common congenital heart disease and resulted from the persistence of ductal patency after birth. Ductus arteriosus closure involves functional and structural remodeling, controlled by many factors. The changes in plasma protein levels associated with PDA closure are not known. Here we for the first time demonstrate six key differential plasma proteins in human patent ductus arteriosus patients using proteomic technology and present a model to illustrate the constriction and closure of ductus arteriosus. Differentially expressed proteins were analyzed by using isobaric tags for relative and absolute quantification and validated by enzyme-linked immunosorbent assay in new samples. The proteomic data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD008568. We found 74 upregulated and 98 downregulated proteins in the plasma of patients with PDA. Five decreased proteins (platelet factor 4, fibrinogen, von Willebrand factor, collagen, and mannose binding lectin-associated serine protease-2) and one increased protein (fibronectin) may increase the risk of patent ductus arteriosus. Those proteins are closely related to platelet activation and coagulation cascades, complement mannan-binding-lectin, and other systemic signaling pathways. Our findings for the first time indicate that the differential proteins involved in different pathways may play key roles in the nonclosure of the ductus arteriosus in humans and may be developed as biomarkers for diagnosis. All those findings may be served as the basis of understanding the etiology and pathogenesis of patent ductus arteriosus.
1 INTRODUCTION
The ductus arteriosus (DA) is a normal and essential structure in fetal circulation. It is highly patent and shunts more than half of the deoxygenated cardiac output into the descending aorta (Backes & Smith, 2016; Leonhardt et al., 2003; Matsushita, Endo, & Fujita, 2000). Fetal patency of the DA is controlled by many factors, including low fetal oxygen tension (Heymann & Rudolf, 1975), nitric oxide (NO; Echtler et al., 2010; Kajino et al., 2000), and cyclooxygenase-mediated products of arachidonic acid metabolism, particularly prostaglandin E2 (PGE2; Coggins et al., 2002). Locally produced and circulating PGE2 in the fetus causes vasodilation of the DA via interaction with ductal prostanoid receptors (Michelakis et al., 2000).
After birth, the process of ductal closure occurs in two sequential but overlapping phases. First, provisional closure is accomplished by smooth muscle cell constriction and DA constriction, which result in functional closure (Clyman, 2006; Echtler et al., 2010). Subsequently, proliferation of cells within the former DA lumen leads to anatomical remodeling of the DA and permits permanent closure, which result in structural remodeling (Clyman et al., 2002; Clyman, 2006; Echtler et al., 2010; Natarajan et al., 2013).
Persistence of ductal patency is abnormal. Patent ductus arteriosus (PDA) is one of the most common congenital heart diseases (CHD); it is found in about 57 full-term neonates (Hoffman, Kaplan, & Liberthson, 2004) per 100,000. Its incidence increases to about 33% in preterm neonates (Listed, 1993) and rises even higher in those with very low-birth weight (< 1,000 g; Fanaroff et al., 2007). PDA in full-term neonates can have their PDA last for their childhood, adolescence, or even adulthood.
PDA can result in right ventricular dysfunction, pulmonary hypertension, and other serious complications (Echtler et al., 2010; Schneider & Moore, 2006).
Closure of the DA requires prenatal formation of intimal cushions. A gene transfer strategy was developed in utero using fetal lamb to test the role of fibronectin in DA intimal cushion formation, which inhibited fibronectin translation and prevented intimal cushion formation (Mason et al., 1999). Other studies demonstrated that prostaglandin E2-activated Epac (Yokoyama et al., 2008), T-type voltage-dependent Ca2+ channel (Akaike et al., 2009), and growth hormone (Jin et al., 2011) play an important role in the vascular remodeling of the rat DA.
There are no reports on human plasma proteins related to DA closure by using proteomic method although there was a study in tissue (Hong et al., 2015). In recent years, hundreds of proteins have been tested for their importance as potential biomarkers in different diseases. We have used proteomic methods to demonstrate the plasma protein changes in complex cyanotic CHD such as tetralogy of Fallot or noncyanotic CHD such as ventricular septal defect (Xuan et al., 2014; Zhang et al., 2015), in pulmonary arterial hypertension associated with CHD (Zhang et al., 2016), in valvular heart disease (Gao et al., 2013), and in coronary artery disease (Guo et al., 2017). These studies may reveal the possible mechanisms and genetic deficiency in the process of diseases and may have strong clinical implications.
In the current study, we used proteomic methods to identify the differential plasma proteins from patients with PDA at early childhood, compared with normal children as the control. We anticipated that identification of the differential proteins may help understanding the etiology and pathogenesis of PDA.
2 METHODS
2.1 Study population
From January 2013 to December 2016, 60 patients with CHD with isolated PDA without other major CHD were enrolled at TEDA International Cardiovascular Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China. The study protocol was approved by the Ethics Committee (Institutional Review Board) of the hospital and informed consent was obtained from parents or guardians of the children with CHD. Routine clinical assessment of the patients was performed and diagnosis of patients was verified by preoperative echocardiography and/or Computed Tomography angiography. All PDA patients underwent corrective surgery.
In addition, 60 normal children with same ethnicity, gender, and age were recruited in the study as normal control. The control group was chosen from normal body checking or CHD-screening program at the hospital. All control subjects were confirmed either by clinical screening or plus echocardiography that confirmed no cardiac diseases.
2.2 Sample preparation
Blood samples were taken from patients before surgery. From each sample, 2 ml blood was harvested in collection tubes with ethylene diamine tetraacetic acid and then centrifuged at 1,500 g for 10 min. The plasma was separated from the blood cells. Plasma was then collected, divided into aliquots, and stored frozen at − 80°C until the analysis was carried out.
2.3 Plasma high-abundance protein depletion
The plasma samples from patients with PDA and normal controls were processes to deplete the top-two (albumin and IgG) high abundance proteins using the ProteoExtractTM Albumin/IgG Removal Kit (Calbiochem, La Jolla, CA). Samples were processed according to the manufacturer's instructions.
2.4 Solution digestion and isobaric tags for relative and absolute quantitation (iTRAQ) labeling
The eluted samples were mixed in 0.5 M triethylammonium bicarbonate (TEAB) buffer with 1 mM phenylmethyl sulfonyl fluoride and 0.1% sodium dodecyl sulfate (SDS), followed by sonication for 5 min and centrifugation at 20,000 g for 30 min. The supernatant was transferred to another tube, 0.5 M TEAB buffer was added to the pellet to repeat the protein extraction, and the sample was again centrifuged at 20,000 g for 30 min. Proteins in the combined supernatant were reduced (10 mM DTT, 56℃ for 60 min), alkylated (55 mM iodoacetamide, room temperature for 60 min), precipitated by precooled acetone at 220℃ for 30 min, and then centrifuged at 20,000 g for 30 min. The pellet was washed twice with acetone, and the final pellet was dissolved in 0.5 M TEAB buffer with 0.1% SDS, sonicated for 5 min, and centrifuged at 20,000 g for 30 min. The supernatant was used for liquid digestion, and the protein concentration was determined using the Bradford assay.
For the iTRAQ/Shotgun experiment, 3.3 μg of trypsin was added to 100 μg of the protein solution for protein digestion at 37℃ for 24 hr. Then, 1 μg of trypsin was added again, and the sample was digested for 12 more hours. The digests were dried in a Speedvac. Each precipitate was dissolved in 30 μl of 0.5 M TEAB and mixed with 70 μl of isopropanol. The protein digests obtained from 6, 12, 18, 24, and 30 days after pollination (DAP) were labeled with an iTRAQ reagent (AB SCIEX, Framingham, MA) 113, 114-115, 116, 117, 118, 119, and 121, respectively. The labeling reaction was conducted for 2 hr at room temperature, and then the eight labeled peptides were pooled together.
2.5 SCX and RP nano LC-MS/MS analysis of labeled peptide
The peptides were dried in a Speedvac and dissolved in 1 ml of buffer A (10 mM KH2PO4 in 25% ACN at pH 2.8). After adjusting the pH to 3 with H3PO4, the sample was fractionated using strong cation-exchange chromatography (SCX) on an HPLC (Shimadzu, Kyoto, Japan) equipped with a silica-based SCX column (250 mm x 4.6 mm, Phenomenex, Torrance, CA). A total of 28 fractions were collected at a rate of 1 ml/min with a buffer B (10 mM KH2PO4 and 2 M KCl in 25% ACN, pH 2.8) gradient as following: 0% for 50 min, 5% for 51 min, 30% for 71 min, 50% for 76 min, 50% for 81 min, 100% for 86 min, and 100% for 96 min. The fractions were desalted with a strata-X 33 mm PolyRevStage SPE (Phenomenex) following the manufacturer's instructions and dried in a Speedvac. Then, 30 μl of 0.1% formic acid (FA) was added to each dried fraction tube, and 1 μl of the re-dissolved solution was spotted on the target well of an Anchor-chip plate for MALDI-TOF testing. After the MALDI-TOF (Bruker Daltonics, Germany) testing, the peptides in the tubes with few peaks were pooled, resulting in 16 pooled SCX-separated fractions. Each SCX fraction was loaded on a Prominence Nano HPLC system (Ultimate 3000, Germering, Germany) mounted with a 10 cm reversed phase C18 column (ID 75 mm, 5 μm particles, 300A aperture) and separated over a 40 min acetonitrile gradient from 5% to 35% in 0.1% FA combined with a Q Exactive mass spectrometer (Thermo Fisher Scientific, MA, USA). The data were acquired using a data-dependent data acquisition mode in which, for each cycle, the 20 most abundant multiply charged peptides (2+ to 4+) with an m/z between 350 and 2,000 were selected for MS/MS with the 15-s dynamic exclusion setting.
2.6 iTRAQ data analysis and bioinformatics analysis
Protein identification was performed by using Mascot search engine (Matrix Science, London, UK; version 2.3.0) against Uniprot-Human Database containing 216,686 sequences. Proteins identified as showing expression changes were tested for conformity to the following conditions: (1) a false discovery rate < 1% (false discovery rate was estimated by “decoy database searching” using the Proteome Discoverer 1.3); and (2) protein confidence > 99% (“unused ProtScore” > 2). Unused ProtScore was defined as −log (1−% confidence/100). Proteins fulfilling these criteria were considered to have “statistical significance.” Blast2GO software was used to get the proteins GO annotation. The expect value < 0.001 was used to cut off the Blast result and the GO term with Blast2GO's score > 30 to be considered. Kyoto Encyclopedia of Genes and Genomes (KEGG) was used to identify pathway analysis. The Fisher exact test was used for the pathway enrichment. The mass spectrometry proteomics data were deposited to the ProteomeXchange Consortium via the PRIDE (Vizcaíno et al., 2016) partner repository with the data set identifier PXD008568.
2.7 Enzyme-linked immunosorbent assay
Further validation of candidate proteins was performed by using human ELISA kits (CUSABIO BIOTECH, Life Sciences Advanced Technologies Inc., Houston, TX) to detect plasma levels in a new cohort of 80 subjects (40 patients with PDA and 40 controls). The methods followed the manufacturer's instructions. The differentially expressed proteins (DEPs) for further validation was based on (1) expressed differently in PDA and normal controls; (2) potential functional or pathological significance in PDA; and (3) not been reported before in previous proteomics study.
2.8 Statistical analysis
SPSS 17.0 software (SPSS Inc, Chicago, IL) and GraphPad Prism 5 Demo software (GraphPad Software, San Diego, CA) were used for statistical analysis. Data were expressed as mean ± SEM. Unpaired t test was used to compare values between PDA and control group. Statistical significance was defined as p < 0.05.
3 RESULTS
3.1 Patients characteristics and proteomic analysis
The demographics of the PDA patients and controls in the study of iTRAQ and ELISA are shown in Table 1. iTRAQ labeling and mass spectrometry identified 1,012 peptides. Among those, 172 DEPs were found to be statistically significant. The proteins fulfilled both the false discovery rate < 1% and protein confidence > 95% criteria. The ratio of differential proteins was >1.2 or <0.83. All 172 DEPs identified are provided in Supporting Information Supplemental Material Table 1. Of these, platelet factor 4 (PF4), fibrinogen, von Willebrand factor (vWF), collagen, fibronectin, and mannose binding lectin-associated serine protease-2 (MASP-2) listed in Table 2 were possibly associated with PDA and selected for validation by ELISA.
| Stage | Groups | Number | Sex (M/F) | Age (years) p |
|---|---|---|---|---|
| iTRAQ | PDA | 20 | 10/10 | 4.4 ± 0.8 |
| Controls | 20 | 10/10 | 3.8 ± 0.6 > 0.05 | |
| ELISA | PDA | 40 | 20/20 | 4.0 ± 0.5 |
| Controls | 40 | 20/20 | 4.2 ± 0.8 > 0.05 |
- Note. CHD: congenital heart disease; ELISA: enzyme-linked immunosorbent assay; iTRAQ: isobaric tags for relative and absolute quantitation;
- M: male; F: female; PDA: patent ductus arteriosus.
| Accession | Protein | Coverage (%) | Unique peptides | Peptides | MW (kDa)a | Calc. pIb | PDA/ control |
|---|---|---|---|---|---|---|---|
| 4505733 | Platelet factor 4 | 34.65 | 1 | 3 | 10.8 | 8.62 | 0.492 |
| 1769552 | Von Willebrand factor | 3.73 | 1 | 1 | 27.1 | 6.93 | 0.679 |
| 160858157 | Collagen | 1.31 | 1 | 1 | 68.9 | 6.77 | 0.69 |
| 119584204 | Fibrinogen | 12.36 | 2 | 9 | 21 | 5.97 | 0.661 |
| 5459317 | Mannose binding lectin-associated serine protease-2 | 28.0 | 4 | 4 | 19.5 | 5.73 | 0.829 |
| 194390508 | Unnamed protein product (fibronectin) | 40.93 | 1 | 24 | 111.2 | 6.21 | 1.892 |
- Note. iTRAQ: isobaric tags for relative and absolute quantitation; PDA: patent ductus arteriosus.
- a MW = molecular weight.
- b pI = isoelectric point.
3.2 Gene Ontology (GO), KEGG, and STRING analysis of DEPs
Gene Ontology (GO) enrichment and KEGG pathway analysis (Kanehisa, Furumichi, Tanabe, Sato, Morishima, 2017) were performed for an overview of the function of all DEPs and the potential linkage among them. Figure 1 shows the amount of GO enriched terms (biological process, cellular component, and molecular function, Figure 1a) and KEGG pathways based on the DEPs (Figure 1b). The p value < 0.05 represents the significant GO terms and pathways in Homo sapiens from GO and KEGG database. Heatmap (Figure 1c) analysis represents the ratio levels and functional cluster analysis of the DEPs (top 50 from 172, see Supporting Information Supplemental Material Figure 1) in patients with PDA compared with normal controls. The heatmap was constructed using hierarchical clustering algorithm based on Euclidean distance. These results clearly show the differences in the protein profile between patients with PDA and normal controls. The GO analysis may identify the genes that are included in the most significant GO terms in the Homo sapiens (not the number of genes involved) as well as the highest percentage of involved genes (DEPs) in a GO term from a study. In the current study, the most significant GO terms in Homo sapiens based on the DEPs were introduction of bacterial agglutination in biological process, pentametic lgM immunoglobulin complex in cellular component, and calcidiol binding in molecular function (Figure 1d). However, the highest percentage of involved genes (DEPs) were defense response in biological process, extracellular space in cellular component, and binding in molecular function. Further KEGG pathway enrichment and STRING analysis (Figure 2) revealed that these proteins are mainly involved in platelet activation and complement or coagulation cascades that may be related to the pathology of PDA.
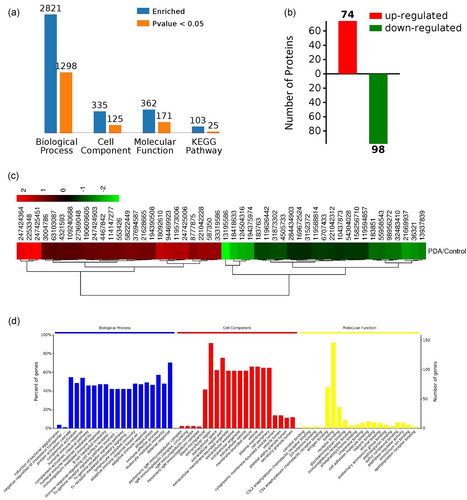
Summary of the protein profile in patent ductus arteriosus and controls. (a,b) Enriched total protein numbers and differentially expressed proteins based on Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. The amount of GO enriched terms (biological process, cellular component, and molecular function) and KEGG pathways are shown in (a). The number of DEPs is shown in (b). The p value < 0.05 represents the significant GO terms and pathways in Homo sapiens from GO and KEGG database. (c) Heatmap represented the ratio levels and functional cluster analysis of the differentially expressed proteins (DEPs, top 50 from 172) in patent ductus arteriosus compared with controls. The heatmap is constructed using hierarchical clustering algorithm based on Euclidean distance. The top of heatmap showed the access number of NCBI. Proteins with similar functions have a relative shorter Euclidean distance (bottom of heatmap). The numbers in the top left represented the fold change of proteins. The upregulation and downregulation are colored by red and green respectively. (d) The top 20 significant GO terms in biological process, cellular component, and molecular function respectively in Homo sapiens from GO and KEGG database based on the DEPs. The most significant GO terms were introduction of bacterial agglutination in biological process, pentametic lgM immunoglobulin complex in cellular component, and calcidiol binding in molecular function. However, the highest percentage of involved genes (DEPs) was defense response in biological process, extracellular space in cellular component, and binding in molecular function [Color figure can be viewed at wileyonlinelibrary.com]
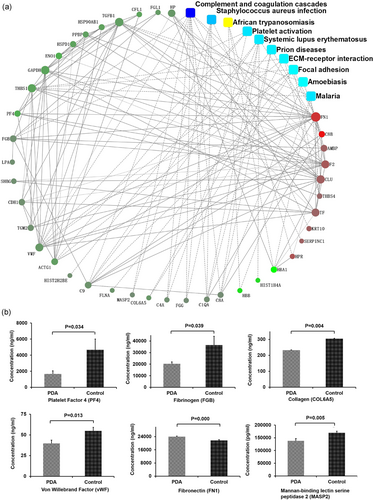
Most significantly associated Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of differentially expressed proteins and key regulation network model of protein–protein interaction in patent ductus arteriosus and controls. (a) Circle nodes indicate proteins, filled color represents expression fold change using gradient color from blue to red; rectangles indicate KEGG pathways, filled color represents p-value using gradient color from yellow to blue to red. A solid line between two proteins indicates a known interaction annotated in the STRING database; a dashed line between proteins indicates indirect interaction. (b) ELISA was used to measure plasma levels of platelet factor 4, von Willebrand factor, collagen, fibrinogen and mannose binding lectin-associated serine protease-2 in patients with patent ductus arteriosus, and controls as validation. Data are shown as mean ± SEM [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Validation of the candidate proteins by ELISA
Further validation of the six chosen proteins were performed in the new cohort of patients with PDA by ELISA (n = 40).
Among the six proteins validated, PF4 and fibronectin were at top 50 of 172 DEPs. The reminder of four proteins (fibrinogen, von Willebrand factor, collagen, and mannose binding lectin-associated serine protease-2) from the 172 proteins was chosen for validation due to their possible association with thrombosis (Wang et al., 2014), platelet thrombus formation (Wu et al., 2002), low immunological reactivity (targeting the microbial world for complement attack and opsonophagocytosis; Jack, Klein, & Turner, 2001), and influence of infection on PDA (Gonzalez et al., 1996). All the above mechanisms are possibly correlated or associated with PDA. The result (Figure 2b) shows that the level of PF4 (1,677.7 ± 385.1 ng/ml), von Willebrand factor (39.6 ± 4.0 ng/ml), collagen (231.9 ± 2.6 ng/ml), fibrinogen (20,364.1 ± 1,563.3 ng/ml), and MASP-2 (138,383.8 ± 8,712.2 pg/ml) in patients with PDA was significantly lower than those in normal controls (4,668.9 ± 1,334.8 ng/ml, p = 0.034; 54.8 ± 4.4 ng/ml, p = 0.013; 313.1 ± 1.3 ng/ml, n = 40, p = 0.004; 36,563.4 ± 7,548.7 ng/ml, n = 40, p = 0.039; 169,584.2 ± 6,620.6 pg/ml, n = 40, p = 0.005; by unpaired t test), as shown in Table 3. However, fibronectin was 23,944.9 ± 413.1 ng/ml, significantly higher than that in the control (21,689.5 ± 459.0 ng/ml, n = 40, p = 0.000; unpaired t test; Table 3).
| Protein | Concentration (PDA) | Concentration (Control) | p value |
|---|---|---|---|
| Platelet factor 4 (ng/ml) | 1,677.7 ± 385.1 | 4,668.9 ± 1,334.8 | 0.034 |
| Von Willebrand factor (ng/ml) | 39.6 ± 4.0 | 54.8 ± 4.4 | 0.013 |
| Collagen (ng/ml) | 231.9 ± 2.6 | 313.1 ± 1.3 | 0.004 |
| Fibrinogen (ng/ml) | 20,364.1 ± 1,563.3 | 36,563.4 ± 7,548.7 | 0.039 |
| Mannose binding lectin-associated Serine protease-2(pg/ml) | 138,383.8 ± 8,712.2 | 169,584.2 ± 6,620.6 | 0.005 |
| Unnamed protein product (fibronectin) | 23,944.9 ± 413.1 | 21,689.5 ± 459.0 | 0.000 |
- Note. ELISA: enzyme-linked immunosorbent assay.
In the KEGG analysis in combination with our own analysis coagulation cascade, complement cascade, platelet activation pathway, and PI3K-AKT pathway are the main cascades/pathways (Figure 3). All of the six proteins validated are included in these cascades/pathways. In addition, among the 172 DEPs, HSP90AB1 (downregulated, GI: 10437873, Uniport: P08238) and ITGA8 (upregulated, GI: 11122875, Uniport: P53708) are involved in the PI3K-AKT pathway and these two proteins were also included in Figure 3.
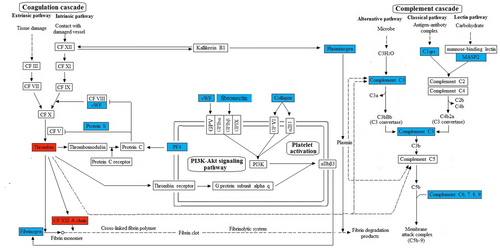
The molecular mechanism analysis of platelet factor 4, fibrinogen, von Willebrand factor, collagen, fibronectin, and mannose binding lectin-associated serine protease-2 involved in coagulation cascades, complement cascades, platelet activation, and PI3K-Akt signaling pathway. The up- and downregulation were colored by red and blue respectively. α2β1, integrin subunit alpha 2; αIIbβ3, integrin subunit alpha 2b; CF, coagulation; GPV, glycoprotein V platelet; GPIbα, glycoprotein Ib platelet alpha subunit; GPIbβ, glycoprotein Ib platelet beta subunit; GPIX, glycoprotein IX platelet; GP VI, glycoprotein VI platelet; HSP90AB1, heat shock protein 90 alpha family class B member; 1ITGA8, integrin subunit alpha 8 [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
The current study has for the first time by using the iTRAQ proteomic technology and validation methods found in patients with PDA that: (1) there are 74 upregulated and 98 downregulated proteins in the plasma of patients with PDA that are related to molecular function, biological processes, and cellular components and (2) five decreased proteins and one increased protein may increase the risk of PDA. These proteins are closely related to platelet activation and coagulation cascades, complement mannan-binding-lectin, and other systemic signaling pathways.
There are different groups of patients with PDA. Keeping the DA open in neonates with other complex congenital heart diseases such as hypoplastic left heart syndrome, transposition of the great vessels, and pulmonary stenosis may be life-saving. However, there is a group of patients who are born with isolated PDA without any other congenital heart diseases, often seen in premature but also seen in full-term babies. The PDA often closes within the first 2 years of life (Dice & Bhatia, 2007). For those with PDA remaining after this period, the PDA may last from childhood to adolescence, and even to adulthood, causing pulmonary arterial hypertension, and other complications. The current study was designed to investigate the mechanism(s) of PDA in patients after the neonate and infancy period but still in their early childhood. In fact, the average age of this group of patients was around 4 years. The mechanism of opening of the DA at this age is probably different from that of premature neonates with PDA, with or without other complex CHDs.
According to GO, KEGG, protein–protein interaction (PPI;Figure 1 and 2) analyses between PDA and controls, the involved various biological processes include coagulation, adhesion, aggregation, proliferation, binding, inflammation, thrombus, and cell growth. The analyses suggest that the functional and structural remodeling in ductal closure formation is likely caused by a combination of different molecular mechanisms.
PF4 is a 70-amino acid heparin-binding protein released from the alpha-granules of activated platelets. PF4 plays important role in chemotaxis, coagulation, inflammation, and cell growth (Eisman, Surrey, Ramachandran, Schwartz, & Poncz, 1990; Guo et al., 2017; Nguyen, Greinacher, & Delcea, 2015). It has been demonstrated that PF4 modulates coagulation by inhibiting heparin–antithrombin interactions, promoting protein C activation, and attenuating the activity of activated protein C (Egan et al., 2017). Accumulating evidence (Alyamac et al., 2012; Clyman & Chemtob, 2010; Dani, Poggi, & Fontanell, 2013; Murphy, Lee, Payton, & Powers, 2016; Olukman et al., 2017) demonstrates that low-platelet counts might result in increased risk of PDA in preterm infants. Likewise, a retrospective analysis in preterm human neonates revealed that low-platelet counts are associated with a 13-fold increase in the risk of DA patency (Echtler et al., 2010). However, there have been no reports on the PF4 level in patients with PDA. The current study has found that decreased concentration of PF4 is associated with PDA in children (Figure 2). In fact, it has been reported that low-platelet counts may increase the risk of resistance to pharmacological closure by preventing the thrombosis of the ductal lumen during anatomic closure (Echtler et al., 2010). In addition, platelets are also key targets of both NO (Radomski & Moncada, 1993) and PGE2 (Fabre et al., 2001). NO causes vasodilation and is one of the most potent endogenous platelet antagonists whereas PGE2 acts on the platelet prostacyclin receptor and inhibits agonist-induced platelet activation (Echtler et al., 2010). The roles of NO and PGE2 in maintaining DA patency are generally attributed to their dilating action on smooth muscle cells in the DA wall. Low-platelet counts may relate to high concentrations of NO and PGE2, which would prevent DA constriction and platelet adhesion (Echtler et al., 2010). As mentioned above, PF4 is released from alpha-granules of activated platelets during platelet aggregation and promotes blood coagulation (Eisman et al., 1990). The current study is the first reporting the association between the low-plasma concentration of PF4 and PDA in children.
vWF, a mosaic protein (Zhou et al., 2012) and an unique coagulation factor (Zahr & Lentz, 2016), is released by activated or dysfunctional endothelial cells (Savage, Saldívar, & Ruggeri, 1996; Theilmeier et al., 2002). Fibrinogen is a large and complex plasma glycoprotein that helps in the formation of blood clots (Adamczyk, Struwe, Ercan, Nigrovic, & Rudd, 2013) and can support both platelet–surface and platelet–platelet interactions, adhesion, and aggregation, respectively (Savage et al., 1996). Collagen is a major subendothelial matrix protein in the various connective tissues and can be easily exposed to blood components after the denudation of endothelial cells following injury (Di Lullo, Sweeney, Korkko, Ala-Kokko, & San Antonio, 2002; Wang et al., 2014). Collagen represents a continuous torsional force opposed to the fluid mechanics of blood pressure emitted from the heart and the thin collagen membrane with a natural microstructure is analogous to the in vivo extracellular matrix structure and functions, supporting the function of the engineered cardiac tissue (Wang et al., 2016). Interestingly, vwf, fibrinogen, and collagens are the most adhesive matrices and key proteins for platelet adhesion and aggregation in large arteries (Massberg et al., 2003; Wang et al., 2014).
In this study, although both PF4 and vWF were downregulated, such changes did not make downregulation of thrombin. The mechanism of this is unknown and worth further studies. Nevertheless, as mentioned before, it has been reported that low-platelet (releasing PF4) counts may increase the risk of resistance to DA closure (Echtler et al., 2010). Further, the interaction between vWF and collagen is the first step in hemostasis and thrombosis, blockade of vWF-collagen interaction reduces thrombus formation in the injured and stenosed baboon femoral arteries (Wu et al., 2002). It is suggested that the downregulation of both PF4 and vWF certainly play a role in the mechanism against the closure of the DA.
It has been demonstrated that platelets and those major substrates are crucial for DA closure by promoting thrombic sealing of the constricted DA (Ali Engür & Engür, 2014), as mentioned above (Echtler et al., 2010; Savage et al., 1996). In fact, fibrinogen, vWF, and collagen are all abundantly expressed in mouse DA lumen using immunohistochemical analysis (Echtler et al., 2010). However, whether in the circulation of patients with PDA these factors present a low concentration is unknown. In the current study, we have demonstrated in patients with PDA that those matrices were downregulated and present in low-plasma concentrations (Figure 2), similar to our findings for PF4.
Fibronectin, a dimer of 250-kDa subunits, is a key component of the extracellular matrix and is required for embryonic development (Wang et al., 2014). Fibronectin exists in the plasma and in the cell (Wang et al., 2014). Plasma fibronectin may play dual roles by switching its soluble form (inhibit platelet aggregation and thrombogenesis) to insoluble form (support platelet aggregation and thrombogenesis) by either self-assembly or nonself covalent linkage with vWF or fibrin or other matrix proteins (Reheman et al., 2009). Plasma fibronectin is synthesized in the liver and secreted into blood and platelets may serve as a transporter of plasma fibronectin (Wang et al., 2016). Further, plasma fibronectin is rapidly deposited and initiated hemostasis when vessels are injured (Reheman et al., 2009). The deposited plasma fibronectin on the vessel wall may induce more platelet adhesion through a GPIb–plasma fibronectin interaction (Beumer et al., 1995), which could lead to more plasma fibronectin release from the platelets. Interestingly, it was also reported that plasma fibronectin content was markedly increased in mice and human patients (Ni et al., 2000, Xu et al., 2006, Zhai et al., 2007), and inhibited platelet aggregation and thrombogenesis in the absence of fibrinogen, vWF, and fibrin (Wang et al., 2014). A model was proposed to explain the dual role of plasma fibronectin between maintenance of hemostasis and prevention of vessel occlusion (Wang et al., 2014). Interestingly, in the current study, we found high concentration of plasma fibronectin and low concentration of fibrinogen and vWF in patients with PDA. This finding supports the above notion that in the absence of fibrinogen, vWF, and fbrin, the role of fibronectin is actually inhibiting rather than enhancing platelet aggregation and thrombogenesis (Wang et al., 2014) and this is probably why in patients with PDA the elevated level of plasma fibronectin is associated with inhibited platelet aggregation and thrombogenesis that finally lead to the nonclosure of the DA.
The complement system is part of the innate immune system and contributes to the establishment of adaptive immune responses (Walport, 2001). The mannan-binding lectin (MBL) pathway of the complement system is activated when MBL binds to carbohydrate structures (Gadjeva, Thiel, & Jensenius, 2001, Matsushita et al., 2000), which happens through autoactivation of the MASP-2 (Stengaard-Pedersen et al., 2003). MASP2 is capable of activating complement by cleavage of C4 and C2, converting C3 (Huber-Lang et al., 2006; Koroglu et al., 2010; Krarup, Wallis, Presanis, Gál, & Sim, 2007; Stengaard-Pedersen et al., 2003). MASP2 also generates thrombin via prothrombin and may contribute to localized fibrinogen activation (Huber-Lang et al., 2006; Krarup et al., 2007). Low-MBL and -MASP-2 levels are associated with increased susceptibility to infections and with the development of immunological disease (Jack et al., 2001), particularly in infants and young children, whose immunity is already compromised (Koroglu et al., 2010; Worthley, Bardy, & Mullighan, 2005). A previous study reported that infection increased the risk of late ductal reopening and PDA closure failures (Gonzalez et al., 1996). It is also reported that PDA frequency was higher in infants with MBL gene polymorphism (Koroglu et al., 2010). In this study, we for the first time revealed low-plasma MASP-2 levels in patients with PDA, suggesting that in humans MASP-2 is associated with the patency of the DA.
In summary, the reduced PF4, fibrinogen, vWf, collagen, MASP-2, and increased fibronectin may result in impaired DA closure in neonates with malfunctioning platelet adhesion or aggregation or with defective platelet biogenesis, which may persistently exist in young children, even in adults. Figure 3 is the molecular mechanism analysis that demonstrates the molecular mechanism of PF4, fibrinogen, vWf, collagen, and MASP2 involved in coagulation cascades, complement cascades, platelet activation, and PI3K-Akt signaling pathways. All of the pathways were found to be significant in our study and may be interacted in many ways. Based on the above findings and previous findings from others (Echtler et al., 2010), we propose a model (Figure 4) to illustrate DA closure of normal life (Figure 4a–c), PDA in pathology conditions (Figure 4d), and the role of the five DEPs for sealing of the constricted DA. In normal conditions, smooth muscle cell and DA constriction occur after birth because of the increased oxygen tension and endothelial injury, which result in the deposition of fibrinogen, collagen, and fibronectin. This triggers the adhesion and accumulation of platelets circulating in the residual DA lumen. Subsequently, proliferation of cells within the former DA lumen leads to anatomical remodeling of the DA and permits permanent closure.
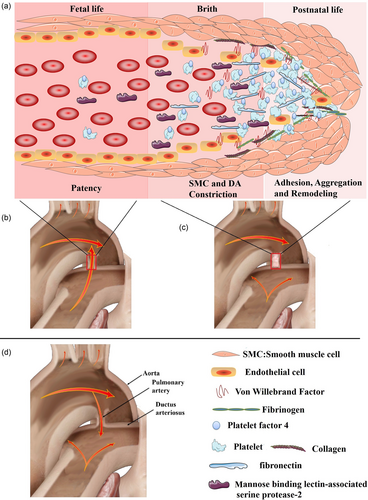
The model of ductus arteriosus closure of normal life (a–c) and patent ductus arteriosus in pathology conditions (d). (a) The role of platelet factor 4 (released from platelet), fibrinogen, von Willebrand factor (released from endothelial cells), collagen, fibronectin (bind to platelet surface integrins), and mannose binding lectin-associated serine protease-2 for sealing of the constricted ductus arteriosus. (b–d) The blood flow in fetal, normal postnatal life, and patent ductus arteriosus, respectively. In normal conditions, smooth muscle cell and ductus arteriosus constriction occur after birth because of the increased oxygen tension and endothelial injury, which result in the deposition of fibrinogen, collagen, and fibronectin. This triggers the adhesion and accumulation of platelets circulating in the residual ductus arteriosus lumen. Subsequently, proliferation of cells within the former ductus arteriosus lumen leads to anatomical remodeling of the ductus arteriosus and permits permanent closure (the model is established based on Echtler et al. (2010) and the current study.) [Color figure can be viewed at wileyonlinelibrary.com]
In conclusion, our study for the first time identified differential plasma proteins by using the iTRAQ and other proteomic technologies in isolated patients with PDA, which are associated with the patency of the DA and reflect the mechanisms of the nonclosure of the DA. Our results demonstrated that DA closure might be regulated by complement and coagulation cascades, platelet activation, PI3K-Akt signaling pathway, mannan-binding-lectin, and other systemic signaling pathways. Our findings indicate that the differential proteins involved in these pathways may play key roles in the nonclosure of the ductus arteriosus and may be developed as biomarkers for clinical diagnosis. All those findings may be served as the basis of understanding the etiology and pathogenesis of PDA.
ACKNOWLEDGEMENT
This study was supported by grants from National Natural Science Foundation of China (81870288, 81870227), National Central Grants for Research Institute (2017NL31001), Natural Science Foundation of Zhejiang Province (LY15H020008), Binhai New Area Health Bureau (2016BWKY007 and 2016BWKZ003).
CONFLICTS OF INTEREST
Authors declare that they have no conflicts of interest.




