Challenges facing antiangiogenesis therapy: The significant role of hypoxia-inducible factor and MET in development of resistance to anti-vascular endothelial growth factor-targeted therapies
Abstract
It is now fully recognized that along with multiple physiological functions, angiogenesis is also involved in the fundamental process and pathobiology of several disorders including cancer. Recent studies have fully established the role of angiogenesis in cancer progression as well as invasion and metastasis. Consequently, many therapeutic agents such as monoclonal antibodies targeting angiogenesis pathway have been introduced in clinic with the hope for improving the outcomes of cancer therapy. Bevacizumab (Avastin®) was the first anti-vascular endothelial growth factor (VEGF) targeting monoclonal antibody developed with this purpose and soon received its accelerated US Food and Drug Administration (FDA) approval for treatment of patients with metastatic breast cancer in 2008. However, the failure to meet expecting results in different follow-up studies, forced FDA to remove bevacizumab approval for metastatic breast cancer. Investigations have now revealed that while suppressing VEGF pathway initially decreases tumor progression rate and vasculature density, activation of several interrelated pathways and signaling molecules following VEGF blockade compensate the insufficiency of VEGF and initially blocked angiogenesis, explaining in part the failure observed with bevacizumab single therapy. In present review, we introduce some of the main pathways and signaling molecules involved in angiogenesis and then propose how their interconnection may result in development of resistance to bevacizumab.
1 INTRODUCTION
Blood vessels sprouting from the already existing ones, also referred as angiogenesis, is a vital process during many physiological processes including embryonic and adult development, wound healing, and so on (Ahluwalia & Tarnawski, 2012; Gacche & Meshram, 2014; Martínez-Corral et al., 2012; Siekmann, Affolter, & Belting, 2013). Alongside, angiogenesis is also involved in numerous pathological conditions such as ophthalmic disorders, rheumatoid arthritis, and cancer (Dimova, Popivanov, & Djonov, 2014b; Konisti, Kiriakidis, & Paleolog, 2012; Yoo & Kwon, 2013). Among different cytokines and growth factors, vascular endothelial growth factors (VEGF) and their corresponding receptors play the most crucial role in regulating tumors angiogenesis (Mittal, Ebos, & Rini, 2014) and their antibody-mediated suppression is presently the most prevailing method of antiangiogenic therapy. Other common groups of antiangiogenic agents are the ones suppressing the function of VEGF receptor tyrosine kinases (RTK; Sitohy, Nagy, & Dvorak, 2012).
The first targeted anti-VEGF antibody and perhaps the most studied one in the field, which succeeded to obtain the Food and Drug Administration (FDA) approval was bevacizumab (Avastin®). Bevacizumab is capable of targeting VEGF-A and all its isoforms (Buss, Henderson, McFarlane, Shenton, & de Haan, 2012). Nevertheless, a latent notice was that, although anti-VEGF-targeted therapy with bevacizumab, decreased tumor burden and ameliorated survival in several cancers, the results were not as prosperous as the ones obtained in preclinical studies (Lambrechts, Lenz, de Haas, Carmeliet, & Scherer, 2013). Continuance of bevacizumab failure to meet expecting results in different follow-up studies, finally convinced US FDA to remove approval of bevacizumab for metastatic breast cancer treatment, In the late 2010 (Kieran, Kalluri, & Cho, 2012).
Further preclinical evaluations revealed that while suppressing VEGF pathway initially decreases tumor progression rate and vasculature density, a rapid enhancement in revascularization and tumor invasiveness occurs after a while (Bottsford-Miller, Coleman, & Sood, 2012). This may, in part, take place as a response to hypoxia, induced by bevacizumab and many other antiangiogenic therapies. Hypoxia activates different VEGF-independent angiogenesis pathways including vascular mimicry, enhances tumor cells invasion potency, recruits precursors of bone marrow-derived endothelial cells for promoting angiogenesis, activates adaption mechanisms to hypoxic condition in tumor cells and finally and perhaps more important promotes several alternative proangiogenic pathways (Giuliano & Pagès, 2013).
In the latter effect, hypoxia results in upregulation of hypoxia-inducible factor 1α (HIF-1α) expression, which in turn results in overexpression of VEGF together with upregulation of MET receptor, permitting tumor cells to compensate with the hypoxic condition through exciting angiogenesis (Schito et al., 2012; Tsai & Wu, 2012). Here, we mainly focused on outlining different pathways involved in angiogenesis to clarify how these pathways and their connections may result in neutralization of antiangiogenic therapy with bevacizumab.
2 VEGF PATHWAY IS THE MOST IMPORTANT PATHWAY IN ANGIOGENESIS
VEGF, also referred as VEGF-A, is one of the members of growth factors superfamily, which was initially isolated as a specific mitogenic factor for endothelial cells with the ability to induce pathological or physiological angiogenesis. Other members of this superfamily include VEGF-B, -C, -D, and placental growth factor. Members of this superfamily are unique in their biological functions, expression pattern, and receptor specificity (Chang et al., 2012). Several cell types like fibroblasts, inflammatory cells, and many cancer cells are capable of synthetizing and secreting VEGF in response to hypoxia through the HIF-1α-signaling pathway (Semenza, 2013).
RTKs, VEGF receptor (VEGFR), and neuropilins (NRPs) are the specific receptors responsible for transducing VEGF signals in tumor cells (Koch, 2012). VEGFRs are the classical receptors for VEGFs, dividing in to three different subtypes namely, VEGFR1 (FLT-1), VEGFR2 (FLK-1 or KDR), and VEGFR3 (FLT4; Koch & Claesson-Welsh, 2012). Although VEGFRs share multiple regulatory mechanisms with RTKs including receptor dimerization, activation of the tyrosine kinases, and development of docking site for signal transducers, they seem unique for their capability to transduce signals leading in swelling of tissues and development of edema (Dimova et al., 2014b). In many cell types, VEGFR2 is the prominent receptor, responsible for transducing VEGF-mediated angiogenesis signals. However, in some others, signal transduction seems to happen in an independent manner (Shibuya, 2013). In addition, VEGFR1 has a regulatory role on VEGFR2 and VEGFR3 function and plays a pivotal role in lymphangiogenesis (Matsumoto et al., 2013).
NRPs are a group of 140 kDa cell surface receptors, which were initially identified as neuronal receptors for class 3 semaphorins, providing specific function in development of nervous system (Guo & Vander Kooi, 2015). These receptors have also shown to be involved in initiation and progression of tumor cells (Parker, Guo, Li, Linkugel, & Vander Kooi, 2012; Prud'homme & Glinka, 2012) and can modulate angiogenesis mediated by VEGF. NRP1 and NRP2 are two subclasses of NRPs in vertebrates (Goel & Mercurio, 2013), demonstrating 44% homology at amino-acid level. These transmembrane glycoproteins consist of four distinct extracellular domains a short cytoplasmic domain without any catalytic activity (Parker, Guo et al., 2012; Parker, Xu, Li, & Vander Kooi, 2012). NRPs mostly act as coreceptors. As an example, they can negotiate with plexins to form functional semaphorins receptors in neural cell or can form complexes with VEGFR1 and VEGFR2 and further enhance these receptors affinity for VEGF. Alongside, NRPs can enhance activity of many receptors critical in tumor cell's activity and also it has been proposed that in some circumstances they can even transduce signals individually.
3 VEGF-SIGNALING PATHWAY
Coupling with extracellular domain of VEGFR2 on the surface of endothelial cells, VEGF results in dimerization of VEGFR2 and initiation of autophosphorylation of tyrosine moieties. Phosphorylated tyrosines either directly interact with specific proteins involved in signal transduction pathway like VEGFR-associated protein (VRAP), phosphoinositide phospholipase Cγ, and Sck or serve as docking sites for multiple Src-homology-2 domain (SH2 domains) containing proteins, which in turn couple with proteins involved in signal transduction pathways including RAS. Protein kinase Cγ (PKCγ) plays a critical role in mediating VEGF-A mitogenic activities through activation of the mitogen-activated protein kinase (MAPK) pathway. Phosphoinositide 3-kinase (PI3K) mediates cell survival through activation of AKT/protein kinase B (PKB). Also PI3K can lead in agglomeration of phosphatidylinositol-3,4,5-triphosphate, which in turn phosphorylates AKT/PKB via binding to its PH domain. Furthermore, activation of VEGFR2 results in several cellular activities, which angiogenesis and vasculogenesis are among them (Dimova, Popivanov, & Djonov, 2014a; Waldner & Neurath, 2012; Figure 1).
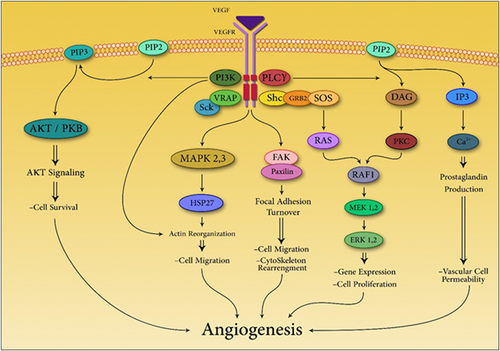
VEGF mediates many cell mechanisms through several paths which cause angiogenesis eventually. DAG: 1,2-diacylglycerol; ERK: extracellular signal-regulated kinase; FAK: focal adhesion kinase; GRB: growth factor receptor-bound protein 2; HSP27: heat shock protein 27; IP3: inositol trisphosphate; MAPK: mitogen-activated protein kinase; MEK: MAPK/ERK kinases; PIP2: phosphatidylinositol 4,5-bisphosphate; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; PI3K: phosphoinositide 3-kinase; PKB: protein kinase B; PKC: protein kinase C; PLCγ: phosphoinositide phospholipase Cγ; SCk: a protein from the Shc family of cell signaling proteins; Shc: a adaptor protein; SOS: Son of sevenless protein; RAS: a small GTPase; RAF: rapidly accelerated fibrosarcoma [Color figure can be viewed at wileyonlinelibrary.com]
4 REGULATION OF VEGF SIGNALING IN TUMOR CELLS
Significant differences exist between VEGF expression levels in physiological compared with the pathophysiological condition of cancer. Although VEGF levels extensively decline in certain situations such as wound healing, tumors highly preserved VEGF levels can conserve development of hypersprouting and obstructing maturation and has been shown to be associated with reduced free and overall survival rates in several cancer types (Chatterjee et al., 2013). As tumors possess large reservoirs of VEGF, anti-VEGF therapy seems to be a rational approach for cancer treatment and multiple drugs in this field have received approval in treatment of many types of tumors (Scott, Wolchok, & Old, 2012).
In most cases, VEGF-signaling pathways have shown to be either autocrine (Cao et al., 2012; Hamerlik et al., 2012) or paracrine (Clarkin & Gerstenfeld, 2013) and accessibility of ligands mostly regulates VEGFR function. During hypoxia, VEGF-A expression is straightly regulated by HIFs. This mediator specifically sticks to a particular promoter element on the promoter region of VEGF-A (Ahluwalia & S Tarnawski, 2012; Fraga et al., 2012). Likewise, VEGFR2 expression is also upregulated during hypoxia, nevertheless, the exact role of different HIFs in this regulation still remains unknown. Hypoxia has also been shown to be a boosting up factor for autocrine-signaling networks in tumor cells. Aggressive tumor cells mostly preserve HIF-mediated transcription (Mimeault & Batra, 2013), and procedures involved in the induction of VEGF expression, such as epithelial–mesenchymal transition (Li et al., 2013), RAS transformation (Shen et al., 2012), and the ones straightly affecting HIF activation or expression.
Regulatory mechanisms of VEGF expression, specifically HIF-mediated ones, are crucial traits of a stem-cell phenotype (Philip, Ito, Moreno-Sanchez, & Ralph, 2013; Singh, Franke, & Wielockx, 2012). Therefore, autocrine expression of VEGF is a consequence of HIF activation and correlates with the cancer stem cells generation, which is an essential characteristics of high-grade tumors (Mimeault & Batra, 2013; Philip et al., 2013). Furthermore, VEGF, HIF-1α, and NRP expression levels are significantly higher in high-grade prostate carcinoma cells compared with more differentiated tumor cells (Mak et al., 2010).
5 HIF-1 REGULATES 1–5% OF ALL HUMAN GENES
HIF-1 is the key factor in adapting cells response to oxygen deprivation by adjusting expression of genes involved in different cellular processes, including angiogenesis, cell proliferation, erythropoiesis, glucose uptake, and apoptosis (Xia, Choi, & Lee, 2012). So far, three members of HIF family, namely HIF-1, -2, and -3 have been identified (Agani & Jiang, 2013). The first member of this family, HIF-1, was initially identified utilizing DNA affinity purification technique. HIF-1 is a heterodimer consisting of two protein subunits HIF-1α and HIF-1β (Semenza, 2016). In response to hypoxia the α subunit of HIF-1, translocates to the nucleus and initiates transcription of different targeted genes. Different aspects of this pathway is mostly determined by the developmental and physiological states of each cell. During hypoxia, about 1–5% of all human genes specifically the ones responsible for expression of VEGF and MET are mostly induced by HIF-1 (Semenza, 2003). HIFs coupling with DNA results in formation of a heterodimer complex consisting of an α subunit (the oxygen-sensitive subunit) and a constitutively expressed β subunit, also known as aryl hydrocarbon receptor nuclear translocator (ARNT; Z.-G. Zhang, Zhang, Wang, & Tian, 2013). Due to an oxygen-dependent degradation (ODD) domain deficiency in ARNT, this subunit is constitutively expressed in all tissues under normoxic condition (Koh & Powis, 2012).
In normoxic condition, HIFs couple with pVHL (the von Hippel–Lindau [VHL] tumor suppressor) and become subjected for further proteasomal degradation. This is mostly due to the fact that pVHL is recognized by a component of an E3 ubiquitin ligase complex, which interact with HIF-α in an oxygen-dependent manner. Prolyl-4-hydroxylase domain-containing proteins hydroxylate conserved proline residues in the HIF-α ODD domain facilitating pVHL binding with HIF and induction of further degradation (Foxler et al., 2012; Xia et al., 2012).
During hypoxia, HIF-α subunits become stable and translocate from the cytoplasm to the nucleus. There, they form heterodimers with ARNTs and bind with hypoxia-response elements (HREs) located in regulatory sites of HIF target genes. Based on in vitro studies, as the oxygen concentration drops below 6% when maximal oxygen tension is 0.5%, HIFs become stabilize and gain DNA-binding activity (Koh & Powis, 2012). The HIF-α/ARNT heterodimer further triggers transcription of different genes through the involvement of p300 and CREB binding protein (CBP), which are two important transcriptional activators. Additionally, factor inhibiting HIF-1 (FIH-1), a member of the 2-oxoglutarate and Fe(II)-dependent oxygenase superfamily, can also regulate the HIF-P300/CBP interactions through an oxygen-dependent mechanism. FIH hydroxylates asparagine residues located on the C-terminal transactivation domain (CTAD) of HIF-α and prohibits p300/CBP binding with it. Therefore, both stabilization of HIFα and CTAD activation are required for full activation of HIF transcriptional activity (Masoud & Li, 2015).
Based on a recent study, expression of both HIF-1α and HIF-2α are raised in a variety of human tumors compared with normal tissues, such as breast, colon, bladder, hepatocellular, glial, renal, prostate, ovarian, and pancreatic tumors (Semenza, 2012).
Furthermore, high expression levels of HIF-1 is associated with poor patient outcome in nasopharyngeal carcinoma, head and neck, breast, colorectal, cervical, pancreatic, osteosarcoma, bladder, glioblastoma, endometrial, ovarian, and gastric carcinomas (Blick et al., 2013; Chen et al., 2014; Cheng, Klausen, & Leung, 2013; Fu et al., 2012; Hu et al., 2015; Isa, Ward, West, Slevin, & Homer, 2014; Masoud & Li, 2015; Qiang et al., 2012; Spirina et al., 2012; H. Zhang et al., 2012; Zhao et al., 2014; Zighelboim et al., 2013).
As mentioned earlier, hypoxia is the key inducer of HIF activation in tumors. It has been shown that about 50–60% of solid tumors contain hypoxic and even anoxic regions due to imbalancement in oxygen content and rate of tumor proliferation (Agani & Jiang, 2013). Deficient oxygen contents is mainly the consequence of raised metabolic activity and oxygen consumption or distancing from capillaries and blood product (Bailey-Serres et al., 2012). Additionally, evidence suggest that activation of the PI3k-signaling pathway can also induce HIF activation. Based on Mazure, Chen, Laderoute and Giaccia (1997) inactivation of PI3k remarkably inhibits hypoxic induction of VEGF in Ha-ras-transformed cells. Consequently, activation of the PI3k/Akt pathway could increase HIF activity as well as inactivation of negative regulators as well as to promote growth factor signaling comprising TSC2 or PTEN (Carpenter & Jiang, 2013; E. Zhang et al., 2014). Activation of the PI3K and MAPK pathways can regulate HIF-1α synthesis. Signaling via G-protein-coupled receptors, RTKs, and non-RTKs could activate these pathways (Masoud & Li, 2015).
“Angiogenic switch” is considered as tumor cells ability to induce angiogenesis through a multistep process in response to proangiogenic factors. Expression of multiple proangiogenic factors, including VEGF, VEGFRs FLT-1 and FLK-1, angiopoietins (ANG-1 and -2), plasminogen activator inhibitor-1, platelet-derived growth factor B, matrix metalloproteinases (MMP)-2 and -9 and the TIE-2 receptor could straightly activate by HIF (Shahneh, Baradaran, Zamani, & Aghebati-Maleki, 2013; Zepeda et al., 2013).
As mentioned earlier, HIF signaling is required for the regulation of VEGF expression and tumor angiogenesis in both murine and human cell lines. Nonetheless, the existing controversy is the relative association of individual members of HIF family in this process. It has been reported that HIF-1 has a proangiogenic role as well. Remarkably, ES cells deficient in HIF-1 expression, form significantly smaller teratocarcinomas compare with the ones derived from wild-type ES cells (Hiraki et al., 2012). More clues also exist proving HIF-1 role in mediated angiogenesis. Angiogenesis and VEGF expression were also discovered to be HIF-1 dependent in hypoxic astrocytes (Pan, Chen, Liu, & Liu, 2013).
6 INHIBITING HIF-1 ACTIVITY
Multiple lines of evidence have demonstrated that hypoxic cancer cells are more probable for developing resistant to chemotherapy and/or radiation, and have increased capability for metastasis, invasion, and patient mortality (Semenza, 2012). Recent studies have also shown that HIF-1 can also result in development of resistance to chemotherapy and radiation. Interference in HIF-1 activity could represent a great component for antiangiogenesis therapies (Soda, Myskiw, Rommel, & Verma, 2013).
So far, two main approaches have been developed for inhibiting HIF-1 function in human cancer cells. The first strategy is based on the fact that deletion of the transactivation and DNA-binding domains results in a dominant-negative structure of HIF-1α that can create an inactive heterodimer with HIF-1β. As an example, in PCI-43 pancreatic cancer cells expressing high levels of HIF-1α, Overexpression of dominant-negative structure of HIF-1α, caused an enhancement in apoptosis during oxygen and glucose deprived condition. Furthermore, tumor formation was also significantly reduced in severe combined immunodeficient mice (Kawaguchi & Kataoka, 2014).
The second approach is based on inhibiting HIF1-α binding with its coactivators p300 and CBP. HIF-1α have two transactivation domains, namely C-terminal TAD (TAD-C) and N-terminal TAD (TAD-N). the TAD-C is responsible for binding with p300 and CBP (Hong et al., 2014; Yang, Lin, Wang, Guo, & Wang, 2012). Fusion proteins containing GAL4 can fuse to TAD-C transactivation domains, inhibit the interaction between these coactivators with HIF-1α and block HIF-1α-dependent transcription consequently. In a recent study, MDA-MB-435 and HCT116 cell lines were infected with a retrovirus capable of encoding one of these specific fusion proteins. Interestingly, the rate of tumor growth after injection of these transfected cells to nude mice compare with control growth was significantly slower. Nonetheless, the most restricting point of this approach is that these fusion protein can interrupt interaction of many other transcription factors with CBP/p300 as well (Kung, Wang, Klco, Kaelin, & Livingston, 2000).
7 HYPOXIA CAN RESULT IN OVEREXPRESSION OF MET RECEPTOR
Hepatocyte growth factor (HGF), the ligand which binds to MET tyrosine kinase receptor (also known as the HGF receptor), is one of the major environmental inducers of invasive growth (Kawaguchi & Kataoka, 2014). MET receptor is required for various morphogenetic events and also malignant progression of several different types of tumors (Graveel, Tolbert, & Vande woude, 2013). Activation of MET pathway results in formation of a complex signaling network that end in extensive changes in transcription of several genes (Cecchi, Rabe, & Bottaro, 2012). MET-signaling pathway only shares some of the common features of RTKs and includes many other unique characteristics which have been reviewed elsewhere. Also different components and subdivisions of MET-dependent-signaling network have been identified (Cecchi et al., 2012; Feng, Thiagarajan, & Ma, 2012; Gherardi, Birchmeier, Birchmeier, & Woude, 2012; Goyal, Muzumdar, & Zhu, 2013; Jung, Park, & Hong, 2012; Lam, Dai, & Qin, 2016; Peters & Adjei, 2012).
HGF is initially secreted as a single-chain precursor and after modification with different extracellular proteases transforms into a two-chain functional heterodimer. In most tissues, HGF is extensively distributed in extracellular matrix, where it can be isolated by heparin-like proteoglycans in its inactive form. This growth factor is primarily secreted by mesenchymal cells and demonstrate its effects on epithelial cells which express the MET receptor in a paracrine manner (Fajardo-Puerta, Mato Prado, Frampton, & Jiao, 2016; Xu et al., 2016).
During, cancer invasion or tissue repair, many cytokines presented in interstitial compartment can regulate expression of HGF and its corresponding receptor. For instance, transforming growth factor-β, interleukin-1 and -6, and tumor necrosis factor-α can upregulate transcription of HGF in fibroblasts and resident macrophages and MET in epithelial cells respectively (Giannoni et al., 2014). Furthermore, overexpressed proteases like matriptase and the plasminogen activation system in tumor stroma during inflammation are mostly involved in activation of pro-HGF molecules (Owen et al., 2010). Consequently, MET activation in target cells is mostly regulated by a combination of transcriptional and posttranslational factors, which is contemplated as part of a common mechanism of physiological defense to tissue damage.
Overall, this overexpression results in effective activation of downstream signal transducing pathways components including MAPK cascade, signal transducer and activator of transcription (STATs) proteins, the PI3K–Akt axis, and the nuclear factor-κB inhibitor-α–nuclear factor-κb (Iκbα–NF-κb) complex. All of which result in further suppression of apoptosis, increase in cell proliferation, and migration (Burris, 2013; Feng et al., 2012; Garajová, Giovannetti, Biasco, & Peters, 2015; Matsumura et al., 2013; Tsou et al., 2013).
8 CENTRAL SIGNALING PATHWAYS REGULATED BY MET
A series of signaling coreceptors and amplifiers being physiologically correlated with MET pathway, can adjust the strength, versatility, and duration of signals initiated by MET. MAPK cascade is one of these pathways which consists from three subfamilies, each of which includes three protein kinases, which can phosphorylate and activate each other respectively. The Grb2–SOS complex, is one of the primary factors which is activated by the Ras. GTPase activating proteins (GAPs), like p120 Ras-GAP (p120) are capable of switching ras off and terminating this pathway. Terminal effectors of this pathway consist of extracellular signal-regulated kinases, c-Jun N-terminal kinases, and p38s. These factors further translocate into the nucleus and modulate activity of several transcription factors (Jung et al., 2012; Raghav & Eng, 2012). In other pathway PI3K activate by MET. This protein creates a docking site for Akt activation and then various factors activate by Akt phosphorylate. These factors involve in the regulation of cell size, proliferation and survival. (Porta, Paglino, & Mosca, 2014). The third pathway that is in association with activation of MET is STAT3. Through their SH2 domain, STAT3 monomers bind to MET and become trans-phosphorylated. After homodimerization STAT3 translocates to the nucleus and modulates transcription of different target genes (Trovato et al., 2013). Finally, IκB kinase (IKK) can be activated as a consequence of MET stimulation as a consequence of Src-dependent and PI3K pathways activation. This factor phosphorylates the IκB which is bound to NF-κB. These interactions results in translocation of NF-κB to the nucleus and activation of downstream genes transcription (Figure-2; Hao et al., 2015; Lee & Kim, 2012). VEGFR2 regulates MET signaling in response to VEGF stimulation. VEGFR2 can form complexes with MET in glioblastoma cells and further inhibits mesenchymal transition and HGF-mediated invasion (Lu et al., 2012). Moreover, NRP1 interacts with MET receptor and enhance survival and proliferation of gliomas as well as the invasion of pancreatic carcinoma cells (Taniguchi, 2016).
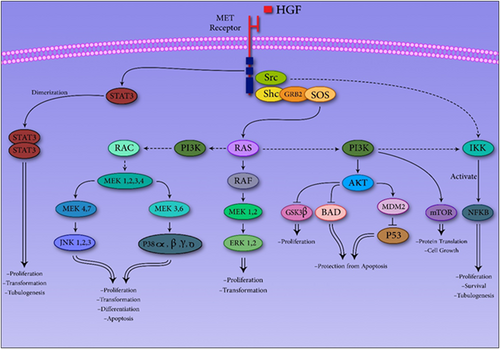
MET Pathway could increase tumorigenesis through several pathways, which increase proliferation, transformation, tubulogenesis, and so on in cancer cells. AkT: protein kinase B; BAD: a member of the BCL-2 family; ERK: extracellular signal-regulated kinase; GRB2: Growth factor receptor-bound protein 2; GSK3: glycogen synthase kinase 3; HGF: hepatocyte growth factor; IKK: IκB kinase; JNK: c-Jun N-terminal kinase; MDM2: murine double minute 2; MEK: Mitogen-activated protein kinase kinase; MET: mTOR: mechanistic target of rapamycin; NF-κB: nuclear factor κB; PI3K: phosphoinositide 3-kinase; P38: a mitogen-activated protein kinase; P53: tumor suppressor protein; RAC: a small GTP binding protein; RAF: Rapidly Accelerated Fibrosarcoma; RAS: a small GTPase; Shc: a adaptor protein; SOS: Son of sevenless protein; STAT: signal transducer and activator of transcription; Src: a non-receptor protein tyrosine kinase [Color figure can be viewed at wileyonlinelibrary.com]
9 CONCLUSION
As mentioned in this study, VEGF is not the sole factor in modulation of angiogenesis, rather multiple factors and receptors negotiate in a complex network for controlling it. Thus with prevention and suppression of VEGF, it is only possible to inhibit the VEGF pathway. Nevertheless other pathways remain intact, which could lead in angiogenesis through other pathways, exactly what may happen during antiangiogenic therapy with bevacizumab. As it was mentioned before, MET and HIF are the pathways which have important roles in angiogenesis just like VEGF pathway. Consequently, combination therapy seems to be a better choice regarding antiangiogenic therapy. Cabozantinib is a newly developed bispecific antibody which targets VEGF–MET axis simultaneously and has demonstrated relatively good results in clinic (Xiang et al., 2014). At the end, due to the wide range of HIF effects in body, determining a proper biomarker for evaluating the effects of targeted anti-HIF therapy seems to be essential.




