Cyclooxygenase-2 in cancer: A review
Abstract
Cyclooxygenase-2 (COX-2) is frequently expressed in many types of cancers exerting a pleiotropic and multifaceted role in genesis or promotion of carcinogenesis and cancer cell resistance to chemo- and radiotherapy. COX-2 is released by cancer-associated fibroblasts (CAFs), macrophage type 2 (M2) cells, and cancer cells to the tumor microenvironment (TME). COX-2 induces cancer stem cell (CSC)-like activity, and promotes apoptotic resistance, proliferation, angiogenesis, inflammation, invasion, and metastasis of cancer cells. COX-2 mediated hypoxia within the TME along with its positive interactions with YAP1 and antiapoptotic mediators are all in favor of cancer cell resistance to chemotherapeutic drugs. COX-2 exerts most of the functions through its metabolite prostaglandin E2. In some and limited situations, COX-2 may act as an antitumor enzyme. Multiple signals are contributed to the functions of COX-2 on cancer cells or its regulation. Members of mitogen-activated protein kinase (MAPK) family, epidermal growth factor receptor (EGFR), and nuclear factor-κβ are main upstream modulators for COX-2 in cancer cells. COX-2 also has interactions with a number of hormones within the body. Inhibition of COX-2 provides a high possibility to exert therapeutic outcomes in cancer. Administration of COX-2 inhibitors in a preoperative setting could reduce the risk of metastasis in cancer patients. COX-2 inhibition also sensitizes cancer cells to treatments like radio- and chemotherapy. Chemotherapeutic agents adversely induce COX-2 activity. Therefore, choosing an appropriate chemotherapy drugs along with adjustment of the type and does for COX-2 inhibitors based on the type of cancer would be an effective adjuvant strategy for targeting cancer.
1 INTRODUCTION
Cyclooxygenases (COXs) have three isoforms: COX-1, COX-2, and COX-3 (B. Li, Lu, et al., 2017; Wong et al., 2017). COX-2 is a membrane-bound (W. Xu, Huang, et al., 2018), short-living (Obermoser et al., 2018), and rate-limiting enzyme (W. Xu, Huang, et al., 2018) discovered in 1991 (Goswami & Sharma-Walia, 2016) and has long been known as a target for relief of pain and treatment of inflammation (H.-Y. Qiu et al., 2016). COX-2 is a prostaglandin (PG)–endoperoxide synthase 2 (X. Xu, Huang, et al., 2018) enzyme responsible for generation of prostanoids like prostaglandin E2 (PGE2) that are contributed to the modulation of multiple procarcinogenic effects (Dannenberg, Lippman, Mann, Subbaramaiah, & DuBois, 2005; F. Li & Zhu, 2015). COX-2 expression is negligible in normal cells (Gurram et al., 2018) in which its basal expression only occurs within stomach (Su, Zhang, & Zhu, 2016), kidney, central nervous system, and in organs of female reproduction (Obermoser et al., 2018). However, it is expressed frequently at the tumorigenic nests in most types of cancers (Gurram et al., 2018; Raj et al., 2018), including adenocarcinoma, squamous cell carcinoma (SCC), cholangiocarcinoma, transitional cell carcinoma, endometrial carcinoma (Dannenberg et al., 2005), and hepatocellular carcinoma (HCC; Mortezaee, 2018). In fact, the tumor microenvironment (TME) is an inducer for COX-2 overexpression (Ohtsuka et al., 2018). This overexpression is due to dysregulated control over its transcriptional or post-transcriptional levels (Y. Liu, Borchert, Surazynski, & Phang, 2008), so it could be a promising marker for identification of the tumor from normal cells (Gurram et al., 2018; Yue, Nguyen, Zellmer, Zhang, & Zorlutuna, 2018). COX-2 is also a constituent of the tumor-derived exosomes (Theodoraki, Hoffmann, & Whiteside, 2018). Oncogenic viruses (Charalambous, Maihöfner, Bhambra, Lightfoot, & Gooderham, 2003), cancer promotors (Y. Xu, Yang, et al., 2018), radiation (Kirk et al., 2018), and chemotherapy (Ikeya, Sakabe, Yamada, Naito, & Tokura, 2018) and proinflammatory cytokines (H.-Y. Qiu et al., 2016) are inducers of COX-2 expression in cancer cells. COX-2 overexpression promotes carcinogenesis (Hung et al., 2004), increases the rate of cancer recurrence (Montezuma et al., 2018) and reduces survival (Höing et al., 2018; Sharma et al., 2005; H. Sun et al., 2017) in affected patients, and it is related to cancer cell resistance to chemo- and radiotherapy (J. Li, Zhou, et al., 2017).
COX-2 via its pleiotropic effects could evolve a wide range of activities (Noda et al., 2002). Expression of this rapid response gene (Y. Hao et al., 2017) occurs as probably an early event (Charalambous et al., 2003) promoting cancer cell survival, growth, and angiogenesis (Noda et al., 2002), and that COX-2 transgene has been recognized to be a sufficient initiator of cancers like HCC (H. Chen et al., 2017). There is also evidence for the involvement of COX-2 in cancer development (Dong et al., 2018) and invasion (Greenhough et al., 2009), and its contribution to tumor aggressiveness and poor patient prognosis (Esbona et al., 2018).
The COX-2 blockade has been shown to provide chemopreventive for a number of cancers (Harris, Beebe-Donk, & Alshafie, 2007), so targeting this enzyme might be a promising approach for cancer therapy. To write this review, we intended to summarize roles for COX-2 in cancer focusing on its upstream or downstream signaling cascades and targeting for cancer therapeutic approaches. PubMed database was searched for recent and novel articles in this context. The criteria for article selection was based on the novelty, time of publication, quality of the journals, and number of citations. A total number of 1,100 papers were scanned for this review with the keywords “COX-2” and “cancer.”
2 COX-2 STRUCTURE, ACTIVATION, AND DEGRADATION
COX-2 gene is located on the chromosome 1q25.2-q25.3 in human subjects (W. Xu, Huang, et al., 2018). Degradation of COX-2 occurs in the proteasome in association with endoplasmic reticulum (ER; Sample, Zhao, Qiang, & He, 2017). Due to existence of many polymorphisms in the COX-2 gene, susceptibility among individuals to cancer is different (W. Xu, Huang, et al., 2018). Proteins specifically binding to the COX-2 promotor would take an important role in promotion of tumorigenesis (Xuan et al., 2016). COX-2 promoter contains a 3-untranslated region (3-UTR) that could be a target for microRNAs (miRNAs) like miR-144 for suppression of cancer progression (Yao, Gu, Wang, & Xue, 2018). There are AU-rich elements (AREs) in the 3-UTR of COX-2. These AREs could influence messenger RNA (mRNA) decay and protein translation (Dannenberg et al., 2005) probably through interaction with Hu antigen-R (HuR; Janakiraman et al., 2017). Stability in the COX-2 mRNA is regulated by p38 mitogen-activated protein kinase (MAPK; Hung et al., 2004) and K-ras (Araki et al., 2003).
-
Two sites for nuclear factor-κβ (NF-κβ; Charalambous et al., 2003) by which this nuclear factor can influence COX-2 gene transcription (X. Zheng et al., 2018). P300 and NF-κβ work as co- and transactivator for COX-2 promoter, respectively (Mortezaee, 2018). Recruitment of p300 to the COX-2 promoter is mediated by tonicity-responsive enhancer binding protein (J.H. Lee et al., 2018), while suppression of nuclear localization for this coactivator is mediated by N-myc (and STAT) interactor (NMI; J. Wang, Zou, et al., 2017). Factors like thyroid hormone receptor interactor 4 (TRIP4) have synergy with p300 for inducing COX-2 promoter directly (J. Hao et al., 2018). Oxidative stress is an inducer of the inflammatory enhanceosome sites on the COX-2 promoter (J.H. Lee et al., 2018).
-
Sites for several cytokines like interleukin (IL)-6 (J. Wang, Zou, et al., 2017).
-
A site for cyclic AMP response element (CRE). CRE is a target for protein binding (CREB) via activation by p38 MAPK, so this site is for transcriptional activation of COX-2 (Yadav et al., 2018). CRE is also a binding site for c-Jun (Qin et al., 2015).
-
A site for TCF-4-binding element through which activation of COX-2 promoter is mediated by β-catenin (Araki et al., 2003).
-
TEAD binding sites for interaction with factors like YAP contributed to the regulation of COX-2 transcription favoring drug resistance in cancer cells (W. Li, Cao, et al., 2017).
-
Activator protein-2 (AP-2) and specificity protein binding sites are responsive to lipopolysaccharide (Wong et al., 2017). AP-2 is a transactivator for COX-2 (X. Wang, Yu, et al., 2017; Figure 1).
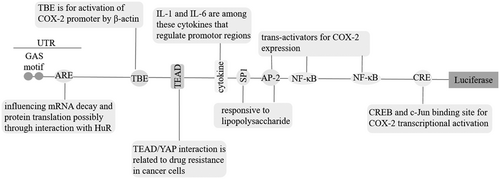 Figure 1
Figure 1Cyclooxygenase-2 (COX-2) promoter. AP-2: activator protein-2; ARE: AU-rich elements; CRE: cyclic AMP response element; CREB: CRE protein binding; HuR: Hu Antigen-R; IL: interleukin; mRNA: messenger RNA; NF-κβ: nuclear factor-κβ; 3-UTR, 3-untranslated region; TBE: Tcf-4-binding element; TEAD: TEA domain
3 PGs ARE IMPORTANT TARGETS FOR COX-2 IN THE PROMOTION OF CANCER
It is accepted that COX-2 contribution to carcinogenesis is mediated through overproduction of PGs. Metabolites of these PGs released into the urine are now considered as approved biomarkers for prediction of cancer risk (Cui et al., 2017). PGE2 is a cardinal mediator of inflammation playing a key role in carcinogenesis (Cui et al., 2017). There is a positive-feedback loop between COX-2 expression and PGE2 (D. Wang, Buchanan, Wang, Dey, & DuBois, 2005). The effects of PGE2 are exerted through interaction with its putative transmembrane G-protein-coupled EP receptors (EP1–EP4), especially EP1 and EP4 (Majumder et al., 2018; Majumder, Landman, Liu, Hess, & Lala, 2015; Pan et al., 2016). PGE2 is responsible for induction of COX-2 expression through activation of ROS/AKT/activator protein-1 (E.-H. Kim et al., 2008), Ras (D. Wang et al., 2005), and Ras subfamily RhoC (Lang et al., 2017).
- (1)
Apoptosis resistance via upregulation of Bcl-2 (Hosseini et al., 2018; Todoric, Antonucci, & Karin, 2016).
- (2)
Angiogenesis through upregulation of vascular endothelial growth factor (VEGF; Hosseini et al., 2018; Todoric et al., 2016).
- (3)
Metastasis by upregulation of matrix metalloproteinases (MMPs) type 2 and 9 (Hosseini et al., 2018; Todoric et al., 2016) and IL-11 (Singh, Berry, Shoher, & Lucci, 2006) along with activation of oncogenic miR526b (Majumder et al., 2015).
- (4)
Tumor growth via upregulation of epidermal growth factor receptor (EGFR; Hosseini et al., 2018; Todoric et al., 2016).
- (5)
Maintenance of cancer stem cell (CSC)-like activity via activation of WNT/β-catenin/TCF (Greenhough et al., 2009) and miR526b (Majumder et al., 2015), and suppression of dual specificity phosphatase 2 (Hou et al., 2017).
- (6)
Cancer cell survival (Majumder et al., 2018) and inflammation (Ramu et al., 2018) through interaction with PI3K/AKT.
- (7)
Cancer cell invasion via upregulation of extracellular signal-regulated kinase (ERK; Majumder et al., 2018) and membrane proteases (C. Ko et al., 2017).
- (8)
Modulation of the immune system through increase of the activity for regulatory T cells (Tregs; Sharma et al., 2005; via induction of Nr4a expression; Hibino et al., 2018) and the activity for protumor M2 cells (Esbona et al., 2018; Figure 2).
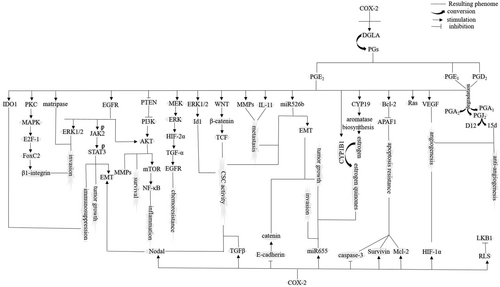 Figure 2
Figure 2Cyclooxygenase-2 (COX-2) acting as a procancer enzyme. AKT: protein kinase B; APAF: apoptosis protease-activating factor; CSC: cancer stem cell; CYP19: aromatase cytochrome P450; DGLA: dihomo-γ-linolenic acid; EGFR: epidermal growth factor receptor; EMT: epithelial–mesenchymal transition; ERK1: extracellular signal-regulated kinase-1; HIF: hypoxia inducible factor; IDO1: indoleamine 2,3-dioxygenase 1; LKB1: liver kinase B1; MAPK: mitogen-activated protein kinase; MMP: matrix metalloproteinases; mTOR: mechanistic target of rapamycin; NF-κβ: nuclear factor-κβ; PG: prostaglandin; PI3K: phosphoinositide 3-kinase; PKC: protein kinase C; PTEN: phosphatase and tensin homologue; RLS: reactive lipid species; STAT3: signal transducer and activator of transcription-3; TCF: T-cell factor; TGF-α: tumour growth factor-α; TGF-β: tumour growth factor-β; VEGF: vascular endothelial growth factor
4 CONTRIBUTION OF COX-2-EXPRESSING TUMOR-ASSOCIATED MACROPHAGES (TAMs) TO TUMOR PROGRESSION
TAMs are part of inflammatory circuits that exhibit antitumor (M1) or protumor (M2) features depending on the cytokine concentration in the tumor milieu. COX-2 is not a specific determinator of macrophage polarity toward either type. In human and animal intestinal adenomas, it has been attested no conspicuous phonotypic alterations in macrophages expressing COX-2 (Hull et al., 2017). Although it has been asserted that COX-2 inhibition could make a switch in the TAM phenotype from M2 to M1 (Nakanishi et al., 2011), this is not persuasive to come to the conclusion of COX-2 acting as TAM switcher toward M2 cells. A point in this context is that M1 is a proinflammatory phenotype, while M2 is an anti-inflammatory cell type (Xiang et al., 2018). Another point is that polarity toward M1 cell type is stimulated by NF-κβ that influences inflammatory states in macrophages in an active heterodimer form (p50-p65; Genard et al., 2018). As it has been discussed, NF-κβ is a transactivator for COX-2 promoter contributed to activation of COX-2 in both cancer cells (Charalambous et al., 2003) and TAMs (Hung et al., 2004). There is also evidence for protumoral activity of COX-2 in macrophages (Lu Gan et al., 2016). Taken together, it could be speculated that macrophage polarity is not influenced by COX-2. Rather, COX-2 could act as a promoter of macrophage protumoral activity.
Upon irradiation, infiltration of the COX-2 expressing TAMs to the tumor milieu occurs. This infiltration promotes tumor growth (Esbona et al., 2018), reduces patient survival (Esbona et al., 2018), and influences normal epithelial cells around the tumor by relaying protumorigenic signals (S.C. Ko et al., 2002). In addition, release of COX-2 from M2 cells is also contributed to tumor invasion (Tjiu et al., 2009), angiogenesis (Rong et al., 2016), and metastasis (Lu Gan et al., 2016). COX-2 expression in M2 cells induces protein kinase B (AKT) pathway in cancer cells for secretion of MMPs (mediators of invasion and metastasis; Lu Gan et al., 2016; Tjiu et al., 2009). M2 macrophages are also able to induce EP1 expression in cancer cells for the promotion of angiogenesis (Rong et al., 2016). In addition, TAMs express sphingosine kinase-1 (SphK1)/sphingosine 1-phosphate (S1P; Furuya et al., 2017) and a disintegrin and metalloproteinase 17 (ADAM17; Bohrer et al., 2016) that are known for their role in regulation of COX-2 expression in cancer cells (Figure 3).
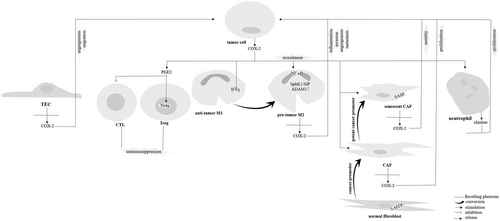
Interaction between cyclooxygenase-2 (COX-2) derived from cancer cells with cells in the tumor microenvironment. 5-MTP: 5-methoxytryptophan; ADAM17: A disintegrin and metalloproteinase 17; CAF: cancer-associated fibroblast; CTL: cytotoxic T lymphocyte; IFN: interferon; NF-κβ: nuclear factor-κβ; PGE2: prostaglandin E2; TEC: tumor endothelial cell; Sp1: specificity protein; SASP: senescence-associated secretory phenotype; Treg: regulatory T cell
5 CONTRIBUTION OF COX-2-EXPRESSING CANCER-ASSOCIATED FIBROBLASTS (CAFs) TO TUMORIGENESIS
There is a positive relation between COX-2 and collagen synthesis in tumor cells attested by either pharmacologic inhibition of COX-2 (Esbona et al., 2016; Krishnamachary et al., 2017) or using proline oxidases (Y. Liu et al., 2008), and that this relation is considered to be a driving force for reducing the time of cancer metastasis (Esbona et al., 2018). CAFs are responsible for production of the high amount of collagen in cancer cells (Krishnamachary et al., 2017). There is a positive relation between COX-2 expression with the number of CAFs (Krishnamachary et al., 2017). CAFs are major producers of COX-2 to the TME (Su et al., 2016). COX-2 release by cancer cells to this milieu could also influence CAFs into attaining a senescence-associated secretory phenotype (SASP) called senescence CAFs that are inducers of cancer cell motility (Kabir et al., 2016). Therefore, COX-2 provides a link between cancer cells with CAFs for the promotion of cancer, and that increasing in the number of CAFs mediated by COX-2 would remove a possible barrier made by normal fibroblasts against tumor progression. For example, normal fibroblasts release factors like 5-methoxytryptophan acting as inhibitors for COX-2 expression in cancer cells (Cheng et al., 2016; Figure 3).
6 COX-2 AND AUTOPHAGY IN CANCER
Autophagy is a process of waste disposal for degradation of damaged organelles/proteins. A dysfunction (dysregulation) in this process is contributed to tumorigenesis (Mortezaee, 2018). Autophagy-related (ATG) gene ATG6 activates COX-2 in a CREB-depended mechanism (Qiang et al., 2017). COX-2 is also regulated by p62 (Sample et al., 2017). P62 is an intracellular ubiquitin-binding protein responsible for the generation of autophagosome after interaction with LC3 (Mortezaee, 2018; Figure 4).
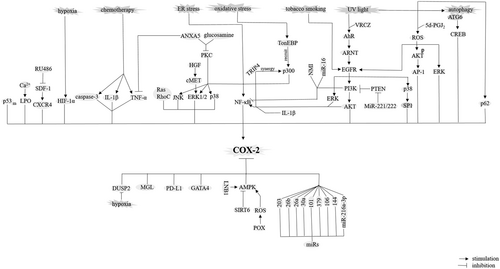
Signaling pathways or factors contributed to positive or negative regulation of cyclooxygenase-2 (COX-2) in cancer cells. AhR: arylhydrocarbon receptor; AKT: protein kinase B; AMPK: AMP-activated protein kinase; ANXA5: annexin A; ARNT: aryl hydrocarbon receptor nuclear translocator; CREB: cyclic AMP response element protein binding; DUSP2: dual specificity phosphatase 2; EGFR: epidermal growth factor receptor; ER: endoplasmic reticulum; ERK: extracellular signal-regulated kinase; GATA-4: GATA-binding protein 4; HGF: hepatocyte growth factor; HIF: hypoxia inducible factor; IL: interleukin; JNK: c-Jun NH2-terminal kinase; LKB1: liver kinase B1; m: mutant; MGL: monoglyceride lipase; NF-κβ: nuclear factor-κβ; NMI: N-myc (and STAT) interactor; PI3K: phosphoinositide 3-kinase; PKC: protein kinase C; POX: proline oxidase; PTEN: phosphatase and tensin homologue; ROS: reactive oxygen species; SIRT: sirtuin; Sp1: specificity protein; TRIP4: thyroid hormone receptor interactor 4; TNF-α: tumour necrosis factor-α; UV: ultraviolet; VRCZ: voriconazole; wt: wild type
7 COX-2 AND OXIDATIVE STRESS IN CANCER
Reactive oxygen species (ROS) are among COX-2 derived paracrine mediators for inducing mutation in cells (S.C. Ko et al., 2002). COX-2 is involved in peroxidation of lipids (Y. Xu, Yang, et al., 2018) taking a role for the generation of reactive lipid species (RLS; Wagner, Mullally, & Fitzpatrick, 2006). Cyclopentenone PGs are an example for these COX-2-derived RLS (Higdon, Diers, Oh, Landar, & Darley-Usmar, 2012; Wagner et al., 2006) contributed to inhibition of the tumor-suppressing liver kinase B1 (LKB1; Wagner et al., 2006). ROS have two diverse effects on COX-2 in cancer cells by being either inducer (E.-H. Kim et al., 2008) or inhibitor (Jeong, Kim, Lee, & Kim, 2017; Y. Liu et al., 2008) of this enzyme. Induction of COX-2 by ROS is mediated by activation of AKT (at Ser473), EGFR (at Tyr1068; J. Lee et al., 2017) and ERK (J. Qiu, Shi, & Jiang, 2017), and inhibition of COX-2 is mediated by the stimulatory effect of ROS on AMP-activated protein kinase (AMPK; Jeong et al., 2017). The ROS/ERK/COX-2 is contributed to cancer cell migration and invasion (J. Qiu et al., 2017; Figure 4).
CSCs and bulk cancer cells are vulnerable to alterations in the intracellular redox state (C. Lu, Laws, Eskandari, & Suntharalingam, 2017) depended on the ROS concentration. Low ROS concentration promotes cell proliferation via compensatory increase of the activity for antioxidative enzymes, while high ROS concentration induces mitochondrial pathway of apoptosis through inducing release of proapoptotic proteins from mitochondria into cytosol (Mortezaee, 2018).
8 COX-2 IS A MEDIATOR OF INFLAMMATION IN CANCER
Inflammation is an inducer of carcinogenesis in which an inflammatory microenvironment is considered as a promoter of cancer development and invasiveness (Echizen, Oshima, Nakayama, & Oshima, 2018). COX-2 is a proinflammatory enzyme (Pollock et al., 2018) overexpressed at the inflammatory site of cancer (Raj et al., 2018). There is a reciprocal positive feedback between COX-2 and inflammatory mediators with either one inducing another (Desai, Prickril, & Rasooly, 2018; Hosseini et al., 2018; Figure 4).
9 COX-2 AND TUMOR IMMUNITY
COX-2 is able to instruct an immune phenotype through inducing immune cell recruitment into the tumor tissue to induce an immunosuppressive state in the tumor milieu (Lang et al., 2006) in favor of cancer cell activation (Höing et al., 2018). COX-2/PGE2 released from cancer cells to this milieu suppresses host immunological responses to tumor-derived antigens through blockade of the activity for cytotoxic T lymphocytes (Miao et al., 2017). Another way for COX-2-mediated immune resistance is through inducing indoleamine 2,3-dioxygenase 1 in cancer cells (Hennequart et al., 2017; Figure 3).
10 COX-2 IS A MEDIATOR OF CANCER CELL GROWTH
There is a strong evidence for the contribution of COX-2 in the promotion of cancer cell growth (Raj et al., 2018). There are several ways for COX-2-mediated cancer cell proliferation as follows: (a) Induction of aromatase gene activity (aromatase cytochrome P450 [CYP19]; Esbona et al., 2018). Activation of CYP19 is contributed to estrogen biosynthesis (Harris et al., 2007). COX-2/PGE2 also induces cytochrome P450 1B1 (CYP1B1) that is responsible for conversion of the estrogen into estrogen quinones (Esbona et al., 2018) involved in tumor mitogenic and proliferative features (Harris et al., 2007). (b) Activation of neutrophils. COX-2 derived from cancer cells is able to activate neutrophils for the release of elastase relying proliferative signals on cancer cells (Hattar et al., 2014; Figure 3). (c) Activation of stromal CAFs. CAFs also send proliferative signals on cancer cells (Hull et al., 2017; Figure 3). Finally, (d) inactivation of adhesion molecules involved in regulation of cancer cell proliferation. An example for these molecules is E-cadherin that it makes a complex with catenin (Noda et al., 2002).
11 COX-2 IS AN INHIBITOR OF APOPTOSIS IN CANCER CELLS
COX-2 is related to apoptosis suppression in many cancers. COX-2 causes apoptosis resistance in cancer cells by making a delay in G1 phase (Gungor, Ilhan, & Eroksuz, 2018), induction of the expressions for Survivin (Krysan, Dalwadi, Sharma, Põld, & Dubinett, 2004), Mcl-2 (W. Chen et al., 2010) and Bcl-2 (Hosseini et al., 2018; Todoric et al., 2016), and repression of caspase-3 (Janakiraman et al., 2017;Figure 2).
The apoptosis mediator wild-type p53 is a suppressor for COX-2 expression (Mortezaee, 2018; Ohtsuka et al., 2018). In cancer cells, however, mutations in the p53 occurs, so a positive-feedback loop between COX-2 and the mutant p53 is an interesting event that might be a target for chemotherapeutic agents (Chin et al., 2018; Ohtsuka et al., 2018).
12 COX-2 IS A MEDIATOR OF TUMOR MIGRATION, INVASION, AND METASTASIS
COX-2 has been identified as one of the key metastasis progression genes (Greenhough et al., 2009) involved in the metastasis into lymph nodes (Höing et al., 2018), bone (Singh et al., 2006), liver (Sorski et al., 2016), brain (Soto et al., 2016), and so forth. COX-2 induces factors like IL-11 that are related to cancer metastasis (Singh et al., 2006). Epithelial–mesenchymal transition (EMT) in cancer cells is a promoter of cancer invasiveness (Mortezaee, 2018). COX-2 induces EMT through increasing the activity for miR526b (Majumder et al., 2015) and factors like signal transducer and activator of transcription-3 (STAT3; Tong et al., 2017), and that inhibition of COX-2-mediated EMT occurs after application of cannabinoids for cancer (Xian et al., 2016). COX-2 also induces β1-integrin (Pan et al., 2016) and membrane proteases like matripase that are responsible for cancer cell invasion (C. Ko et al., 2017; Figure 2).
13 COX-2 IS A MEDIATOR OF ANGIOGENESIS IN CANCER
COX-2 is expressed in tumor endothelial cells (Hull et al., 2017) and it is related to an increase in the number of blood vessels in human cancers (Montezuma et al., 2018) for cancer cell migratory purposes (Rüegg, Dormond, & Mariotti, 2004; Figure 3). COX-2 inhibitors like c-phycocyanin are able to suppress angiogenesis in cancer cells through docking with VEGF (Jiang et al., 2018). Activation of COX-2 for the promotion of angiogenesis is induced by caspase-3 (Feng et al., 2017; Figure 2).
14 COX-2 AS AN INDUCER OF CSC ACTIVITY IN CANCER CELLS
COX-2 is identified to be implicated in maintenance of tissue resistance CSC populations through promotion of CSC-like properties including tumorosphere formation, stemness, and metastatic propensity (C. Lu, Eskandari, Cressey, & Suntharalingam, 2017). Inducible effect of COX-2 on TGF-β is related to the enrichment of CSCs (Tian et al., 2017). Subcellular localization of COX-2 within mitochondria favors mitochondrial recruitment (translocation) of p53 that in turn induces the activity for mitochondrial fission Drp1 for the promotion of stemness (Zhou et al., 2017). In addition, COX-2 induces Id1 that serves as a marker of CSC self-renewal (Cook et al., 2016).
15 MECHANISMS FOR COX-2 CONTRIBUTION TO CANCER PROGRESSION
15.1 Sirtuin (SIRT)/COX-2
SIRT6 induces COX-2 in cancer cells by suppressing AMPK (Yadav et al., 2018). AMPK activation is a downstream target for the tumor suppressor LKB1 (Gao, Ge, & Ji, 2011; Figure 4).
15.2 NF-κβ/COX-2
NF-κβ is contributed to activation of COX-2 in both cancer cells (Charalambous et al., 2003) and TAMs (Hung et al., 2004). There are five related NF-κβ proteins including p50 (NF-κβ1), p52 (NF-κβ2), p65 (RelA), RelB, and c-Rel (Mortezaee, 2018), among them p65 has been identified for its role in the activation of COX-2 in cancer cells (Cai et al., 2017; J. Hao et al., 2018). IKK and IkB are upstream signaling molecules for NF-κβ acting in its nuclear translocation (Mortezaee, 2018). NF-κβ/COX-2 could be a target for chemotherapeutic agents for exerting their anticancer roles (H. Yang, Huang, et al., 2017). NF-κβ/COX-2 signaling is induced by ERK1/2 (Wong et al., 2017), protein kinase C (PKC; H.-S. Baek et al., 2017), TRIP4 (J. Hao et al., 2018), caspase-3 (Feng et al., 2017), cytokines like interleukin 1β (IL-1β; Charalambous et al., 2003), and conditions like ER stress (Hung et al., 2004). Inhibition of this signaling is mediated by miR-16 (X. Liu, Li, Li, et al., 2018) and annexin A5 (H.-S. Baek et al., 2017; Figure 4).
15.3 COX-2/STAT3
COX-2/STAT3 signaling is responsible for the promotion of an immunosuppressive microenvironment (Maturu et al., 2017), EMT (Tong et al., 2017), and proliferation (Ramu et al., 2018) of cancer cells. This signaling could be a part of the extended NF-κβ/COX-2/BCL-2/IL-6/STAT3 pathway (Das et al., 2017; Figure 2).
15.4 PI3K/AKT/COX-2
COX-2 takes a role for activation of PI3K/AKT pathway by suppressing phosphatase and tensin homologue (PTEN, an essential tumor-suppressing factor) in cancer cells either directly (Chang et al., 2018) or indirectly through suppression of TET-1-induced PTEN activation (H. Chen et al., 2017). MiR-221/222 is able to block PTEN resulting in activation of the PI3K/AKT/NF-κβ/COX-2 pathway (B. Li, Lu, et al., 2017). Phosphoinositide 3-kinase (PI3K) acts as both a positive (Rundhaug & Fischer, 2008) or negative (W. Liu et al., 2003) activator of COX-2. Activation of COX-2 by PI3K/AKT is mediated by induction of NF-κβ (B. Li, Lu, et al., 2017; Ramu et al., 2018). Interestingly, PI3K also reduces binding activity of COX-2 for NF-κβ (W. Liu et al., 2003; Figures 2 and 4).
15.5 MAPK/COX-2
Members of MAPK family including p38, ERK1/2, and c-Jun NH2-terminal kinase are contributed to induction of COX-2 (W. Liu et al., 2003). ERK1/2 (Maturu et al., 2017) and p38 (Hu et al., 2017) are also downstream targets for COX-2. These MAPK members are mediator of PKC inducible effects on COX-2 in both cancer cells (Chou et al., 2015) and CAFs (F. Li & Zhu, 2015). In addition, MAPK activity mediates inducible activity of EGFR on COX-2 (Dannenberg et al., 2005; Hu et al., 2017) in which a possible interaction between EGFR/p38 with COX-2 favors angiogenesis in cancer cells (Hu et al., 2017). P38/COX-2 is also involved in cancer cell resistance to apoptosis (Semaan et al., 2016; Figure 4).
15.6 COX-2/hypoxia inducible factor (HIF)
HIF-1α is a marker of angiogenesis in cancer cells (Mortezaee, 2018) and a target for COX-2 (Maturu et al., 2017). An existing interplay between COX-2 and HIF-1α is contributed to induction of hypoxia within the TME. In the hypoxic conditions, HIF-1α acts as a positive inducer for COX-2 expression (Greenhough et al., 2009). COX-2/HIF-2α is associated with cancer cell resistance to chemotherapeutic drugs in hypoxic conditions (Dong et al., 2018; Figure 2).
15.7 Arylhydrocarbon receptor (AhR)/ARNT/COX-2
AhR interaction with its nuclear transporter ARNT is contributed to the expression of COX-2 and further production of PGE2 in cancer cells (Ikeya et al., 2018; Figure 4).
15.8 EGFR/COX-2
There is a dual positive feedback between EGFR and COX-2 in cancer. This cross-talk can amplify the process of carcinogenesis. Tobacco smoking (Dannenberg et al., 2005; probably via its active nicotine component; Dinicola et al., 2018) and ultraviolet light (Fritsche et al., 2007) are among stimulators of this signaling. EGFR is a downstream target for AhR signaling (Fritsche et al., 2007), and it is also a part of the EGFR/COX-2/Nodal pathway for cancer EMT and CSC activity. Nodal is a member of TGF-β superfamily (X. Wang, Reyes, et al., 2017; Figures 2 and 4).
15.9 SDF-1/CXCR4/COX-2
SDF-1a is a member of CXC chemokine family that upon interaction with its receptor CXCR4 and further stimulation of COX-2 is contributed to cancer cell invasion (N. Zheng, Chen, Liu, et al., 2017) and metastasis (N. Zheng, Chen, Li, et al., 2017; Figure 4).
15.10 COX-2/β-catenin
β-Catenin is another signaling for COX-2/PGE2 to exert pro-oncogenic actions. This pathway is activated in lower stages of cancer progression, and it is stimulated by factors like serpinB3 (Terrin et al., 2017; Figure 2).
16 COX-2 AS AN ANTICANCER ENZYME
COX-2 acts a double-edged sword by being as a pro- or anticancer enzyme. COX-2 is able to metabolize dihomo-γ-linolenic acid into two different agents with diverse functions: PGs and 8-hydroxyoctanoic acid (8-HOA; X. Yang, Xu, et al., 2017). COX-2 promotes tumor expansion through regulation of PGs (Gurram et al., 2018). On the other hand, high level of COX-2 can inhibit tumor growth and migration through regulation of 8-HOA (Y. Xu, Yang, et al., 2018; X. Yang, Xu, et al., 2017). Products from COX-2 can also exert both anti- and protumorigenic functions. An example is the 15d-PGJ2 acting as an anti- and proangiogenic ligand (Ciccone, Monti, Monzani, Casella, & Morbidelli, 2018; E.-H. Kim & Surh, 2008). 15d-PGJ2 is an inducer for the proapoptotic peroxisome proliferator-activated receptor γ (PPARγ; Walther, Emmrich, Ramer, Mittag, & Hinz, 2016), while PGE2 is an inhibitor for this proapoptotic factor (Rossi et al., 2018).
Depending on accumulation in which subcellular component, COX-2 could be either a promoter or suppressor of cancer progression. This would be best explained after application of resveratrol for cancer cells. Transresveratrol is a component of the polyphenol extract and a stilbene analog (Regulski et al., 2016) that acts on ERK1/2 (Lin et al., 2017) for induction of nuclear accumulation (shuttling) of COX-2. Nuclear shuttling of ERK/COX-2 complex favors p53 phosphorylation at Ser-15 and the subsequent apoptosis in cancer cells (Chin et al., 2018). In addition, resveratrol represses COX-2 translocation to mitochondria to suppress stemness in cacner cells (Zhou et al., 2017). Eventually, cytosolic retention of COX-2 leads to cancer cell proliferation mediated through inhibition of the attachment between ERK and COX-2 (Chin et al., 2018; Lin et al., 2017).
For some tumors, COX-2 inhibition is considered as a disadvantage for cancer therapy. Overexpression of COX-2 for cancers like osteosarcoma (Z. Xu et al., 2006) favors the suppression of tumor proliferation and induction of apoptosis. Activation of the P38/COX-2 signaling (J.-H. Baek et al., 2018) induces ROS overproduction (Z. Xu et al., 2006) that is further contributed to promotion of apoptosis in cancers like osteosarcoma. For glioma, there are two opposite views for the roles of COX-2 overexpression with either enhancement of cancer promotion through interaction between COX-2 with NF-κβ (X. Wang, Yu, et al., 2017; J. Zhao, Zhu, et al., 2017), WNT (Wu et al., 2017), and EGFR (J. Li, Zhou, et al., 2017) for respective induction of inflammation, CSC activity and survival in cancer cells, or suppression of tumor progression through possible interaction between COX-2 with the endocannabinoid system that further elicits proapoptotic actions in this system (Hinz, Ramer, Eichele, Weinzierl, & Brune, 2004). Evidence for antitumor activity of COX-2 has been shown in the Figure 5. A point in this context is that the protumor activity of COX-2 is far more justifiable than its antitumor activity. Combining the knowledge from Figures 6 to 8 in which COX-2 overexpression is related to tumorigenesis for a wide variety of cancers (Figure 6), targeting COX-2 expression in cancer cells is a common function of a variety of chemotherapeutic agents (Figure 7), and COX-2 induces multiple signaling in cancer cells for the promotion of cancer cell progression (Figure 8), our justification toward the fate for COX-2 would be more into considering COX-2 as a tumor promoter, rather than an antitumor enzyme.
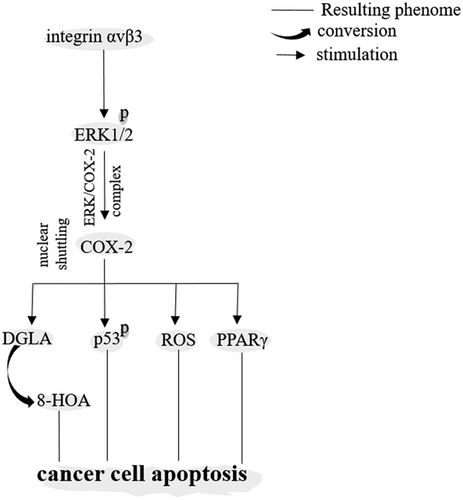
Cyclooxygenase-2 (COX-2) acting as an anticancer enzyme. 8-HOA: 8-hydroxyoctanoic acid; DGLA: dihomo-γ-linolenic acid; ERK1/2: extracellular signal-regulated kinase-1/2; PPARγ: peroxisome proliferator-activated receptor γ; ROS: reactive oxygen species
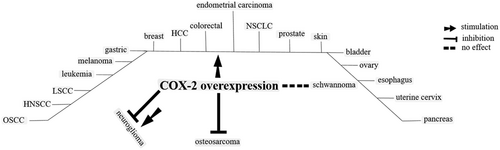
Cancers known to overexpress cyclooxygenase-2 (COX-2). HNSCC: head and neck squamous cell carcinoma; LSCC: laryngeal squamous cell carcinoma; NSCLC: non-small-cell lung cancer; OSCC: oral squamous cell carcinoma
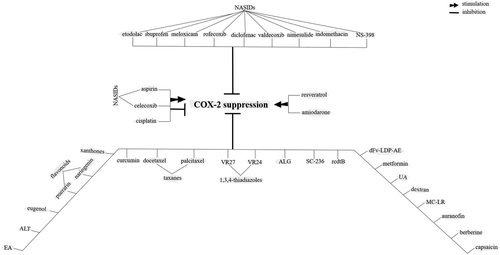
Agents identified for their chemopreventive roles against tumors working through cyclooxygenase-2 (COX-2) blockade in cancer cells. ALG, α-l-guluronic acid; ALT: alantolactone; EA: Ellagic aicd; MC-LR: microcystin-LR; NSAIDs: nonsteroidal anti-inflammatory drugs; rcdtB: clostridium difficile toxin B; UA, uroslic acid
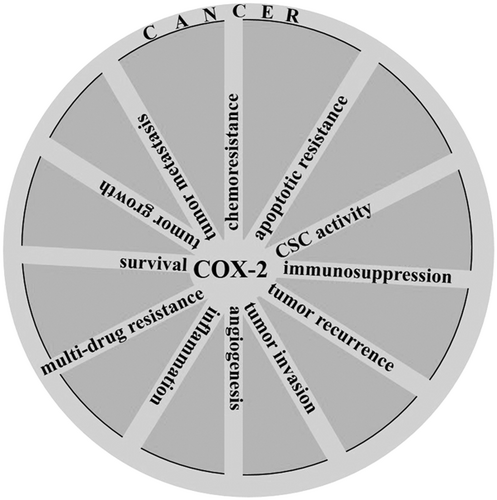
A diagram revealing multitasking roles for cyclooxygenase-2 (COX-2) in promotion of cancer. CSC: cancer stem cell
17 HORMONES AFFECT THE FATE FOR COX-2 EXPRESSION DURING THE PROCESS TUMORIGENESIS
A number of hormones are identified to have interactions with COX-2 for the promotion of tumorigenesis. Thyroxin (T4) stimulates PD-L1 to mediate cytoplasmic retention of COX-2 that further blocks the formation of ERK/COX-2 complex resulting in proproliferative features evolved in thyroid cancer cells (Chin et al., 2018; Lin et al., 2017). Testosterone, DHT, suppresses ERK/COX-2 and COX-2/p53 complexes leading to proproliferative features in breast cancer cells (Lin et al., 2017). Androgen inducible genes and androgen receptors are also positively affected by COX-2 (Brizzolara et al., 2017). In addition, the existence of a positive-feedback loop between estrogen and COX-2 favors aggravation of tumor-promoting effect for this hormone (Chung et al., 2017). Another hormone is epinephrine that has been identified for its role in induction of COX-2 and mediation of an immunosuppressive state in cancer cells (Muthuswamy et al., 2017).
18 COX-2 INHIBITORS AS A PROMISING THERAPEUTIC APPROACH FOR CANCER
Inhibition of COX-2 could provide a high possibility to exert therapeutic outcomes in cancer (Shi et al., 2018). Administration of COX-2 inhibitors for organs like lung, breast, colon, and prostate has been attested to cause a reduction of cancer risk by about 68% (Brizzolara et al., 2017). Interestingly, although it has been estimated that about half of the adenomatic cancers do not express COX-2 (Kashfi & Rigas, 2005; Noda et al., 2002), administration of COX-2 inhibitors has shown promising results for suppression of adenoma development (Hull et al., 2017; Noda et al., 2002). For these types of cancers, there is probably a shift for COX-2 expression in stromal (rather than cancer) cells (Hull et al., 2017).
COX-2 inhibitors are relatively inexpensive in comparison with the standard cancer therapies, having tolerable side effects, and that they are able to sensitize cancer cells to treatments like radiotherapy (Esbona et al., 2018) and chemotherapy (Shi et al., 2018) both of which are recognized to induce COX-2 expression in cancer cells (Ikeya et al., 2018; Kirk et al., 2018). In fact, COX-2 inhibitors are able to relieve COX-2-mediated expression of multidrug resistance proteins in cancer cells (Guo et al., 2017), and when they are administered in a perioperative setting, the inhibitors would reduce the risk of surgical related metastasis (Behrenbruch et al., 2018; Shaashua et al., 2017). COX-2 could be targeted by many types of chemotherapeutic drugs, as it has been shown in the Figure 7.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the drugs used for prevention of cancer. COX-2 inhibition by NASIDs is related to the reduction of cancer recurrence and increase of patient survival (Esbona et al., 2018). Elevation of ROS concentration by NSAIDS is possibly accounted for inhibition of COX-2 in cancer cells (C. Lu, Laws, et al., 2017). Conventional NSAIDs are not specific for inhibition of COX-2, as they are also an inhibitor for COX-1, and that they irritate upper gastrointestinal (GI) system. Example of these nonselective NSAIDs are ibuprofen (Liaras, Fesatidou, & Geronikaki, 2018), sulindac (Dannenberg et al., 2005), nimesulide (Barac et al., 2017), diclofenac, indomethacin, and aspirin (J. Qiu et al., 2017). By contrast, the rate of injury to GI using selective COX-2 inhibitors is low (Dannenberg et al., 2005). Coxibs (Lang et al., 2006; J. Qiu et al., 2017), etodolac (Noda et al., 2002), and NS-398 (J. Qiu et al., 2017) are examples for these selective COX-2 inhibitors (Lang et al., 2006; Figure 7). Recently, copper-bearing NSAIDs has been taken a special focus due to their anti-CSC properties (Eskandari et al., 2016).
Some NSAIDs like aspirin (Amaral, Nery, Leite, de Azevedo Junior, & Campos, 2018) and celecoxib (J. Kim et al., 2018) depending on the type of cancer could either inhibit or stimulate COX-2 expression (Amaral et al., 2018), both of which favors apoptosis in cancer cells. To explain, initial activation of COX-2 by these inhibitors may induce the proapoptotic factor PPARγ (Ramer et al., 2013), while a late and extended deactivation of COX-2 is probably required for activation of PPARγ-mediated apoptosis in cancer cells (Michael, Badr, & Badawi, 2003; W.-H. Sun et al., 2009). This deactivation could blunt the inhibitory roles for COX-2 metabolites like PGE2 on PPARγ activity (Rossi et al., 2018). Therefore, COX-2 inhibitors like celecoxib although induce an initial expression for COX-2 in cancers like lung, this induction favors cancer cell apoptosis (Ramer et al., 2013). Aspirin may also suppress growth of cancer cells via mechanisms independent on COX-2 (Henry et al., 2017).
COX-2 could be a target for hormonal therapy. Melatonin, a secretory product of pineal gland with diverse pharmacological effects including anticancer activity (Mortezaee & Khanlarkhani, 2018; Mortezaee, Khanlarkhani, Beyer, & Zendedel, 2018), is an example in this context showing promising results for suppression of cancer progression by inhibiting NF-κβ/COX-2 signaling in cancer cells (J.-J. Lu et al., 2016; Mortezaee, 2018).
Application of miRNAs could be another approach for suppression of COX-2 in cancer cells. MiRs 16 (X. Liu, Li, Li, et al., 2018), 101 (Y. Liu, Li, Zhao, & Jia, 2018), 379 (O'Brien et al., 2018), and 144 (Yao et al., 2018) are identified for their role in suppression of COX-2 in cancer cells. COX-2 could be an inducer of oncogenic miRs like miR655 and miR526b in cancers like breast (Majumder et al., 2018; Figure 4).
Another strategy for targeting COX-2 in cancer cells is to use carriers delivering siRNA for this enzyme. Agents like dextran would do such work important for suppression of COX-2 expression in cancer cells (Z. Chen, Krishnamachary, Penet, & Bhujwalla, 2018).
COX-2 inhibitors provide a common target for exerting synergistic therapeutic effects after combination with either chemotherapeutic drugs (X. Xu et al., 2017) or chemoradiation therapy (Das et al., 2017) against cancer cells. Chemotherapeutic agents could adversely cause cancer cell repopulation through induction of caspase-3-mediated COX-2 activity (Y. Zhao, Cui, et al., 2017). Irradiation is also an inducer of COX-2-mediated angiogenesis through activation of the same pathway (Feng et al., 2017). Some chemotherapeutic agents adversely induce COX-2 expression indirectly via promotion of an inflammatory setting (Kirk et al., 2018) probably through developing CAFs into senescence CAFs by acquiring a proinflammatory SASP (Kabir et al., 2016). Therefore, it would be advisable to use COX-2 inhibitors as an adjuvant with chemotherapy and/or radiotherapy. Such combination has been reported to synergistically increases the antitumoral activity for chemotherapeutic agents like sorafenib (Dong et al., 2018), 5-fluorouracil, bleomycin, irinotecan (Shi et al., 2018), cisplatin (Barac et al., 2017; F. Wang et al., 2018; C.-C. Yang et al., 2016), paclitaxel, carboplatin (Altorki et al., 2003), sunitinib (Q. Zhao, Guo, Wang, Chu, & Hu, 2017), and cetuximab (an EGFR blocker; Y. Lu, Shi, Qiu, & Fan, 2016) among others. This combination also improves the rate of tolerance to chemoradiation (Araujo-Mino et al., 2018) and overall response rate for advance stages of cancers (Dai et al., 2018), especially when it is administered before radiotherapy (J. Qiu et al., 2017; Salehifar & Hosseinimehr, 2016).
19 FACTORS INFLUENCING COX-2-TARGETED THERAPY FOR CANCER
- (1)
The type of cancer: Some types of cancers like schwannoma do not respond to COX-2 inhibitors despite its high rate of expression (Wahle et al., 2018). In addition, cancer cells have their specific activators for COX-2, as for gastric cancer that the activator is Helicobacter pylori (X. Liu et al., 2017) via JMJD2B (Han et al., 2016).
- (2)
The type of COX-2 inhibitor: As for NSAIDSs, the response would be more potent after application of selective drugs compared to the non-specific agents.
- (3)
The dose for COX-2 inhibitors: High doses for COX-2 inhibitors exert opposite effects by showing anticancer activity (Shi et al., 2018). This might be due to the effects of negative feedback loop or probably upregulation of COX-2 isoforms (Noda et al., 2002). To remove this burden (decreasing the doses demanded for COX-2 inhibitors), these inhibitors are preferred to be administered in combination with conventional anticancer agents (Shi et al., 2018).
- (4)
The type of chemotherapy (a conventional antitumor drug received by patient) used in combination with COX-2 inhibitors. Choosing a right type of chemotherapy as an adjuvant with COX-2 inhibitor is also important.
- (5)
The grade of tumor: Relation between the grade of tumor with COX-2 expression is still a controversial issue. For example, two studies performed on ovarian cancer were reached opposite results with a one reported high expression for COX-2 in the low (mucus) grade (Beeghly-Fadiel et al., 2018), but another the high rate for COX-2 in the high (serous) grade of cancer (Beeghly-Fadiel et al., 2018). This controversy has also been marked for the breast cancer (Tury et al., 2016).
20 CONCLUSION AND PERSPECTIVE
From what has been discussed for most of the cancer types, COX-2 is a marker indicating worse prognosis and a stimulator for a variety of cancers (Figure 6) by taking multitasking roles (Figure 8), so suppression of COX-2 could provide a promising approach for cancer therapy especially when it is used as an adjuvant with appropriate chemotherapeutic agents.
21 SIGNIFICANCE
-
Both activation and suppression of COX-2 is related to tumorigenesis.
-
COX-2 activation could be both pro- or antitumorigenic, but the evidence for its protumor activity is by far more strength.
-
COX-2 could be either activated or deactivated by ROS. This is probably dependent on the concentration of ROS.
-
For most type of cancers, uncontrolled activation of COX-2 is related to worse outcome and its blockade favors cancer prophylaxis and regression.
-
Most of the functions of COX-2 on tumor cells is exerted through engagement of PGE2/EP4, so targeting EP4 would provide an optimistic approach for cancer therapy.
-
Members of MAPK family, EGFR, and p65 NF-κβ are major regulators of COX-2 in cancer cells. ERK acting on COX-2 is contributed to both pro- and antitumorigenic functions of COX-2.
-
Nuclear translocation of COX-2 induces apoptosis in cancer cells, while mitochondrial translocation of COX-2 promotes stemness in cancer cells.
-
TME is a key for the promotion of COX-2-mediated tumorigenesis. Production of COX-2 occurs within this milieu. Macrophage type 2 cells, Tregs, and cancer cells are contributed to COX-2 release to the TME.
-
Due to the complexity of signaling pathways contributed to the regulation of COX-2 in cancer cells and cancer cell resistance to chemotherapy, it is preferred to use a combination of COX-2 inhibitor with a suitable chemotherapeutic agent to achieve appropriate responses.
-
Existence of a negative feedback between COX-2 and mediator of apoptosis favors both apoptosis resistance and angiogenesis in cancer cells.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.




