Chronic kidney disease after 5/6 nephrectomy disturbs the intestinal microbiota and alters intestinal motility
Abstract
Organ–organ crosstalk is involved in homeostasis. Gastrointestinal symptoms are common in patients with renal failure. The aim of this study was to elucidate the relationship between gastrointestinal motility and gastrointestinal symptoms in chronic kidney disease. We performed studies in C57BL/6 mice with chronic kidney disease after 5/6 nephrectomy. Gastrointestinal motility was evaluated by assessing the ex vivo responses of ileum and distal colon strips to electrical field stimulation. Feces were collected from mice, and the composition of the gut microbiota was analyzed using 16S ribosomal RNA sequencing. Mice with chronic kidney disease after 5/6 nephrectomy showed a decreased amount of stool, and this constipation was correlated with a suppressed contraction response in ileum motility and decreased relaxation response in distal colon motility. Spermine, one of the uremic toxins, inhibited the contraction response in ileum motility, but four types of uremic toxins showed no effect on the relaxation response in distal colon motility. The 5/6 nephrectomy procedure disturbed the balance of the gut microbiota in the mice. The motility dysregulation and constipation were resolved by antibiotic treatments. The expression levels of interleukin 6, tumor necrosis factor-α, and iNOS in 5/6 nephrectomy mice were increased in the distal colon but not in the ileum. In addition, macrophage infiltration in 5/6 nephrectomy mice was increased in the distal colon but not in the ileum. We found that 5/6 nephrectomy altered gastrointestinal motility and caused constipation by changing the gut microbiota and causing colonic inflammation. These findings indicate that renal failure was remarkably associated with gastrointestinal dysregulation.
1 INTRODUCTION
Gastrointestinal disorders are known to commonly occur in patients with renal failure (for review see Shirazian & Radhakrishnan, 2010). Uremia and dialysis have long been speculated to increase the risk of lesions in the gastrointestinal tract (for review see Shirazian & Radhakrishnan, 2010). The incidence of gastrointestinal disorders in patients with chronic kidney disease (CKD) has increased around the world, including Japan, and gastrointestinal disorders affect 70% of the US population (Coresh et al., 2007). Urgently, the prevalence of CKD could rise sharply over the next few decades, driven by population aging and an increasing prevalence of diabetes and hypertension (Lozano et al., 2012; White, Chadban, Jan, Chapman, & Cass, 2008; NIH and NIDDK, 2012). Indeed, as the number of patients who develop diabetes and hypertension increases, more patients will develop CKD and associated gastrointestinal disorders. Earlier studies have investigated the gastric emptying and gastrointestinal bleeding in patients with CKD. For instance, gastrointestinal transit time was longer in patients receiving hemodialysis and peritoneal dialysis than in healthy individuals (M. J. Wu et al., 2004). In recent studies, patients with CKD had adversely altered gut microbiota (Mishima et al., 2015; Vaziri et al., 2013). The exploration of the connection between gastrointestinal disorders and CKD has been appreciated over time. However, this connection is incompletely understood. In addition, the mechanism association by which patients with CKD develop gastrointestinal disorders is unclear.
The symptoms of constipation and diarrhea are severe in some patients with CKD, requiring additional medical therapy. Gastrointestinal motility is associated with the brain–gut axis and is easily influenced by body condition. In addition, a high-fat diet delays intestinal transit because of enteric neuronal damage (Nezami et al., 2014). Type I diabetic patients often have disruption of intestinal motility (Jackson, Gordon, & Waterman, 2004). Parkinson's disease is also associated with gastrointestinal motility problems (Jost, 1997).
The movements of the contents of the gastrointestinal tract rely on the coordinated contraction and relaxation of the smooth muscle that surrounds each specialized region (Azuma et al., 2012). Coordinated responses of the smooth muscle depend on the influences of the enteric nervous system in each region, intrinsic pacemaker cells, endocrine and paracrine mediators, and intrinsic spontaneous excitability of the smooth muscle cells (Costa, Hennig, & Brookes, 1998). Excitatory or inhibitory responses to smooth muscle are controlled by the conventional neurotransmitters, such as acetylcholine (ACh) and noradrenaline but also nonadrenergic noncholinergic transmitters (Hidaka, Azuma, Nakajima, & Takeuchi, 2010; Takeuchi, Tanaka, Nakajima, Matsui, & Azuma, 2007).
Our objectives were to attempt to define the association between kidney disease and gastrointestinal disorders. In this study, we focused on constipation and diarrhea among gastrointestinal symptoms and investigated gastrointestinal motility in a CKD mouse model. Furthermore, we sought to identify the mechanism underlying altered gastrointestinal motility.
2 MATERIALS AND METHODS
2.1 Drugs
ACh, atropine, chloramphenicol, doxycycline hydrochloride n-hydrate, lincomycin hydrochloride monohydrate, and spermine were purchased from Wako Pure Chemical (Osaka, Japan). Lipopolysaccharides (LPS) from Escherichia coli [0111:B4] was purchased from Sigma (St. Louis, MO). Indoxyl sulfate potassium salt and hippuric acid were purchased from Nakarai Tesuque (Kyoto, Japan). Transaconitic acid was purchased from Tokyo Chemical Industry (Tokyo, Japan). A goat polyclonal antibody against choline acetyltransferase (ChAT; AB144P) was purchased from Chemicon International (Temecula, CA). An Alexa Fluor 488-labeled goat anti-rat immunoglobulin G (IgG) was purchased from Molecular Probes Inc. (Eugene, OR).
2.2 CKD model by 5/6 nephrectomy
Male C57BL/6 mice (8–9 weeks old) were obtained from CLEA Japan, Inc. (Tokyo Japan). We used an established model to induce CKD by subtotal nephrectomy (5/6 Nx) (Mino et al., 2007). 5/6 Nx was performed using previously described methods (Mino et al., 2007). Through a right dorsolateral incision, the right kidney was pulled out to expose the renal vessels and ureter, which were then ligated with cotton thread and cut between the hilus and the ligated portion to remove the kidney. One week after the operation, the left kidney was pulled out through a left dorsolateral incision to expose the renal vessels and the ureter. The renal vessels were clipped by a clamp, and the cranial and caudal parts of the organ were cut so that one-third of the kidney remained. The cut surfaces were then coated with adhesive gum, and the clamp was released. Finally, the treated left kidney was returned to the abdominal cavity. We measured serum creatinine in mice 5 weeks after the second surgery. Serum creatinine levels were measured by LabAssay Creatinine (Wako Pure Chemical). The mice were used for experimentation as long as serum creatinine values in the 5/6 Nx mice were twice as higher as those in the sham mice (Fu et al., 2011). In addition, renal histopathology was performed in each experiment. All procedures used in this study complied with the institutional policies of the Osaka Prefecture University Animal Care and Use Committee.
2.3 Recording of responses of the longitudinal smooth muscle of the ileum and distal colon
A recording of the responses was performed using previously described methods (Nishiyama et al., 2014; Nishiyama et al., 2016). Briefly, the ileum and distal colon were removed from the mice. Whole-wall strips were prepared in the orientation of the longitudinal muscle layer. These strips were mounted in organ baths and held at a resting tension of 0.5 g. The baths were filled with Tyrode's solution, which was maintained at 37℃ and bubbled with 95% O2 and 5% CO2. Responses were detected using isotonic force transducers (TD-112A; Nihonkohden, Tokyo, Japan). Specifically, the strips were exposed to electric field stimulation (EFS) with 1 Hz for 10 s to evoke smooth muscle contraction and relaxation. Atropine (1 μM) and uremic toxins (100 μM) were directly added to the bath at least 15 min before EFS and ACh. Contractions relative to the baseline tone were analyzed by measuring the extent of the maximal contraction in response to the KCl solution (60 mmol/L). Relaxations relative to the baseline tone was analyzed by measuring the extent of the maximal relaxation in response to the Ca2+-free EGTA solution.
2.4 F4/80 immunofluorescent staining
Immunofluorescent staining for frozen sections was performed as described previously (Fujimoto et al., 2017). Briefly, the ileum and distal colon were removed from the mice and fixed with 4% paraformaldehyde. Frozen sections (5 μm thick) were cut and prepared for immunofluorescent staining. The expression of ChAT was detected using goat anti-ChAT antibody. The immunoreactivity of ChAT was detected using an Alexa Fluor 568-labeled goat anti-rabbit IgG antibody (Azuma et al., 2012). To detect macrophages, the immunoreactivity of F4/80 was detected using a rat anti-F4/80 antibody (1:500) and Alexa Fluor 488-labeled goat anti-rat IgG antibody (1:2,000). Confocal images were obtained under a laser-scanning microscope (C1si; Nikon Corporation, Tokyo, Japan). Positive cells were counted using ImageJ.
2.5 Microbiota analyses
The genomic DNA of the gut microbiota was extracted from mouse feces using NucleoSpin® DNA Stool (MACHEREY-NAGEL). DNA fragments in the variable V3–V4 region of the 16S ribosomal RNA (rRNA) gene were amplified by performing touch-down polymerase chain reaction (PCR) experiments, using a forward/reverse PCR primer set (Illumina). The forward primer sequence was 5′-TCG TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG CCT ACG GGN GGC WGC AG-3′, and the reverse primer sequence was 5′-GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GGA CTA CHV GGG TAT CTA ATC C-3′. The PCR products were purified using AMPure XP beads (Beckman), and their sequences were exhaustively determined by next-generation sequencing. Operational taxonomic units were identified using the UCLUST 30 algorithm in QIIME with a sequence-similarity threshold value of 0.97. The microbiome data were deposited in the DDBJ database.
2.6 Antibiotic treatments
The mice were treated with lincomycin hydrochloride monohydrate (17 mg/kg), doxycycline hydrochloride n-hydrate (43 mg/kg), and chloramphenicol (50 mg/kg) via intraperitoneal injection 2 and 3 days before beginning the experiment.
2.7 RNA isolation and quantitative real-time reverse transcription PCR
Total RNA was isolated as previously described (Azuma et al., 2010). The RNA was used to synthesize complementary DNA using SuperScript Reverse Transcriptase (Roche, Madison, WI). The primers used for the amplification are described in Table 1. Messenger RNA (mRNA) expression levels were quantified using real-time PCR analysis based on the intercalation of SYBR Green (Toyobo, Osaka, Japan). The amplification of HPRT mRNA was used as an endogenous control to account for the differences in the amount and quality of RNA added to each reaction.
| Target | 5′–3′ | |
|---|---|---|
| HPRT | F | GTTGGATACAGGCCAGACTTTGTTG |
| R | GAGGGTAGGCTGGCCTATAGGCT | |
| TNF-α | F | ATGAGCACAGAAAGCATGATCCGC |
| R | CCAAAGTAGACCTGCCCGGACTC | |
| IL-6 | F | AAGGGCCAGGGATCTGTAAG |
| R | TCTCTTGTTGCTCCCCAAAG | |
| iNOS | F | CCAAGCCCTCACCTACTTCC |
| R | CTCTGAGGGCTGACACAAGG | |
- Note. F: Forward; IL-6: interleukin 6; PCR: polymerase chain reaction; R: reverse; TNF-α: tumor necrosis factor-α.
2.8 Macrophage cultures
RAW264.7 cells were a gift from S. Maeda and Y. Yoshioka (Setunan University, Osaka, Japan) (Nishiyama et al., 2017). The RAW264.7 cells were grown in Dulbecco's modified Eagle medium 4.5 g/L (25 mM) glucose supplemented with 10% fetal bovine serum, l-glutamine, and antibiotics/antimycotics (Invitrogen, Waltham, MA) at 37°C in a 5% CO2 humidified incubator. The RAW264.7 cells were placed on a 24-well plate at 1 × 105 cells/cm2 and were treated with the uremic toxin in the presence of LPS at 1 ng/ml for 24 hr. Then, tumor necrosis factor-α (TNF-α) concentrations were determined in the culture supernatants using enzyme-linked immunosorbent assay (eBioscience, San Diego, CA) (Matsuo et al., 2015).
2.9 Statistical analysis
Error bars indicate the standard error of the mean. The statistical significance of the parametric data was evaluated using two-tailed Student's t test (unpaired) for comparisons with the sham mice and two-tailed Student's t test (paired) for comparisons with the control group to detect differences between two groups. Statistical significance was determined by one-way analysis of variance for nonrepeated measures to detect differences among 3–5 groups. The differences between groups were determined using the Tukey–Kramer test. A p value less than 0.05 was considered to indicate statistical significance.
3 RESULTS
3.1 Gastrointestinal motility in mice with CKD
Body weight changes after the nephrectomy are shown in Figure 1 (upper). Serum creatinine values were twofold higher in mice with CKD after 5/6 Nx (5/6 Nx mice) than in the sham mice (Figure 1 [upper]). We found a decreased amount of stool for the 5/6 Nx mice compared with the sham mice (Figure 1 [middle right]). Water intake was increased in the 5/6 Nx mice compared with that of the sham mice (Figure 1). However, food intake in the 5/6 Nx mice was similar to that in the sham mice (Figure 1). Histological analysis of the kidney from the 5/6 Nx mice showed vacuolation and mild mesangial proliferation (Figure 1 [lower]). These results suggested that CKD may contribute to dyschezia through dysregulated intestinal motility.

A decrease in the amount of stool in the 5/6 Nx mice. Percentage weight change, serum creatinine level, food intake, water intake, and amount of stool in the sham (n = 4) and 5/6 Nx (n = 6–8) mice. The H&E staining of representative sections of the kidney is shown. Scale bar, 20 μm. *p < 0.05, **p < 0.01 compared with sham. H&E: hematoxylin and eosin [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Altered gastrointestinal motility in mice with CKD
To study the impact of the dysregulated intestinal motility in CKD, we next analyzed the motility of the longitudinal muscles in the ileum and distal colon ex vivo. Figure 2a shows representative recording traces of responses to EFS in the ileum. The 5/6 Nx mice (mice with CKD after 5/6 Nx) presented with a decrease in EFS-induced contraction. In addition, ACh-induced contraction was not significantly altered in the 5/6 Nx mice compared with the sham group (Figure 2b). These results indicate that attenuated contraction in the 5/6 Nx group is associated with a decreased release of ACh from neurons; however, the attenuation of the sensitivity of the smooth muscle cells to ACh was not observed.
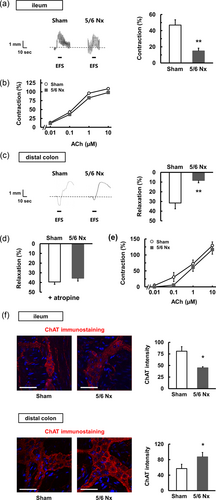
Dysregulated motility in 5/6 Nx mice is shown. The gastrointestinal motility in longitudinal muscle strips isolated from the ileum (a,b) and the distal colon (c–e) in sham (n = 5) and 5/6 Nx (n = 5) mice. (a) EFS-induced contraction. Representative recording traces and quantitative data are shown. (b) ACh-induced contraction. (c) EFS-induced relaxation. Representative recording traces and quantitative data are shown. (d) EFS-induced relaxation with atropine at 1 μM. (e) ACh-induced contraction. **p < 0.01 compared with sham. (f) Immunohistochemical staining in the ileum and distal colon of sham and 5/6 Nx mice is shown. Similar results were obtained in three to four independent experiments. The section was stained for ChAT. Scale bar = 50 μm. Positive cells were counted using ImageJ. *p < 0.05 compared with sham mice. ACh: acetylcholine; ChAT: choline acetyltransferase; EFS: electric field stimulation [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2c shows representative recording traces of responses to EFS in the distal colon. The 5/6 Nx mice (mice with CKD after 5/6 Nx) presented with a decrease in EFS-induced relaxation. During EFS, myenteric neurons release ACh as an excitatory transmitter and nitric oxide (NO), CO, and ATP as inhibitory transmitters in the distal colon (Hidaka et al., 2010). Thus, there are two possibilities for the decreased relaxation observed in the 5/6 Nx group. One possibility is an increase in the excitatory component. Another possibility is a decrease in the inhibitory component. In the presence of atropine, the 5/6 Nx mice showed relaxation in a manner similar to the sham group (Figure 2d), suggesting that the attenuated relaxation observed in the 5/6 Nx mice is associated with an increase in the excitatory component. In addition, the ACh-induced contraction was not significantly altered in the 5/6 Nx mice relative to the sham mice (Figure 2e), suggesting that the attenuated relaxation in the 5/6 Nx mice is associated with an increased release of excitatory mediators, such as ACh from neurons; however, the sensitivity of the smooth muscle cells to ACh was not attenuated. We investigated the expression levels of ChAT to demonstrate the release of ACh contents in the myenteric plexus. As shown in Figure 2f, we observed that the ileum in the 5/6 Nx mice presented a significantly decreased expression of ChAT, whereas the distal colon in the 5/6 Nx mice presented a significantly increased expression of ChAT compared with that in the sham mice. These data support the decreased ACh release in the ileum and increased ACh release in the distal colon. Taken together, the ex vivo experiments show that CKD presented dysregulated intestinal motility in different patterns between the ileum and distal colon.
3.3 The effect of spermine on intestinal motility
Because uremic toxins are increased in the body with CKD, we focused on uremic toxins, such as spermine, indoxyl sulfate, and hippuric acid, to elucidate the mechanism to alter the gastrointestinal motility. Spermine at 100 μM attenuated the EFS-induced contraction in the ileum (Figure 3a). Unlike spermine at 100 μM, indoxyl sulfate, aconitic acid, and hippuric acid at 100 μM had no effect on the EFS-induced responses in the ileum (Figure 3a). However, spermine at 100 μM had no effect on the ACh-induced contraction in the ileum (Figure 3a [lower]). In contrast, these uremic toxins had no effect on the EFS-induced relaxation in the distal colon (Figure 3b). These results suggest that spermine may decrease the release of ACh in the ileum.
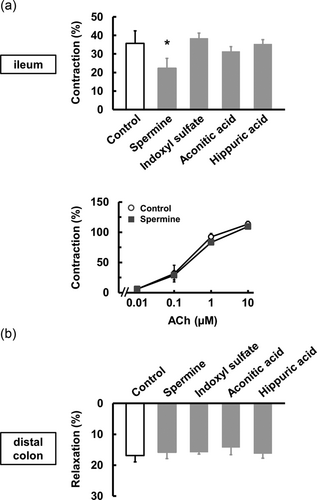
The effect of uremic toxins on motility. (a) The effects of spermine at 100 μM (n = 4), indoxyl sulfate at 100 μM (n = 3), aconitic acid at 100 μM (n = 3), and hippuric acid at 100 μM (n = 3) on EFS-induced contraction in the ileum. The effect of spermine at 100 μM (n = 4) on ACh-induced contraction in the ileum. (b) The effects of uremic toxins at 100 μM (n = 3–4) on EFS-induced relaxation in the distal colon. *p < 0.05 compared with control. ACh: acetylcholine; EFS: electric field stimulation
3.4 The effect of renal impairment on microbiota populations
To further elucidate the mechanism by which gastrointestinal motility is altered, we investigated the gut microbiota. The gut microbiome analysis of bacterial 16S rRNA genes was categorized at the genus level into the sham and 5/6 Nx groups. At the genus level, the quantitative analysis showed a significant increase in Allobaculum, Bifidobacterium, and Turicibacter in the 5/6 Nx mice group (Figure 4a). Interestingly, a Lactobacillus, an Oscillospira, and an unclassified Ruminococcaceae were significantly decreased in the 5/6 Nx mice group. An unclassified Rikenellaceae was also decreased (not significant) in the 5/6 Nx mice group. Firmicutes (F) and Bacteroidetes (B), known as the F/B ratio, can be potentially used as biomarkers for pathological conditions (Sanz & Moya-Pérez, 2014). However, the F/B ratio in the 5/6 Nx group was similar to that in the sham group (Figure 4b). These results suggest that CKD is able to disturb gut microbiota balances in the 5/6 Nx mice.
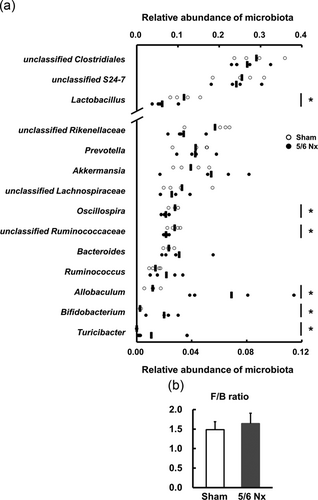
Altered gut microbiota in 5/6 Nx mice. (a) The proportional change in each subfamily at the genus level in the feces of the sham (n = 4) and 5/6 Nx (n = 4) mice. The x-axis indicates the relative abundance of each microbe percentage. The bars indicate each average. *p < 0.05 compared with sham. (b) A ratio (F/B) of firmicutes phylum to bacteroidetes phylum in the feces of the sham (n = 4) and 5/6 Nx (n = 4) mice is shown
3.5 Antibiotic treatment
Microbiota is involved in intestinal motility and inflammation (Anitha, Vijay-Kumar, Sitaraman, Gewirtz, & Srinivasan, 2012; Maslowski et al., 2009). In addition, it is reported that the microbiota change in chronic renal failure patients (Vaziri et al., 2013). To reveal whether the microbiota contribute to intestinal motility dysfunction in the 5/6 Nx mice, we investigated the responses in the longitudinal muscle obtained from the ileum and distal colon of mice treated with antibiotics (Figure 5a [upper left]). Serum creatinine values were still higher in the 5/6 Nx mice treated with antibiotics than in the sham mice treated with antibiotics (Figure 5a). However, the amount of stool for the 5/6 Nx mice treated with antibiotics was similar to that for the sham mice treated with antibiotics (Figure 5a [lower right]). In contrast, the food and water intake was similar to that shown in Figure 1a (Figure 5a).
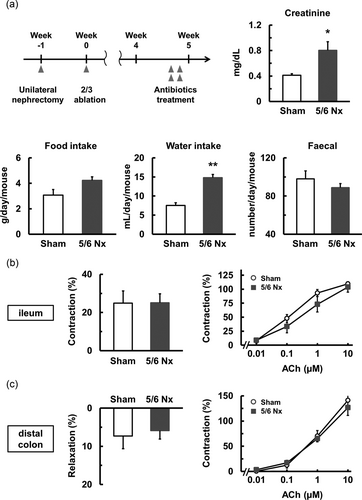
The effect of antibiotic treatment. (a) The protocol of antibiotic treatment is shown. Serum creatinine level, food intake, water intake, and amount of stool in the sham (n = 5) and 5/6 Nx (n = 5) mice treated with antibiotics. (b,c) The gastrointestinal motility in longitudinal muscle strips isolated from the ileum (b) and distal colon (c) in the sham (n = 5) and 5/6 Nx (n = 5) mice treated with antibiotics. (b) EFS-induced contraction and ACh-induced contraction. (c) EFS-induced relaxation and ACh-induced contraction. *p < 0.05, **p < 0.01 compared with sham. ACh: acetylcholine; EFS: electric field stimulation
The EFS-induced contraction of the ileum obtained from the 5/6 Nx mice treated with antibiotics was similar to that observed in the sham group treated with antibiotics (Figure 5b [left]). Moreover, the ACh-induced contraction of the ileum obtained from the 5/6 Nx group treated with antibiotics was similar to that of the sham group treated with antibiotics (Figure 5b [right]). Similar to the ileum, the EFS-induced relaxation of the distal colon obtained from the 5/6 Nx mice treated with antibiotics was similar to that observed in the sham group treated with antibiotics (Figure 5c [left]). Moreover, the ACh-induced contraction of the distal colon obtained from the 5/6 Nx treated with antibiotics was similar to that of the sham group treated with antibiotics (Figure 5c [right]). These results suggested that the microbiota contribute to renal-impairment-related gastrointestinal motility dysregulation in the ileum and distal colon.
3.6 Altered levels of cytokines in the distal colon of 5/6 nephrectomy mice
CKD induces intestinal inflammation, which manifests as gastrointestinal hemorrhage and ulcer formation (Coresh et al., 2007). In addition, intestinal inflammation causes intestinal motility dysfunction through inflammatory mediators such as TNF-α, interleukin 6 ( IL-6), and NO (Ohama et al., 2007). To investigate inflammation, we measured the expression levels of IL-6, TNF-α, and iNOS in the ileum and distal colon. In the distal colon, the expression of IL-6, TNF-α, and iNOS was increased in the 5/6 Nx mice (Figure 6a), whereas the expression of IL-6, TNF-α, and iNOS in the ileum of the 5/6 Nx mice was similar to that observed in the sham mice (Figure 6a). Furthermore, we investigated the same mediators in the distal colon of the 5/6 Nx mice treated with antibiotics. As shown in Figure 6b, the expression of IL-6, TNF-α, and iNOS in the 5/6 Nx mice treated with antibiotics was similar to that observed in the sham mice treated with antibiotics. These results suggest that the 5/6 Nx mice experienced motility dysregulation in the distal colon through microbiota imbalance followed by the increase in inflammatory mediators.
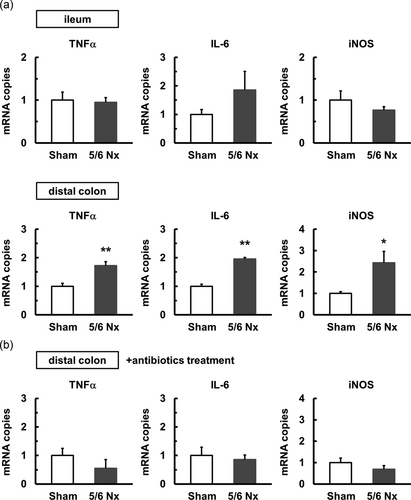
Increased inflammatory cytokine levels in the distal colon of 5/6 Nx mice. (a) The mRNA expression levels of TNF-α, IL-6, and iNOS in the ileum and distal colon of the sham (n = 4–5) and 5/6 Nx (n = 4–6) mice. (b) The mRNA expression levels of TNF-α, IL-6, and iNOS in the distal colon of sham (n = 5) and 5/6 Nx (n = 5–6) mice treated with antibiotics. The mRNA levels were examined using quantitative real-time PCR. The mRNA level is shown as a fold increase relative to the level in the sham group. *p < 0.05, **p < 0.01 compared with sham. IL-6: interleukin 6; mRNA: messenger RNA; PCR: polymerase chain reaction; TNF-α: tumor necrosis factor-α
3.7 The effect of indoxyl sulfate and transaconite acid on LPS-induced TNF-α production in RAW264.7 macrophage cells
In agreement with the increased expression of IL-6, TNF-α, and iNOS in the distal colon, we observed that the distal colon in the 5/6 Nx mice presented more severe inflammation than that in the sham mice, as evidenced by a significant increase in the infiltration of macrophages (Figure 7a). In addition, we investigated whether uremic toxins were involved in the inflammatory response by using RAW264.7 macrophage cells. Indoxyl sulfate and transaconite acid, but not hippuric acid (data not shown), enhanced LPS-induced TNF-α production in RAW264.7 cells (Figure 7b). These results show that some uremic toxins associated with CKD cause colonic inflammation and dysregulated motility.
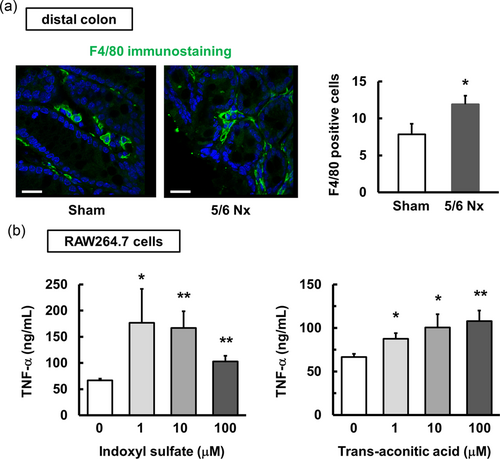
Increased TNF-α production in macrophages treated with uremic toxins. (a) Immunohistochemical staining in the distal colon of the sham and 5/6 Nx mice is shown. Similar results were obtained in three independent experiments. The section was stained for F4/80. Scale bar = 200 μm. Positive cells were counted using ImageJ. *p < 0.05 compared with sham mice. (b) The effect of indoxyl sulfate (n = 4) and aconitic acid (n = 4) on LPS-induced TNF-α production in RAW264.7 macrophages. RAW264.7 cells were stimulated with indoxyl sulfate or transaconitic acid in the presence of LPS at 1 ng/ml for 24 hr. The release of TNF-α into the culture supernatant was measured using ELISA. *p < 0.05, **p < 0.01 compared with 0 μM as the control. ELISA: enzyme-linked immunosorbent assay; LPS: lipopolysaccharides; TNF-α: tumor necrosis factor-α [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Our results showed that the CKD model of 5/6 Nx was associated with dysfunction of gastrointestinal motility through different mechanisms in the ileum and distal colon. This event is associated with composition changes in the gut microbiota, uremic toxins, and colonic inflammation.
The methods used in the study to evaluate gastrointestinal motility have two strengths. First, an isotonic transducer was used as a detector to simultaneously analyze muscle contraction and relaxation. Most researchers use an isometric transducer rather than an isotonic transducer because the operator uses an isometric transducer for a short period of time compared with the time associated with the use of an isotonic transducer. Second, EFS was used as an endogenous stimulus, and ACh treatment was used as an exogenous stimulus, because ex vivo organ strips are regulated by the enteric nervous system and parasympathetic nerves. Our second strength was to evaluate and analyze the motility according to the stimulus. We are confident in the used of these methods as our laboratory has already published many papers involving the use of an isotonic transducer under EFS (Azuma et al., 2016; Azuma, Samezawa, Nishiyama, Nakajima, & Takeuchi, 2016; Fujimoto et al., 2016).
The 5/6 Nx mice revealed decreased contraction through decreased ACh release in the ileum and decreased relaxation through increased ACh release in the distal colon. Although it may be seemingly contradictory, the ileum and distal colon have different functions in gastrointestinal motility. The contraction response is physiologically important for the transport of contents in the ileum, whereas the relaxation response is important for the accumulation of contents in the distal colon. As a result, the 5/6 Nx mice experienced marked constipation.
Our findings indicated that increased spermine, one of the polyamines, CKD after 5/6 Nx inhibited contraction through decreased ACh release in the ileum, although we actually did not detect the spermine level in the serum and/or the gastrointestinal tissues. Recent studies have independently shown that spermine acts as a uremic toxin (Sindhu, 2016) and inhibits the contraction in the ileum of mice (Sánchez et al., 2017). These findings are supported in our results. Of note, spermine regulates gastrointestinal motility through the activation of extracellular calcium-sensing receptors (Rogers et al., 2015; Tang et al., 2016).
Unlike the ileum, uremic toxins tested in this study have no effect on EFS-induced responses in the distal colon. We showed that macrophage infiltration into the distal colon, but not into the ileum, was increased in the 5/6 Nx mice (data not shown). The 5/6 Nx mice also revealed the enhanced expression of TNF-α, IL-6, and iNOS in the distal colon, but not in the ileum. Importantly, indoxyl sulfate and transaconite acid enhanced TNF-α production in macrophage cells in vitro. This is in accordance with previous studies showing that indoxyl sulfate increased inflammatory cytokines in macrophage cell lines THP-1 and J774.1 (Adesso et al., 2013; K. Matsuo et al., 2015; Y. Matsuo et al., 2015). In contrast, indoxyl sulfate and transaconite acid induced reactive oxygen species (ROS) (Enoki et al., 2016; Toyohara et al., 2009). ROS induced cytokine production in macrophages (Wen et al., 2011). Indeed, ROS may contribute to TNF-α production enhanced by indoxyl sulfate and transaconite acid. Our results show that inflammatory mediators suppressed the relaxation response through a decrease in contraction components in the distal colon. Although we did not directly observe ACh release from enteric neurons treated with TNF-α or IL-6, recent studies showed a modulatory interaction between inflammatory mediators and enteric neurons (Buckley, O‘Halloran, Rae, Dinan, & O'Malley, 2014; Di Liddo et al., 2015). We offer the following explanation as to why macrophage infiltration occurred only in the distal colon but not in the ileum. Indoxyl sulfate and transaconite acid were produced by altered components of the gut microbiota and affected macrophages in the distal colonic lamina propria to produce TNF-α. TNF-α likely induced inflammatory responses, such as macrophage infiltration.
Patients with renal failure experience changes in the gut microbiota (Vaziri et al., 2013). The gut microbiota largely contributes to the production of various uremic toxins, such as indole derivatives (e.g., indoxyl sulfate) and glycine conjugates (e.g., hippuric acid) (Wikoff et al., 2009). Therefore, altered gut microbiota and/or an imbalance of gut microbiota lead to the accumulation of uremic toxins (Anders, Andersen, & Stecher, 2013; Mishima et al., 2015). In this study, our gut microbiota analysis showed that Allobaculum genus, Bifidobacterium genus, and Turicibacter genus were significantly increased in the 5/6 Nx mice group. In contrast, a Lactobacillus genus, an Oscillospira genus, and an unclassified Ruminococcaceae genus were significantly decreased in the 5/6 Nx mice group. The Lactobacillaceae family including the Lactobacillus genus was reduced in patients with CKD (Sabatino et al., 2015). The Lactobacillus genus is therapeutically used to treat motility disturbances in humans and experimental animals (R.Y. Wu et al., 2013). The luminal administration of a live Lactobacillus genus improves intestinal motility in mice (Wang et al., 2010). It is likely that the decrease in the Lactobacillus genus in the 5/6 Nx mice contributes to CKD-associated intestinal motility dysregulation. This component alteration contributes to the dysregulation of gastrointestinal motility, because this dysregulation and constipation were resolved by antibiotic treatments. The change in the gut microbiota disturbed gastrointestinal motility by influencing the enteric nervous system including enteric neurons, smooth muscle, and pacemaker cells (Kashyap et al., 2013). Moreover, gut microbiota regulate gastrointestinal motility via toll-like receptor (TLR) signaling (Anitha et al., 2012; Brun et al., 2013). TLRs, one family of pattern-recognition receptors, detect microbial components and produce inflammatory cytokines, such as IL-6 and TNF-α (for review see Akira & Takeda, 2004). IL-6 mediates neurotransmitter release from myenteric plexus neurons (Rühl, Hurst, & Collins, 1994). TNF-α changes the electrophysiological properties of myenteric neurons (Rehn, Hübschle, & Diener, 2004). TLRs are expressed in enteric neurons and smooth muscle cells as well as in immune cells in the intestinal mucosa. Moreover, some gut microbiota produce lactic acids, which control gastrointestinal motility (Cherbut, 2003). Increased expression of IL-6 and TNF-α was abolished by antibiotic treatment, suggesting that gut microbiota affect the function of enteric nerves through inflammatory cytokines in the distal colon. In the ileum, it is likely that gut microbiota contribute to the CKD-associated increased contraction response without inflammation because some uremic toxins including spermine are generated from the gut microbiota (Evenepoel, Meijers, Bammens, & Verbeke, 2009).
It is reported that Clostridia induce Treg accumulation in the colon, indicating that there is a positive link between Clostridia and the accumulation of colonic Treg (Atarashi et al., 2011). A significant decrease in the number of Clostridia was observed in the colon of antibiotic-treated mice. In this study, however, the Clostridia in the 5/6 Nx group was similar to that in the sham group (data not shown). Therefore, it is unlikely that 5/6 Nx affects Treg responses.
From a clinical viewpoint, our findings reveal an important insight. In this study, the 5/6 Nx mice showed twofold higher serum creatinine levels than those in the sham mice. Twofold creatinine is equivalent to approximately Stage 3 of CKD, which involves a moderate decrease in kidney function. The twofold increase in creatinine level in the 5/6 Nx mice did not result in a severe decrease in kidney function or kidney failure. Nevertheless, the 5/6 Nx mice experienced a component change in the gut microbiota and motility dysregulation. Regarding the potential benefits to patients, the treatment of gastrointestinal symptoms from the early stage of CKD would be a better strategy to inhibit the development of a complex disease.
In summary, these findings show that renal failure is associated with gastrointestinal disorders through the component change in the gut microbiota and the production of uremic toxins, followed by the alteration of the enteric nervous system. We found the mechanism of motility dysregulation caused by CKD in the ileum and distal colon. Our findings support important evidence for the kidney–gut axis in CKD. Our results provide valuable insight for both renal failure and gastrointestinal disorders.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




