Invasiveness-triggered state transition in malignant melanoma cells
Abstract
Cancer cells are considered to have high morphological heterogeneity in human melanoma tissue. Here, we report that epithelial cancer cells are dominant in different development stages of human melanoma tissues. The cellular and molecular mechanisms that maintain melanoma cells in the epithelial state are further investigated in the A2058 cell line. We find that micropore (8 µm) transwell invasion, but not superficial migration in the scratch assay, can induce remarkable morphological changes between epithelial and mesenchymal melanoma cells within 4 days. The morphological switch is associated with dynamic changes of epithelial–mesenchymal transition (EMT) hallmarks E-cadherin and vimentin. Further immunoflurencent staining and co-immunoprecipitation assay showed the uncoupling of the M3 muscarinic acetylcholine receptor (mAChR) and the p75 neurotrophin receptor (p75NTR) in epithelial melanoma cells. Specific knockdown of M3 mAChR by small interfering RNA (siRNA) significantly abrogates the transition of spindle-shaped mesenchymal cells to epithelial cells. Collectively, we report a cellular model of invasiveness-triggered state transition (ITST) in which melanoma cell invasion can induce morphological changes between epithelial and mesenchymal cells. ITST is one of the biological basis for maintaining metastatic melanoma cells in the epithelial state. Furthermore, M3 mAChR receptor-mediated ITST provides a novel therapeutic strategy to inhibit the development of malignant melanoma.
1 INTRODUCTION
Melanoma is one of the most aggressive skin cancers and highly heterogeneous diseases. The heterogeneity is present not only in different melanoma patients but also in individual cancer cells within the tumor tissue (De Sousa, Melo, Vermeulen, Fessler, & Medema, 2013). Some unusual melanoma variants highly increase the morphological and histopathological heterogeneity of cancer cells (Magro, Crowson, & Mihm, 2006). Epithelial–mesenchymal transition (EMT) is known to be the rate-limiting step during melanoma metastasis (Alonso et al., 2007; Caramel et al., 2013). It is unclear, however, why epithelial melanoma cell conversion to spindle-shaped mesenchymal cell is rare in vivo and metastatic lesions mostly exhibit epithelial phenotypes (Gao et al., 2012). Recent studies in breast cancer and pancreatic cancer demonstrated a lower contribution of EMT in cancer cell invasion and metastasis (Fischer et al., 2015; Zheng et al., 2015). The correlation between cancer cell state transition and melanoma metastasis needs further evaluation.
Muscarinic acetylcholine receptors (mAChRs), M1–M5, were identified in human (Caulfield & Birdsall, 1998). mAChRs are expressed in the majority of cancers derived from epithelial and endothelial cells (Spindel, 2012) and regulate cellular proliferation and cancer progression (Shah, Khurana, Cheng, & Raufman, 2009). Early evidence showed that the A2058 human melanoma cell line expressed M5 receptors (Kohn et al., 1996). Subsequently, monoclonal antibody M35 staining results confirmed primarily the expression of muscarinic receptors in melanoma cells (Boss, Oppitz, Lippert, & Drews, 2005; Lammerding-Köppel et al., 1997; Noda, Lammerding-Köppel, Oettling, & Drews, 1998; Oppitz, Busch, Garbe, & Drews, 2008). Nagy et al. (2011) showed M3 in different melanoma cell lines by both immunocytochemistry and western blot methods (Nagy et al., 2011). Interestingly, Lammerding-Koppel et al. (1997) found different intensities of the muscarinic receptor in melanoma tissue. Single malignant cells displayed only weak or even no staining in the central part of the tumor. In contrast, the muscarinic receptor was highly positive in the pericentral and peripheral zones of melanoma (Lammerding-Köppel et al., 1997). Muscarinic receptors in the leading edges of tumors and in metastases suggested potential roles in melanoma growth or metastasis. Our previous study indicated that muscarinic receptors dynamically changed in different stages of melanoma progression, especially significant in the later stage of metastasis (Wang et al., 2009). The molecular mechanism of the M3 function in melanoma metastasis remains to be determined.
The p75 neurotrophin receptor (p75NTR), also known as CD271, is a low-affinity receptor and plays important roles in skin functions (Pincelli, 2017). There was one report that showed that p75NTR expression on patient melanoma cells was unstable and unlinked to tumorigenicity (Boyle et al., 2016). A series of studies clearly demonstrated the major contribution of p75NTR in human melanoma initiation (Boiko et al., 2010), maintenance of tumorigenicity and stem-like properties (Redmer et al., 2014), and metastasis (Ngo et al., 2016; Radke, Roßner, & Redmer, 2017). Recently, Restivo et al. (2017) identified that dynamic p75NTR expression enabled melanoma cells to grow at low levels, to reduce growth, and invade at overexpression levels. This interesting study indicated that p75NTR was a key effector of phenotype switching in melanoma (Restivo et al., 2017). An early study found that p75NTR represented more than 50% of cells in the spindle cell melanoma specimens. In contrast, p75NTR in epithelioid melanomas was lower than 10% (Iwamoto, Odland, Piepkorn, & Bothwell, 1996). Taken together, these results strongly suggest the roles of p75NTR in regulating melanoma cell morphology and phenotype switching.
These findings led us to hypothesize that the muscarinic receptor and p75NTR might control melanoma state transition during invasion and metastasis. In the current study, we introduce a cellular model of morphological switch during the process of melanoma invasion. Further data shows the uncoupling of M3 mAChR and p75NTR in the morphological transition of melanoma cells. Then, we investigate the effects of specific knockdown of M3 mAChR on the transition of mesenchymal cells to epithelial cells. Our findings show that M3 mAChR receptor-mediated invasiveness-triggered state transition (ITST) is one of the biological basis maintaining melanoma cells in the epithelial state during invasion and metastasis.
2 MATERIALS AND METHODS
2.1 Human tissue specimens
Studies using tissues from human subjects were approved by the Research Ethics Committee of Tangdu Hospital, Fourth Military Medical University. Formalin-fixed paraffin-embedded tumor blocks from patients with melanoma were retrieved from the Department of Dermatology in Tangdu Hospital. Hematoxylin- and eosin-stained sections (5 μm thickness) were reviewed in each case by two pathologists blinded to the study, along with the pathology reports and case histories. The number of mitosis per 40 high-power fields in the primary skin lesion areas was assessed.
2.2 Melanoma cell lines and culture
The melanoma cell lines A2058, WM793B, and 451Lu were obtained from the American Type Culture Collection (ATCC) and cultured according to a previous report (Wang et al., 2009). Briefly, melanoma cells were cultured in RPMI-1640 medium (GIBCO, NY) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin G, and 100 U/ml streptomycin. Cells were maintained at 37°C in 5% CO2.
2.3 Scratch assays
Scratch assays were performed by plating cells in slide chambers coated with 10 μg/ml collagen (Wang et al., 2009). Different cell suspensions in serum-free RPMI 1640 with 10% FBS were applied to chambers and incubated at 37°C in 5% CO2. After cells reached confluency, a scratch was made through the collagen with a plastic pipette tip. After washing with phosphate-buffered saline (PBS), the scratched monolayers of the cells were allowed to heal for 12 hr in RPMI 1640 containing 10% FBS. Photographs of cells invading the scratch were obtained by an IX50 microscope (Olympus, Tokyo, Japan) at different time points.
2.4 Transwell migration assay
Transwell migration assays were performed using an 8 µm pore polycarbonate membrane (3422; Corning). The lower-chamber side filter was coated for 1 hr with 0.1% gelatin B. Typically, 1 × 105 cells were seeded in the upper chamber, which was placed over the lower chamber containing HT1080-conditioned media. Migration was allowed to proceed for different time points (4 hr, 6 hr, 12 hr, 24 hr, 48 hr, 72 hr and 96 hr) at 37°C, and cells remaining on the upper-chamber side of the filter were removed with cotton swabs. The lower surface of the filter was fixed with 70% methanol and stained with 1% crystal violet solution. The number of migrated cells was determined by counting stained cells from multiple randomly selected microscopic visual fields.
2.5 Small interfering RNA (siRNA)-mediated knockdown of M3 mAChR
The sequences of M3 mAChR siRNA were as follows: siRNA 1, 5′-CCUCGCCUUUGUUUCCAAATT-3′, 5′-UUUGGAAACAAAGGCGAGGTT-3′; siRNA 2, 5′-CCUGGUAAUUGUGUCAUUUTT-3′, 5′-AAAUGACACAAUUACCAGGTT-3′; siRNA 3, 5′-GGUCAUCUCCUUUGUCCUUTT-3′, 5′-AAGGACAAAGGAGAUGACCTT-3′. SiRNA were chemically synthesized by Sangon Biotech (Shanghai, China) using standard phosphoramidate chemistry, annealed, and purified by HPLC to a purity of >97%. Lyophilized siRNAs were dissolved in diethyl pyrocarbonate (DEPC)-treated water to a final concentration of 20 nM. Six-well plates were seeded with 1 × 105 cells/well. CHRM3 or control siRNA was added to each well in the presence of OptiMEM medium with a final concentration of 250 nmol/l. The medium was replaced with full medium 4 hr after transfection and the cells were incubated overnight.
2.6 Western blot analysis
A2058 melanoma cells were incubated for 2 days at 37°C in RPMI-1640 medium containing 10% fetal calf serum. Cells were washed with PBS and scraped into ice-cold lysis buffer (20 mM Tris, 150 mM NaCl, 0.5% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM sodium vanadate, 10 mM NaF, 1 mM EDTA, 1 mM Pefabloc) and incubated on ice for 30 min. Lysates were clarified by centrifugation at 10,000g for 20 min at 4°C. Total proteins (50 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 12.5% polyacrylamide gel. The proteins in the gel were transferred to a 0.45-μm nitrocellulose membrane with a Trans-Blot SD semidry electrophoretic transfer cell (BIO-RAD, CA). Membranes were blocked with 10% nonfat dry milk and were probed with the rabbit anti-mAChR M3 antibody (sc-9108; 1:200; Santa Cruz Biotechnology, CA). β-Actin was used as a loading control. After washing the membranes in TBS-T, blots were incubated with anti-rabbit immunoglobulin G (IgG; 7074; 1:2,000; Cell Signaling Technology, MA). The immunoreaction was visualized using the Immobilon Western Chemiluminescent horseradish peroxidase (HRP) Substrate (Millipore, MA) on an X-ray film (Agfa, Antwerp, Belgium).
2.7 Immunofluorescence and confocal microscopy
Immunofluorescent staining was performed in A2058 melanoma cells. The cells were fixed with ethanol/acetic acid. After blocking with 3% bovine serum albumin (BSA; Sigma-Aldrich, MO) for 30 min, coverslips with the cultured A2058 cells were incubated with different primary antibodies, including mouse anti-p75 nerve growth factor (NGF) receptor (ab8877; 1:200; Abcam), rabbit anti-mAChR M3 (sc-9108; 1:200; Santa Cruz Technology, MA), mouse anti-E-cadherin (sc-21791; 1:200; Santa Cruz Technology, CA), mouse anti-desmoplakin (sc-390975; 1:200; Santa Cruz Technology, CA), mouse anti-N-cadherin (sc-393933; 1:200; Santa Cruz Technology, CA), and mouse anti-Vimentin (sc-6260; 1:200; Santa Cruz Technology, CA). Secondary antibodies were Alexa Fluor 488-labeled donkey F(ab′)2 anti-mouse IgG (ab150101; 1:200; Abcam, MA) and Alexa Fluor 647-labeled donkey anti-rabbit IgG (ab150063; 1:200; Abcam, MA). Nuclei were stained with DAPI (1:2,000; Invitrogen, CA). Coverslips were mounted in antifading embedding medium (BioMeda, CA) and the distribution of the signal was studied using a fluorescence microscope.
2.8 Co-immunoprecipitation
Cell extracts were prepared via the lysis of A2058 cells in RIPA buffer (P0013; Beyotime, Shanghai, China) containing certain protease inhibitors for 15 min on ice and clarified via centrifugation. Protein extracts were diluted fourfold with immunoprecipitation (IP) buffer (50 mM Tris–HCl pH 8.0, 0.01% sodium deoxycholate, 150 mM NaCl, 1% Triton X-100, and a complete protease inhibitor mixture). In all, 2 μg of mouse anti-p75NTR antibody (ab8877; 1:500; Abcam) was incubated with the protein extract for 2 hr at 4°C with rotation before the addition of 20 μl of protein G Plus agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. The immunoprecipitates were incubated for 1 hr at 4°C with rotation to capture immune complexes, and then the beads were pelleted via brief centrifugation and washed three times with WIP buffer. Bound proteins were eluted by boiling with 50 μl of SDS-PAGE loading buffer. Protein content was analyzed via western blot analysis and the HRP-linked secondary antibodies were anti-rabbit IgG (7074; 1:2,000; Cell Signaling Technology, MA) and anti-mouse IgG (7076; 1:2,000; Cell Signaling Technology, MA).
2.9 Statistical analysis
Statistical analyses were performed on the mean values of biological replicates in each group using an unpaired two-tailed Student's t test or one-way ANOVA with Tukey's multiple comparisons test using GraphPad Prism (GraphPad, CA), as described in the figure legends. p < 0.05 were considered significant. Error bars depict SEM.
3 RESULTS
3.1 Epithelial cells rather than mesenchymal cells dominate human melanoma tissue
Melanoma cells are highly heterogeneous in tissue sections of melanoma patients in the different stages, including in situ melanoma confined to the epidermis (Figure 1a), superficial spreading melanoma (SSM; Figure 1b), nodular melanomas in the vertical growth phase (VGP; Figure 1c), and highly aggressive melanoma in the lymph node metastases phase (Figure 1d). The cancer cells have high morphological heterogeneity from pagetoid cells in the epidermis to epithelioid melanocytes with abundant cytoplasm, vesicular nuclei often a brisk mitotic activity to spindle-shaped, hyperchromatic melanocytes that may lack pigment production, and to multinucleated melanoma cells with dense nuclear chromatin and unapparent nucleoli. From the primary tumor to distant metastatic stages, however, more than 97% of melanoma cells are epithelial tumor cells, whereas mesenchymal tumor cells are less than 3% (Figure 1e). These results demonstrate that epithelial rather than mesenchymal tumor cells dominate in the development of human melanoma.
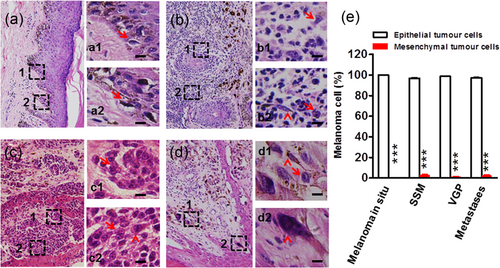
Heterogeneity of melanoma cells in tissue sections of melanoma patients. Representative hematoxylin- and eosin-stained skin melanoma tissue sections showed heterogeneity in different growth phases, including melanoma in situ confined to the epidermis (a) SSM, (b) nodular melanomas in VGP, (c) highly aggressive melanoma in the lymph node metastases phase (insert, metastatic lymph node), (d) the melanoma cells have high morphological heterogeneity in Pagetoid cells within the epidermis (arrows in a1 and a2), epithelioid melanocytes with abundant cytoplasm, vesicular nuclei often a brisk mitotic activity (arrows in b1, b2, c1, c2, d1), spindle-shaped, hyperchromatic melanocytes that may lack pigment production (arrowheads in b2 and c2), and multinucleated melanoma cells with dense nuclear chromatin and unapparent nucleoli (arrowheads in d1 and d2). Scale bar = 200 μm in a, b, c, d; 20 μm in a1, a2, b1, b2, c1, c2, d1, d2, and (e) percentage of epithelial cells and mesenchymal cells in different stages of melanoma cells (n = 4 slices in each stage under 40 high-power fields); ***p < 0.001. Error bars represent means ± SEM. p-Values were obtained with an unpaired t test. SSM: superficial spreading melanoma; VGP: vertical growth phase [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Melanoma cell transwell invasion, but not superficial migration, triggers morphological transition
One possibility of human melanoma in the state of epithelial phenotypes is that cancer cells maintain their original morphology and rarely change during melanoma development. We first compare the cellular morphology of human melanoma tissues (Supporting Information Figure S1a) and cultured cell lines (Supporting Information Figure S1b). The WM793B cell line is established from skin primary SSM. The 451Lu cell line is established from skin nodular melanoma in VGP. These two cell lines are in epithelial states and well mimicked the pathological properties in melanoma patients. The similar morphological states both in vitro and in vivo indicate the poor ability of morphological transition in WM793B and 451Lu melanoma cells. Differently, highly invasive A2058 cell lines derived from lymph node metastases from skin melanoma are present in the spindle-shaped mesenchymal cells. The morphology of this cancer cell line is quite different from the human melanoma tissue, which has a predominantly epithelial phenotype (Figure 1). The results suggest the state transitions during malignant melanoma cells invasion and metastasis in vivo. We further investigate this hypothesis in A2058 cells. In the scratch assay, superficial migration of A2058 cells into the scratch maintained spindle-shaped mesenchymal tumor cells (Figure 2a). However, the original spindle-shaped A2058 melanoma cells showed an epithelioid morphology after vertical invasion through 8 μm-diameter micropores (Figure 2b). The next state transition from epithelial cells to mesenchymal cells lasts for more than 3 days (Figure 2c). Our results strongly indicate that melanoma cell vertical invasion, but not superficial migration, can cause morphological changes between mesenchymal and epithelial cells. ITST might maintain cancer cells in the epithelial state during the early stage of invasion and metastasis.
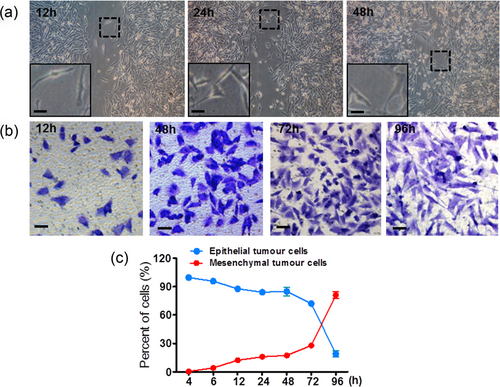
Invasiveness-triggered state transition (ITST) in malignant melanoma cell. (a) In the scratch assay, A2058 melanoma cells migrate; the scratch maintains the spindle-shaped mesenchymal morphology. (b) In the transwell migration assay, A2058 cells transition from spindle-shaped to epithelial cells after transwell micropore invasion. The following state transition occurs from epithelial cells to spindle-shaped mesenchymal cells. (c) Time course of ITST in A2058 melanoma cells. Scale bar = 20 μm [Color figure can be viewed at wileyonlinelibrary.com]
To confirm that the morphological changes were melanoma state transition at the molecular level, we further used the immunofluorescent staining method to detect the expressions of EMT biomarkers, including E-cadherin, desmoplakin, N-cadherin, and vimentin, in A2058 melanoma cells (Supporting Information Figure S2). EMT-related protein E-cadherin is highly expressed in epithelial cells and vimentin is highly expressed in mesenchymal cells. Although desmoplakin and N-cadherin are detectable in A2058 melanoma cells by immunofluorescent staining, the dynamic changes are minimal from epithelial to mesenchymal cells. These results confirm that the morphological transitions during melanoma cancer cells invasion are associated with the dynamic changes of EMT-related proteins.
3.3 Uncoupling of M3 mAChR and p75NTR in the morphological transition of melanoma cells
Our previous study indicated the muscarinic receptors could modulate the functions of different stages of melanoma cells (Wang et al., 2009). To reveal the ITST mechanisms of A2058 melanoma cells, the interactions of M3 mAChR and p75NTR are further investigated. Double immunofluorescence staining showed that M3 mAChR and p75NTR were intensively coexpressed in the cytoplasm and the cell membrane of spindle-shaped mesenchymal cells. However, M3 mAChR not overlapping with p75NTR was present in the cell membrane of epithelial tumor cells (Figure 3a and Supporting Information Figure S3). The results indicate the coupling of M3 mAChR and p75NTR. Therefore, co-IP experiments were conducted to test the interaction. We found three blot bands at about 55, 100, and 250 kD (Supporting Information Figure S4). Except for the known dimers of M3 mAChR (McMillin, Heusel, Liu, Costanzi, & Wess, 2011), the 250 kD band might indicate potential forms other than oligomers and dimers of M3 mAChR. Nevertheless, these results indicate the uncoupling of M3 mAChR and p75NTR in the state transition of melanoma cells.
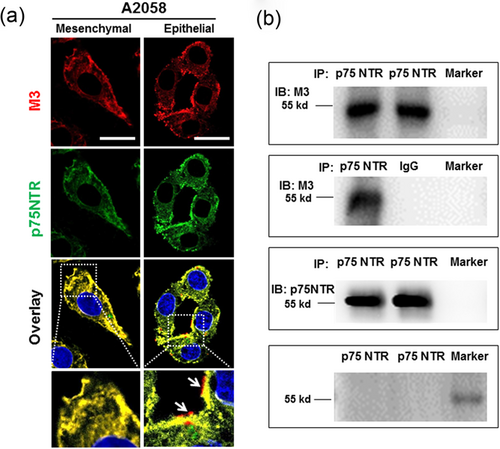
mAChR and p75NTR in the process of ITST in A2058 melanoma cells. (a) Representative confocal images showed the distinguishable expressions of M3 mAChR and p75NTR in spindle-shaped mesenchymal and epithelial cells. Nuclei were visualized with DAPI. Scale bar = 20 μm, (b) Co-IP results showed the interaction between M3 mAChR and p75NTR. PageRuler Plus Prestained Protein Ladder (Thermo Scientific, MA) was used as a marker. Normal mouse IgG was used as a negative control. Western blot source data are shown in Supporting Information Figure 3. IgG: immunoglobulin G, ITST: invasiveness-triggered state transition, mAChR: muscarinic acetylcholine receptor; p75NTR: p75 neurotrophin receptor [Color figure can be viewed at wileyonlinelibrary.com]
To further confirm the contribution of M3 mAChR in melanoma ITST, we designed siRNAs of CHRM3 gene encoding M3 mAChR (Figure 4a). Transwell invasion assay results (Figure 4b) showed that M3 mAChR knockdown markedly decreased the total number of invaded melanoma cells and epithelial cells (Figure 4c). In contrast, the cell number (Figure 4d) and percentage (Figure 4e) of spindle-shaped mesenchymal cell were increased after siRNA treatment. These results support the contribution of M3 mAChR toward the state maintenance of epithelial melanoma cells.
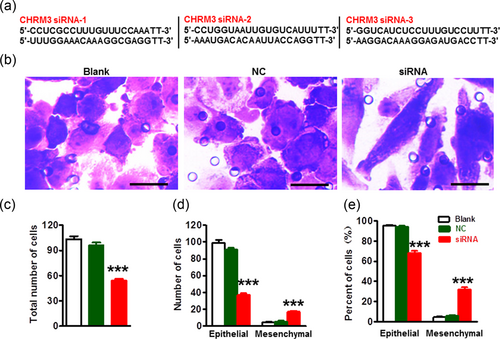
CHRM3 knockdown inhibited ITST of melanoma cells. (a) Sequences were siRNAs targeting the CHRM3 gene, (b) CHRM3 gene knockdown markedly inhibited ITST of A2058 melanoma cells. Scale bar = 20 μm. Quantification of siRNA-mediated knockdown of M3 mAChR on the total number of invaded A2058 melanoma cells (c) and the total number (d) and percentage (e) of epithelial cells and spindle-shaped mesenchymal cells; ***p < 0.001. Error bars represent means ± SEM. Statistical significance was assessed using one-way ANOVA with Tukey's multiple comparisons test. ANOVA: analysis of variance; ITST: invasiveness-triggered state transition, siRNAs: small interfering RNA [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
In the current study, we found a cellular model of ITST in melanoma cells. The model is feasible for the future study of cancer cell state transition without complex exogenous chemical agents. In general, at least two major stages of ITST are involved. In the first stage, invading melanoma cells transit to epithelial cells for about 3 days after vertical invasion. The following stage is the recovering process from epithelial cells to spindle-shaped mesenchymal cells. At present, we still do not know what degree of these two steps in the cultured cells may be reflected in human melanoma tissue. Our data suggested that malignant invading cancer cells tend to maintain the epithelial cell transition during invasion and metastasis into deep tissue. ITST provided one of the biological basis for metastatic lesions of cancer cells mostly exhibiting epithelial phenotypes in patients (Gao et al., 2012). There was one report showing that the EMT transcription factor TWIST actually required intact adherens junctions to mediate local invasion in breast cancer (Shamir et al., 2014). Therefore, transwell micropores might mimic the similar condition of the adherens junctions that would be broken through by invasive cancer cells during melanoma invasion. Recent studies found that EMT was not required for cancer metastasis (Fischer et al., 2015; Zheng et al., 2015). These results suggest that EMT is caused by, but not the reason for, cancer cell invasion and metastasis.
We further found that cancer cell morphological transitions are associated with some EMT-related biomarkers. The immunofluorescent staining results show that E-cadherin is highly expressed in epithelial cells and decreased in mesenchymal melanoma cells. In contrast, vimentin is highly expressed in mesenchymal cells, but not in epithelial melanoma cells. These results support the loss of E-cadherin and gain of vimentin as hallmarks of melanoma EMT (Mitchell, Leone, Feller, Yang, & Mahalingam, 2016; Satelli & Li, 2011; Thiery, 2002). However, EMT biomarkers N-cadherin (Lade-Keller et al., 2013) and desmoplakin (Huber & Petersen, 2015) are not highly expressed in epithelial and mesenchymal A2058 melanoma cells and the switch profiles are subtle. Nevertheless, our results suggest that certain EMT hallmarks, such as E-cadherin and vimentin, are valuable to evaluate morphological transition.
The contribution of p75NTR in melanoma development is not fully understood. Restivo et al. (2017) reported that the cleaved intracellular domain of p75NTR controlled proliferation, whereas the interaction of p75NTR with the NTR TrkA modulated cell adhesiveness through dynamic regulation of a set of cholesterol biosynthesis genes. Interestingly, it was also shown that overexpressing p75NTR could induce cancer cell growth from a spindle to a roundish morphology in the M010817 melanoma cell line (Restivo et al., 2017). In the current study, we further identified the coupling and uncoupling of p75NTR and M3 in melanoma cell morphological transition. Our findings point to another intriguing question on the mechanisms mediating the interactions of p75NTR with membrane receptors during melanoma development.
Here, the ITST in melanoma cells reinforces the concept that the reversible phenotypic switch existed during the growth and metastasis of cancer (Mani et al., 2008; Pinner et al., 2009; Roesch et al., 2010; Quintana et al., 2010). A study showed that single melanoma cells obtained directly from patients that led to the formation of tumors in mice and tumorigenic cells appeared to have unlimited tumorigenic capacity on serial transplantation (Quintana et al., 2010). It would still be interesting to determine whether ITST melanoma cells would acquire or affect the ability of cancer cell tumorigenicity.
In summary, we demonstrate a cellular model of ITST in which melanoma cell invasion can induce morphological changes between epithelial and mesenchymal cells. Furthermore, M3 mAChR contributes to state transition of melanoma cells. At the present stage, our study on the coupling of M3 and p75NTR still has some limitations, and a number of questions remain to be answered. The molecular mechanism of coupling or uncoupling of M3 and p75NTR is unknown. It also needs to be investigated whether the ITST-related invasive mechanism plays roles in the other metastatic cancer cells.
ACKNOWLEDGEMENT
Y. Y. was supported by NSFC grant 81571073, 31871067 and 81102064, the Shaanxi Science Fund for Distinguished Young Scholars 2018JC-014. H. W. was supported by NSFC grant 81102064. The funding organizations played no role in experimental design, data analysis, and manuscript preparation.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.




