Colorectal cancer invasiveness in vitro: Predominant contribution of neonatal Nav1.5 under normoxia and hypoxia
Present address: R. Mine Guzel, Biruni Laboratories, Istanbul, Turkey.
Abstract
Functional expression of voltage-gated Na+ channels (VGSCs) occurs in human carcinomas and promotes invasiveness in vitro and metastasis in vivo. Both neonatal and adult forms of Nav1.5 (nNav1.5 and aNav1.5, respectively) have been reported to be expressed at messenger RNA (mRNA) level in colorectal cancer (CRCa) cells. Here, three CRCa cell lines (HT29, HCT116 and SW620) were studied and found to express nNav1.5 mRNA and protein. In SW620 cells, adopted as a model, effects of gene silencing (by several small interfering RNAs [siRNAs]) selectively targeting nNav1.5 or aNav1.5 were determined on (a) channel activity and (b) invasiveness in vitro. Silencing nNav1.5 made the currents more “adult-like” and suppressed invasion by up to 73%. Importantly, subsequent application of the highly specific, general VGSC blocker, tetrodotoxin (TTX), had no further effect. Conversely, silencing aNav1.5 made the currents more “neonatal-like” but suppressed invasion by only 17% and TTX still induced a significant effect. Hypoxia increased invasiveness and this was also blocked completely by siRNA targeting nNav1.5. The effect of hypoxia was suppressed dose dependently by ranolazine, but its effect was lost in cells pretreated with nNav1.5-siRNA. We conclude that (a) functional nNav1.5 expression is common to human CRCa cells, (b) hypoxia increases the invasiveness of SW620 cells, (c) the VGSC-dependent invasiveness is driven predominantly by nNav1.5 under both normoxic and hypoxic conditions and (d) the hypoxia-induced increase in invasiveness is likely to be mediated by the persistent current component of nNav1.5.
Abbreviations
-
- aNav1.5
-
- “adult” splice variant of Nav1.5
-
- CRCa
-
- colorectal cancer
-
- INaP
-
- persistent current of voltage-gated Na+ channel
-
- INaT
-
- transient current of voltage-gated Na+ channel
-
- nNav1.5
-
- “neonatal” splice variant of Nav1.5
-
- PCR
-
- polymerase chain reaction
-
- TTX
-
- tetrodotoxin
-
- VGSC
-
- voltage-gated Na+ channel
1 INTRODUCTION
The annual incidence of colorectal cancer (CRCa) is expected to increase globally by some 80% (to ~2.2 million) over the next 20 years, with 62% of cases occurring in less-developed countries (Karsa, Lignini, Patnick, Lambert, & Sauvaget, 2010; Torre, Siegel, Ward, & Jemal, 2016). There has also been a trend for CRCa to be diagnosed in younger people and these tend to be late stage (e.g., You, Xing, Feig, Chang, & Cormier, 2012). This is a heterogeneous disease demonstrating varied genetic and epigenetic mechanisms (e.g., Ogino, Chan, Fuchs, & Giovannucci, 2011). Most of the CRCa cases are adenocarcinomas developing in a complex, multistep process known as the “adenoma–carcinoma sequence” (e.g., Fearon, 2011). Major problems remain in clinical management of CRCa, especially for patient subgroups that cannot be treated by surgery alone. This is mainly due to the absence of effective functional biomarkers of disease progression and eventual onset of chemoresistance during available therapies (Van Emburgh, Sartore-Bianchi, Di Nicolantonio, Siena, & Bardelli, 2014). Consequently, novel predictive biomarkers and personalised treatment regimens are urgently needed.
It has been known for some time that several major human carcinomas express functional voltage-gated Na+ channels (VGSCs), which promote their cellular invasiveness in vitro and metastasis in vivo (Campbell, Main, & Fitzgerald, 2013; Driffort et al., 2014; Fraser et al., 2005; House, Vaske, et al., 2010; Laniado, Lalani, et al., 1997; Nelson, Yang, Millican-Slater, & Brackenbury, 2015; Roger, Besson, & Le Guennec, 2003; Yildirim, Altun, Gumushan, Patel, & Djamgoz, 2012). House, Vaske et al. (2010) and House, Wang et al. (2015) initially investigated this phenomenon in human CRCa and showed that the Nav1.5 subtype of VGSC occurred functionally in CRCa cell lines. In biopsies, also, Nav1.5 protein expression was upregulated. Importantly, computational analysis revealed SCN5A (the gene encoding Nav1.5) to be an upstream “key regulator” of CRCa invasiveness, driving a network of canonical genes, including those for Ca2+ signalling, mitogen-activated protein (MAP) kinase and proteases (House, Vaske et al., 2010).
Nav1.5 is developmentally regulated via alternative splicing of exon 6, giving rise to “adult” and “neonatal” forms that differ in the S3–S4 region of domain I by several amino acids (Fraser et al., 2005). This difference enabled a polyclonal antibody (NESOpAb) specific for the neonatal splice form of Nav1.5 (nNav1.5) to be produced (Chioni et al., 2005). In breast cancer, the functional VGSC was shown to be nNav1.5 (Brackenbury, Chioni, Diss, & Djamgoz, 2007; Fraser et al., 2005). This is in line with the expression being “oncofetal” (e.g., Ben-Porath et al., 2008). Originally, House, Vaske, et al. (2010) stated that the Nav1.5 in CRCa was the “adult” form (aNav1.5). Subsequently, nNav1.5 messenger RNA (mRNA) was also shown to be expressed in vitro (Baptista-Hon et al., 2014).
It is well known that an important characteristic of growing tumours is the development of internal hypoxia, which promotes their metastatic potential (e.g., Krishnamachary et al., 2003). This is most apparent as increased invasiveness (e.g., Hongo et al., 2013). However, most work on control of cancer cell behaviour by ion channels has been carried out under normoxic conditions. In particular, the possible involvement of VGSC (nNav1.5) activity in the effects of hypoxia is not known.
The main aims of the current study were (a) to quantify nNav1.5 mRNA and protein expression in several CRCa cell lines, to compare the relative contributions of nNav1.5 versus aNav1.5 (b) to the VGSC current and (c) to the VGSC-dependent control of invasiveness. In addition, (d) we determined the impact of hypoxia on invasiveness and its dependence on nNav1.5. Finally, (e) we evaluated the possible anti-invasive effects of ranolazine, a blocker of hypoxia-associated VGSC activity.
2 MATERIALS AND METHODS
2.1 Cell lines and basal culture conditions
Three different human CRCa cell lines were used: HT29, HCT116 and SW620 (Brattain, Fine, Khaled, Thompson, & Brattain, 1981; Fogh, 1975; Leibovitz et al., 1976). Most of the experiments were carried out on the SW620 cell line derived originally from a lymph-node metastasis and later shown to have “stemness” (Kawamoto et al., 2010; Leibovitz et al., 1976). All cells were cultured in the Roswell Park Memorial Institute formulation 1640 (RPMI 1640) medium (Invitrogen, Paisley, UK) supplemented with 4 mM l-glutamine and 10% foetal bovine serum (FBS; Invitrogen). Culturing was in a humidified incubator at 37°C with 100% relative humidity and 5% CO2 (Fraser et al., 2005). For hypoxia, cells were maintained as above but in 1% O2 for up to 120 hr in a dedicated incubator (Micro Galaxy; RS Biotech Laboratory Equipment Ltd., Irvine, UK).
2.2 Electrophysiology and curve fitting of data
Details of the patch pipettes, solutions and the whole-cell recording protocols were as described previously (e.g., Laniado, Fraser, & Djamgoz, 2001; Laniado, Lalani et al., 1997; Fraser, Grimes et al., 2003; Grimes et al., 1995). In brief, patch pipettes (tip resistances, ~5 MΩ) were filled with a solution designed to block the outwards K+ currents; the composition was as follows: 5 mM NaCl, 145 mM CsCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 11 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), adjusted to pH 7.4 with 1 M CsOH. The estimated intracellular-free Ca2+ concentration was ~15 nM (Laniado, Fraser et al., 2001). Whole-cell membrane currents were recorded from cells that appeared ‘isolated’ in culture, using an Axopatch 200B amplifier (Axon Instruments, San Jose, CA). Analogue signals were filtered at 10 kHz using a low-pass Bessel filter, and series resistance errors were compensated by >90%. Electrophysiological signals were sampled at 50 kHz and digitised using an interface (Digidata 1200; Axon Instruments). Data acquisition and analysis of whole-cell currents were performed using pClamp software (Axon Instruments). A holding potential of −100 mV was applied, unless indicated otherwise. Standard voltage-clamp protocols were used to study the electrophysiological properties of the VGSC currents. There was a noticeable decrease in current amplitude with time in culture, also apparent in immunocytochemistry (Supporting Information Figure S1). All routine recordings were carried out 24 hr after (re)plating and 24 hr of serum starvation (to match the condition of the invasion assays).
2.3 Pharmacology
All pharmacological agents were obtained from Sigma-Aldrich (Poole, UK), except where specified. Tetrodotoxin (TTX; Alomone Labs, Jerusalem, Israel) was prepared as a stock solution of 3,132 µM in the normal culture medium and used at a final working concentration of 20 μM. Since Nav1.5 is TTX resistant and neonatal and adult isoforms do not differ in their TTX sensitivity, this concentration of TTX would block >> 80% of currents generated by either Nav1.5 splice variant (Onkal et al., 2008). Ranolazine was prepared as a stock solution of 2 mM in the normal culture medium and used in the concentration range 1–10 μM. Aconitine, used in preliminary experiments, was prepared in dimethyl sulfoxide (DMSO) at a stock concentration of 100 mM. The final working concentration of 100 μM contained 0.1% DMSO. The control solution was 0.1% DMSO.
2.4 Polymerase chain reactions (PCRs)
- (1)
Neonatal Nav1.5 (SCN5A): 5′-CTGCACGCGTTCACTTTCCT-3′ (F); 5′- GACAAATTGCCTAGTTTTATATTT-3′ (R; J. K. J. Diss, unpublished).
- (2)
General Nav1.5 (SCN5A): 5′-CTGCACGCGTTCACTTTCCT-3′ (F); 5′-CAGCCAGCTTCTTCACAGACT-3′ (R; J. K. J. Diss, unpublished). These targeted the spliced region (DI:S3–S4) encapsulating both nNav1.5 and aNav1.5. The resulting PCR products were sequenced (MWG-Biotech). More details of the primers can be found in Guzel (2012).
- (3)
Control gene-1 (CYB5R3): 5′-TATACACCCATCTCCAGCGA-3′ (F); 5′- CATCTCCTCATTCACGAAGC-3′ (R; Fitzsimmons et al., 1996; Marin et al., 1997).
- (4)
Control gene-2 (SDHA): 5′-TGGGAACAAGAGGGCATCTG-3′ (F); 5′-CCACCACTGCATCAAATTCATG -3′ (R; Jacob et al., 2013).
The mRNA levels were quantified using the comparative method (Livak & Schmittgen, 2001).
2.5 Small interfering RNAs (siRNAs)
- (1)
Control (c-siRNA): AGGUAGUGUAAUCGCCUUG (supplied by Eurofins).
- (2)
Neo1 (n1-siRNA): CUAGGCAAUUUGUCGGCUC (R. M. Guzel, unpublished).
- (3)
Neo2 (n2-siRNA): UAUCAUGGCGUAUGUAUCA (R. M. Guzel, unpublished).
- (4)
NESO (n3-siRNA): GAGUCCUGAGAGCUCUAAA (Brackenbury et al., 2007).
- (5)
ADULT (a-siRNA): GUCUCAGCCUUACGCACCU (R. M. Guzel, unpublished).
Lipofectamine 2000 reagent (Invitrogen) was used as the transfection agent, and the protocol was applied according to the manufacturer’s instructions. Transfection of nontargeting control and targeting siRNAs was performed in parallel, and a final concentration of 40 nM siRNA was achieved for each condition. Electrophysiological recordings of siRNA-treated cells were also performed in parallel at given time points and in random order. For these treatments, recordings were made from 19 to 37 cells from ≥4 different transfections with matching controls.
2.6 Western blot analysis
Cells were washed with phosphate-buffered saline (PBS) containing 0.5 mM NaF, 0.1 mM Na3VO4 (Sigma-Aldrich) and lysed in radioimmunoprecipitation assay (RIPA) buffer (0.5 M Tris–HCl, pH 7.4, 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mM ethylenediaminetetraacetic acid [EDTA]; Merck-Millipore Ltd., Watford, UK) supplemented with an EDTA-free protease inhibitor cocktail (Roche Products Ltd., Welwyn Garden City, UK). Then, cells were vortexed for 30 min at 4°C. The lysate was centrifuged (15,000g) at 4°C and supernatant collected for measurement of the protein concentration by a standard Bradford assay (Bio-Rad protein assay; Bio-Rad). After 15 min at 70°C in sodium dodecyl sulphate (SDS) loading buffer (62.5 mM Tris–HCl, 20% glycerol, 2% SDS, 100 mM dithiothreitol [DTT], 0.0025% bromphenol blue, 10% B-mercaptoethanol; pH 6.8), 50 µg of protein/lane was separated by 7.5% SDS-polyacrylamide gel electrophoresis and electroblotted onto 0.45 µm nitrocellulose membrane (Thermo Fisher Scientific, Waltham, MA). Equal protein loading was controlled by Ponceau S staining (Sigma-Aldrich). Membranes were “blocked” with 5% bovine serum albumin (BSA) in TBS + 0.1% Tween-20 for 1 hr and probed for 20 hr with the following primary antibodies (diluted in TBS + 0.1% Tween-20 + 1% BSA): (a) NESOpAb antibody for nNav1.5 (1 µl/ml) and (b) antiactinin antibody (1 µl/ml) as loading control (Sigma-Aldrich). Secondary antibodies were horseradish peroxidase-conjugated anti-rabbit IgG for (a) and anti-mouse IgG for (b) (Vector Laboratories Ltd., Peterborough, UK). Protein bands were visualised by enhanced chemiluminescence (Fujifilm Imaging Colorants Ltd., Manchester, UK) using Super-Signal West Dura ECL substrate (Thermo Fisher Scientific). Signal intensity of nNav1.5 was normalised to antiactinin and averaged from five independent biological repeats. For each antibody, linearity of signal intensity with respect to protein concentration in the range of 20–80 µg was ensured.
2.7 Immunocytochemistry
This protocol was as described previously (Fraser et al., 2005). The cells were plated on poly-l-lysine-coated (10 μg/ml) coverslips for 24–72 hr before brief fixation (10 min) with 4% paraformaldehyde. The primary antibody was NESOpAb, specific for nNav1.5 (Fraser et al., 2005). The secondary antibody was swine anti-rabbit conjugated to Alexafluor-568 (Invitrogen).
2.8 Cell viability and proliferation
Cellular viability (toxicity) and proliferation were quantified as described previously (Fraser, Ding, Liu, Foster, & Djamgoz, 1999; Fraser, Salvador et al., 2003; Grimes et al., 1995). Briefly, cells were seeded into 35 mm plates at 3.5 × 104/plate (for toxicity) or 24-well plates at 2 × 104/well (for proliferation) and allowed to settle overnight. The cells were incubated under control conditions or treated with the drug, with a change of medium every 24 hr. Cell viability was determined by trypan blue exclusion assay. Proliferation was determined by the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. A “standard curve” was constructed showing linearity over the experimental range. Each biological repeat (treatment and control) was performed in triplicate.
2.9 Matrigel invasion assay
Invasiveness was measured as described before (Fraser et al., 2005), following optimisation (Supporting Information Figure S2). Thus, (a) insert filters (with 8 µm pores) were coated with 50 μl of 0.21 mg/ml of Matrigel (BD Biosciences, Bedford, MA), (b) the chemotactic gradient was 0.1–10% FBS, (c) the cells were serum starved for 24 hr and (d) 105 cells were seeded onto each filter. After 48 hr, the insert was swabbed and then stained with crystal violet. The invaded cells in 12 nonoverlapping fields of view were counted under ×400 magnification. “Invasiveness” was calculated as the number of invaded cells normalised to the largest value observed amongst the different treatment conditions in given experimental sets. Each treatment condition was tested 5–8 times.
2.10 Data analysis
Quantitative data were analysed using the statistical software Origin 8.5 (OriginLab Corporation, Northampton, MA). The Shapiro–Wilk test was used to test for normality. Parametric data are presented as mean ± standard error (SE); nonparametric data are presented as median and 25% and 75% interquartile ranges (data in text) and/or 5% and 95% confidence intervals (data in figures). Data were then analysed by either Student’s t test or Mann–Whitney U test, respectively. Proportion of cells with and without VGSCs was analysed by Fisher’s exact test. Statistical significance is presented as follows: *p < 0.05, **p < 0.01 or ***p < 0.001.
3 RESULTS
Data were obtained from CRCa cell lines mainly under normoxic conditions. Further characterisations were performed under hypoxia.
3.1 nNav1.5 mRNA and protein expression in CRCa cells
Conventional PCRs using nNav1.5-specific primers were performed on the three CRCa cell lines (Figure 1a). The strongly metastatic human breast cancer MDA-MB-231 cells were used as positive control (data not shown; Fraser et al., 2005). nNav1.5 mRNA was detected in all three CRCa cell lines tested (Figure 1a). Compared with HT29 cells, SW620 and HCT116 cells expressed significantly higher levels of nNav1.5 mRNA (Figure 1a). As a confirmation, a further PCR was carried out on SW620 cells using “general” Nav1.5 primers targeting the “spliced” (DI:S3–S4) region (Fraser et al., 2005). When aligned, the sequence of the purified PCR product showed 99% similarity to nNav1.5 and only 88% similarity to the “adult” form (aNav1.5; not shown). nNav1.5 protein expression in the CRCa cells was investigated using the nNav1.5-specific polyclonal antibody, NESOpAb (Chioni et al., 2005). The antibody was first revalidated for its nNav1.5 versus aNav1.5 specificity (Supporting Information Figure S3). Immunoblots showed that nNav1.5 protein (~220 kDa) was present in all three CRCa cell lines, again SW620 and HCT116 cells expressing significantly higher levels (Figure 1b,c). Immunocytochemistry of nonpermeabilised cells also revealed nNav1.5 protein expression in all three CRCa cell lines (Figure 1d). There was some heterogeneity in the immunostaining, but both the cell-surface fluorescence intensity and the percentage of cells stained were highest for SW620 cells (Figure 1e,f). It was concluded that nNav1.5 mRNA and protein expression was a feature of the three CRCa cell lines tested. For the remaining experiments, SW620 cells were adopted as a model for more detailed characterisation.
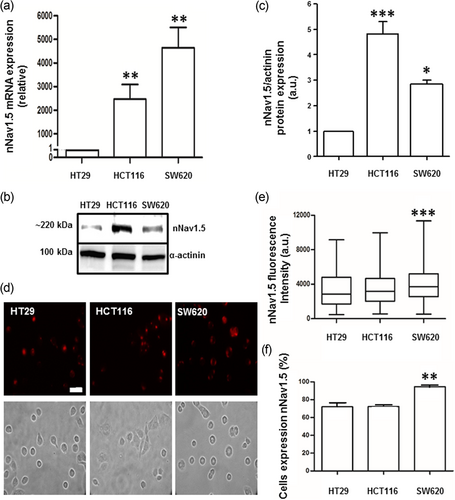
Neonatal Nav1.5 (nNav1.5) mRNA and protein expression in human CRCa cell lines (HT29, HCT116 and SW620). (a) mRNA expression levels of neonatal SCN5A, normalised to SDHA by the 2−ΔΔC(t) method and plotted relative to the level in HT29 cells. Each histobar indicates mean ± SE (n = 6); **p < 0.01. (b) Western blot analyses carried out on total protein (50 µg) from the same panel of CRCa cell lines, using the nNav1.5-specific NESOpAb antibody. Upper bands indicate protein of the expected size (220 kDa). α-Actinin was used for loading control (lower bands). (c) The levels of nNav1.5 protein expression in the three cell lines. Data (mean ± SE; n = 5) are plotted relative to the level in HT29 cells. Statistics: ***p < 0.001 for HT29 versus HCT116, *p < 0.005 for HCT116 versus SW620, p = 0.058 for HT29 versus SW620 (not indicated). (d) Immunocytochemical staining of HT29, HCT116 and SW620 cells using the NESOpAb antibody at 1:100 dilution of a 0.7 mg/ml stock. Cells were not permeabilized. Both immunofluorescence and matching phase contrast images are shown. Scale bar (30 µm) applies to all panels. (e) Immunocytochemistry data quantified as immunofluorescence intensity normalised to cell area. (f) Percentage of cells stained. Error bars represent SE (n = 350 cells for each cell; three independent biological repeats). Statistical significance was determined relative to HT29 cells: **p < 0.01 and ***p < 0.001. CRCa: colorectal cancer; mRNA: messenger RNA [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Electrophysiological effects of differential knockdown of nNav1.5 and aNav1.5
SW620 cells were transfected with siRNAs selectively targeting either nNav1.5 or aNav1.5 (with corresponding controls), and the results were analysed individually and comparatively (Figure 2; Supporting Information Figure S4; Table 1).
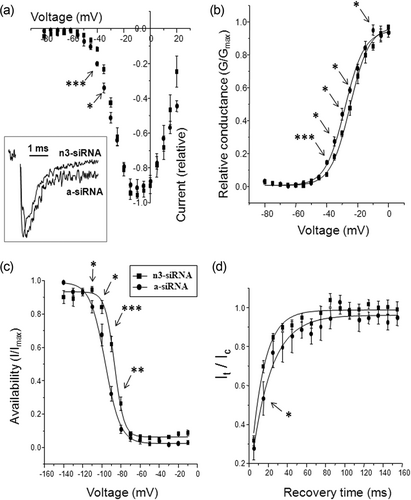
Comparison of electrophysiological effects of treating SW620 cells with siRNAs targeting either nNav1.5 (with n3-siRNA) or aNav1.5 (with a-siRNA). Square symbols: n3-siRNA treatment; circles: a-siRNA treatment (key applies to all parts of figure). (a) Current–voltage relationships. Inset shows normalised representative current traces obtained following treatment with either n3-siRNA or a-siRNA. (b) Conductance–voltage relationships. (c) Steady-state inactivation (“availability”). (d) Recovery from inactivation (It/Ic). For all the data, recordings were from cells following 96 hr of transfection and 24 hr of serum starvation. Each data point denotes mean ± SE (n = 5–9 cells). (All symbols are defined in the text.) Statistical significance between individual n3-siRNA or aNav1.5 data points are given by *p < 0.05, **p < 0.01 and ***p < 0.01. siRNA: small interfering RNA
| Parameter | n3-siRNA | a-siRNA | n | p |
|---|---|---|---|---|
| Activation V1/2 (mV) | −24.7 ± 2.0 | −28.1 ± 1.7 | 5, 5 | <0.05 |
| Activation k (mV) | 6.1 ± 0.3 | 6.7 ± 0.6 | 5, 5 | 0.4 |
| Inactivation V1/2 (mV) | −86.5 ± 1.2 | −95.6 ± 2.1 | 5, 9 | <0.01 |
| Inactivation k (mV) | −5.0 ± 0.4 | −6.7 ± 0.7 | 5, 9 | <0.05 |
| Trecovery (ms) | 12.9 ± 0.6 | 20.9 ± 3.0 | 6, 7 | <0.05 |
| Tpeak (ms) | 0.66 ± 0.04 | 0.90 ± 0.06 | 10, 10 | <0.01 |
| Tinactivation (ms) | 0.80 ± 0.10 | 1.35 ± 0.19 | 9, 10 | <0.01 |
- Note. V1/2: half-(in)activation voltage; k: (in)activation slope factor; Trecovery: recovery from inactivation time constant; Tpeak: time to peak; Tinactivation: inactivation time constant. Data are shown as mean ± SEM. For the number of measurements (n), the first and second values relate to n3-siRNA and a-siRNA, respectively. Unpaired t tests were used to determine statistical “p” values.
- siRNA: small interfering RNA.
Three different siRNAs targeting the nNav1.5 sequence were used. For each siRNA, mRNA levels were compared with cells treated with control c-siRNA 90 hr after transfection. Real-time reverse transcription (RT)-PCRs revealed a significant decrease in nNav1.5 mRNA levels by 50 ± 12%, 85 ± 10% and 32 ± 10% for n1/n2/n3-siRNAs in comparison to the c-siRNA (p < 0.05 for all). A similar trend was observed at protein level by immunocytochemistry (not shown). Patch-clamp recordings confirmed significant reduction in peak VGSC current density for all three siRNAs: 0.0 (0–3.1) cf. 5.0 (2.2–16.8) pA/pF for n1-siRNA (p < 0.001), 0.0 (0–2.4) cf. 5.3 (3.7–10.2) pA/pF for n2-siRNA (p < 0.001) and 1.0 (0–6.8) cf. 8.4 (2.9–13.0) pA/pF for n3-siRNA (p < 0.01), relative to the respective controls. In addition, in all cases, the proportion of cells demonstrating VGSC currents was significantly reduced from 90% to 42% (n1-siRNA), 91% to 33% (n2-siRNA) and 82% to 51% (n3-siRNA; p < 0.01 cf. c-siRNA for all).
Patch-clamp recordings were also performed on SW620 cells transfected with a-siRNA targeting aNav1.5. This had noticeably less effect on the VGSC activity. Relative to controls, there was no significant reduction in peak VGSC current density: 3.5 (0–7.5) cf. 5.3 (0–10.7) pA/pF (p = 0.18). Similarly, the proportion of cells expressing functional channel was not affected (58 cf. 71%; p = 0.43).
We also analysed the comparative effects of the neonatal (n3) versus adult siRNA treatments on additional characteristics of the VGSC current. These analyses demonstrated significant differences in the shifts of all parameters tested (Table 1): (a) current–voltage relationships (Figure 2a), (b) conductance–voltage (G−V) relationships (Figure 2b), (c) steady-state inactivation (Figure 2c) and (d) recovery from inactivation (Figure 2d). In addition, there were significant differences in the effects on half-activation voltage, half-inactivation voltage, inactivation slope factor, recovery from inactivation time constant, time to peak and inactivation time constant (Table 1). In all cases, except one (activation V1/2 for a-siRNA), the directions of the shifts were as expected, that is, n3-siRNA produced more adult-like characteristics and vice versa. The greatest differential shifts (>3-fold) were for time for peak and inactivation time constant, consistent with these parameters being characteristic of nNav1.5 (Onkal et al., 2008).
In overall conclusion, the electrophysiological data taken together confirmed that the functional VGSC expressed in SW620 cells was primarily nNav1.5.
3.3 Effects of differential knockdown of nNav1.5 and aNav1.5 on invasiveness of SW620 cells
Compared with the control c-siRNA transfections, the number of invaded cells with nNav1.5 “silenced” was reduced significantly by all three siRNAs: 64% (n1-siRNA), 45% (n2-siRNA) and 73% (n3-siRNA; p < 0.001 cf. c-siRNA for all; Figure 3a). When a-siRNA was used, there was a much smaller (17%) but significant reduction in invasion (p < 0.05 cf. c-siRNA; Figure 3a). Importantly, subsequent treatment with TTX (20 μM) significantly reduced invasiveness of the cells treated with (i) c-siRNA by 32% or (ii) a-siRNA by 34% (Figure 3b; p < 0.01 for both). There was no difference in the effects of TTX in reducing invasion in cells transfected with c-siRNA or a-siRNA (p = 0.23). In contrast, a similar treatment with TTX had no effect on the cells following silencing of nNav1.5 with n3-siRNA (Figure 3b). None of the treatment conditions had any effect on the cells’ viability or proliferative activity (not shown).
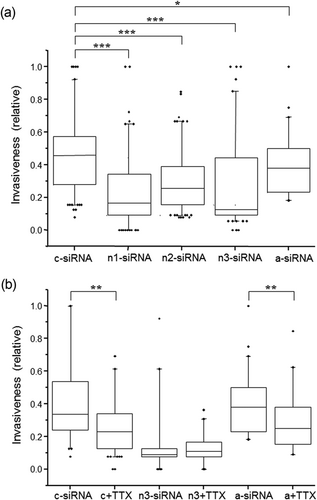
Effects targeting neonatal or adult Nav1.5 with specific siRNAs on Matrigel invasion of SW620 cells with and without TTX cotreatment. Cells were serum starved for 24 hr before treatments and then allowed to invade over 48 hr. Invasiveness is presented as “box plots” relative to the largest value observed between treatment conditions for each individual experiment. (a) Data from cells treated with (i) control siRNA (c-siRNA), (ii) three different siRNAs targeting nNav1.5 (n1,n2,n3-siRNA) and (iii) siRNA targeting “adult” Nav1.5 (a-siRNA). (b) Similar to (a), cells were treated with control siRNA (c-siRNA), control siRNA + 20 μM TTX (c + TTX), siRNA targeting nNav1.5 (n3-siRNA), n3-siRNA with 20 μM TTX (n3 + TTX), siRNA targeting “adult” Nav1.5 (a-siRNA) and a-siRNA + 20 μM TTX (a + TTX). Statistical significance: xp > 0.05, *p < 0.05, **p < 0.01 and ***p < 0.001. Each experiment was performed 4–8 times. siRNA: small interfering RNA; TTX: tetrodotoxin
These results suggested, again, that the VGSC-dependent invasiveness of SW620 cells was driven primarily by nNav1.5 activity. This agreed with the electrophysiological characterisation.
3.4 Effects of hypoxia and ranolazine on invasiveness
We next tested whether the predominance of nNav1.5 would prevail under hypoxia, a condition inherent to growing tumours and known generally to increase invasiveness (e.g., Krishnamachary et al., 2003). Indeed, exposing SW620 cells to hypoxia continuously for 72 hr caused a significant (22%) increase in invasiveness (p < 0.01 cf. normoxia; Figure 4). c-siRNA treatments had no effect under either condition (Figure 4). Interestingly, proliferation was significantly reduced by hypoxia treatment in a time-dependent manner (Supporting Information Figure S5). Thus, as determined by the MTT assay, hypoxia reduced proliferation over 72 hr by 48% (p < 0.05 cf. normoxia). There was no effect on cell viability (not shown). This suggested that the effect of hypoxia on invasiveness was underestimated. The hypoxia-induced increase in invasiveness was completely suppressed following treatment of the cells with n3-siRNA (Figure 4). Importantly, the levels of cellular invasiveness attained under normoxic and hypoxic conditions with nNav1.5 silenced were the same (p = 0.53). In conclusion, the hypoxia-induced increase in invasiveness was driven solely by nNav1.5 activity.
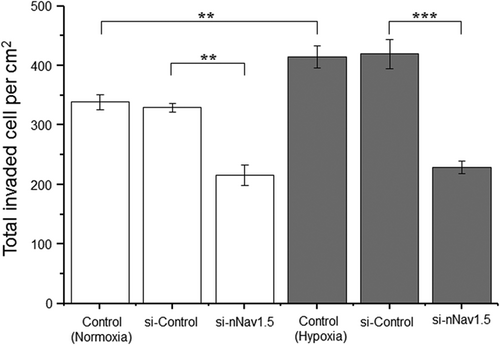
Effect of hypoxia on SW620 cell invasiveness. Cells were serum starved for 24 hr before treatments and then allowed to invade over 48 hr. Data are presented as mean ± SE (n = 5): normoxia (white bars) and hypoxia (grey bars). Hypoxia (1% O2; 72 hr) caused a significant increase in invasiveness compared with the normoxia control. The hypoxia-induced increase in invasiveness was completely suppressed following treatment of the cells with siRNA targeting nNav1.5. Statistical significance: **p < 0.01 and ***p < 0.001. siRNA: small interfering RNA
We then questioned the possible involvement in invasiveness of the channel’s persistent current (INaP), itself known to be promoted by hypoxia (e.g., Ju, Saint, & Gage, 1996). For this, we used ranolazine, a well-known blocker of INaP (e.g., Antzelevitch et al., 2004; Belardinelli, Shryock, & Fraser, 2006). In this set of experiments, we first confirmed that treating the cells with TTX (20 μM) under normoxic conditions caused inhibition of invasiveness (by 37%; p < 0.001 cf. control; Figure 5a). As expected, lowering the concentration of TTX to 1 μM had no effect (not shown). As before, exposing the cells to hypoxia for 72 hr caused a significant increase in invasiveness (by 33%; p < 0.001 cf. normoxia); TTX (20 μM) reduced it by 45% (p < 0.001 cf. hypoxia control; Figure 5a). Under normoxia, ranolazine (5 μM) caused only a small (9%) but significant reduction in invasion (p < 0.01 cf. control; Figure 5a). Under hypoxia, however, the effect of 5 μM ranolazine was significantly increased to 37% (p < 0.001 cf. both 5 μM ranolazine under normoxia and the hypoxia control). Proliferation was not affected by 20 μM TTX or up to 10 μM ranolazine under normoxia or hypoxia (not shown).
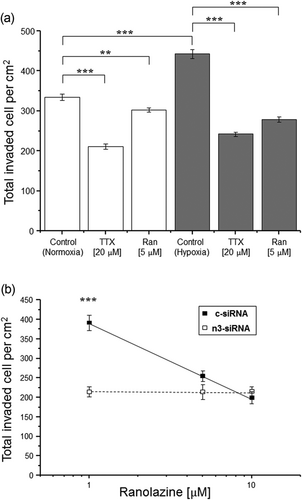
Ranolazine reduced the hypoxia-induced increase in invasiveness in SW620 cells through nNav1.5. (a) SW620 cell invasion (over 72 hr) was significantly reduced by 20 μM TTX and by 5 μM ranolazine, compared with the respective controls. Data are presented as mean ± SE (n = 5): normoxia (white box) and hypoxia (grey box). (b) Dose-dependent inhibition of c-siRNA-treated SW620 cell invasion (over 72 hr under 1% O2) by ranolazine (1, 5 and 10 μM; closed symbols/solid line). The effect of ranolazine was lost in cells transfected with n3-siRNA (open symbols/dotted line). Ranolazine-treated cells (>1 μM) showed significant reduction in invasiveness compared with c-siRNA + 1 μM ranolazine-treated cells. Neither n3-siRNA + 5 μM ranolazine nor n3-siRNA + 10 μM ranolazine-treated cells showed significant reduction in invasiveness compared with the respective controls. Data are presented as mean ± SE (n = 3). Statistical significance: **p < 0.01 and ***p < 0.001. siRNA: small interfering RNA; TTX: tetrodotoxin
We also tested whether the effect of ranolazine under hypoxia was mediated by nNav1.5. In control/c-siRNA-treated cells, ranolazine inhibited invasion in a dose-dependent manner with two-fold difference over 1–10 μM (Figure 5b). In cells pretreated with n3-siRNA, however, invasiveness was suppressed, as shown before, and ranolazine had no additional effect even at the highest concentration (10 μM) used (Figure 5b).
It was concluded that (a) hypoxia promoted invasiveness via nNav1.5 activity and (b) ranolazine blocked this effect.
3.5 Effects on nNav1.5 mRNA and protein expression
Finally, we questioned whether nNav1.5 mRNA and protein expression would change under hypoxia. Under normoxic conditions, neither TTX (20 μM) nor ranolazine (5 μM) had any effect on the mRNA expression (Figure 6a). On the other hand, exposing the cells to hypoxia for 48 hr increased nNav1.5 mRNA expression by 49% (p < 0.01) and both TTX and ranolazine blocked this increase. In contrast, hypoxia had no effect on nNav1.5 protein expression and TTX and ranolazine also had no effect (Figure 6b).

Effects of VGSC inhibition on nNav1.5 mRNA and protein expression in SW620 cells under normoxia and hypoxia. (a) Lack of effect of TTX (20 μM) and ranolazine (5 μM) on nNav1.5 mRNA expression in normoxia. Hypoxia (1% O2 for 48 hr) significantly increased nNav1.5 mRNA expression. This was inhibited by the same treatments with TTX and ranolazine, as above. Data are presented as mean ± SE (n = 5): normoxia (white box) and hypoxia (grey box). Statistical significance: ***p < 0.001. (b) Lack of effect of hypoxia and cotreatment with TTX (20 μM) or ranolazine (5 μM) on nNav1.5 protein expression. Typical western blot analyses are shown, using the nNav1.5-specific NESOpAb antibody (~220 kDa) and α-actinin as loading control (~100 kDa). Quantitative data are presented as mean ± SE (n = 4): normoxia (white box) and hypoxia (grey box). mRNA: messenger RNA; TTX: tetrodotoxin; VGSC: voltage-gated Na+ channel
4 DISCUSSION
The main results were as follows: (1) nNav1.5 mRNA and protein were expressed commonly in all three CRCa (HT29, HCT116 and SW620) cell lines tested. Expression levels were generally higher in the SW620 cells and comparable to the MDA-MB-231 cells also expressing nNav1.5 functionally. (2) Electrophysiology revealed that silencing nNav1.5 caused shifts towards aNav1.5-like characteristics and vice versa. However, the effect of silencing nNav1.5 was significantly greater. (3) Three different siRNAs suppressing nNav1.5 expression in SW620 cells all reduced Matrigel invasiveness. (4) TTX had no effect on invasiveness of cells pretreated with siRNA targeting nNav1.5. (5) Silencing aNav1.5 also caused an apparent inhibition of invasiveness. In contrast to nNav1.5, however, TTX still significantly reduced invasiveness in cells treated with aNav1.5 siRNA. (6) Hypoxia increased cellular invasiveness. This effect was lost in cells treated with nNav1.5 siRNA. (7) Ranolazine significantly reduced invasiveness much more under hypoxia than normoxia, but had no effect on invasiveness in hypoxic cells pretreated with siRNA targeting nNav1.5. (8) During hypoxia, nNav1.5 mRNA expression increased (blocked by ranolazine), but there was no effect at protein level.
4.1 Prevalence of nNav1.5 expression in CRCa cells
Conventional PCRs performed with specific primers detected nNav1.5 mRNAs in all three CRCa cell lines tested. Primers targeting the developmentally regulated DI:S3–S4 region of Nav1.5 and sequencing the PCR products confirmed that nNav1.5 mRNA was present. A polyclonal antibody, NESOpAb, that specifically recognises nNav1.5 protein with high selectivity over its “nearest neighbour,” aNav1.5, was produced earlier (Chioni et al., 2005) and revalidated. Furthermore, NESOpAb would bind to an external epitope thus enabling expression in plasma membrane to be assessed in nonpermeabilized cells. Western blot analyses and immunocytochemistry using NESOpAb showed that nNav1.5 protein was also present in all cell lines. Expression was generally higher and more consistent in the metastatic/poorly differentiated SW620 and HCT116 cell lines compared with the relatively differentiated HT29 cells. This was especially so for the immunocytochemistry data, which showed higher levels of nNav1.5 protein in the plasma membrane, where the channel would be functional. However, some inconsistency was noted between the mRNA/protein levels in the cell lines. Such mismatch between mRNA/protein levels has been reported previously, including in cancer cells (e.g., Tian et al., 2004; Zhang et al., 2014). Overall, these results extend the results of House, Vaske, et al. (2010) and Baptista-Hon et al. (2014). In human breast cancer cells also, nNav1.5 was found to be expressed and dominant (Brackenbury et al., 2007; Fraser et al., 2005). The expression of a “neonatal” splice variant fits well with the phenomenon of embryonic gene expression in cancer (e.g., Ben-Porath et al., 2008).
Treatments of SW620 cells with three different siRNAs targeting nNav1.5 (n1/2/3-siRNAs) all resulted in significant reduction of nNav1.5 mRNA levels, compared with the control treatment (c-siRNA). Concurrently, both the proportion of cells expressing functional nNav1.5 and the associated current density were reduced significantly. In contrast, the quantitative effects of silencing aNav1.5 were significantly less. Comparing the residual inwards currents in cells treated with n3-siRNA versus a-siRNA revealed significant differences in the shifts of the following characteristics: (a) current–voltage relationship, (b) voltage dependence of conductance, (c) steady-state inactivation and (d) recovery from inactivation. In addition, there were significant differences for half-activation voltage, half-inactivation voltage, inactivation slope factor, recovery from inactivation time constant, time to peak and the inactivation time constant. These effects agree generally with the reported differential characteristics of nNav1.5 and aNav1.5 (Onkal et al., 2008). Taken together, these analyses suggested that nNav1.5 made by far the greatest contribution to the VGSC current in the SW620 cells.
4.2 Control of invasiveness predominantly by nNav1.5
The three siRNA treatments targeting nNav1.5 suppressed invasiveness of the SW620 cells by 45–73%, comparable to the effect of TTX. Indeed, when the n3-siRNA transfected cells were additionally treated with TTX, there was no further decrease in invasion. In agreement with the electrophysiology, therefore, it was concluded that nNav1.5 was predominant in controlling the VGSC-dependent component of invasiveness. However, a-siRNA also had an inhibitory effect on invasiveness, consistent with the presence of some functional aNav1.5 as seen in the differential electrophysiological effects of a-siRNA versus n3-siRNA (Figure 3). The size of the a-siRNA effect on invasiveness (17%) was rather surprising considering that the corresponding inhibition of current density (as well as the percentage of cells expressing functional channel) was, in fact, nonsignificant. This could indicate that the relationship between VGSC expression/activity and its contribution to invasiveness is steep (Djamgoz, 2011). Consistent with this notion, it was shown previously that ~30% reduction in nNav1.5 protein expression was sufficient to eliminate the VGSC-dependent invasiveness of breast cancer MDA-MB-231 cells where, similarly, nNav1.5 is dominant (Brackenbury et al., 2007).
Interestingly, the specific neonatal nature of the VGSC may not be essential for its contribution to invasiveness. Such a splice variant could just be a by-product of the overall dedifferentiation process inherent to cancer. Even the subtype of VGSC expressed may not be significant, reflecting merely tissue specificity of expression. This point was demonstrated directly in a study on nonmetastatic prostate cancer cells, in which overexpression of a “nondominant” VGSC (Nav1.4 rather than the dominant Nav1.7) was found to be “necessary and sufficient” for invasiveness (Bennett, Smith, & Harper, 2004). Instead, it may be the influx of Na+ that the channels mediate that is important (Brackenbury & Djamgoz, 2006). This agrees with previous work showing that VGSC “openers” (e.g., aconitine and veratridine) increase metastatic cell behaviours, including invasiveness (Fraser, Salvador et al., 2003; Fraser et al., 2005; House, Wang, et al., 2015). Furthermore, the Na+ content of tumour cells and tissues is known generally to be higher than normal tissues (e.g., Ouwerkerk et al., 2007; Roger, Rollin et al., 2007).
Finally, the pathway(s) through which nNav1.5 activity enhances invasiveness need further study. House, Vaske et al. (2010) revealed SCN5A (the gene encoding Nav1.5) to be an upstream “key regulator” of a network of genes, including those for Ca2+ signalling, MAP kinase and proteases. In addition, evidence from breast cancer, where nNav1.5 has been more extensively studied, has shown channel activity to be linked to pericellular acidification and activation of cathepsin B and MMP9 (Gillet et al., 2009; Nelson et al., 2015). For CRCa, it may be worth exploring the link between nNav1.5 activity and MMP7 expression, since earlier research has shown positive correlation between increased MMP7 expression and tumour invasion in HCT116 and SW620 cell lines compared with the HT29 cell line (Banskota, Regmi, & Kim, 2015).
4.3 Hypoxia-induced increase in invasiveness: Control by nNav1.5 and inhibition by ranolazine
The effects of hypoxia on CRCa cell behaviour appear to be cell-type dependent (Tatrai et al., 2017). As regards invasiveness, most evidence indicates an enhancement (e.g., Hongo et al., 2013). This was also the case here. Thus, exposing SW620 cells to hypoxia significantly increased their invasiveness. It is of interest to note that normal colorectal tissue may only be at 6.8% O2 and CRCa cells are exposed to 2–4% O2 (Mckeown, 2014). It could be argued, therefore, that the “hypoxia” induced by 1% O2 in our experiments might actually correspond to the basal tumour condition, whilst the “atmospheric” (ca. 20%) O2 in the normal cultures represents “hyperoxygenation.” Nevertheless, subjecting the SW620 cells to relative hypoxia (i.e., ~20% to 1%) enhanced their invasiveness and this was blocked completely by pretreatment with n3-siRNA suggesting that it was nNav1.5 that was the underlying VGSC. Thus, the predominance of nNav1.5 in controlling SW620 invasiveness that was demonstrated under normoxia was maintained under hypoxia.
The effect of hypoxia on the invasiveness of SW620 cells was also suppressed by ranolazine. Thus, treatment of the cells with 5 μM ranolazine inhibited invasion significantly by 37%. Importantly, this effect of ranolazine was lost after silencing nNav1.5 demonstrating that the key role played by nNav1.5 in promoting invasiveness manifests itself also in pharmacological control. Ranolazine has been shown independently to block the hypoxia-induced persistent current, INaP, of VGSC, especially Nav1.5, with an IC50 of 5.9 µM (Antzelevitch et al., 2004). Taken together, our results are consistent with INaP mediating the hypoxia-induced increase in invasiveness. Under normoxia, however, the effect of ranolazine was much smaller than the effect of TTX (9% vs. 37%). This would imply that the transient current (INaT) of the VGSC made the significant contribution to invasiveness. More research is required to understand the differential proinvasive roles of these separate current components under normoxia versus hypoxia. Interestingly, hypoxia concurrently promoted nNav1.5 mRNA (but not protein expression) and this was also inhibited by ranolazine. It would appear, therefore, that further effects of hypoxia may occur in the longer term.
4.4 Future perspective
Our results and other available evidence support the case for nNav1.5 being a viable functional biomarker and target for managing invasive CRCa. First, as suggested initially by House, Vaske et al. (2010), it is expressed early in metastasis and is upstream of several canonical signalling mechanisms of invasiveness, consistent with its pathophysiology. Approximately 50% of CRCa patients relapse, some with distant metastases, even after surgery and/or chemotherapy and, thus, earlier diagnosis is vital (Young et al., 2014). Second, as a neonatal splice variant, it has the potential to be “cancer specific” in the adult body and can be targeted with antibody, for both diagnosis and therapy (Brackenbury et al., 2007; Chioni et al., 2005; Yamaci et al., 2017). In this light, a recent study has reported that high Nav1.5 expression levels correlated with unfavourable disease-free survival in patients with nonmetastatic CRCa (Peng et al., 2017). Third, finally, ranolazine has been shown in this study to reduce CRCa cell invasiveness at clinical doses (<10 μM) and was found previously to reduce metastatic dissemination in a breast cancer xenograft model (Driffort et al., 2014). Accordingly, ranolazine and other available VGSC blockers could be “repurposed” as novel antimetastatic drugs (Djamgoz & Onkal, 2013; Koltai, 2015; Nelson et al., 2015).
ACKNOWLEDGEMENTS
We are grateful to the Pro-Cancer Research Fund (PCRF) and the Robert Luff Foundation Ltd. for support (M. B. A. D. and S. P. F.).
CONFLICTS OF INTEREST
M. B. A. D. is involved in a spinout company (Celex Oncology Ltd.) focused on ion channels and cancer.




