USP49 inhibits ischemia–reperfusion-induced cell viability suppression and apoptosis in human AC16 cardiomyocytes through DUSP1–JNK1/2 signaling
Abstract
Dual-specificity protein phosphatases (DUSP) also known as mitogen-activated protein kinase (MAPK) phosphatases (MKPs) can dephosphorylate MAPKs, including extracellular signal-regulated kinase, c-Jun N-terminal kinase (JNK), and p38. DUSP1-mediated JNK dephosphorylation has been found to play an antiapoptotic role against cardiac ischemia–reperfusion (I/R) injury. However, the regulation of DUSP1–JNK pathway remains unclear. In the current study, ubiquitin-specific peptidase 49 (USP49) expression in human AC16 cardiomyocytes following I/R injury was measured by real-time polymerase chain reaction and western blot analysis. Cell viability, apoptosis, the Bax, Bcl-2, and DUSP1 expression, and the activity of MAPKs in AC16 cardiomyocytes following indicated treatment was measured by CCK-8, flow cytometry, and western blot analysis. The direct interaction between USP49 and DUSP1 was measured by coimmunoprecipitation and ubiquitination analysis. The effect of USP49 on apoptosis and JNK activity in rat cardiomyocytes following I/R injury was also measured by TUNEL and western blot analysis. Here, we found that USP49 expression was time-dependently increased in AC16 cardiomyocytes following I/R. I/R-induced cell apoptosis and JNK1/2 activation both in in vivo and in vitro reversed by USP49 overexpression in AC16 cardiomyocytes. Inhibiting JNK1/2 activation significantly inhibited USP49 knockdown-induced the cell viability inhibition, apoptosis and the JNK1/2 activation in AC16 cardiomyocytes. Moreover, USP49 positively regulated DUSP1 expression through deubiquitinating DUSP1. Overall, our findings establish USP49 as a novel regulator of DUSP1–JNK1/2 signaling pathway with a protective role in cardiac I/R injury.
1 INTRODUCTION
Acute myocardial infarction is a major cause of death in cardiovascular diseases (Zuhaid et al., 2014). Timely opening the artery with infarct and restoring the blood supply of ischemic myocardium is the key to rescue the death of myocardium. However, reperfusion for a period of time can aggravate the injury in the structure and function of myocardium and cause myocardial stunning, cardiac dysfunction and malignant arrhythmia, which are known as I/R injury (Siu, Lotz, Ping, & Cai, 2015; H. Wu et al., 2015). At present, I/R injury is still a major problem in the process of heart ischemia and has attracted more and more attention. The pathophysiological process of I/R injury is complex, including autophagy, calcium overload, energy metabolism disorder, inflammatory cell infiltration, oxidative stress, myocardial cell apoptosis, vascular endothelial dysfunction, and no vessel reflow caused by microcirculation obstruction (Kaplan, O’Connor, Hake, & Zingarelli, 2005; White, Giordano, & Anantharamaiah, 2016). The occurrence of apoptosis in myocardial I/R injury may be related to the oxygen free-radical production, calcium overload and mitochondrial damage, which directly or indirectly activate apoptosis signaling pathway and regulate apoptosis-related gene expression. Abnormal cell apoptosis is involved in the pathophysiological abnormalities of the cardiovascular system (Prescimone et al., 2013). The imbalance of apoptosis is related to the mechanism of many stress responses and the accumulation of myocardial apoptosis and the gradual decrease of cell numbers result in the incomplete development of heart function (Ge et al., 2014).
Mitogen-activated protein kinase (MAPK) signaling pathway, such as extracellular signal-regulated kinase (ERK)1/2, c-Jun N-terminal kinase (JNK)1/2, and p38, participates in the apoptosis, cell cycle, cell growth, and differentiation through transmitting extracellular signals into the cell (Sun et al., 2015). ERK1/2 activation can protect myocardium against I/R injury by its antiapoptotic role and JNK1/2 promotes I/R injury-induced cardiomyocyte apoptosis (Lin et al., 2011; H. Xu et al., 2011). JNK1/2 and ERK1/2 are mediated by MAPK kinases such as dual-specificity protein phosphatase 1 (DUSP1), DUSP2, DUSP4, and DUSP16 that regulate the activity of each other in a step-by-step fashion (Fey, Croucher, Kolch, & Kholodenko, 2012). DUSP4/16-mediated ERK1/2 activation and DUSP2-mediated JNK1/2 inactivation were associated with myocardial I/R injury (T. Xu et al., 2014). DUSP1 also known as MAP kinase phosphatase 1 (MKP-1) regulates cell growth, apoptosis, and chemoresistance through dephosphorylation of JNK1/2 (Feng et al., 2016; Rincon et al., 2016). DUSP1 was downregulation in H9C2 cells and in the infarct area of rats following cardiac I/R injury, and DUSP1 alleviated cardiac I/R injury via the JNK1/2 signaling pathway (Jin et al., 2018). However, the regulation of DUSP1–JNK1/2 signaling in I/R injury is largely unknown.
E3 ubiquitin ligase is an important ubiquitin molecule that can promote proteasome-mediated protein degradation through ubiquitination or affect the activity of substrate proteins through regulating the interaction between substrate proteins and other proteins (Ci et al., 2018; Y. Xie et al., 2013). Therefore, it plays an important role in the inflammation, cell growth, proliferation, apoptosis, and survival (Ashida et al., 2010; Bernassola, Karin, Ciechanover, & Melino, 2008). Increasing evidence have been shown that the reversal of ubiquitination modification mainly accomplished by deubiquitinating enzymes (DUBs) plays a key role in intracellular signal transduction. Ubiquitin-specific protease 18 (USP18) is a DUB induced in liver following hepatic I/R injury and enhances lymphocytic choriomeningitis replication (MacParland et al., 2016). USP14 is associated with the cerebral I/R-induced neuronal injury (Min, Lu, Freeling, Martin, & Wang, 2017), and USP14 and USP4 suppress the JNK1/2 activation (Vaden et al., 2015; Zhao et al., 2018).
Except for the report suggesting USP49 deubiquitinates histone H2B, p53, and FKBP51, regulates DNA-damage response, cotranscriptional pre-messenger RNA (pre-mRNA) splicing, chemoresistance, and tumorigenesis (Tu et al., 2018), the cellular function of USP49 remains largely unknown. In this study, our data demonstrated that the expression of USP49 was time-dependently decreased in human AC16 cardiomyocytes following I/R injury. USP49 overexpression inhibited I/R-induced cell viability reduction, apoptosis, and JNK1/2 activation. JNK1/2 inhibitor SP600125 treatment inhibited USP49 silencing-induced cell viability reduction, apoptosis, and JNK1/2 activation. USP49 promoted DUSP1 protein expression through deubiquitinating DUSP1 suggesting USP49 inhibits cardiac I/R-induced injury in AC16 cardiomyocytes through DUSP1–JNK1/2 signaling.
2 MATERIALS AND METHODS
2.1 Cell culture
Human AC16 cardiomyocytes purchased from American Type Culture Collection (ATCC, Rockville, MD) were seeded in gelatin-coated six-well plates, cultured in DMEM/F-12 medium (Hyclone, Logan, UT) supplemented with 12.5% fetal calf serum (Hyclone) and 1% penicillin–streptomycin solution (100×; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), and incubated in an incubator containing 5% CO2 at 37°C.
2.2 Cell transfection
AC16 cardiomyocytes were cultured in six-well plates for 24 hr and then transfected with three small interfering RNA (siRNAs; siRNA-1, position 105–123, 5′-GGATAGATGCAAACATGTA-3′; siRNA-2, position 343–361, 5′-GACTACGTGCTCAATGATA-3′; siRNA-3, position 1705–1723, 5′-GAAGCTAGAAAGCAGTTAA-3′) targeting human USP49 (NM_001286554.1) or siRNA negative control (siNC) by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the instruction of the manufacturer for 48 hr. USP49 overexpression lentiviral vector was constructed by integrating the coding sequence (CDS) of USP49 into pLVX-Puro (Clontech, Mountain View, CA). he primers used to synthesize the CDS were as follows: forward, 5′-GCGAATTCATGGATAGATGCAAACATGTAGGGC-3′; reverse, 5′-CGGGATCCTCAGGAAAATGTCTGTGGTCTGC-3′. 293 T cells were transfected with pLVX-Puro-USP49 or blank pLVX-Puro lentiviral vector for 4–6 hr using Lipofectamine 2000 according to the manufacturer’s instruction. Viruses were collected 48 hr posttransfection and infected AC16 cardiomyocytes. The blank pLVX-Puro (vector) was used as negative control.
2.3 In vitro I/R model
AC16 cardiomyocytes underwent simulated ischemia for 3 hr by replacing the cell medium with serum-free DMEM/F-12 and incubating in an incubator containing 5% CO2 and 1% O2 at 37°C, and reperfusion was done under normoxia conditions using DMEM/F-12 medium containing 0.1 mg/ml of bovine serum albumin (Thermo Fisher Scientific, Waltham, MA) for 6, 12, and 24 hr.
2.4 CCK-8
AC16 cardiomyocytes were seeded in 96-well plates (3 × 103 cells/well). Then, the wells were divided into seven groups: AC16 cardiomyocytes cultured in normal medium (control), AC16 cardiomyocytes following I/R (I/R), AC16 cardiomyocytes transfected with blank pLVX-Puro vector following I/R (I/R + blank vector), AC16 cardiomyocytes transfected with pLVX-Puro-USP49 vector following I/R (I/R + USP49), AC16 cardiomyocytes transfected with siNC, AC16 cardiomyocytes transfected with USP49-siRNA (siRNA), AC16 cardiomyocytes transfected with USP49-siRNA and treated with 1 μM SP600125 (siRNA + SP600125). After cultured for 0, 24, 48, and 72 hr, CCK-8 solution (Dojindo Laboratories, Kumamoto, Japan) was added to each well. Optical density value at 450 nm was detected using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA) to evaluated cell viability.
2.5 Flow cytometry
AC16 cardiomyocytes were seeded in six-well plates (5 × 105 cells/well) for 24 hr until 50% confluent. Then, the wells were divided into seven groups as previously described in CCK-8 analysis. After transfected for 48 hr and simulated ischemia for 3 hr and reperfusion for 24 hr, AC16 cardiomyocytes were centrifugated at 1000g for 5 min and incubated with 5 μl Annexin V-FITC for 15 min and 5 μl propidium iodide for 5 min at 4°C. The cell apoptosis was assayed on flow cytometer (FACSCalibur; Becton-Dickinson, San Joes, CA).
2.6 In vivo myocardial I/R model
Sprague-Dawley rats (weight 260–310 g, 13–15-week-old) were anesthetized by intraperitoneal injection, followed by myocardial I/R injury or sham operation as previously described (Song et al., 2014; Y. Zhang et al., 2011). Myocardial I/R injury in rats was completed by coronary artery ligation. After occlusion for 30 min, the suture was loosened and the myocardium was reperfused for 24 hr. Rats in sham operation without coronary artery ligation. When the experiment ended, rats got euthanasia by cervical dislocation and the myocardia were collected and the H&E and TUNEL staining were performed as previously described (D. W. Luo et al., 2015; Qu, Zhang, & Wu, 2015). All procedures were performed in accordance with China Animal Welfare Legislation. Protocols were approved by the Ethics Committee of Shanghai Tenth People’s Hospital.
2.7 Real-time polymerase chain reaction
Total RNA was isolated and purified using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. RNA was reverse transcripted using a complementary DNA synthesis kit (Thermo Fisher Scientific, Saint Louis, MO). mRNA expression was assessed using an Applied Biosystems (Thermo Fisher Scientific) Prism 7300 sequence detection system with Maxima SYBR Green/ROX qPCR Master Mix (Thermo Fisher Scientific) according to the manual, using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal normalized reference. The primers used were as follows: USP49 (NM_001286554.1), forward: 5′-TCCCACAAAGGAAGTAACC-3′ and reverse: 5′-TATGACAGCAGCAAGTAGG-3′; GAPDH (NM_001256799.2), forward: 5′-AATCCCATCACCATCTTC-3′ and reverse: 5′-AGGCTGTTGTCATACTTC-3′. The relative quantification was calculated using cycle threshold method.
2.8 Western blot analysis
Protein samples were extracted from AC16 cardiomyocytes or rat myocardia by radioimmunoprecipitation lysis buffer (Solarbio) and quantified using a BCA Protein Assay Kit (Thermo Fisher Scientific). Target proteins were transferred to nitrocellulose membranes (Millipore, Burlington, MA) after separated with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Protein bands were incubated with primary antibodies and paired secondary antibodies. Tanon-5200 Imaging System (Tanon, Shanghai, China) was used to analyze the protein levels. The antibodies used were as follows: USP49 (18066-1-AP, diluted 1:500; Proteintech Group, Inc., Rosemont, IL), DUSP1 (M02276, diluted 1:500; Bosterbio, Pleasanton, CA), Bax (ab32503, diluted 1:1000; Abcam, Cambridge, MA), Bcl-2 (ab32124, diluted 1:1000; Abcam), p-ERK1/2 (#4370, diluted 1:1000; Cell Signaling Technology, Inc., Danvers, MA), ERK1/2 (#4695, diluted 1:1000; Cell Signaling Technology), p-JNK1/2 (#9251S, diluted 1:1000; Cell Signaling Technology), JNK1/2 (#9252, diluted 1:1000; Cell Signaling Technology), p-p38 (#9211, diluted 1:1000; Cell Signaling Technology), p38 (#9212, diluted 1:1000; Cell Signaling Technology), and GAPDH (#5174, diluted 1:2000; Cell Signaling Technology). The membrane was visualized using the ECL kit (Pierce Chemical Co., Rockford, IL) and signals determination used software of the ImageJ software version 1.46 (National Institutes of Health, Bethesda, MD).
2.9 Enzyme-linked immunosorbent assay
The serum levels of interleukin (IL)-1β (R&D Systems, Inc., Minneapolis, MN), IL-6 (R&D Systems, Inc.), TNF-α (R&D Systems, Inc.), creatine kinase-muscle/brain fraction (CK-MB; X-Y Biotechnology, Shanghai, China), and cardiac troponinI (cTnI; Yuanmu Biotechnology, Shanghai, China) in rat were determined by ELISA method following the instructions of ELISA kits.
2.10 Coimmunoprecipitation and ubiquitination analysis
Briefly, cold phosphate-buffered saline (PBS) solution was used to wash the cells for three times, and the cells were scraped into lysis buffer containing complete protease inhibitors and centrifuged at 14,000g for 20 min at 4°C. The supernatants were incubated with anti-USP49 (1:1000; Proteintech), anti-DUSP1 (1:1000; Bosterbio) or normal IgG (1:1000; Abcam) antibody, and the immunocomplexes were then associated with protein A-sepharose (Roche Molecular Biochemicals, Mannheim, Germany). Anti-USP49 (1:1000; Proteintech), anti-DUSP1 (1:1000; Bosterbio) and antiubiquitin (1:2000; Abcam) antibody were used for western blot analysis.
2.11 Statistical analysis
All values were expressed as means ± SD. All experiments and measurements were performed at least in triplicate. Statistical analyses were evaluated with the GraphPad Prism software Version 6.0 (San Diego, CA) using the one-way analysis of variance followed by Tukey’s post hoc comparisons. The level p < 0.05 was considered as the cut-off value for significance.
3 RESULTS
3.1 USP49 expression in AC16 cardiomyocytes following I/R injury
To investigate the function of USP49 in cardiac I/R injury, the USP49 expression in AC16 cardiomyocytes was detected. The results showed that AC16 cardiomyocytes following I/R injury led to a time-dependent decrease in the expression of USP49. These data suggest that USP49 may associate with I/R injury in AC16 cardiomyocytes (Figure 1).
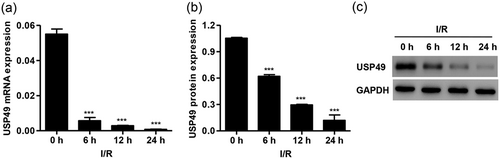
USP49 expression in AC16 cardiomyocytes following I/R injury. The expression of USP49 at both mRNA (a) and protein level (b,c) in AC16 cardiomyocytes following 0, 6, 12, and 24 hr I/R injury was measured. Data are expressed as mean ± SD (n = 3 per group). ***p < 0.001 compared with 0 hr. I/R: ischemia–reperfusion; mRNA: messenger RNA
3.2 USP49 overexpression inhibited I/R-mediated cell viability and apoptosis in AC16 cardiomyocytes
To elucidate the effect of USP49 on I/R-mediated cell viability and apoptosis in vitro, the AC16 cardiomyocytes were transfected with pLVX-Puro-USP49 or blank vector. We found that USP49 overexpression markedly increased the mRNA and protein level of USP49 by 66.4- and 2.12-fold compared with blank vector, respectively (Figure 2a–c). AC16 cardiomyocytes following I/R injury also demonstrated decreased cell viability at 24, 48, and 72 hr compared with control, which was inhibited by pLVX-Puro-USP49 transfection (Figure 2d). Moreover, AC16 cardiomyocytes simulated ischemia for 3 hr and reperfusion for 24 hr showed increased cell apoptosis and Bax expression, but decrease in the Bcl-2 expression compared with control, which was inhibited by pLVX-Puro-USP49 transfection (Figure 2e–g).
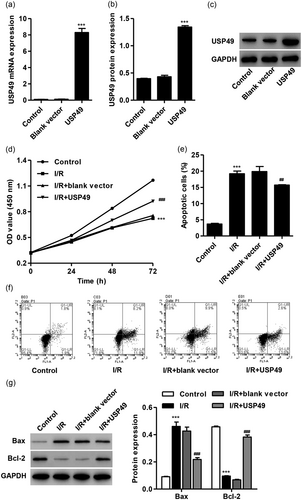
USP49 overexpression inhibited I/R-induced the cell viability suppression and apoptosis in AC16 cardiomyocytes. AC16 cardiomyocytes were transfected with blank vector or pLVX-Puro-USP49, and the expression of USP49 at both mRNA (a) and protein level (b,c) was measured. AC16 cardiomyocytes following I/R injury were transfected with blank vector or pLVX-Puro-USP49, and the cell viability (d), apoptosis (e,f) and the Bax and Bcl-2 protein expression (g) was measured by CCK-8, flow cytometry, and western blot analysis, respectively. Data are expressed as mean ± SD (n = 3 per group). ***p < 0.001 compared with control; ##p < 0.01, ###p < 0.001 compared with I/R + blank vector. I/R: ischemia–reperfusion; mRNA: messenger RNA
3.3 USP49 overexpression inhibited I/R-mediated p38 MAPK, JNK1/2, and ERK1/2 activation in AC16 cardiomyocytes
Next we measured the expression of p-p38, p-JNK1/2, p-ERK1/2, p38, JNK1/2, and ERK1/2 in AC16 cardiomyocytes following I/R injury and USP49 overexpression. The expression of p-p38, p-JNK1/2, and p-ERK1/2 was significantly increased in AC16 cardiomyocytes simulated ischemia for 3 hr and reperfusion for 24 hr, suggesting that I/R-induced JNK1/2, ERK1/2, and p38 activation (Figure 3). However, pLVX-Puro-USP49 transfection significantly inhibited I/R-mediated activation of p38, JNK1/2, and ERK1/2 in AC16 cardiomyocytes. Moreover, JNK1/2 signaling showed the most obvious alternation response to I/R injury and USP49 overexpression, and was therefore used in our following experiments.
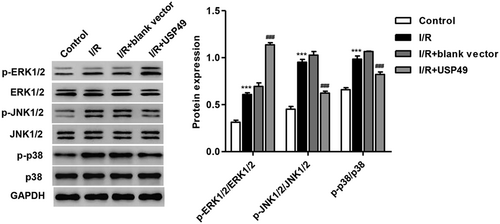
Effect of USP49 overexpression on MAPKs activity in AC16 cardiomyocytes following I/R injury. AC16 cardiomyocytes following I/R injury were transfected with blank vector or pLVX-Puro-USP49, and the protein expression of p-p38, p-JNK1/2, p-ERK1/2, p38, JNK1/2, and ERK1/2 were measured by western blot analysis. Data are expressed as mean ± SD (n = 3 per group). ***p < 0.001 compared with control; ###p < 0.001 compared with I/R + blank vector
3.4 I/R injury-induced cell apoptosis, USP49 expression decrease and JNK1/2 activation in rats
Compared with the sham rats, the rats following I/R injury showed that cardiomyocytes were arranged in disorder, rupture and dissolution, the necrotic cells were visible, some of the myocardial fibers were broken, the nuclei were concentrated, dissolved and disappearing, and the interstitial edema and infiltration of inflammatory cells were seen (Figure 4a). Moreover, rats following I/R injury also demonstrated increased cell apoptosis and JNK1/2 activation and decreased USP49 expression (Figure 4a,b). Additionally, the level of proinflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor (TNF)-α in serum was measured by ELISA. We found that I/R injury significantly induced IL-1β, IL-6, and TNF-α secretion (Figure 4c). CK-MB and cTnI as serum marker of myocardial cellular damage were also measured by ELISA and it was found that I/R injury significantly increased the CK-MB and cTnI levels compared with control rats (Figure 4d).
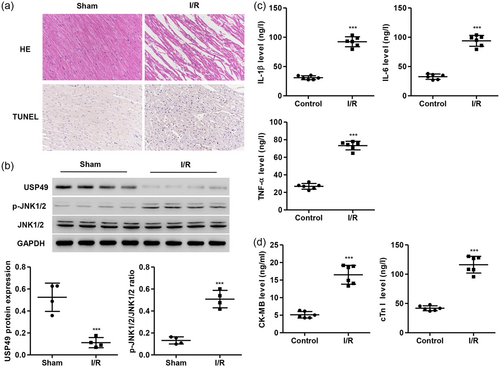
I/R injury-induced USP49 expression reduction and JNK1/2 activation in rat cardiomyocytes. Following I/R injury in rat cardiomyocytes, the myocardia were collected and analyzed by H&E and TUNEL staining (n = 6; a), the USP49, p-JNK1/2, and JNK1/2 protein expression were measured by western blot analysis (n = 4; b), and the levels of IL-1β, IL-6, TNF-α, CK-MB, and cTnI were measured by ELISA (n = 6; c,d). Data are expressed as mean ± SD. Magnification, ×200. ***p < 0.001 compared with control. CK-MB: creatine kinase-muscle/brain; cTnI: cardiac troponin I; ELISA: enzyme-linked immunosorbent assay; IL-1β: interleukin-1β; I/R: ischemia–reperfusion; TNF-α: tumor necrosis factor α; USP49: ubiquitin-specific peptidase 49 [Color figure can be viewed at wileyonlinelibrary.com]
3.5 SP600125 treatment inhibited USP49 silencing-mediated cell viability and apoptosis in AC16 cardiomyocytes
To further examine the effect of USP49 and the involvement of JNK1/2 signaling in I/R injury, AC16 cardiomyocytes were transfected with USP49-siRNA with or without SP600125 (1 μM) treatment. As shown in Figure 5a–c, USP49-siRNA-1, USP49-siRNA-2, and USP49-siRNA-3 transfection significantly inhibited the expression of USP49 at both mRNA and protein levels, with the lowest expression detected in AC16 cardiomyocytes with USP49-siRNA-2 transfection. Therefore, USP49-siRNA-2 was used in our following experiments. we found that USP49 silencing significantly inhibited cell viability at 24, 48, and 72 hr compared with negative control (siNC), which was inhibited by SP600125 treatment (Figure 5d). Meanwhile, USP49 silencing also significantly promoted cell apoptosis, Bax expression, and JNK1/2 activation, but decreased Bcl-2 expression, which was inhibited by SP600125 treatment (Figure 5e,f). These results suggest that USP49 regulates cell viability and apoptosis in AC16 cardiomyocytes through JNK1/2 signaling.
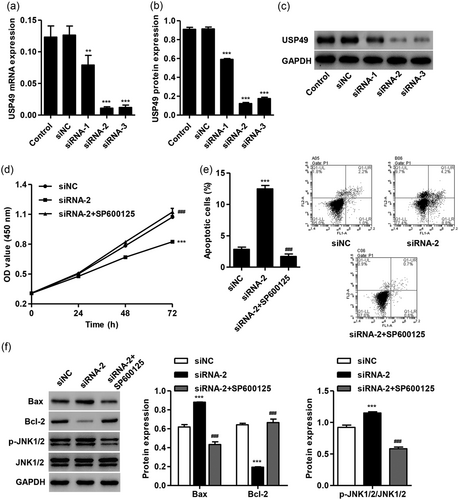
SP600125 treatment-inhibited USP49 knockdown induced the cell viability suppression and apoptosis in AC16 cardiomyocytes. AC16 cardiomyocytes were transfected with siNC, USP49-siRNA-1, USP49-siRNA-1, or USP49-siRNA-1, and the expression of USP49 at both mRNA (a) and protein level (b,c) was measured. AC16 cardiomyocytes were transfected with siNC or USP49-siRNA in the absence or presence of SP600125 (1 μM), and the cell viability (d), apoptosis (e) and the protein expression of Bax, Bcl-2 p-JNK1/2, and JNK1/2 (f) was measured by CCK-8, flow cytometry and western blot analysis, respectively. Data are expressed as mean ± SD (n = 3 per group). ***p < 0.001 compared with siNC; ##p < 0.01, ###p < 0.001 compared with siRNA-2. mRNA: messenger RNA; siNC: siRNA negative control; siRNA: small interfering RNA; USP49: ubiquitin-specific peptidase 49
3.6 USP49 promoted DUSP1 protein expression through binding and deubiquitinating DUSP1
DUSP1-mediated JNK dephosphorylation has been found to play an antiapoptotic role against I/R injury (P. Xie et al., 2009). However, the regulation of DUSP1–JNK1/2 in USP49-mediated I/R injury is still unclear. Our data found that USP49 overexpression significantly increased DUSP1 protein expression (Figure 6a), but not the mRNA expression (data not shown). In addition, the decreased DUSP1 protein expression was reversed by treatment of MG132, a proteasome inhibitor, indicating that USP49 regulates DUSP1 protein expression in a proteasome-dependent manner (Figure 6b). As shown in Figure 6c,d, USP49 coimmunoprecipitated with DUSP1. Reciprocal immunoprecipitation with DUSP1 antibodies also brought down USP49, suggesting an interaction between USP49 and DUSP1 in cells. USP49 overexpression decreased polyubiquitination of DUSP1, suggesting that USP49 deubiquitinates DUSP1 in cells.
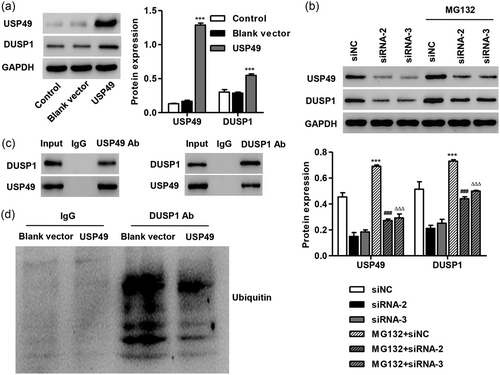
USP49 deubiquitinated DUSP1. (a) AC16 cardiomyocytes were transfected with blank vector or pLVX-Puro-USP49, and the expression of USP49 and DUSP1 was measured by western blot analysis. (b) AC16 cardiomyocytes transfecting siNC or USP49 siRNAs were treated with MG132 (50 μM) for 4 hr, and the USP49 and DUSP1 protein expression were measured by western blot analysis. (c,d) DUSP1 was immunoprecipitated and immunoblotted with the indicated antibodies. Data are expressed as mean ± SD (n = 3 per group). ***p < 0.001 compared with control or siNC; ###p < 0.001 compared with siRNA-2; ΔΔΔp < 0.001 compared with siRNA-3. DUSP: dual-specificity protein phosphatases; siNC: siRNA negative control; siRNA: small interfering RNA; USP49: ubiquitin-specific peptidase 49
4 DISCUSSION
Myocardial I/R injury is an extremely complex pathophysiological process, that is, the phenomenon of myocardial injury aggravated when blood flow and oxygen transport are restored. DUSP1-mediated JNK1/2 signaling was associated with the myocardial I/R injury (Jin et al., 2018). In the current study, we demonstrated that USP49 expression was decreased in AC16 cardiomyocytes following I/R injury in a time-dependent manner. USP49 overexpression inhibited I/R-induced cell viability reduction, apoptosis, and activation of JNK1/2 and p38. USP49 silencing-induced cell viability reduction, apoptosis, and activation of JNK1/2, which were reversed by SP600125 treatment. USP49 overexpression promoted DUSP1 protein expression through deubiquitinating DUSP1. Our study has provided novel information that USP49 inhibits cardiac I/R-induced cell viability suppression and apoptosis in AC16 cardiomyocytes through DUSP1–JNK1/2 signaling.
As a member of the USP family, the functions of USP49 are still poorly understood. USP49 bound to and stabilized p53 by deubiquitination (Tu et al., 2018) and that p53 expression was increased and associated with apoptosis in H9C2 cells and in adult cardiac myocytes (Wang et al., 2014), suggesting that USP49 may participant in the cardiac I/R injury. Here, we showed that USP49 expression was decreased in AC16 cardiomyocytes 6, 12, and 24 hr following I/R injury and in myocardial I/R rats. USP49 expression was decreased in pancreatic cancer tissues and that USP49 silencing inhibited pancreatic cancer cell proliferation and colon through activating AKT signaling (K. Luo et al., 2017). Similarly, USP49 overexpression promoted cell viability and Bcl-2 expression and inhibited apoptosis and Bax expression in AC16 cardiomyocytes following I/R injury. While, USP49 silencing inhibited cell viability and Bcl-2 expression and induced apoptosis and Bax expression in AC16 cardiomyocytes.
To elucidate the mechanism of USP49 regulates the viability and apoptosis in AC16 cardiomyocytes following I/R, we therefore, examined the p38 MAPK, JNK1/2, and ERK1/2 activity, which are of great importance in regulating apoptosis, in I/R-induced cardiomyocytes and in ischemic hearts. p38 MAPK, ERK1/2, as well as JNK1/2 signaling was activated in AC16 cardiomyocytes following I/R injury and in myocardial I/R rats, which were in line with the previous study (Jiang et al., 2015; Y. Zhang et al., 2011). However, ERK1/2 was inactivated after I/R in cardiomyocyte and in mice (Song et al., 2014), while the phosphorylation level of JNK1/2 was not significantly altered in human umbilical vein endothelial cells following I/R injury (T. Zhang, Yang, Fan, Xie, & Li, 2009). Among the selected three MAPK signaling, JNK1/2 showed the most significantly altered in response to I/R injury and USP49, and was therefore used in our following study. In our in vivo experiments, I/R injury also decreased USP49 expression and induced JNK1/2 activation along with the increase of IL-1β, IL-6, TNF-α, CK-MB, and cTnI levels in serum, suggesting the inflammation and myocardial cellular damage occurred in rats following I/R. CK-MB and cTnI have been suggested as specific and sensitive biomarker for detection of myocardial damage and elevated in patients with acute ischemic stroke (Suleiman et al., 2017). Inhibition of JNK1/2 signaling by SP600125 significantly inhibited USP49 knockdown-induced cell viability reduction, apoptosis and JNK1/2 activation, suggesting that USP49 regulates the viability and apoptosis of AC16 cardiomyocytes following I/R through JNK1/2 signaling pathway.
DUSP1 (MKP-1) can inactivate all three MAPKs including ERK, p38 MAPK, and JNK with physiological functions that regulates resistance to cellular stress in mouse fibroblasts, metabolic homeostasis, and immune function, and protects mice from lethal endotoxic shock (Keyse, 2008; G. S. Wu, 2007). In the current study, USP49 overexpression promoted DUSP1 protein expression, but had no effect on its mRNA expression, and the degradation of DUSP1 was attenuated by proteasomal inhibitor MG132, suggesting that USP49 regulates DUSP1 protein expression through deubiquitination. Given that the protein degradation systems in cells include the ubiquitin–proteasome and the lysosome–autophagy pathways, the partial restoration of DUSP1 suggests the possible role of lysosome/autophagy pathway on the DUSP1 degradation, which needs further investigation (Niendorf et al., 2007). Previous studies suggested USP49 deubiquitinates p53 that has also been shown to regulate DUSP1 protein abundance during the cellular response to oxidative stress (Liu et al., 2008; Tu et al., 2018). Moreover, DUSP1 was downregulation in H9C2 cells and in the infarct area of rats following cardiac I/R injury, and DUSP1 alleviated cardiac I/R injury via the JNK1/2 signaling pathway (Jin et al., 2018). These suggest that USP49 may regulate I/R-induced cell viability suppression and apoptosis in AC16 cardiomyocytes through DUSP1–JNK1/2 signaling.
In conclusion, USP49 is increased and promotes the cell viability and inhibits cell apoptosis and JNK1/2 activation in AC16 cardiomyocytes following I/R injury. Myocardial I/R injury induces cell apoptosis, USP49 decrease and JNK1/2 activation in rats. USP49 promotes DUSP1 protein expression through deubiquitination. USP49 regulates I/R-induced cell viability suppression and apoptosis in AC16 cardiomyocytes through DUSP1–JNK1/2 signaling. Our study may lead to the development of new therapeutic approaches.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




