Melatonin and pancreatic cancer: Current knowledge and future perspectives
Abstract
Pancreatic cancer has a high mortality rate due to the absence of early symptoms and subsequent late diagnosis; additionally, pancreatic cancer has a high resistance to radio- and chemotherapy. Multiple inflammatory pathways are involved in the pathophysiology of pancreatic cancer. Melatonin an indoleamine produced in the pineal gland mediated and receptor-independent action is the pancreas and other where has both receptors. Melatonin is a potent antioxidant and tissue protector against inflammation and oxidative stress. In vivo and in vitro studies have shown that melatonin supplementation is an appropriate therapeutic approach for pancreatic cancer. Melatonin may be an effective apoptosis inducer in cancer cells through regulation of a large number of molecular pathways including oxidative stress, heat shock proteins, and vascular endothelial growth factor. Limited clinical studies, however, have evaluated the role of melatonin in pancreatic cancer. This review summarizes what is known regarding the effects of melatonin on pancreatic cancer and the mechanisms involved.
1 INTRODUCTION
Pancreatic cancer is often fatal and is recognized as the eighth and ninth leading cause of cancer death among men and women, respectively (Jemal et al., 2011). The risk of pancreatic ductal adenocarcinoma is increased during aging (D. Li et al., 2009). Pancreatic cancer risk factors include smoking (Lynch et al., 2009), obesity (Preziosi, Oben, & Fusai, 2014), and alcohol consumption (Talamini et al., 1999). In addition, other chronic conditions including diabetes mellitus (Talamini et al., 1999), pancreatitis (Lowenfels et al., 1993), and hereditary tumor syndromes (Hruban, Canto, & Yeo, 2001) way augment the risk of pancreatic cancer. Late symptoms of pancreatic cancer include abdominal pain, weight loss, nausea and vomiting, changes in bowel habits, back pain, and jaundice. Surgical resection followed by chemotherapy are the principal treatments (Kamisawa, Wood, Itoi, & Takaori, 2016; Keane, Horsfall, Rait, & Pereira, 2014). Currently, gene therapy and supplementation with a potential anticancer agent are known as new approaches for the treatment of pancreatic cancer (Barber, Fearon, Tisdale, McMillan, & Ross, 2001; Sato-Dahlman, Wirth, & Yamamoto, 2018).
Melatonin (N-acetyl-5-methoxytryptamine) is secreted by the pineal gland. The circadian pattern of its secretion is regulated by the biological clock within the hypothalamic suprachiasmatic nucleus (Klein & Moore, 1979). In addition to pineal gland, melatonin is produced in many organs including the gastrointestinal tract (Bubenik, 1980), retina (Cardinali & Rosner, 1971), skin (Slominski, Tobin, Zmijewski, Wortsman, & Paus, 2008), lymphocytes (Lardone et al., 2011), and so on (Acuña-Castroviejo et al., 2014). Some of the major functions of the blood melatonin rhythm include regulation of sleep, modulation of circadian rhythms (Cajochen, Kräuchi & Wirz-Justice, 2003), and involvement in the immune response (Maestroni, Conti, & Pierpaoli, 1986). Melatonin is also a powerful endogenous antioxidant (D.-X. Tan, Manchester, Esteban-Zubero, Zhou, & Reiter, 2015) and its supplementation has radical-scavenging and anti-inflammatory actions (Esposito & Cuzzocrea, 2010; D. Tan et al., 2002). Several studies have reported the beneficial effects of melatonin on different cancer including, head and neck cancer (Yeh et al., 2017), non-small-cell lung cancer (Ma et al., 2016), and gastric cancer (W. Li et al., 2017) and many others (R. Reiter et al., 2017) through its preapoptotic effects (Rodriguez et al., 2013). Studies that have evaluated the role of melatonin on pancreatic cancer are common. The aim of this review is to assess the current knowledge related to the role of melatonin on pancreatic cancer.
2 THE PHYSIOLOGICAL ROLES OF MELATONIN IN EXOCRINE AND ENDOCRINE PANCREAS
Melatonin is involved in different gastrointestinal tract functions including its mobility, inflammation, and pain suppression (Bubenik, 2002). Following food consumption, melatonin concentration is increased in the gastrointestinal system, which promotes the secretion of pancreatic enzymes (Jaworek et al., 2007).
2.1 Endocrine pancreas
Melatonin receptors (MT1 and MT2) are expressed on the pancreatic islets cells (Ramracheya et al., 2008). Melatonin affects α cells of the pancreas and stimulates the secretion of glucagon (Bähr, Mühlbauer, Schucht, & Peschke, 2011). Its effects on glucagon perform through phosphatidylinositol 3-kinase signaling pathway (Bähr, Mühlbauer, Albrecht, & Peschke, 2012). Melatonin reduces insulin secretion by pancreas, in response to glucose, through the down regulation of the protein kinase A signaling pathway (Peschke, Bähr, & Mühlbauer, 2013; Peschke, Peschke, Hammer, & Csernus, 1997; Picinato et al., 2002). Moreover, melatonin might inhibit insulin secretion through the activation of MT1 receptor (Mühlbauer, Albrecht, Bazwinsky-Wutschke & Peschke, 2012; Peschke, Bach & Muhlbauer, 2006). Stumpf, Mühlbauer, and Peschke (2008) reported that melatonin role in inhibiting insulin secretion might be through the modulation of MT2 receptor and the activation of cyclic guanosine 3′,5′-monophosphate (cGMP)-signaling pathway. Likewise, melatonin inhibits cGMP through the inhibition of the soluble guanylate cyclase (sGC) in pancreatic β cells. It also might inhibit cyclic nucleotide-gated channels (Stumpf, Bazwinsky, & Peschke, 2009).
2.2 Exocrine pancreas
Melatonin stimulates pancreatic enzyme secretion, such as amylase. The stimulatory impact of melatonin may be mediated by the cholecystokinin release and vagal sensory nerve stimulation. Melatonin function on exocrine pancreas is independent of the MT2 receptor (Jaworek et al., 2004). The exocrine pancreas also expresses the melatonin MT1 receptor (Gonzalez, del Castillo-Vaquero, Miro-Moran, Tapia, & Salido, 2011).
Melatonin has been involved in a variety of pancreatic cell pathways (Bazwinsky-Wutschke, Mühlbauer, Albrecht & Peschke, 2014; Y. Li et al., 2018) in different pathological conditions including human pancreatic carcinoma (Leja-Szpak, Jaworek, Pierzchalski, & Reiter, 2010), diabetes (Stumpf et al., 2008). Simsek et al. (2012) have shown that the administration of melatonin might lead to the inhibition of apoptosis, promoting β-cell differentiation, increased B-cell lymphoma-extra-large (Bcl-xL) activation and the inactivation of caspase-3 in diabetic animal model. However, in another study melatonin induced apoptosis in pancreatic cancer cells (Leja-Szpak et al., 2010).
3 MELATONIN ROLE IN PANCREATIC CANCER
3.1 Melatonin, as an inflammation inhibitor
The activation of inflammatory pathways has the crucial role in the pathophysiology of pancreatic cancer (Farrow et al., 2004). Nuclear factor-κB (NF-κB) and CCAAT-enhancer-binding protein (C/EBP) are the major transcription factors for the expression of proinflammatory transition (Shi et al., 2012). Both classical and nonclassical NF-κB pathways interact with a number of signaling pathways including KRAS, transforming growth factor-β and notch pathways to remain constitutively activated in pancreatic cancer cells and promotes tumorigenesis and metastasis (Prabhu, Mundade, Korc, Loehrer, & Lu, 2014). C/EBP positively regulates LINC01133 expression by binding to the response elements within the LINC01133 promoter (Huang et al., 2018). Higher expression of C/EBP was observed in pancreatic adenocarcinoma tissues, and this overexpression was also correlated with the poorer prognosis (Huang et al., 2018). In addition, the LINC01133 knockdown decreased cyclin G1 expression, which in turn attenuated the LINC01133 silencing-induced impairment of proliferation in pancreatic adenocarcinoma cells (Huang et al., 2018). Also, inflammatory factors might be activated following the induction of transcription 3 signal transducer and activator of transcription 3 (STAT3) in cancer cells such as pancreatic cancer (Lesina et al., 2011; Yu, Pardoll, & Jove, 2009). Therefore, the inhibition of NF-κB, C/EBP, and STAT3 may be an ideal approach for the treatment of pancreatic cancer. There are few studies focused on the effects of melatonin on inflammatory pathways in pancreatic cancer. Melatonin was found to inhibit NF-κB and other inflammatory factors including TNF-α, interleukin (IL)-1b, IL-6, IL-10, and inducible nitric oxide synthase (iNOS; Jung et al., 2010). Melatonin also inhibited phosphorylation of IκB and translocation of NF-κB/p65 to nuclei. Also, melatonin decreased its downstream gene expression including cell proliferation (cyclin D1), invasion (MMP-2, MMP-9, and CXCR-4), apoptosis (Bcl-xL) and angiogenesis vascular endothelial growth factor C (VEGF-C; Ju et al., 2016).
3.2 Melatonin, an oxidative stress regulator
The imbalance between cellular oxidants and antioxidants direct radical scavenger and indirect antioxidant (R. J. Reiter et al., 2016) when it plays a major role in the pathophysiology of pancreatic cancer (Kodydkova et al., 2013). Melatonin when combined with chemotherapy increased reactive oxygen species (ROS), mitochondrial membrane depolarization and apoptosis as well as inhibited cell viability of pancreatic tumors in pancreatic tumor cell line AR42J (Uguz et al., 2012). However, the administration of melatonin in animal models of pancreatic cancer induced by BOP (N-nitrosobis(2-oxopropyl)amine) resulted in the inhibition of ROS. The administration of melatonin reduced the size of cancerous nodules and increased glutathione, catalase, and superoxide dismutase in pancreatic cancer cells in the hamster (J. F. Ruiz-Rabelo et al., 2007). In addition, the combination of melatonin and capecitabine exaggerated the antioxidant potential in an animal model of pancreatic cancer (J. Ruiz-Rabelo et al., 2011). Also, the coadministration of melatonin with celecoxib increased antioxidant processes and decreased tumor cell number in an animal model of pancreatic cancer (Padillo et al., 2010). The amount of data on the effects of antipancreatic cancer melatonin in animal studies is clearly not abundant and not entirely consistent.
3.3 Melatonin, a regulator of Bcl-2/Bax balance
Proapoptotic factors, such as Bax, in contrast to antiapoptotic factors like Bcl-2, are mainly involved in the pathophysiology of pancreatic cancer (Miyamoto et al., 1999). Bax is a regulator of cytochrome c release from mitochondria (Jurgensmeier et al., 1998) and interacts with apoptosis protease-activating factor 1 (Apaf-1), caspase-9, and caspase-3, which leads to apoptosis (P. Li et al., 1997). Mitogen-activated protein kinases (MAPK), p38, and c-Jun N-terminal kinase (JNK) regulate the ratio of Bcl-2/Bax and the activity of caspase-3 in cancer cells. The upstream of MAPKs is apoptosis signal-regulating kinase 1 (ASK1). This pathway is of interest for pharmaceutical reasons (Guo et al., 2016; C.-C. Hsieh & Papaconstantinou, 2006; Tobiume et al., 2001). In addition to caspases, other Ca2+-dependent proteases including calpains might play an important role in cell apoptosis (Momeni, 2011). The calpain stimulates Bax and promotes cytochrome c release, the activation of caspase-3 and finally cellular apoptosis. This pathway is independent of Bcl-2 (Gao & Dou, 2001; Oh, Lee, & Lim, 2004; Wood et al., 1998; Yeo et al., 2002). Calpain modulates myocyte enhancer factor 2 through activating cyclin-dependent kinase 5, an important pathway in cell apoptosis (Smith et al., 2006; Verdaguer et al., 2005). Current evidence has demonstrated that some supplementations including vitamin D, genistein, and resveratrol induces cell apoptosis through the activation of calpain and caspases (Mathiasen et al., 2002; Sareen, Darjatmoko, Albert, & Polans, 2007; Sergeev, 2004). To determine the effects of melatonin on the apoptotic process, an in vitro study revealed the inhibitory effect of melatonin on cancer cell proliferation and reduced cell survival. Also, melatonin decreased gene and protein expression of Bcl-2 and augmented Bax expression. Also, melatonin decreased tumor volume and the expression of Bcl-2 and increased Bax expression in mice (Xu et al., 2013). This was documented in another study where melatonin elevated Bax expression and stimulated caspase-9 (Leja-Szpak et al., 2010). Moreover, W. Li et al. (2016) reported that melatonin supplementation led to the activation of MAPKs pathway, increased Bax, caspase-3, and inhibited Bcl-2 in pancreatic cancer cells. An in vitro study showed that melatonin supplementation led to the activation of caspase-3, depolarization and changes in the oxidative state of the mitochondria and the reduction in the viability of a pancreatic tumor cells (Gonzalez et al., 2011).
3.4 Melatonin, a heat shock proteins (HSP) inhibitor
Cells respond to different stressors through the activation of a group of proteins known as HSPs; these include HSP27, HSP60, HSP70, HSP90, and HSP100. These proteins have antiapoptotic functions (Beere, 2004; Lanneau, de Thonel, Maurel, Didelot, & Garrido, 2007) and can inhibit different steps in apoptosis signaling pathways including Bax translocation (Stankiewicz, Lachapelle, Foo, Radicioni, & Mosser, 2005), the release of cytochrome c from mitochondria (Bruey et al., 2000; Mosser et al., 2000) and the release of Apaf-1 (Beere et al., 2000; Saleh, Srinivasula, Balkir, Robbins, & Alnemri, 2000). HSPs are expressed in pancreatic cancer cells and have crucial roles in the pathogenesis of pancreatic cancer (Gress et al., 1994). Dutta et al. (2012) demonstrated that serum levels of HSP70 are elevated in patients with pancreatic cancer. HSP27 has been suggested as a marker for pancreatic cancer (Melle et al., 2007). Heinrich, Tuukkanen, Schroeder, Fahrig, and Fahrig (2011) reported that RP101 might be beneficial for the treatment of pancreatic cancer through the HSP27 pathway. Consistent with these findings, (Leja-Szpak, Jaworek, Szklarczyk, Konturek, & Pawlik, 2007) reported that melatonin stimulated protein expression of HSP27 in pancreatic cancer.
3.5 Melatonin, a VEGF inhibitor
VEGF with its angiogenesis properties is highly expressed in pancreatic cancer, and this overexpression is correlated with disease progression (Hoeben et al., 2004; Itakura, Ishiwata, Shen, Kornmann & Korc, 2000; Tang et al., 2001). Human pancreatic cancers have the capacity to overexpress VEGF/VEGF receptor and suggest that in some instances VEGF may directly promote pancreatic cancer growth via the MAPK pathway (Hoeben et al., 2004; Itakura et al., 2000; Tang et al., 2001). As upstream factors, hypoxia-inducible factor 1 and STAT3 stimulated gene expression of VEGF in pancreatic cancer cells (Büchler et al., 2003; Gray et al., 2005; Niu et al., 2002). VEGF also upregulates the gene expression of Bcl-2 in cancer cells (Beierle, Strande, & Chen, 2002; Pidgeon, Barr, Harmey, Foley, & Bouchier-Hayes, 2001). Melatonin administration inhibits VEGF messenger RNA expression in pancreatic cancer cells (Lv, Cui, Yao, Xu, & Yang, 2012). Therefore, melatonin may induce apoptosis in pancreatic cancer cells through reducing VEGF production and reducing blood flow to the tumor.
3.6 Melatonin and death or survival of cancer cells
Melatonin induces apoptotic cell death in the human pancreatic carcinoma cell line MIA PaCa-2 via the suppression of NF-κB and activation of extracellular signal-regulated kinase (ERK) and JNK (W. Li et al., 2016). Some reports suggest that ERK pathway activation affects a survival signal that weakens proapoptotic effects via activating JNK (Hsiang, Tunoda, Whang, Tyson, & Ornstein, 2006; Mukherjee, Gupta & Kumar, 2009). The MAPK pathway plays an important role in cell survival and proliferation (Chang & Karin, 2001). The MAPK family has been categorized into three groups: JNK, extracellular-regulated kinase 1/2, and p38 MAPK. Some studies have demonstrated that the JNK substrate is involved in cell growth and apoptosis (M. H. Hsieh & Nguyen, 2005), which take part in tumor progression and are influenced by melatonin (Joo & Yoo, 2009).
4 CONCLUSION
Current evidence supports the therapeutic benefits of melatonin in the treatment of pancreatic cancer. Melatonin has been shown to influence multiple pathways related to cell proliferation, invasion, angiogenesis, and apoptosis (Figure 1). The major focus has been on the beneficial effects of melatonin supplementation on pancreatic cancer through apoptosis pathways. Clinical trials and more in vivo studies are regained to evaluate the therapeutic potential of melatonin in the treatment of patients with pancreatic cancer.
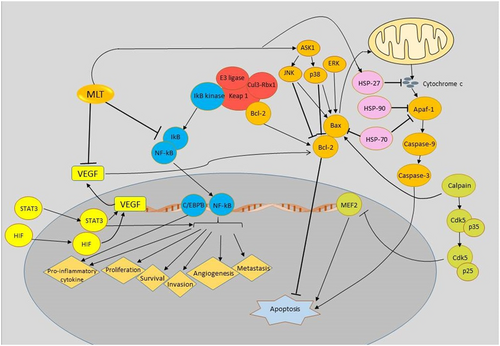
Schematic representation of targeting different signaling pathways using melatonin is a novel therapeutic strategy in the treatment of pancreatic cancer. Apaf-1: apoptotic protease-activating factor 1; ASK1: apoptosis signal-regulating kinase 1; Bax: Bcl-2-associated X protein; Bcl-2: B-cell lymphoma 2; CDK5: cyclin-dependent kinase 5; C/EBP: CCAAT-enhancer-binding protein; ERK: extracellular signal-regulated kinase; HSP: heat shock proteins; HIF: hypoxia-inducible factors; IκB: inhibitor of κB; JNK: c-Jun N-terminal kinases; Keap 1: Kelch-like ECH-associated protein 1; Mef2: myocyte enhancer factor 2; MLT: melatonin; NF-κB: nuclear factor-κB; STAT3: signal transducer and activator of transcription 3; VEGF: vascular endothelial growth factor [Color figure can be viewed at wileyonlinelibrary.com]




