1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one induces lung cancer cell apoptosis via the PI3K/Akt and ERK1/2 pathways
Abstract
1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one (EB30) is a diarylheptanoid-like compound isolated from Viscum coloratum. This curcumin analog exhibits significant cytotoxic activity against HeLa, SGC-7901, and MCF-7 cells. However, little is known about the anticancer effects and mechanisms of EB30 in human lung cancer. The current study reports that EB30 significantly reduced the cell viability of A549 and NCI-H292 human lung cancer cells. Further examination revealed that EB30 not only induced cell cycle arrest and promoted the generation of reactive oxygen species (ROS) but also induced cell apoptosis through the intrinsic and extrinsic signaling pathways. Furthermore, EB30 upregulated the expression levels of p-ERK1/2 and p-P90RSK, whereas downregulating the phosphorylation of Akt and P70RSK. Cell viability was further inhibited by the combination of EB30 with LY294002 (a specific PI3K inhibitor) or U0126 (a MEK inhibitor). The current study indicates that EB30 is a potential anticancer agent that induces cell apoptosis via suppression of the PI3K/Akt pathway and activation of the ERK1/2 pathway.
1 INTRODUCTION
Lung cancer is the most common type of cancer in China and the leading cause of death due to cancer worldwide; 1,688,780 new cancer cases and 600,920 cancer deaths in the US are projected for 2017 (Chen et al., 2016; Siegel, Miller, & Jemal, 2016; Torre, Siegel, & Jemal, 2016). Most individuals with lung cancer are diagnosed at an advanced stage. In delayed treatment protocols, the therapeutic effect is modified by drug resistance, which can result in poor prognosis (Siegel, Miller, & Jemal, 2015; Vilmar & Sorensen, 2011). Therefore, the development of new chemotherapeutic drugs to improve survival in patients with lung cancer is needed.
Natural products, which have received significant attention for use in herbal medicine, are an important source of anticancer agents (Zhao et al., 2016). Viscum coloratum (Kom.) Nakai, a medicinal herb listed in Pharmacopoeia of the People's Republic of China, is mainly used for the treatment of cancer and cardiovascular disease. V. coloratum is a semiparasitic plant, and its cauline leaf has been used as a medicinal plant in China for hundreds of years, exhibiting favorable anticancer effects in colorectal carcinoma (Li, Zhang, Zhi, & Yuan, 2011), lymphoblastic leukemia (Seifert et al., 2008), breast cancer (Hong, Park, & Lyu, 2014), and osteosarcoma (Ge et al., 2016). Its active constituents are small molecular flavonoid compounds, alkaloids, organic acids, and macromolecular compounds, such as phallotoxins, lectins, proteins, and polysaccharides (Jiang et al., 2014; Jung, Yoo, Lee, Kim, & Song, 2004; Kim et al., 2000; Liang et al., 2011). EB30, a curcumin analogue, can be extracted from V. coloratum. A previous study identified that EB30 exhibited significant cytotoxic activity against HeLa, SGC-7901, and MCF-7 cells (Zhao et al., 2012). Nevertheless, the molecular mechanism underlying the chemopreventive effects of EB30 has not been elucidated.
In the current study, we estimated the effects of EB30 on human lung cancer cells A549 and NCI-H292. We sought to evaluate the roles of EB30 in affecting changes in cell viability and apoptosis in lung cancer cells and to assess the mechanism mediated by EB30. Our results revealed that EB30-induced lung cancer cell apoptosis, which was regulated by the PI3K/Akt and ERK1/2 signaling pathways.
2 MATERIALS AND METHODS
2.1 Plant
V. coloratum (Kom.) Nakai was purchased from the Pharmacy Department of Shandong Provincial Hospital. A plant sample was deposited at the School of Pharmaceutical Sciences, Shandong University.
2.2 Extraction and separation
Air-dried V. coloratum material (10 kg) was first crushed into powder. Next, four treatments with 95% ethanol were used for extraction (20 L, 7 days each). After distillation evaporation and rotary evaporation, the crude extract (800 g) was suspended in H2O and successively treated with petroleum ether, ethyl acetate, and n-butanol. The ethyl acetate part (150 g) was isolated by silica column chromatography using CH2Cl2–MeOH (300:1-2:1, v/v) as a mobile phase for the gradient elution to obtain nine fractions. Fraction 6 (20 mg) was further separated successively by chromatography using a gel column and a silica column. The mobile phase consisted of CH2Cl2–MeOH (1:1) and petroleum ether-ethyl acetate (8:1). Finally, we obtained compound EB30 (8 mg).
2.3 Antibodies and reagents
Cisplatin (CDDP) was purchased from Sigma-Aldrich (St. Louis, MO). Dimethyl sulfoxide (DMSO) was purchased from Solarbio (China). Carbonyl cyanide 3-chlorophenylhydrazone (CCCP) was obtained from Beyotime (China). LY294002 and U0126 were purchased from Cell Signaling Technology (Beverly, MA). The antibodies for Bcl-2-associated X protein (Bax), B-cell lymphoma protein 2 (Bcl-2), Bcl-2-related protein, long isoform (Bcl-xL), cysteinyl aspartic acid protease-9 (Caspase-9), cleaved caspase-9, cysteinyl aspartic acid protease-3 (caspase-3), cleaved caspase-3, cysteinyl aspartic acid protease-8 (caspase-8), cleaved caspase-8, and cytochrome c were purchased from Proteintech Group (Wuhan, China). Apoptosis-inducing factor (AIF), Akt, p-Akt, p-ERK1/2, and β-actin were purchased from Santa Cruz Biotechnology (Dallas, TX). ERK1/2 was purchased from Abcam (ab184699, Abcam, 1:10,000). Poly (ADP-ribose) polymerase (PARP), P70S6K and P90RSK were purchased from Cell Signaling Technology (Beverly, MA).
2.4 Cell culture
Human lung cancer cell line A549 cells, NCI-H292 cells (originating from adenocarcinoma) and human bronchial epithelial (HBE) cells were purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). A549 and NCI-H292 cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Grand Island, NY) supplemented with 10% (v/v) fetal bovine serum (HyClone, Logan, UT), 100 U/ml penicillin, and 100 U/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. HBE cells were maintained in Dulbecco’s modified Eagle medium (DMEM) (HyClone, Logan, UT), supplemented with 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 U/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37°C.
2.5 Cell viability assay
Cell viability was assessed with a Cell Counting Kit-8 (CCK-8; CK04–500, Dojindo, Kumamoto, Japan) assay. Briefly, cells were seeded in 96-well plates and incubated for 24 hr at a density of 1.0 × 104 cells/well. After treatment with EB30 for 48 hr, the cells were incubated for an additional 4 hr with 100 μl of serum-free RPMI 1640/DMEM with 10 μl of CCK-8 solution at 37°C. The absorbance of each well was then read at 450 nm using a microplate reader (Bio-Rad, Model 680).
2.6 Cytotoxicity assay using the xCELLigence system
A cytotoxicity assay was conducted using the xCELLigence system (ACEA Biosciences, San Diego, CA), which monitors cell growth in response to treatment in real time. The system uses impedance as a readout. Cells grow on top of electrodes so that the impedance varies based on the number of cells attached and the quality of the cell–electrode interaction. Electrode impedance, which is displayed as the cell index (CI), can be used to monitor cell viability, number, morphology, and adhesion. In this experiment, A549, NCI-H292, and HBE cells were seeded onto E-plates at 8,000 cells per well. After incubating overnight, the cells were exposed to EB30 and CDDP. The impedances were measured every 30 min for 4 days and expressed as CI values.
2.7 DAPI (4,6-diamidino-2-phenylindole) staining
A549 and NCI-H292 cells were seeded in 12-well plates at a density of 4 × 104 cells/well and allowed to adhere overnight. Subsequently, A549 (5, 10, and 20 μM) and NCI-H292 (2.5, 5, and 10 μM) cells were treated with EB30. Twenty-four hours after the treatment, the cells were fixed and incubated with 2.5 μg/ml DAPI (Genview Scientific Inc.) solution for 10 min, protected from light. Images of the stained nuclei of the cells were taken by an inverted florescence microscope system (Axiovert 200, Zeiss, Germany).
2.8 Apoptosis assays
Cells (4 × 105) were seeded in 6-well plates and incubated overnight. Cells were harvested by trypsinization after the treatment with EB30 for 48 hr and washed with phosphate-buffered saline (PBS) at 3,000 rpm. The CDDP treatment served as a positive control. Then, an Annexin V-FITC/propidium iodide (PI) kit (BD Biosciences, San Jose, CA) was used for cell staining according to the manufacturer's instructions. Finally, flow cytometric analysis was performed using a flow cytometer.
2.9 Measurement of intracellular ROS
2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma) was used to detect intracellular reactive oxygen species (ROS) levels. After incubating cells with EB30 for 24 hr, the cells were washed with PBS and incubated in fresh medium containing DCFH-DA (10 µg/ml) at 37°C for 30 min. Then, the cells were collected and analyzed at an excitation wavelength of 488 nm and an emission wavelength of 530 nm by a flow cytometer.
2.10 Cell cycle analysis
To determine whether EB30 had an impact on the cell cycle, cells were seeded in 6-well plates and treated with EB30 for 24 hr. After exposure to EB30, cells were collected and fixed overnight in 70% ethanol at −20°C. The ethanol was discarded, and the cells were incubated with RNase A (Invitrogen) solution (10 mg/ml) for 30 min, stained with 1 mg/ml PI for 1 hr in the dark at 4℃. For each sample, 1 × 104 cells were analyzed using a FACS Calibur flow cytometer system (BD Biosciences, San Jose, CA).
2.11 Analysis of mitochondrial membrane potential (MMP)
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl benzimidazolyl-carbocyanine iodide (JC-1) was used as a fluorescence probe to determine MMP. Cells (4 × 105) were seeded in 6-well plates and incubated for 24 hr. After treating with EB30 for 24 hr, cells were collected and stained with JC-1 for 45 min. Cells were subsequently stained with CCCP as a positive control. Finally, cells were analyzed using a flow cytometer.
2.12 Mitochondrial extraction
Mitochondria were extracted using a Cell Mitochondria Isolation Kit (Beyotime, China). Cells were seeded in D-100 dishes for 24 hr. After incubation with EB30 for another 24 hr, cells were homogenized with mitochondrial extraction reagent and centrifuged at 600 g and 4°C for 10 min, and then, the supernatants were centrifuged for an additional 15 min (4°C, 12,000 g). The resulting pellet sediments contained the mitochondria.
2.13 Immunofluorescence
Dissociated cells were seeded onto coverslips for 24 hr and treated with EB30 for another 24 hr. Next, cells were fixed with methanol for 10 min at room temperature. After removing the methanol, cells were incubated with AIF primary antibody (1:100) at 4°C overnight followed by Alexa Fluor 594-conjugated secondary antibody for 1 hr. Then, nuclei were stained with DAPI (2.5 μg/ml) for 10 min. Fluorescent images were captured with a fluorescence microscope.
2.14 Western blot analysis
EB30-treated cells were collected and lysed in a sample buffer (50 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate (SDS), 10% glycerol, 100 mM DTT, and 0.1% bromophenol blue). Lysates were sonicated and electrophoresed on SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (Millipore). After blocking with 5% non-fat milk in PBS buffer, the membranes were incubated with the appropriately diluted primary antibodies at 4°C overnight, followed by horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 hr at room temperature. Proteins were visualized by enhanced chemiluminescence detection reagents (Millipore, Billerica, MA). GAPDH and β-actin were used as internal controls.
3 RESULTS
3.1 The structure of EB30
The structure of EB30 (Figure 1a) was identified by 1H and 13C nuclear magnetic resonance (NMR) measurements. The spectroscopic data were verified by a previous study (Zhao et al., 2012). 1H-NMR (δ ppm, DMSO-d6, 600 MHz): δH 7.61 (2H, d, J = 8.4 Hz, H-2′, 6′), 7.61 (1H, d, J = 15.6 Hz, H-1), 7.04 (1H, d, J = 15.6 Hz, H-2), 7.01 (1H, d, J = 15.6 Hz, H-4), 7.10 (2H, d, J = 8.4 Hz, 3′/5′), 6.92 (2H, d, J = 8.4 Hz, H-2”/6”), 6.79 (2H, d, J = 8.4 Hz, H-3”/5”), and 6.51 (1H, d, J = 15.6 Hz, H-5); 13C-NMR (δ ppm, DMSO- d6, 150 MHz): δC 143.4 (C-1), 126.1 (C-2), 188.7 (C-3), 147.0 (C-4), 129.6 (C-5), 34.6 (C-6), 33.4 (C-7), 131.5 (C-1′), 131.0 (C-2′/6′), 115.5 (C-3′/5′), 155.9 (C-4′), 128.5 (C-1”), 130.0 (C-2”/6”), 116.2 (C-3,” 5”), and 160.4 (C-4”).
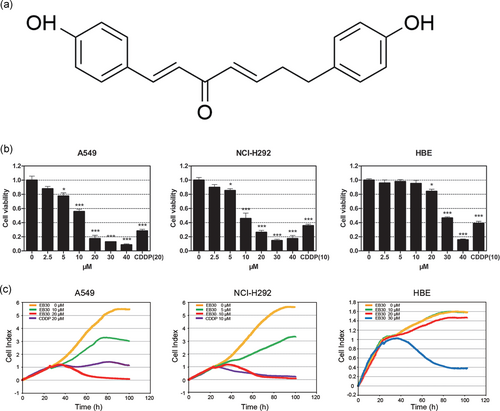
EB30 inhibits cell viability of A549 and NCI-H292 cells. (a) Chemical structure of EB30. (b) A549, NCI-H292, and HBE cells were treated with EB30 (2.5, 5, 10, 20, 30, and 40 μM) in 96-well plates for 48 hr. Data are expressed as mean ± SD, n = 3 (*p < 0.05, **p < 0.01, and ***p < 0.001), compared with control group. (c) Real-time monitoring of cell viability (A549, NCI-H292, and HBE cells) treated with EB30 or CDDP [Color figure can be viewed at wileyonlinelibrary.com]
3.2 EB30 reduces human lung cancer cell viability
A CCK-8 assay was used to screen the drug extracted from V. coloratum (Kom.) Nakai. The screening outcomes showed that EB30 suppressed lung cancer cell proliferation in a dose-dependent manner (Figure 1b). EB30 reduced survival of A549 and NCI-H292 cells when between at 5 (p < 0.05) and between 10 and 40 µM (p < 0.001). The IC50 values of EB30 treatment of 48 hr were 8.61 μM and 12.71 μM, respectively. To demonstrate the specific cytotoxicity of EB30 on tumor cells, the effects on the viability of HBE cells were determined (Figure 1b). In HBE cells, the IC50 value of EB30 treatment of 48 hr was 28.58 µM, which was 2.25 and 3.32 times less toxic than in A549 and NCI-H292 cells, indicating the selective effect of EB30. A real-time cell analyzer (RTCA) was used to further validate this result, as shown in Figure 1c. A549 and NCI-H292 cells treated with 20 μM and 10 μM EB30 evidently suppressed cell growth, respectively. HBE cell growth did not demonstrate obvious suppression at 20 μM and 10 μM.
3.3 EB30 induces apoptosis in A549 and NCI-H292 cells
A DAPI assay was performed to further validate that apoptosis contributed to the reduction in cell viability (Figure 2a). Apoptotic bodies and fragmented nuclei were observed in EB30-treated cells, specifically in cells that were treated with a high dose. All of the above-mentioned results were confirmed by Annexin V-FITC and PI double staining (Figure 2b). Compared with control cells, A549 and NCI-H292 cells treated with 20 μM and 10 μM EB30 had an increased percentage of late apoptotic cells. In A549 cells, apoptosis triggered by EB30 (20 μM) was increased by 311% compared with untreated control cells, and the percentage of apoptotic NCI-H292 cells was increased by 1,326% compared with untreated control cells (Figure 2c). The results from these analyses indicated that EB30-induced cell apoptosis in both lung cancer cell lines.
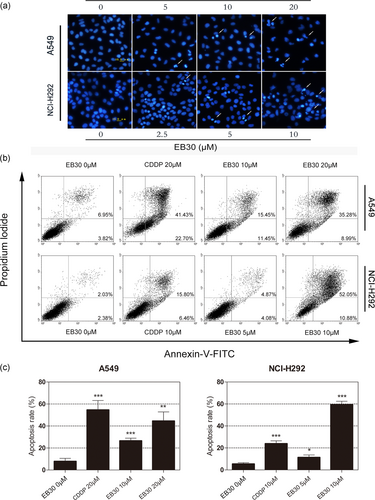
EB30 induces apoptosis in A549 and NCI-H292 cells. (a) A549 cells were treated with EB30 (5, 10, and 20 μM) for 24 hr and NCI-H292 cells were treated with EB30 (2.5, 5, and 10 μM) for 24 hr, the changes in nuclear morphology were evaluated by fluorescence microscopy of DAPI stained nuclei. (b) A549 cells were incubated with EB30 (10 and 20 μM) and CDDP (20 μM), and NCI-H292 cells were treated with EB30 (5 and 10 μM) and CDDP (10 μM) for 24 hr, analyzed by flow cytometry after annexin v-FITC and PI staining. (c) Quantification of apoptotic cells are from three independent experiments. Values are expressed as mean ± SD, n = 3 (*p < 0.05, **p < 0.01, and ***p < 0.001), compared with control group [Color figure can be viewed at wileyonlinelibrary.com]
3.4 EB30 induces lung cancer cell cycle arrest
To investigate the effect of EB30 on the cycle progression of lung cancer cells, both A549 and NCI-H292 cells were cultured in 3.5 cm dishes without (control) or with EB30 for 24 hr. As shown in Figure 3a, EB30 contributed to the accumulation of A549 cells in the S phase, whereas contributed to the accumulation of NCI-H292 cells in the G0/G1 phase in a dose-dependent manner. The percentage of A549 cells in the S phase increased from 33.69% to 47.18%, 49.63% and 53.75% upon the treatment with EB30 5, 10, and 20 μM, respectively (Figure 3b). Similarly, the percentage of NCI-H292 cells in the G0/G1 phase increased from 43.13% (control) to 53.76%, 52.57%, and 58.69% upon the treatment with EB30 2.5, 5, and 10 μM, respectively (Figure 3b). Together, these results show that EB30 induces cell cycle arrest in A549 and NCI-H292 cells at the S and G0/G1 phases, respectively.
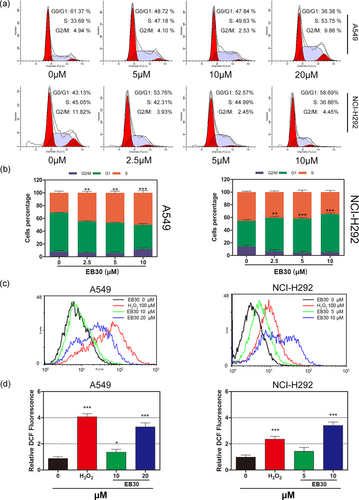
EB30 induces cell cycle arrest in A549 and NCI-H292 cells. (a) A549 and NCI-H292 cells were treated with EB30 at 5, 10, and 20 μM and 2.5, 5.0, and 10 μM for 24 hr, propidium iodide staining and flow cytometry analysis were used to illustrate the distribution of each phase. (b) Representative percentage of each cell cycle for A549 and NCI-H292 cells after exposure to EB30. Data are expressed as mean ± SD, n = 3 (*p < 0.05, **p < 0.01, and ***p < 0.001), compared with control group. EB30 accelerates the generation of intracellular ROS in A549 and NCI-H292 cells. (c) A549 cells were treated with EB30 at 10 and 20 μM, and NCI-H292 cells were treated with EB30 at 5 and 10 μM for 24 hr. The fluorescence obtained by the oxidation of DCFH-DA was determined by flow cytometry. (d) The relative fluorescence intensity is presented as mean ± SD of three independent experiments (*p < 0.05, **p < 0.01, and ***p < 0.001), compared with control group. H2O2 treatment was used as positive control [Color figure can be viewed at wileyonlinelibrary.com]
3.5 EB30 induces the generation of ROS
ROS overproduction is strongly associated with apoptosis. To clarify whether ROS may be involved in the process of apoptosis induced by EB30, flow cytometry was used to determine cellular ROS levels. As shown in Figure 3c,d, high concentrations of EB30 promoted the generation of ROS. The level of ROS was significantly elevated in the NCI-H292 cell line compared with the A549 cell line. Cells treated with 100 μM H2O2 were used as a positive control.
3.6 EB30-induced cell apoptosis is mediated through intrinsic and extrinsic pathways in A549 and NCI-H292 cells
Mitochondrial outer membrane permeabilization and MMP depolarization occur during the early stages of cell apoptosis and, subsequently, result in the release of proapoptotic proteins, including cytochrome c and AIF. JC-1, a fluorescent probe, was used to detect the loss of the MMP in A549 and NCI-H292 cells. As shown in Figure 4a, the JC-1 staining of the control groups of A549 and NCI-H292 cells exhibited higher red/green fluorescence ratios, whereas the fluorescence ratio markedly decreased in the high EB30-treated group. Cells treated with CCCP showed a lower red/green fluorescence ratio and were used as a positive control. Based on these findings, we propose that EB30-induced mitochondrial depolarization in a concentration-dependent manner and the collapse of the MMP in these two cancer cell lines.
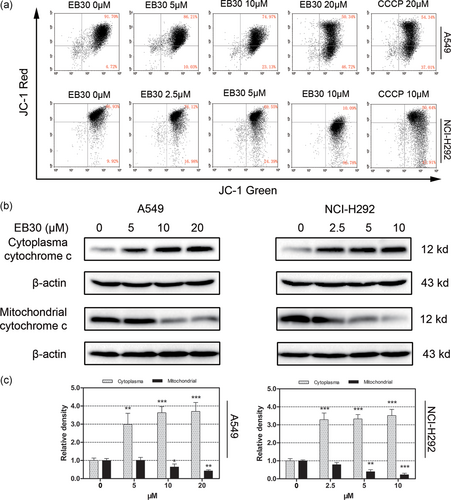
Effects of EB30 on mitochondrial membrane potential in A549 and NCI-H292 cells. (a) Flow cytometry detection of MMP in A549 and NCI-H292 cells in response to EB30. Cell populations indicating JC-1 aggregates are in the upper right quadrant and the JC-1 monomer is represented in the lower right part. The relative signal intensities JC-1 aggregates were normalized to JC-1 monomers and presented as a percentage compared with values for control cells. (b) The cytosolic extracts and mitochondrial fraction were analyzed by immunoblotting to detect mitochondrial cytochrome c release. β-actin was used as a loading control. (c) Results were from three separate experiments. Values are expressed as mean ± SD, n = 3 (*p < 0.05, **p < 0.01, and ***p < 0.001), compared with control group [Color figure can be viewed at wileyonlinelibrary.com]
Cytochrome c release from the mitochondria to the cytoplasm with mitochondrial membrane dysfunction. Western blot analysis demonstrated that EB30-induced upregulation of cytochrome c in the cytoplasm and downregulated cytochrome c in the mitochondria (Figure 4b,c).
AIF is generally located in the inner mitochondrial membrane and can translocate from the cytosol to the nucleus upon stimulation by various apoptotic factors. Free AIF contributed to chromatin condensation and oligonucleosomal DNA fragmentation. As shown in Figure 5b, after the treatment of 24 hr, AIF significantly increased inside the nucleus in EB30-treated cells compared with control cells in both cell lines. Western blot data also suggested that treatment with EB30 induced high expression levels of AIF and cleaved AIF (Figure 5a).
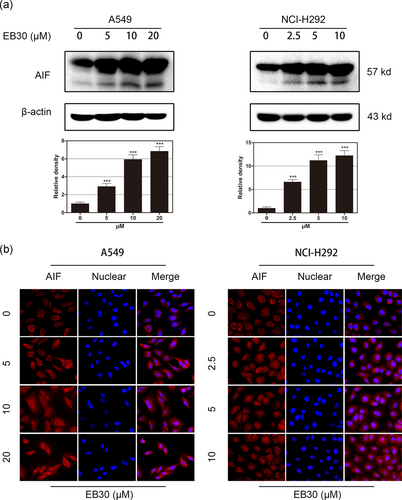
EB30 promotes AIF release. (a) Effects of EB30 treatment on AIF protein levels in A549 and NCI-H292 cells. The cell lysates were subjected to immunoblot analysis for AIF. β-Actin was used as a loading control. Results were from three separate experiments. Values are expressed as mean ± SD, n = 3 (*p < 0.05, **p < 0.01, and ***p < 0.001), compared with control group. (b) A549 cells were treated with EB30 at 5, 10, and 20 μM and NCI-H292 cells were treated with EB30 at 2.5, 5, and 10 μM for 24 hr. Stained with anti-AIF antibody and viewed by fluorescent microscopy [Color figure can be viewed at wileyonlinelibrary.com]
The flow cytometry results demonstrated that mitochondrial depolarization was involved in EB30-induced apoptosis. Bcl-2 family proteins play critical roles in the mitochondria-dependent apoptosis pathway. Hence, immunoblot analysis was used to measure Bcl-2 family protein expression. As shown in Figure 6a, the EB30 treatment induced robust upregulation of the antiapoptotic protein Bcl-2 and Bcl-xL and downregulation of the proapoptotic protein Bax in both cell lines.
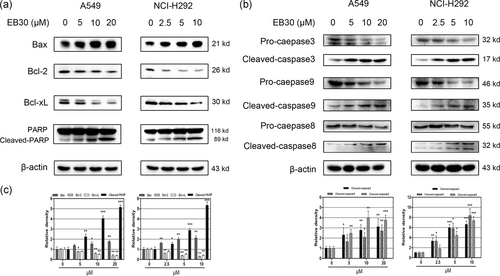
Effects of EB30 on apoptosis-associated proteins in A549 and NCI-H292 cells. Cells were incubated with EB30 for 24 hr, cell lysates were subjected to immunoblotting for Bax, Bcl-2, Bcl-xL,and PARP (a); caspase-3, caspase-9, and caspase-8 (b). (c) Results were from three separate experiments. Values are expressed as mean ± SD, n = 3 (*p < 0.05, **p < 0.01, and ***p < 0.001), compared with control group
A decrease in the Bax/Bcl-2 ratio regulates the delivery of cytochrome c, which further facilitates caspase activation. Thus, we examined whether EB30 could initiate caspase activation. As shown in Figure 6b, the expression levels of caspase-3, caspase-9, and PARP (Figure 6a) were determined by western blot. The EB30 treatment increased the expression of cleaved caspase-3 and cleaved caspase-9. The expression of PARP is highly correlated with DNA injury and repair. In our study, cleavage of PARP was observed in both cell lines treated with EB30. To confirm whether the extrinsic apoptosis pathway is involved in EB30-induced cell apoptosis, caspase-8 was detected. Interestingly, the expression of cleaved caspase-8 was strongly upregulated in the EB30 treatment groups, whereas the level of pro-caspase-8 did not significantly vary with the EB30 treatment. Our results indicated that EB30-induced lung cancer cell apoptosis through intrinsic and extrinsic pathways.
3.7 EB30-induced cell apoptosis via regulating the PI3K/Akt and ERK1/2 pathways
PI3K/Akt and ERK1/2 cell signaling are among the most important intracellular pathways and are closely related to cancer occurrence and cell survival (Janku et al., 2012). Therefore, the searching for effective PI3K/Akt ERK1/2 inhibitors has received significant attention. The level of p-Akt was much lower in the EB30-treated groups (Figure 7a) than in the untreated groups. However, clear stimulation of p-ERK was observed in the EB30-treated groups (Figure 7b). Similarly, the levels of p-P70S6K and p-P90RSK, which are downstream molecules of Akt and ERK1/2, were broadly consistent with these trends (Figure 7c,d). Notably, the expression of p-P90RSK had no significant increase in NCI-H292 cells (Figure 7d), and the protein levels of Akt and t-ERK showed no substantial changes. To further validate the involvement of the PI3K/Akt pathway in the proapoptotic effect of EB30, LY294002 (20 μM, a specific PI3K inhibitor), and U0126 (20 μM, a MEK inhibitor) were used. As shown in Figure 8a,b, EB30 alone decreased both A549 and NCI-H292 cell viability by more than 50% (p < 0.01 and p < 0.001, respectively). Compared with the effects of each pharmacological inhibitor alone, the combination of EB30 with LY294002 or U0126 induced synergistic antiproliferative effects against lung cancer cells (P < 0.001). Based on those results, we investigated the expression of signaling proteins in A549 and NCI-H292 cells treated with EB30 and signaling inhibitors by western blot analyses. The results (Figure 8c,e) showed that LY294002 significantly enhanced the decreases in p-Akt and p-P70S6K induced by EB30, and U0126 weakly enhanced the synergistic action. However, the combination of U0126 with EB30 almost completely blocked the phosphorylation of ERK and P90RSK induced by EB30 (Figure 8d,f). LY294002 only slightly decreased p-ERK and p-P90RSK due to EB30. These results indicated that EB30 regulated lung cancer cell proliferation and transcription via the PI3K and ERK1/2 signaling pathways.
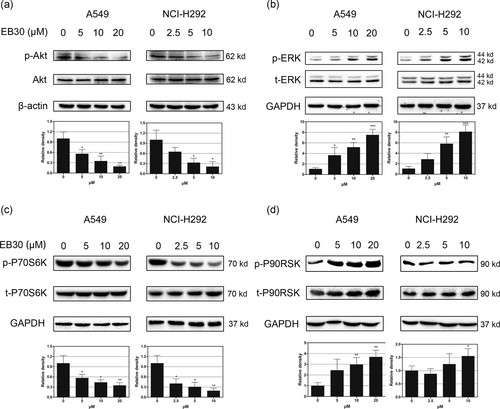
Effects of EB30 on signal transduction cascades in A549 and NCI-H292 cells. EB30 regulated the expression of phosphorylation of AKT (a), ERK1/2 (b), P70S6K (c), and P90RSK (d) in dose-dependent manner, with respect to EB30 at 0, 5, 10, and 20 μM in A549 cells and 0, 2.5, 5, and 10 μM in NCI-H292 cells. Results were from three separate experiments. Values are expressed as mean ± SD, n = 3 (*p < 0.05, **p < 0.01, and ***p < 0.001), compared with control group
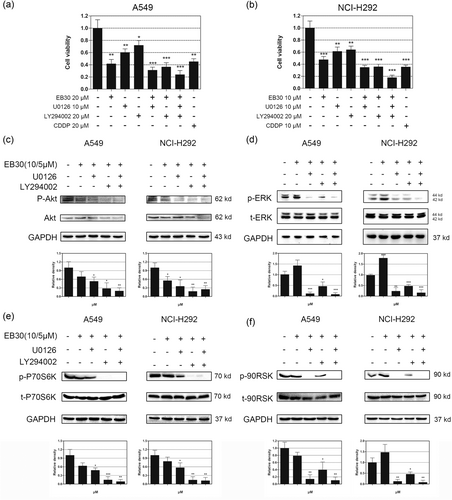
EB30 suppresses proliferation of A549 and NCI-H292 cells in concert with pharmacological inhibitors of cell signaling. Starved A549 (a) and NCI-H292 (b) cells were treated with EB30 (20/10 μM) and LY294002 (20 μM) or U0126 (10 μM) or their combinations for 48 hr. Cell viability was estimated by CCK-8 assays, and data are expressed as a percentage relative to nontreated control A549 and NCI-H292 cells (100%). Values are expressed as mean ± SD, n = 3 (*p < 0.05, **p < 0.01, and ***p < 0.001), compared with control group. Synergetic effects of EB30 and pharmacological inhibitors of cell signaling molecules in A549 and NCI-H292 cells. Starved A549 and NCI-H292 cells were preincubated in serum-free medium with LY294002 (20 μM) and U0126 (10 μM) for 1 hr and then incubated with 10 μM and 5 μM EB30 for 1 hr, respectively. Changes in phosphorylation of Akt (c), ERK1/2 (d), P70S6K (e), and P90RSK (f) treatment with or without EB30, PI3K/AKT and ERK1/2 inhibitors were determined by western blot analyses. Results were from three separate experiments. Values are expressed as mean ± SD, n = 3 (*p < 0.05, **p < 0.01, and ***p < 0.001), compared with control group
4 DISCUSSION
Lung cancer accounts for 19% of cancer-related deaths (Torre et al., 2015). Patients receiving long-term chemoradiation treatment eventually develop resistance to multiple chemotherapeutics, which severely limits therapeutic efficiency (de Bruin & Medema, 2008). Thus, there is an urgent need to develop efficient and safe antitumor agents.
Curcumin can inhibit lung adenocarcinoma cell invasion and metastasis through induction of c-Jun NH(2)-kinase (JNK) phosphorylation and activation of the tumor suppressor DnaJ-like heat shock protein 40 (HLJ1; Chen et al., 2008). A previous study documented that curcumin causes endoplasmic reticulum (ER) stress and mitochondrial-dependent-induced apoptosis through activation of caspase-3 in human lung cancer (Lin et al., 2008). Several groups have demonstrated that curcumin regulates cancer cell apoptosis by inhibiting PI3K/Akt (Reuter, Eifes, Dicato, Aggarwal, & Diederich, 2008). Therefore, we isolated EB30, a structurally related compound from V. coloratum.
Apoptosis, known as programmed cell death, occurs in both physiological and pathological conditions (Shimada et al., 2012). Mitochondrial functions are strongly associated with cell proliferation and apoptosis (Greenfield et al., 2017). The Bcl-2 protein family regulates mitochondrial-mediated apoptosis by inducing loss of the MMP, followed by the release of cytochrome c into the cytosol (Pena-Blanco & Garcia-Saez, 2017). Then, caspase-9 and caspase-3, an apoptosis executioner, are activated by cytochrome c in the cytosol (Hengartner, 2000; Tait & Green, 2010). The extrinsic apoptotic pathway involves binding of the Fas ligand (FasL) to the Fas receptor (FasR; also termed CD95; Ashkenazi, 1998). Caspase-8 is the executor of extrinsic apoptosis, and activation of caspase-8 directly induces cleavage of caspase-3; consequently, activated capase-3 cleaves DNA molecules, leading to apoptosis (Houston, 2004). The current study indicated that EB30-induced depolarization of the MMP, increased the ratio of Bax/Bcl-2 and promoted cytochrome c translocation from the mitochondria to the cytosol. In the present work, caspase-3, caspase-9, and caspase-8 were dramatically activated.
It has been shown that activation of the PI3K/Akt signaling pathway inhibits mitochondrial-dependent apoptosis (Cardone, 1998). MAPK/ERK1/2 and PI3K/Akt signaling are significantly correlated with tumorigenesis, playing central roles in cell growth, apoptosis, migration, differentiation, and metabolism (Burotto, Chiou, Lee, & Kohn, 2014; Datta, Brunet, & Greenberg, 1999). CT120, a novel membrane-associated gene, has been found to be overexpressed in most primary lung cancer tissues. CT120 was considered to be a key modulator of lung carcinogenesis by activating the ERK1/2 signaling pathway (He et al., 2004). Enhancer of zeste homolog 2 (EZH2) is the catalytic subunit of polycomb repressive complex 2. Overexpression of EZH2 in lung cancer is indicative of poor outcomes. A previous study suggested that EZH2 expression is regulated by activation of the MEK-ERK and PI3K/Akt signaling pathways (Riquelme et al., 2016). Simultaneously, a growing number of studies has indicated that overexpression of Akt results in drug resistance of non-small-cell lung cancer (NSCLC) cells and causes ovarian cancer cells to be more highly resistant to paclitaxel (Lu, Shi, Wang, Gu, & Zeng, 2011; Page et al., 2000). Therefore, the PI3K/Akt and ERK1/2 signaling pathways were investigated.
Our results indicated that EB30-induced cell apoptosis by suppressing the PI3K/Akt pathway, whereas activating ERK1/2 signaling (Figure 9). A previous study demonstrated the dual potential of ERK in regulating cell viability. Transient upregulation of ERK is believed to be involved in apoptosis (Ishikawa & Kitamura, 1999). Moreover, apigenin induced cell death via activating the ERK1/2 MAPK signaling pathway (Lim, Park, Bazer, & Song, 2016). Inhibition of mTORC1 led to activation of the MAPK pathway via a PI3K-dependent feedback loop (Carracedo et al., 2008). Furthermore, PI3K/Akt/mTOR pathway inhibitor treatment activated ERK1/2 expression in acute myeloblastic leukemia (AML) cells (Su et al., 2017). U0126 and LY294002 were tested to better elucidate the involvement of the signaling pathway. The combination of EB30 with U0126 or LY294002 not only inhibited the corresponding pathway but also slightly suppressed the expression of p-Akt and p-ERK. The levels of P70S6K and P90RSK, which are downstream targets of Akt and ERK, were altered in the same direction as their upstream proteins. These results indicated that cross-regulation exists between the PI3K and MAPK pathways in lung cancer cells in response to EB30. Together, these results suggest that EB30 may be a potent anticancer agent. However, additional preclinical and clinical trials are required for further validation of antitumor effects of EB30 applicable to patients with cancer in the future.
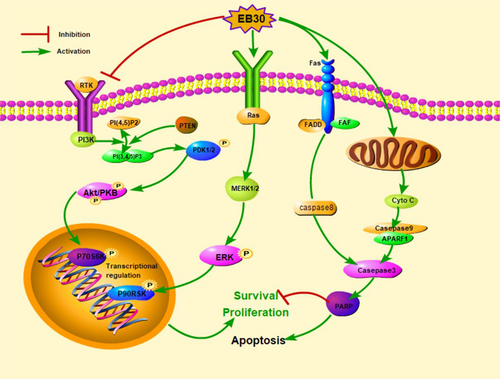
Schematic diagram showing the proposed signaling pathways involved in EB30-induced apoptosis in A549 and NCI-H292 cells [Color figure can be viewed at wileyonlinelibrary.com]
ACKNOWLEDGEMENT
This study was supported by the Science and Technology Development Project of Shandong Province (2015GGH318004).
CONFLICTS OF INTEREST
Authors declare that there are no conflicts of interest in this study.




